Abstract
Insulin-like growth factors (IGFs) stimulate myoblast proliferation and differentiation. It remains elusive how these mutually exclusive cellular responses are elicited by the same growth factor. Here we report that whereas IGF promotes myoblast differentiation under normoxia, it stimulates proliferation under hypoxia. Hypoxia activates the HIF-1 transcriptional program and knockdown of HIF-1α changes the mitogenic action of IGF into myogenic action under hypoxia. Conversely, overexpression of HIF-1α abolishes the myogenic effect of IGF under normoxia. Under normoxia, IGF activates the Akt-mTOR, p38, and Erk1/2 MAPK pathways. Hypoxia suppresses basal and IGF-induced Akt-mTOR and p38 activity, whereas it enhances and prolongs IGF-induced Erk1/2 activation in a HIF-1–dependent fashion. Activation of Akt-mTOR and p38 promotes myogenesis, and p38 also inhibits proliferation. Activation of Erk stimulates myoblast proliferation but inhibits differentiation. These results suggest that hypoxia converts the myogenic action of IGFs into mitogenic action by differentially regulating multiple signaling pathways via HIF-1-dependent mechanisms. Our findings provide a mechanistic explanation for the paradoxical actions of IGFs during myogenesis and reveal a novel mechanism by which cells sense and integrate growth factor signals and oxygen availability in their microenvironments.
Keywords: Akt, hypoxia-inducible factor, muscle cells, MAPK, mTOR
Insulin-like growth factors (IGFs) play critical roles in skeletal muscle differentiation and growth. IGF ligand or receptor knockout mice exhibit muscle hypoplasia (1). Mice with muscle specific overexpression of IGF1 have larger muscle fibers and enhanced muscle strength as they age (2). During myogenesis, the expression of IGFs in muscles, particularly IGF-II, increases dramatically (3) due to the auto-regulation by IGF-II of its own gene expression (4). In vitro, IGF-II antisense oligonucleotides abolish, and IGF-II overexpression accelerates myoblast differentiation (1, 5). Intriguingly, IGFs have been shown to not only promote myogenic differentiation but also stimulate myoblast proliferation in cultured myoblast cells, and these actions are mediated through the same IGF-I receptor (IGF1R) (1, 6). The IGF1R uses two major intracellular signaling pathways, namely the phosphatidylinositol 3-kinase (PI3K)–Akt cascade and the Raf-MEK-mitogen–activated protein kinase (MAPK)–extracellular signal-regulated kinase (Erk) cascade (7). Several laboratories have reported that activation of the PI3K-Akt-mTOR pathway promotes myogenic differentiation (3, 4, 8). Despite extensive knowledge of the intracellular cascades that transmit the IGF signal to the nucleus, it remains elusive as to how the IGF ligands, acting through the same IGF1R, stimulate two mutually exclusive cellular responses.
In multicellular organisms, cell growth, proliferation, differentiation, and survival are not only regulated by hormonal or growth factor signaling, but are also influenced by the availability of oxygen and nutrients in their microenvironments. Adaptation to low oxygen, or hypoxia, is a critical event for cells under numerous physiological and pathological situations. Hypoxia-inducible factor–1 (HIF-1) regulates many of the hypoxic responses (9). HIF-1 is composed of a stable β subunit and an oxygen-regulated α subunit (10). The HIF-1 complex binds to hypoxia-response elements (HREs) of its target genes and regulates their transcription. Recent studies have shown that hypoxia influences proliferation and differentiation of various stem/progenitor cell populations in mammals, in addition to its well-established roles in altering cellular energy metabolism and angiogenesis (11).
In this study, we tested the hypothesis that oxygen availability plays a critical role in specifying the cellular responses to IGF signaling in skeletal muscle cells. We show that whereas IGF signaling promotes muscle cell differentiation under normoxia, it stimulates proliferation under hypoxia. Our further analyses revealed that the activated HIF-1 complex and its transcriptional program converts the myogenic action of IGFs into mitogenic action, and it does so by differentially regulating multiple signaling pathways.
Results
Hypoxia Changes Myogenic Action of IGFs into Mitogenic Action.
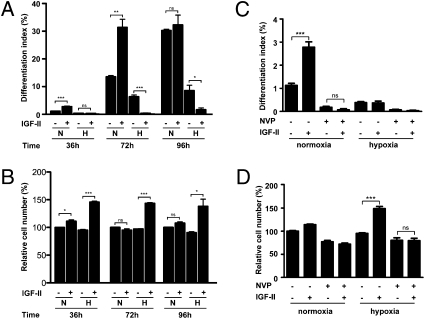
The effects of IGF-II on muscle cell differentiation and proliferation in differentiating C2C12 myoblasts were examined under normoxia (N) and hypoxia (H). 36 h treatment with IGF-II caused a 2.5-fold increase (P < 0.001) in the differentiation index (Fig. 1A and Fig. S1A) and an 11.2% increase (P < 0.05) in total cell number under normoxia (Fig. 1B). Under hypoxia, 36-h treatment with IGF-II had no effect on differentiation but caused a 45.7%, highly significant increase (P < 0.001) in total cell number (Fig. 1B). This IGF-II-induced increase in cell number under hypoxia was mostly due to elevated cell proliferation, as indicated by a significant increase in BrdU labeling (Fig. S1B). At 72 h, IGF-II treatment caused a 2.32-fold increase (P < 0.01) in cell differentiation but had no effect on cell number under normoxia (Fig. 1A). Under hypoxia, IGF-II actually strongly suppressed differentiation (P < 0.001). IGF-II increased the cell number by 47.6% (P < 0.001) at this time point (Fig. 1B). Cell differentiation reached its maximal levels at 96 h under normoxia, and IGF-II did not cause a further increase, but it still suppressed cell differentiation under hypoxia (Fig. 1A). At this time point, IGF-II caused a significant increase in cell number under hypoxia (Fig. 1B). Addition of NVP-AEW541, a specific IGF1R inhibitor (12), abolished the IGF-II–induced increase in differentiation (Fig. 1C) under normoxia, as well as the IGF-II–induced increase in cell number under hypoxia (Fig. 1D). These results indicate that whereas IGF-II promotes myogenic differentiation under normoxia, it stimulates myoblast proliferation under hypoxia by activating the same IGF1R. This is not unique to IGF-II, because we observed a similar effect of hypoxia in changing IGF-I from a myogenic signal into a mitogenic one in these cells (Fig. S2).
Fig. 1.
Hypoxia converts the myogenic action of IGF-II into mitogenic action. (A and B) C2C12 myoblasts were induced to differentiate by switching to differentiation medium supplemented with or without IGF-II (300 ng/mL). After culturing under normoxic (N) or hypoxic (H) conditions for 36, 72, and 96 h, cells were fixed and the differentiation index, defined as the percentage of MHC-positive cells, was determined (A). Total cell number was also quantified and shown in B. Data are mean ± SE, n = 3–9. (C and D) IGF1R inhibitor NVP-AEW541 abolishes myogenic action (C) and mitogenic action (D) of IGF-II. Cells were cultured in differentiation medium supplemented with or without IGF-II (300 ng/mL) and/or NVP-AEW541 (1 μM) under normoxic or hypoxic conditions for 36 h. Data are means ± SE, n = 4–9. *P < 0.05, ** P < 0.01; *** P <0.001. ns, Not significant.
Hypoxia Alters Cellular Responses to IGFs Through HIF-1–Dependent Mechanisms.
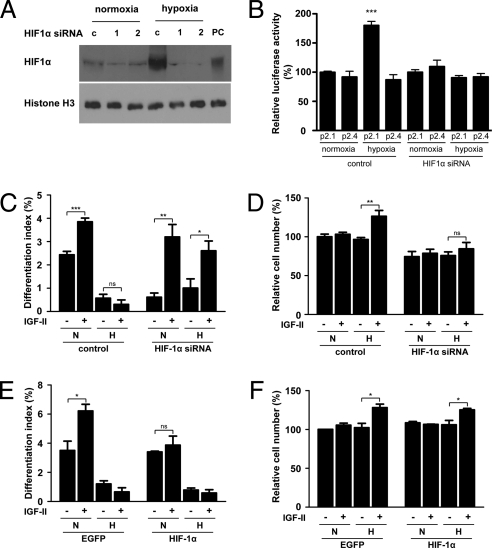
Hypoxia activates the HIF-1 complex in differentiating C2C12 myoblasts, as indicated by increased nuclear HIF-1α levels, increased HIF-1–dependent transcription activity, and increased HIF-1 target gene expression (Fig. S3). To determine whether the effect of hypoxia in specifying IGF actions is dependent on HIF-1, an HIF-1α siRNA construct was generated. Introduction of this construct into cultured myoblasts resulted in a marked reduction in the levels of nuclear HIF-1α protein under hypoxia, whereas the control vector had no such effect (Fig. 2A). We further tested the effectiveness of the HIF-1α siRNA by co-transfecting it with a HIF-1 reporter construct, p2.1. Whereas hypoxia caused a significant increase in the HIF-1 reporter gene expression in the control siRNA transfected cells, this increase was abolished in the HIF-1α siRNA transfected cells (Fig. 2B). No difference was seen in the control reporter gene expression (Fig. 2B). Next, the possible role of HIF-1 in specifying IGF actions was examined. In cells transfected with the control vector, IGF-II increased differentiation only under normoxia (Fig. 2C). In the HIF-1α knockdown cells, IGF-II caused a significant increase in the differentiation index under both normoxia and hypoxia (Fig. 2C). Whereas IGF-II caused a significant increase (38%, P < 0.01) in cell number under hypoxia in the control cells, this effect was abolished in the HIF-1α knockdown cells (Fig. 2D), which suggests that HIF-1α is required for converting the myogenic action of IGF-II into mitogenic action under hypoxia. We next tested the effect of HIF-1α overexpression on IGF actions. In the control vector transfected cells, IGF-II increased differentiation under normoxia but not hypoxia (Fig. 2E). Overexpression of HIF-1α did not change basal differentiation levels, but it abolished the IGF-II–induced increase in differentiation under normoxia (Fig. 2E). There was no significant difference in the cell number between the control and HIF-1α vector transfected cells in the presence or absence of IGF-II (Fig. 2F). These data suggest that HIF-1 plays a critical role in specifying IGF-II actions in C2C12 myoblast cells.
Fig. 2.
Effect of hypoxia in changing IGF actions is HIF-1–dependent. (A) Knockdown of HIF-1α by siRNA. Two days after transfection with 2 μg pSUPER (c) or pSUPER HIF-1α plasmid (1 and 2), cells were subjected to hypoxia for 8 h. Cells treated with CoCl2 were used as positive control (PC). Nuclear fraction was prepared from normoxia and hypoxia groups and analyzed by Western blot. (B) HIF-1α siRNA abolishes HRE-dependent expression. C2C12 myoblasts were transfected with pSUPER (control) or pSUPER HIF-1α together with the HIF-1 reporter gene, p2.1, or the control p2.4 gene (which has a mutated HRE) and were grown under normoxia or hypoxia. At 24 h later, cells were lysed and used for luciferase activity assay. Data are means ± SE, n = 4. (C and D) Knockdown of HIF-1α abolishes mitogenic action of IGF-II but restores myogenic response to IGF-II under hypoxia. Cells transfected with pSUPER or pSUPER HIF-1α plasmid were cultured in differentiation medium with or without IGF-II (300 ng/mL) under normoxic (N) or hypoxic (H) conditions. Differentiation index (C) and total cell number (D) were determined. Data are mean ± SE, n = 4. (E and F) Overexpression of HIF-1α abolishes myogenic response to IGF-II under normoxia. Cells transfected with control or HIF-1α expression plasmid were cultured in differentiation medium with or without IGF-II (300 ng/mL) under normoxic or hypoxic conditions. Differentiation index and total cell number were determined. Data are mean ± SE, n = 3.
Hypoxia Inhibits Myogenic Action of IGF by Suppressing Akt-mTOR Signaling.
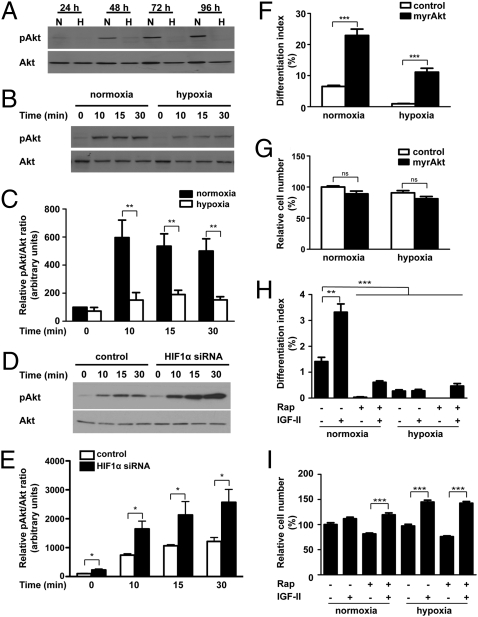
As mentioned above, IGF promotes myogenic differentiation via the PI3K-Akt-mTOR pathway. We therefore examined the possible impact of hypoxia on this signaling pathway. Whereas the levels of phospho-Akt increased as differentiation progressed under normoxia, they remained extremely low under hypoxia (Fig. 3A). Total Akt levels were also decreased under hypoxia, but the reduction in phospho-Akt levels caused by hypoxia was much more pronounced. Hypoxia also suppressed mTOR activity in differentiating C2C12 myoblasts (Fig. S4). We next examined the IGF-induced Akt signaling activity by stimulating cells with a low dose of IGF-I, which is a more potent ligand for the IGF1R. IGF-I stimulation resulted an increase in Akt phosphorylation at 10 min, and this activation lasted for at least 30 min under normoxia. Under hypoxia, the degree of Akt activation was significantly diminished (P < 0.01) (Fig. 3 B and C). These results suggest that hypoxia represses basal as well as IGF-induced Akt-mTOR activation in differentiating myoblasts. To determine whether this reduced Akt activation under hypoxia is HIF-1–dependent, cells transfected with control or HIF-1α siRNA were examined. As shown in Fig. 3 D and E, the levels of IGF-induced phosphorylated Akt in the HIF-1α knockdown cells were significantly higher than those of the control cells under hypoxia. These results show that hypoxia represses basal and IGF-stimulated Akt signaling activity via a HIF-1–mediated mechanism.
Fig. 3.
Hypoxia inhibits myogenic action of IGF-II by suppressing Akt-mTOR signaling through HIF-1–dependent mechanisms. (A) Hypoxia represses Akt signaling activity. C2C12 cells were induced to differentiate under normoxic or hypoxic conditions. Total and phosphorylated Akt levels were determined by Western blot at the time points indicated. (B and C) Hypoxia decreases IGF-induced Akt activation. After switching to DM for 72 h under normoxic or hypoxic conditions, IGF-I (50 ng/mL) was added. Cell lysates were harvested at the time points indicated and blotted for phospho- and total Akt (B). The phospho-Akt/total Akt ratio was quantified (C). Values are expressed as relative to that of the 0-h normoxia group. Data are mean ± SE, n = 6. (D and E) Effect of hypoxia on Akt activation is HIF-1-–dependent. C2C12 cells were transfected with pSUPER or pSUPER HIF-1α and switched to differentiation medium and incubated under hypoxia. After 72 h, cells were stimulated with IGF-I (50 ng/mL), and lysates were collected at different time points as indicated. Western blots were quantified (E). Values are expressed as relative to that of the 0-min control group. Data are mean ± SE, n = 4. (F and G) Expression of myrAkt restores myogenesis under hypoxia. C2C12 cells were transfected with control or myrAkt plasmid and induced to differentiate under normoxic or hypoxic conditions, and differentiation index (F) and cell number (G) were measured. Data are mean ± SE, n = 4. (H and I) Inhibition of mTOR activity by rapamycin decreases the myogenic action of IGF-II. Cells were induced to differentiate in the absence or presence of IGF-II (300 ng/mL) and/or rapamycin (200 nM) under normoxic or hypoxic conditions. After 36 h, cells were fixed and the differentiation index (H) and cell number (I) were determined. Data shown are means ± SE, n = 4–6.
We postulated that this repression down-regulates mTOR activity and thereby suppresses the myogenic action of IGFs. To test this idea, myrAkt, a constitutively active form of Akt, was introduced into C2C12 cells. Overexpression of myrAkt alleviated the hypoxia-induced reduction in mTOR signaling activity (Fig. S4). Hypoxia decreased the differentiation index by 7.0-fold (P < 0.05 compared with the normoxia control), whereas expression of myrAkt restored differentiation to a level comparable to the normoxia control group (Fig. 3F). Expression of myrAkt also increased the differentiation index by 3.5-fold (P < 0.001) under normoxia. However, myrAkt expression did not result in any increase in cell number (Fig. 3G). Rapamycin inhibited basal as well as myrAkt-induced S6 phosphorylation (Fig. S4). These results indicate that myrAkt rescues myoblast differentiation under hypoxic conditions via restoration of mTOR activity.
Next, we inhibited mTOR activity using rapamycin. Under normoxia, IGF-II increased cell differentiation by 2.4-fold (P < 0.01) (Fig. 3H). Rapamycin abolished the differentiation-promoting effect of IGF-II and reduced basal differentiation levels (P < 0.001) (Fig. 3H). Thus, mTOR suppression abolishes the myogenic action of IGF-II. Of note, the group treated with IGF-II and rapamycin had a higher differentiation index value compared with the group treated with rapamycin alone (0.61% vs. 0.03%, P < 0.01), suggesting potential contributions by other signaling pathways. As shown in Fig. 3I, rapamycin caused a modest decrease in basal cell number under both normoxia and hypoxia, but it had little effect on the IGF-II–induced increase in cell number under hypoxia. These results suggest that the reduced Akt-mTOR signaling activity is responsible for the loss of myogenic action of IGF-II under hypoxia.
Hypoxia Promotes Mitogenic Action and Inhibits IGF Myogenic Action by Altering Erk1/2 and p38 MAPK Activities.
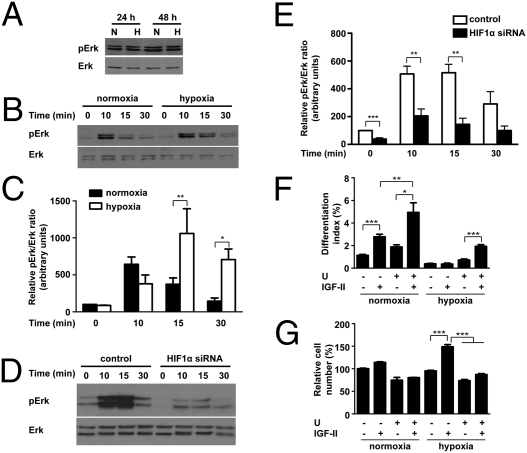
Unlike the case of Akt, hypoxia did not decrease basal Erk1/2 MAPK signaling activity (Fig. 4A). Under normoxia, IGF stimulation caused a rapid and transient increase in phospho-Erk1/2 levels (Fig. 4B). Under hypoxia, the levels of phosphorylated Erk1/2 induced by IGF-I were significantly higher at 15 min (2.8-fold, P < 0.01). At 30 min, the Erk1/2 activity returned to basal levels under normoxia, but it remained significantly elevated under hypoxia (Fig. 4C). The increased Erk activation under hypoxia was HIF-1–dependent, because knockdown of HIF-1α caused significant reduction in Erk activation at 10 min and 15 min (Fig. 4 D and E).
Fig. 4.
Hypoxia promotes mitogenic action of IGFs by enhancing Erk1/2 MAPK signaling. (A) Hypoxia does not repress Erk1/2 activity during myogenesis. C2C12 cells were induced to differentiate under normoxic (N) or hypoxic (H) conditions. Total and phosphorylated Erk1/2 levels were determined at time points indicated. (B and C) Hypoxia prolongs IGF-induced Erk1/2 activation. After induction of differentiation for 72 h under normoxic or hypoxic conditions, cells were subjected to IGF-I treatment. Cell lysates were harvested at time points indicated, and phospho-Erk/total Erk1/2 ratio was quantified (C). Values are expressed as relative to that of 0-h normoxia group. Data are mean ± SE, n = 4–6. (D and E) Effect of hypoxia on Erk activation is HIF-1–dependent. C2C12 cells were transfected with pSUPER or pSUPER HIF-1α, switched to differentiation medium, and incubated under hypoxia. After 72 h, cells were stimulated with IGF-I (50 ng/mL), and lysates were collected at different time points as indicated. Western blots were quantified (E). Values are expressed as relative to that of the 0-min control group. Data are mean ± SE, n = 4. (F and G) Inhibition of Erk1/2 activity by U0126 (10 μM) enhances the myogenic action but abolishes the mitogenic action of IGF-II. Data are means ± SE, n = 4–6.
U0126, an Erk1/2 inhibitor, was used to investigate the role of Erk1/2. IGF-II significantly increased myoblast differentiation under normoxia, and inhibition of Erk1/2 by U0126 resulted in a further increase in differentiation in response to IGF-II (1.8-fold, P < 0.01) (Fig. 4F). Under hypoxia, IGF-II had no myogenic effect. Inhibition of Erk1/2 by U0126, however, restored its myogenic effect. An opposite trend was observed with the mitogenic action. IGF-II caused a significant increase in cell number under hypoxia (56% increase, P < 0.001). Inhibition of Erk1/2 by U0126 not only reduced basal cell number, but also inhibited the mitogenic action of IGF-II (Fig. 4G). These data suggested that Erk1/2 MAPK mediates IGF-induced cell proliferation and inhibits the myogenic action of IGF-II.
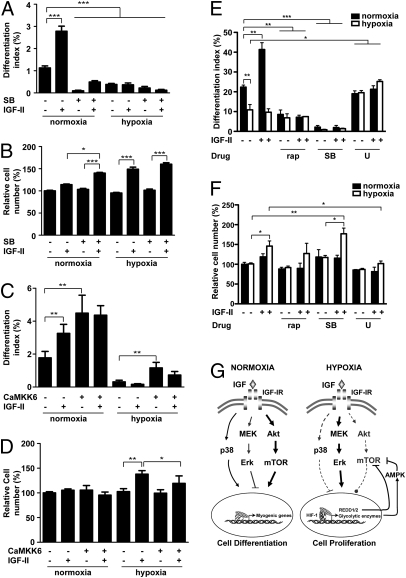
We next investigated the p38 MAPK pathway, which has been shown to play an essential role in myogenesis by activating myogenic transcription factors such as MEF2 and MyoD (13). Hypoxia caused a significant reduction in phospho-p38 MAPK levels in differentiating myoblasts (55%, P < 0.001; Fig. S5 A and B). IGF stimulation increased p38 MAPK activity in the early stages of myogenesis (Fig. S5 C and D). Pharmacological blockade of p38 MAPK α and β was achieved using the p38 inhibitor SB203580. Under normoxia, SB203580 inhibited the basal differentiation and blocked the myogenic action of IGF-II (Fig. 5A). Inhibition of p38 MAPK by SB203580 alone did not affect the cell number, but it increased the mitogenic effect of IGF-II under normoxia. The group treated with IGF-II and SB203580 had significantly more cells compared with the group treated with IGF-II alone (P < 0.05) (Fig. 5B). Consistent with the low p38 MAPK activity under hypoxia, addition of SB203580 did not affect basal or IGF-II-induced changes in differentiation or cell number under hypoxia (Fig. 5 A and B). To further examine the role of p38 MAPK, we overexpressed CaMKK6, a constitutively active form of MKK6 that activates p38 MAPK (14). Overexpression of CaMKK6 caused a 2.54-fold, highly significant increase in differentiation in the absence of IGF-II under normoxia and restored cell differentiation under hypoxia to a level comparable to normoxia control (Fig. 5C). Expression of CaMKK6 did not cause significant changes in cell number under normoxia, but it did inhibit the IGF-II–induced cell number increase under hypoxia (Fig. 5D). Taken together, the data show that hypoxia strongly represses p38 MAPK activity, which contributes to the conversion of the myogenic action of IGF-II into mitogenic action under hypoxia.
Fig. 5.
Hypoxia alters IGF action by suppressing p38 MAPK. (A and B) Inhibition of p38 MAPK activity by SB203580 (20 μM) abolishes myogenic action (A) but enhances mitogenic action (B) of IGF-II. Data are means ± SE, n =4–6. (C and D) Activation of p38 MAPK increases differentiation but inhibits proliferation. C2C12 cells were transfected with control plasmid or constitutively active MKK6 (CaMKK6) plasmid and induced to differentiate under normoxic or hypoxic conditions. At 24 h after differentiation was induced, differentiation index (C) and total cell number (D) were determined. Data are mean ± SE, n = 3. (E and F) Hypoxia converts myogenic action of IGF-II into mitogenic action in primary muscle cells. Primary murine skeletal myoblasts were induced to differentiate under normoxia or hypoxia with or without 100 nM rapamycin, 10 μM SB203580, or 10 μM U0126, and supplemented with or without IGF-II (400 ng/mL). At 14–21 h after induction of differentiation, cells were fixed and quantified for differentiation (E) and cell number (F). Data are mean ± SE, n = 3. (G) Schematic diagram illustrating mechanisms by which hypoxia specifies IGF actions. There are at least three signaling pathways downstream of the IGF1R: Akt-mTOR, p38 MAPK, and Erk1/2 MAPK in myoblasts. Activation of Akt-mTOR pathway strongly promotes myogenic differentiation (arrow) but has little effect on proliferation (circle). Activation of p38 MAPK promotes differentiation and inhibits proliferation (horizontal line). In contrast, activation of the Erk1/2 MAPK stimulates proliferation but inhibits differentiation. Under normoxia, binding of the IGF1R by IGF strongly activates the Akt-mTOR signaling pathway and p38 MAPK pathway (solid lines). Both Akt-mTOR and p38 MAPK positively contribute to myogenesis by up-regulating myogenic genes. IGF also activates Erk1/2 MAPK signaling, which results in a modest increase in cell number. Hypoxia alters cellular response to IGF signaling by suppressing Akt-mTOR and p38 MAPK signaling activities (broken lines). Hypoxia, through the activation of HIF-1, up-regulates REDD and several glycolytic enzymes expression, which inhibit Akt and mTOR activity. Under hypoxia, binding of the IGF-1R by IGF preferentially activates the Erk1/2 MAPK signaling pathway, which in turn stimulates cell proliferation and suppresses differentiation.
Hypoxia Converts Myogenic Action of IGFs into Mitogenic Action in Primary Cells.
Next, we tested our hypothesis in primary myoblasts isolated from neonatal mice. Primary myoblasts were induced to differentiate under normoxia or hypoxia with or without IGF-II and various pharmacological inhibitors. As shown in Fig. 5 E and F, hypoxia inhibited basal differentiation and abolished IGF-II–induced differentiation. IGF-II increased differentiation by 1.85-fold (P < 0.01) under normoxia, whereas it increased cell number under hypoxia (46%, P < 0.05). Addition of rapamycin and SB203580 decreased basal differentiation levels and abolished IGF-II–induced increases in cell differentiation under normoxia. Inhibition of Erk1/2 activity by U0126 had no effect under normoxia, but it significantly increased differentiation in the presence or absence of IGF-II under hypoxia (Fig. 5E). Addition of U0126 abolished the mitogenic effect of IGF-II under hypoxia. In comparison, inhibition of mTOR or p38 MAPK did not abolish the mitogenic effect of IGF-II (Fig. 5F). These data demonstrate the physiological relevance of the observations made in C2C12 cells.
Discussion
In this study, we show that whereas IGFs promote muscle cell differentiation under normoxia, they stimulate proliferation under hypoxia. Our genetic and pharmacological evidence suggest that the suppression of Akt and mTOR activity is responsible for the loss of the myogenic action of IGFs under hypoxia. Hypoxia strongly represses the basal and the IGF-stimulated Akt-mTOR signaling activity in differentiating myoblasts. Overexpression of the constitutively active myrAkt restores the ability of IGF to induce myogenic differentiation under hypoxia. This effect is specific to the myogenic action of IGF, as overexpression of myrAkt did not result in any significant changes in cell number. Inhibition of mTOR activity by rapamycin abolished the differentiation promoting effect of IGF under normoxia, whereas it had no effect on the IGF-induced increase in cell number. How hypoxia represses Akt signaling in muscle cells is not yet clear, but it has been reported in a number of mammalian cell types that hypoxia exerts its effect on mTOR signaling by inducing negative regulatory factors, such as REDD1 and AMPK (15, 16). In differentiating myoblasts, we also found that hypoxia increases REDD1 levels (Fig. S4). Therefore, the elevated REDD1 may inhibit mTOR signaling in muscle cells.
In addition to inhibiting Akt-mTOR, hypoxia also inhibits p38 MAPK signaling in differentiating myoblasts. Although the p38 MAPK was discovered based on its activation by stress and proinflammatory cytokines, there is now compelling evidence for its myogenic role during myogenesis (13). In this study, we observed that IGF activates p38 MAPK in the early stages of myogenesis. Pharmacological blockade of p38 MAPK inhibited the IGF-induced increase in muscle differentiation. Importantly, inhibition of p38 MAPK by SB203580 increased the mitogenic effect of IGF under normoxia, suggesting that p38 MAPK plays dual roles during myogenesis, promoting the myogenic actions of IGF while inhibiting its mitogenic actions. Consistent with the low p38 MAPK activity under hypoxia, pharmacological blockade of the p38 MAPK did not change basal or IGF-induced changes in differentiation or cell number under hypoxia. At present, there are contradictory viewpoints concerning the relationship between the PI3K-Akt and p38 MAPK pathways in muscle cells. Many studies suggest that a positive feedback loop may exist between these two pathways (17–20). Other studies, however, have suggested that inhibition of the PI3K did not affect the phosphorylation state and activity of p38 MAPK (21–24), indicating that they may be two parallel pathways that regulate common myogenic genes. Our studies have clearly demonstrated that these two pathways have overlapping yet distinct roles during myogenesis. Whereas both Akt and p38 MAPK are required for myogenic differentiation, p38 MAPK, but not Akt, affects proliferation. Taken together, these findings indicate that hypoxia strongly represses Akt-mTOR and p38 MAPK activities during myogenesis, and that the reduced activities of Akt-mTOR and p38 MAPK contribute to the altered cellular responses to IGFs.
Another key finding in this study is that hypoxia increases and/or prolongs the IGF stimulated Erk1/2 activation in differentiating myoblasts. Whereas IGF caused a rapid and transient increase in phospho-Erk1/2 levels under normoxia, it caused a greater and more sustained activation of Erk1/2 under hypoxia. It is clear that IGF preferentially activates the Erk1/2 MAPK signaling pathway under hypoxia, as both the Akt-mTOR and p38 MAPK pathways are repressed by hypoxia. This rewiring of the signaling network has important functional significance. Inhibition of Erk1/2 activity by U0126 abolishes the mitogenic action of IGFs in myoblasts. Unexpectedly, inhibition of Erk1/2 activity not only resulted in an increase in the myogenic action of IGF-II under normoxia, but also restored the myogenic action of IGF-II under hypoxia. These data suggest that the activation of the Erk1/2 signaling pathway not only stimulates myoblast proliferation, but also suppresses myogenic differentiation.
There are several possible mechanisms that could explain how hypoxia alters the ability of IGF-II to activate Erk1/2 MAPK and how the activated Erk1/2 inhibits muscle cell differentiation. A recent study by Carracedo et al. has shown that inhibition of mTOR by rapamycin leads to the activation of Erk1/2 through an IRS-1/PI3K–dependent feedback loop in human cancer cells in vitro and in vivo (25). Intriguingly, these authors also reported that rapamycin enhanced the ability of IGF-I and insulin to activate Erk1/2 MAPK in MCF7 human breast cancer cells (25). Koyama et al. have reported that IGF-I activates Erk1/2 via the formation of the Gab1 and SHP2 complex in C2C12 myoblasts under normoxia (26). Importantly, the Gab1-SHP2-Erk1/2-signaling pathway inhibits IGF-I–dependent myogenic differentiation in C2C12 myoblasts (26). It is plausible that hypoxia may alter the expression of Gab1 directly or indirectly. Future experiments are needed to determine whether hypoxia/HIF-1 regulates Gab1 gene expression in myoblasts.
Based on these findings, we propose a model of how oxygen tension determines muscle cell responses to the IGF signal (Fig. 5G). The link between hypoxia and IGF signaling illustrated in Fig. 5G may represent a normal developmental program by which muscle stem/precursor cells respond to different oxygen tensions in their microenvironments. The physiological relevance of our findings is supported by the fact that hypoxia has a similar effect in primary murine skeletal myoblasts. Our conclusion is also supported by recent in vivo findings in nonmammalian vertebrate model organisms. It has been reported that hypoxia decreases the rate of somitogenesis in zebrafish embryos through the inhibition of IGF signaling (27). The interplay between hypoxia/HIF-1 and the IGF signaling pathway unraveled in this study may also have important implications in muscle hypertrophy, atrophy, and regeneration. There is in vitro and in vivo evidence that the activation of the PI3K/Akt signaling pathway by IGFs increases skeletal muscle hypertrophy and prevents muscle atrophy (28, 29). The in vivo functional importance of the IGF1/ PI3K/Akt pathway in muscle regeneration has also been demonstrated (30, 31). Likewise, the p38 MAPK pathway is also activated in satellite cells in response to locally released soluble inflammatory factors, and promotes cell-cycle arrest (32) and terminal differentiation (33, 34). IGFs are mitogens and potent survival factors for a variety of cancer cells, and the up-regulation of IGFs is found to be positively correlated with tumor progression and metastasis (35). One aspect of the microenvironment that differs in normal tissue versus tumor tissue is oxygen tension. For example, solid tumors often have poorly formed vasculature, which is associated with local hypoxia. The presence of HIF-1 is often associated with poor prognosis and has been linked to tumor cell survival, proliferation, and migration; many HIF-1 target genes are involved in crucial aspects of cancer biology (9), and efforts are underway to develop specific molecules targeting the HIF pathway as anticancer therapeutics. Therefore, the specification of IGF actions by oxygen tension unraveled in this study may also have implications in cancer biology.
Experimental Procedures
Materials.
All chemicals were reagent grade and were purchased from Fisher Scientific unless otherwise noted. The IGF-1R inhibitor NVP-AEW541 was kindly provided by Novartis Institutes for Biomedical Research. HIF-1a expression vector and reporter genes were purchased from ATCC.
Plasmid Construction.
Mouse IGF-II, PGK1, GLUT1, and PDK1 partial cDNA were amplified by RT-PCR and cloned into the pGEM-T Easy vector (Promega). The pCS2?Akt construct, which expresses a constitutively active, membrane-localized full-length mouse Akt1 (36), was provided by Anne Vojtek, University of Michigan. pSUPER-HIF-1α was constructed following a published sequence (37). CaMKK6 was kindly provided by Jiahuai Han, Scripps Research Institute.
Cell Culture and Transfection.
The cell culture and transfection were done as previously reported (38). For differentiation experiments, the cells were washed with SFM and then transferred to differentiation medium (DM) consisting of DMEM containing 0.5–2% equine serum. Cells were subjected to hypoxia (1% O2) or normal oxygen (20% O2) in a humidified modular incubation chamber (Billups-Rothenberg). Primary murine myoblasts were isolated from 2- to 5-day-old CD1 mice (Charles River Laboratories) and were cultured following a previously reported method (4).
RT-PCR and Quantitative Real-Time PCR.
Total RNA was extracted from cells using the TriPure Reagent. After treatment with DNase, RNA was subjected to reverse transcription using SuperScript II reverse transcriptase (Invitrogen) according to the supplier's instructions. qRT-PCR was carried out as reported previously (4).
Western Immunoblot and Immunohistochemistry.
Western blot and immunohistochemical staining were carried out as previously reported (4). Cell number was quantified as the number of DAPI-stained nuclei per microscopic field of view. Differentiation index (%) was defined as the percentage of MHC positive nuclei divided by total nuclei. Differentiation index (%) for primary cells was defined as the percentage of nuclei number in differentiated myotubes (harboring more than two nuclei per myotube) divided by total nuclei number.
Luciferase Reporter Assay.
The transcriptional activity of HIF-1 was determined as described previously (39).
Proliferation Assays.
Cell proliferation was determined by bromo-2-deoxyuridine (BrdU) incorporation assay as reported previously (40). Proliferation index is defined as the percentage of BrdU-labeled nuclei divided by total nuclei.
Statistical Analysis.
Differences among groups were analyzed by Student's t test or ANOVA using Prism (GraphPad Software). Significance was accepted at P < 0.05 or better.
Supplementary Material
Acknowledgments
We are grateful to Drs. J. Han, G. Sutcliffe, and A. Vojtek for providing reagents for this work. We also thank J. Allard and E. Anderson for critical reading of this manuscript. This study was supported by National Institutes of Health Grant 2RO1HL60679 and National Science Foundation Research Grant IOB 0110864 (to C.D.) and by a Rackham Graduate Student Research Grant (to H.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909570107/DCSupplemental.
References
- 1.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 2.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Wilson EM, Tureckova J, Rotwein P. Permissive roles of phosphatidyl inositol 3-kinase and Akt in skeletal myocyte maturation. Mol Biol Cell. 2004;15:497–505. doi: 10.1091/mbc.E03-05-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren H, Yin P, Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J Cell Biol. 2008;182:979–991. doi: 10.1083/jcb.200712110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart CE, James PL, Fant ME, Rotwein P. Overexpression of insulin-like growth factor-II induces accelerated myoblast differentiation. J Cell Physiol. 1996;169:23–32. doi: 10.1002/(SICI)1097-4652(199610)169:1<23::AID-JCP3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal SM, Cheng ZQ. Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc Natl Acad Sci USA. 1995;92:10307–10311. doi: 10.1073/pnas.92.22.10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 8.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 10.Bruick RK. Oxygen sensing in the hypoxic response pathway: Regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 11.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Echeverria C, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 13.Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;252:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuenda A, Cohen P. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- 18.Conejo R, de Alvaro C, Benito M, Cuadrado A, Lorenzo M. Insulin restores differentiation of Ras-transformed C2C12 myoblasts by inducing NF-kappaB through an AKT/P70S6K/p38-MAPK pathway. Oncogene. 2002;21:3739–3753. doi: 10.1038/sj.onc.1205469. [DOI] [PubMed] [Google Scholar]
- 19.Cabane C, Coldefy AS, Yeow K, Derijard B. The p38 pathway regulates Akt both at the protein and transcriptional activation levels during myogenesis. Cell Signal. 2004;16:1405–1415. doi: 10.1016/j.cellsig.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez I, et al. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serra C, et al. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Jiang B, Ensign WY, Vogt PK, Han J. Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell Signal. 2000;12:751–757. doi: 10.1016/s0898-6568(00)00120-0. [DOI] [PubMed] [Google Scholar]
- 23.Sarker KP, Lee KY. L6 myoblast differentiation is modulated by Cdk5 via the PI3K-AKT-p70S6K signaling pathway. Oncogene. 2004;23:6064–6070. doi: 10.1038/sj.onc.1207819. [DOI] [PubMed] [Google Scholar]
- 24.Tamir Y, Bengal E. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J Biol Chem. 2000;275:34424–34432. doi: 10.1074/jbc.M005815200. [DOI] [PubMed] [Google Scholar]
- 25.Carracedo A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyama T, et al. Interaction of scaffolding adaptor protein Gab1 with tyrosine phosphatase SHP2 negatively regulates IGF-I-dependent myogenic differentiation via the ERK1/2 signaling pathway. J Biol Chem. 2008;283:24234–24244. doi: 10.1074/jbc.M803907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kajimura S, Aida K, Duan C. Insulin-like growth factor-binding protein-1 (IGFBP-1) mediates hypoxia-induced embryonic growth and developmental retardation. Proc Natl Acad Sci USA. 2005;102:1240–1245. doi: 10.1073/pnas.0407443102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rommel C, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 29.Bodine SC, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 30.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musaro A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 32.Puri PL, et al. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, et al. p38 and Extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol. 2000;20:3951–3964. doi: 10.1128/mcb.20.11.3951-3964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zetser A, Gredinger E, Bengal E. p38 Mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- 35.Clemmons DR. Modifying IGF1 activity: An approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- 36.Kohn AD, et al. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 37.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin P, Xu Q, Duan C. Paradoxical actions of endogenous and exogenous insulin-like growth factor-binding protein-5 revealed by RNA interference analysis. J Biol Chem. 2004;279:32660–32666. doi: 10.1074/jbc.M401378200. [DOI] [PubMed] [Google Scholar]
- 39.Kajimura S, Aida K, Duan C. Understanding hypoxia-induced gene expression in early development: In vitro and in vivo analysis of hypoxia-inducible factor 1-regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol Cell Biol. 2006;26:1142–1155. doi: 10.1128/MCB.26.3.1142-1155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan C, Bauchat JR, Hsieh T. Phosphatidylinositol 3-kinase is required for insulin-like growth factor-I-induced vascular smooth muscle cell proliferation and migration. Circ Res. 2000;86:15–23. doi: 10.1161/01.res.86.1.15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.