Abstract
The soil-transmitted helminths or nematodes (hookworms, whipworms, and Ascaris) are roundworms that infect more than 1 billion of the poorest peoples and are leading causes of morbidity worldwide. Few anthelmintics are available for treatment, and only one is commonly used in mass drug administrations. New anthelmintics are urgently needed, and crystal (Cry) proteins made by Bacillus thuringiensis are promising new candidates. Combination drug therapies are considered the ideal treatment for infectious diseases. Surprisingly, little work has been done to define the characteristics of anthelmintic combinations. Here, by means of quantitative assays with wild-type and mutants of the roundworm Caenorhabditis elegans, we establish a paradigm for studying anthelmintic combinations using Cry proteins and nicotinic acetylcholine receptor (nAChR) agonists, e.g., tribendimidine and levamisole. We find that nAChR agonists and Cry proteins, like Cry5B and Cry21A, mutually display what is known in the HIV field as hypersusceptibility—when the nematodes become resistant to either class, they become hypersensitive to the other class. Furthermore, we find that when Cry5B and nAChR agonists are combined, their activities are strongly synergistic, producing combination index values as good or better than seen with antitumor, anti-HIV, and insecticide combinations. Our study provides a powerful means by which anthelmintic combination therapies can be examined and demonstrate that the combination of nAChR agonists and Cry proteins has excellent properties and is predicted to give improved cure rates while being recalcitrant to the development of parasite resistance.
Keywords: Caenorhabditis elegans, crystal proteins, nAChR agonists, soil-transmitted nematodes, helminth
Intestinal roundworms or nematodes are among the most prevalent parasites of humans today, infecting upwards of 2 billion persons (1–3). The main classes of intestinal nematodes include hookworms (Ancylostoma duodenale, Necator americanus), whipworms (Trichuris trichiura), and roundworms (Ascaris lumbricoides). The cumulative impacts of these parasites are tremendous, causing anemia and growth retardation in children, low nutritional status, loss of appetite, impairment of cognitive function and mental performance (e.g., lower working memory), damage to school performance and increased absenteeism, reduction in future wage earning capacity, anemia in pregnant women, increased proportions of stillbirths/perinatal deaths/very-low-birthweight babies, increases in infant mortality, increases in maternal mortality, impaired worker productivity and productive capacity, and weak adults (2, 4–7). Infection by these parasites also indirectly results in tremendous disease burden via impairment of the immune system, leading to increased severity of HIV/AIDS (lower CD4 counts, higher viral load), increased susceptibility to malaria, increased probability of having active tuberculosis (TB) and poor response to TB vaccine, and decreased immune response/failure of vaccine against cholera (8–14). Intestinal roundworms are thus one of the great diseases of our time and play a significant role in keeping infected peoples impoverished (15).
That the effects of intestinal nematodes are more hidden than many other diseases, and that they affect the poorest peoples, have led to a lack of research and commitment for these diseases and a virtually complete lack of new drug (anthelmintic) development. Since 1981, only China has taken a new drug, tribendimidine, into human clinical trials (16). Of the handful of anthelmintics in our arsenal, just two—tribendimidine, a nicotinic acetylcholine receptor, or nAChR, agonist (17), and albendazole, a benzimidazole—are adequate for single-dose mass drug administrations (MDAs). Both have excellent activity against Ascaris, moderate (but not ideal) activity against hookworms, and poor activity against whipworm and threadworms (18, 19). Only albendazole is used globally for MDAs, as tribendimidine is not yet approved worldwide (20). In addition, both benzimidazoles and nAChR agonists are susceptible to the development of parasite resistance, in both veterinary and human applications (21–25). There is thus an urgent need for new anthelmintics.
Better yet would be the development of anthelmintic combination therapies. Drug combinations are considered the ideal therapy for treatment of important infectious diseases, including HIV, malaria, and TB (26–28) and play a major role in improving therapeutic efficacy and delaying resistance. For example, the drug combinations found in highly active antiretroviral therapy (HAART) have revolutionized treatment of HIV/AIDS and allowed significant improvements in prolonging the duration and improving the quality of life of infected individuals (28). To date, however, there have been no systematic and quantitative studies of anthelmintic combinations.
Fortunately, an inexpensive and powerful laboratory nematode exists that, in principle, can be used to facilitate the study of anthelmintics—Caenorhabditis elegans. C. elegans has played a pivotal role in the discovery of the mechanism of action and resistance of virtually all anthelmintics, namely nAChR agonists, benzimidazoles, aldicarb, ivermectin, pore-forming crystal (Cry) proteins made by Bacillus thuringiensis (Bt), and amino-acetylnitriles (29–33). Such studies are far more difficult with parasitic nematodes. Despite these fundamental anthelmintic discoveries made with C. elegans, and despite the powerful genetic and toxicological tools available, C. elegans has been ignored for studying anthelmintic combinations.
Here we use wild-type and mutant C. elegans to characterize in detail how two different classes of anthelmintics interact. We find that nematodes resistant to either Bt Cry proteins or nAChR agonists are reciprocally hypersusceptible to the other class relative to wild-type nematodes. Furthermore, when combined, Cry proteins and nAChR agonists have strong synergy that is on a par with or better than some of the best combination therapies against other diseases. Thus, nAChR agonists and Cry proteins constitute a new and potentially powerful combination therapy against intestinal nematode diseases.
Results
nAChR Agonist Resistant Mutants Are Hypersusceptible to Cry Protein Anthelmintics.
Our laboratory has pioneered work on natural Bt Cry proteins that kill nematodes. Cry proteins are intensively used in the control of insect pests, including in aerial eradication campaigns, mosquito control programs, transgenic crops, and organic farming (34, 35). We have characterized Cry proteins lethal to free-living, animal-parasitic and plant-parasitic nematodes (29, 36–38). Although deadly to insects and nematodes, Bt Cry proteins are nontoxic to vertebrates (35) and are ideal candidates for a new class of anthelmintic. To date, one Cry protein, Cry5B, has been demonstrated to have anthelmintic activity in vivo against a hookworm parasite infection in hamsters (29).
As part of our development of Cry proteins as anthelmintics, we decided to quantitatively assay how various anthelmintic-resistant nematodes respond to Cry proteins. This information is important for determining the future usefulness of Cry proteins against anthelmintic-resistant nematodes that might appear during MDAs. Although it is known that various anthelmintic classes (e.g., nAChR agonists, benzimidazoles) have different mechanisms of action, how mutants resistant to one class respond to another class has not been quantitated in detail. We reasoned that C. elegans, for which mutants resistant to almost all different anthelmintic classes exist, provides a unique and powerful means to study this important question.
We tested how nematode mutants resistant to nAChR agonists and benzimidazoles (the two classes useful for single-dose MDAs against intestinal nematodes) respond to Cry proteins, using three different intoxication assays and the following mutants: lev-8(ye493), ben-1(e1880), and bre-5(ye17). lev-8 Encodes an nAChR subunit that mutates to levamisole, pyrantel, and tribendimidine resistance; ye493 is a null allele (17, 32). ben-1 Encodes a beta-tubulin conserved in parasitic nematodes and mutates to benzimidazole resistance in C. elegans and parasitic helminths (32). It is the only benzimidazole resistance gene identified to date. bre-5 Encodes a protein involved in the biosynthesis of the invertebrate-specific Cry5B receptor; ye17 is a null allele (30, 39).
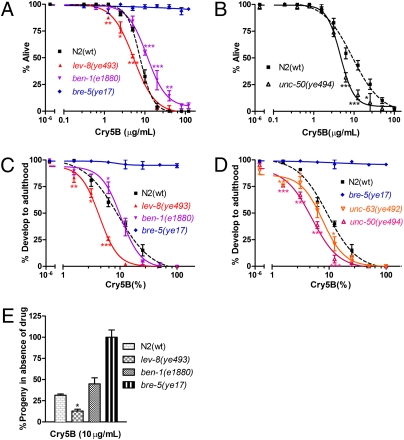
We unexpectedly find that nematodes resistant to nAChR agonists are hypersusceptible (HS) to Cry5B. Using a dose-dependent mortality assay (40), we find that nAChR agonist-resistant lev-8(ye493) nematodes are more susceptible than wild-type nematodes to Cry5B (dose range 1.25–5.0 μg/mL) (Fig. 1A). Conversely, benzimidazole-resistant ben-1(e1880) nematodes are more resistant to Cry5B than wild-type nematodes (doses 10–40 μg/mL). We calculated LC50 values (lethal concentration 50% or dose at which half of the nematodes are dead) from each of these curves, and find that the LC50 value of lev-8(ye493) nematodes on Cry5B is lower (i.e., 50% of lev-8 animals are killed at a lower dose) than that of wild type, confirming hypersusceptibility (Table S1). Hypersusceptibility to killing by Cry5B was also seen with unc-50(ye494) nematodes, a different nAChR agonist-resistant mutant that alters receptor assembly (32) (Fig. 1B and Table S1).
Fig. 1.
nAChR agonist–resistant mutants are hypersusceptible to crystal proteins. (A) Dose-dependent mortality response of wild-type N2 nematodes and ben-1, lev-8, and bre-5 mutant nematodes to purified Cry5B. (B) Dose-dependent mortality response of wild-type N2 and unc-50(ye494) animals to purified Cry5B. (C) Dose-dependent developmental inhibition response of wild-type N2 nematodes and ben-1, lev-8, and bre-5 mutant nematodes to E. coli–expressing Cry5B. (D) Dose-dependent developmental inhibition response of wild-type N2 nematodes and unc-63(ye492) and unc-50(ye494) mutant nematodes to E .coli–expressing Cry5B. The smaller effect seen with unc-63 may be due to the possible nonnull nature of this allele. (E) Effect of 10 μg/mL purified Cry5B on the 64-h brood sizes of N2 wild-type, lev-8, ben-1, and bre-5 mutant nematodes. For A–D and in similar figures below, each data point represents mean ± SEM. LC50 and IC50 values for exposures to Cry5B can be found in Table S1. For E, each bar shows 64-h broods from 15 worms; error bars represent SD. For A–E and in all similar figures below, *P < 0.05 relative to N2; **P < 0.01 relative to N2; and ***P <0.001 relative to N2. The alleles used here and in all other figures are ben-1(e1880), lev-8(ye493), and bre-5(ye17).
Similar striking results were obtained using a developmental inhibition assay. We put first staged larvae from the same mutants on varying concentrations of Escherichia coli (E. coli)–expressed Cry5B and counted the number of nematodes that were able to develop to adulthood in 60 h at 20 °, a time by which all wild-type nematodes in the absence of toxin develop to adulthood. In this assay, nAChR agonist-resistant lev-8(ye493) nematodes are also very HS to Cry5B over a range of doses (1.56–6.25%; Fig. 1C). We calculated the IC50 values (inhibitory concentration 50% or dose at which half the nematodes fail to develop) for wild-type and lev-8(ye493) nematodes, and found that lev-8 mutants are more than 2-fold more susceptible to Cry5B than wild type (Table S1). ben-1 Nematodes were not appreciably resistant to Cry5B in this assay (Fig. 1C) but appear HS based on IC50 values (Table S1). Using this developmental inhibition assay, we found that two other mutants, unc-63(ye492) and unc-50(ye494), resistance to nAChR agonists were also HS to Cry5B (Fig. 1D and Table S1).
As a third measure of intoxication, we looked at the response to Cry5B of each of the mutants with respect to nematode fecundity. We found that, normalized to the amount of progeny produced on no-toxin controls, lev-8(ye493) adults produce ~60% fewer progeny on Cry5B than wild-type adults (Fig. 1E; P < 0.05). No significant difference between wild-type and ben-1(e1880) nematodes was seen. Thus, based on three different measures of intoxication relevant to anthelmintic control and using up to three different nAChR-resistant mutants, we observed that nematodes resistant to nAChR agonists were HS to Cry5B relative to wild-type nematodes.
To see whether the hypersusceptibility applied to Cry proteins other than Cry5B, we tested the response of nAChR-resistant nematodes to Cry21A. Like Cry5B, Cry21A has anti-nematode activity against many free-living nematodes (38) but has only 41% amino acid identity to Cry5B from the N terminus until the end of the toxin domain. Cry21A is distinct from other anthelmintics in that we find that it is more difficult for mutagenized C. elegans to evolve resistance to Cry21A than albendazole, ivermectin, levamisole, or Cry5B (Table S2). Cry5B also has excellent properties in this regard relative to albendazole and levamisole.
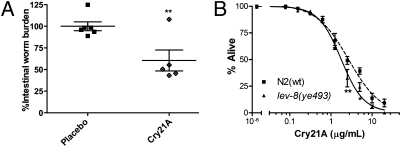
To date, however, no Cry protein other than Cry5B has been shown to have in vivo anthelmintic activity (29). To see if Cry21A is an anthelmintic, we tested the effects of Cry21A on Heligmosomoides bakeri roundworm infections in mice [H. bakeri is a natural intestinal parasite of mice and is an important model of roundworm infections in humans (41)]. We infected mice with H. bakeri larvae and then delivered per os three doses of Cry21A protein or placebo. Five days later, the mice were euthanized and the number of intestinal adult parasites counted. Cry21A treatment resulted in a 40% reduction in parasite burden relative to placebo (Fig. 2A, P = 0.005), demonstrating in vivo anthelmintic activity.
Fig. 2.
Effects of Cry21A on H. bakeri and on C. elegans wild-type and mutant strains. (A) In vivo anthelmintic activity of Cry21A against the small intestinal parasite H. bakeri. Shown are intestinal roundworm burdens normalized to average in the placebo control (41.7 worms) following treatment of infected mice with placebo (spore lysates, no Cry21A) or Cry21A spore crystal lysate (SCL). Normalized worm burdens in each mouse are shown (n = 6 for placebo; n = 5 for Cry21A; one animal in the Cry21A group died with a tumor before treatment). Long horizontal bar shows mean worm burdens; smaller bars indicate SEM. (B) Dose-dependent mortality response of wild-type N2 and nAChR agonist-resistant lev-8 mutant nematodes to Cry21A SCLs at 20 ° for 3 days. LC50 values for exposure to Cry21A can be found in Table S1.
We then performed dose-dependent mortality assays with Cry21A and lev-8 mutant nematodes. As with Cry5B, we found that lev-8 nematodes were HS to Cry21A (range 2.5–5 μg/mL) and were also HS to Cry21A based on LC50 values (Fig. 2B and Table S1). Thus, nAChR agonist-resistant mutants are likely to be HS to pore-forming Cry proteins in general.
Cry5B-Resistant Mutants Are Reciprocally Hypersusceptible to nAChR Agonists.
Because nematodes resistant to nAChR agonists are HS to Cry proteins, we asked what would happen to nematodes resistant to Cry proteins when exposed to nAChR agonists. We therefore quantified the effects of nAChR agonists, such as tribendimidine, levamisole, and pyrantel, on the same set of anthelmintic-resistant nematodes as above. We observed that bre-5(ye17) Cry5B-resistant nematodes were more sensitive than wild-type nematodes to tribendimidine (dose range 0.39–100 μg/mL; Fig. 3A), levamisole (dose range 24–48 μg/mL; Fig. 3B), and pyrantel (Fig. S1A). Based on LC50 values, bre-5(ye17) nematodes relative to wild-type nematodes are ~5-fold more HS to tribendimidine and ~2-fold more HS to levamisole (Table S1). To see how broadly this result applies to other Cry5B-resistant mutants, we also tested the Cry5B-resistant mutant, bre-2(ye31). These nematodes are also HS to tribendimidine, levamisole, and pyrantel (Fig. 3 C and D and Fig. S1B). Based on LC50 values, bre-2 nematodes are ~8-fold more sensitive to both tribendimidine and levamisole (Table S1). Results with ben-1(e1880) nematodes are different for different nAChR agonists. ben-1 Nematodes are HS to tribendimidine (albeit to a far lesser degree than that of Cry5B-resistant nematodes to tribendimidine; Fig. 3A and Table S1) but are resistant to levamisole based on LC50 (Table S1).
Fig. 3.
Cry5B-resistant mutants are hypersusceptible to nAChR agonists. (A) Dose-dependent mortality response of wild-type N2 animals and ben-1, lev-8, and bre-5 mutant animals to tribendimidine. (B) Dose-dependent mortality response of wild-type N2 nematodes and ben-1, lev-8, and bre-5 mutant nematodes to levamisole. (C) Dose-dependent mortality response of wild-type N2 animals and bre-2(ye31) mutant animals to tribendimidine. (D) Dose-dependent mortality response of wild-type N2 animals and bre-2(ye31) mutant animals to levamisole. (E) Dose-dependent developmental inhibition response of wild-type N2 nematodes and ben-1, lev-8, and bre-5 mutant nematodes to tribendimidine. (F) Effect of 25 μg/mL tribendimidine on 64-h brood sizes of N2 wild-type, lev-8, ben-1, and bre-5 nematodes.
As above, we tested whether this hypersusceptibility extended to other measures of intoxication of clinical relevance tested above, namely developmental and reproductive inhibition. We found that the development of Cry5B-resistant bre-5(ye-17) larvae were HS, relative to wild-type nematodes, to the nAChR agonist tribendimidine in the dose range of ~5–20 μg/mL and based on LC50 values (Fig. 3E and Table S1). Cry5B-resistant nematodes were also HS, relative to wild-type animals, to the sterilizing effects of tribendimidine (Fig. 3F; P < 0.05). Benzimidazole-resistant ben-1(e1880) nematodes, albeit less than bre-5(ye17) animals, showed some HS to tribendimidine in the developmental assay (Fig. 3E and Table S1), but not in the reproductive assay (Fig. 3F).
Cry5B and nAChR Agonists Are Strongly Synergistic.
The above data demonstrate that development of nematodes resistant to nAChR agonists actually improves Cry protein nematicidal activity, and that development of nematodes resistant to Cry proteins improves nAChR agonist nematicidal activity. These data, however, do not measure how each class of drug might modulate the activity of the other if the two were combined. That is to say, when combined, are the effects of the two classes antagonistic, additive, or synergistic? A synergistic effect is one that is more than additive; an antagonistic effect is one that is less than additive (i.e., the drugs inhibit each other). Synergistic interactions are sought after because they can result in increased efficacy, decreased dosage, and reduced side toxicity, and can perhaps minimize development of resistance when used clinically (42). Antagonistic effects are generaly avoided.
To quantitate the effects of the two anthelmintics on each other, we subjected wild-type C. elegans nematodes to increasing doses of Cry proteins and nAChR agonists individually and in combination at a constant mass (μg/mL) ratio (Fig. 4). For example, wild-type C. elegans were subjected to various doses of Cry5B, tribendimidine, and a 1:1 mass ratio (e.g., 1 μg/mL Cry5B + 1 μg/mL tribendimidine) mixture of the two. The worms were assayed for viability under each of these three conditions, which are plotted in Fig. 4A.
Fig. 4.
Cry5B and nAChR agonist combinations are strongly synergistic. (A) Dose-dependent mortality response of wild-type N2 animals to purified Cry5B, tribendimidine, and 1:1 ratio of Cry5B:tribendimidine based on mass. The x axis is plotted in terms of total dose (e.g., 1 μg/mL = 1 μg/mL of Cry5B, 1 μg/mL of tribendimidine, or combination of 0.5 μg/mL of each). (B) Dose-dependent mortality response of wild-type N2 animals to purified Cry5B, levamisole, and 1:1 ratio of Cry5B:levamisole based on mass. (C) Dose-dependent mortality response of wild-type N2 animals to purified Cry5B, tribendimidine, and 1:1 ratio of Cry5B:tribendimidine based on LC50 values. (D) Dose–response curve of wild-type N2 animals to tribendimidine without and with a low dose of Cry5B (2 μg/mL), which normally gives ~5% mortality. LEV, levamisole; Trib, tribendimidine.
From these data, we can calculate the degree of synergy using the combination index (CI) algorithm of Chou and Talalay (42). In general, CI values <1 indicate synergy; >1 indicate antagonism. The CI algorithm has been used to calculate the degree of synergy between drug combinations in cancer chemotherapy [typical synergistic CI values 0.1–0.8 (43–46)], viral therapy [synergistic CI values 0.5–0.8 (47, 48)], and insecticides [synergistic CI values of 0.3–0.9 (49, 50)]. To be somewhat conservative, we chose CI values <0.7 as indicative of synergy and <0.3 as indicative of a strong synergy (42, 51).Using this algorithm, we calculated CI values at the ED50 (median-effect dose), ED75, ED90, and ED95 of the combination and found CI values that ranged between 0.52–0.12, indicating strong synergy between Cry5B and tribendimidine when mixed at equal mass doses (Table 1). Synergy between the two compounds was also indicated using isobolograms at this mixture ratio (Fig. S2).
Table 1.
Combination index values of Cry5B and nAChR agonists at different effect dose levels
| Combination index values |
||||
| Combination | ED50 | ED75 | ED90 | ED95 |
| Cry5B + tribendimidine (1:1 mass) | 0.52 | 0.30 | 0.17 | 0.12 |
| Cry5B + levamisole (1:1 mass) | 0.38 | 0.26 | 0.19 | 0.15 |
| Cry5B + tribendimidine (1:1 LC50) | 0.48 | 0.38 | 0.31 | 0.27 |
A similar experiment was also carried out with Cry5B and the nAChR agonist levamisole combined in a fixed ratio based on mass (e.g., 1 μg/mL Cry5B mixed with 1 μg/mL levamisole) as shown in Fig. 4B. CI values of 0.38–0.15 were calculated from these data, indicative of strong synergy (Table 1).
To determine whether Cry5B and nAChR agonists are synergistic at other drug combination ratios, we combined Cry5B and tribendimidine at a 1:1 ratio based on their LC50 values (and 2-fold dilutions up and down from there; Fig. 4C). Again, excellent synergy with CI values ranging from 0.48–0.27 (EC50 – EC95) were seen (Table 1). Thus, Cry5B and tribendimidine also synergize when combined at a ratio based on similar efficacies.
We also calculated the dose-reduction index (DRI) for anthelmintics in different combinations at specific effect levels (Table S3). DRI is a measure of how many fold the dose of each drug in a synergistic combination may be reduced relative to the dose of each drug alone while still achieving the same effect. For example, at the ED90 for the Cry5B and levamisole combination, Cry5B can be reduced ~6-fold and levamisole ~39-fold relative to what it would take for each of these drugs to achieve the same effect on their own. These data suggest that Cry proteins and nAChR agonists mutually potentiate each other and that, for example, inclusion of a small amount of Cry5B might permit significant reductions in the amounts of nAChR agonists to achieve comparable effects when used alone. To test this conclusion, we “spiked” dose-dependent tribendimidine mortality assays with a small dose of Cry5B (2 μg/mL), which normally permits ~95% viability, and in parallel ran nonspiked assays. The small amount of Cry5B produced a synergistic interaction with tribendimidine (Fig. 4D). For example, the same lethality was seen with 20 μg/mL tribendimidine + Cry5B spike than was seen with 200 μg/mL tribendimidine alone.
Discussion
Here we quantitatively analyze two important aspects of anthelmintic interactions—how nematodes resistant to one anthelmintic class behave on other classes, and how two anthelmintic classes behave in combination with each other. These results are more readily achievable with the use of C. elegans than with parasitic nematodes. Each data point on our graph represents ~100–200 nematodes, and each data point is taken under culture conditions in which the nematodes are fully healthy and capable of completing a full life cycle, unlike in vitro assays with parasitic nematodes. Intense quantitative assays such as these are also not practical with in vivo parasite assays, which would require hundreds of vertebrate hosts per graph.
The results are striking—we find that nematodes resistant to nAChR agonists are hypersusceptible (HS) (relative to wild-type nematodes) to two Cry protein anthelmintics and, reciprocally, nematodes resistant to a Cry protein are HS to three nAChR agonists. Hypersusceptibility was seen with three different clinically relevant measures of anthelmintic activity—mortality, developmental inhibition, and sterilization of the nematodes. Why resisting an anthelmintic that acts at the neuromuscular junction should make nematodes more susceptible to pore-forming toxins that attack the intestine and vice versa is unexpected, and suggests large gaps in our understanding of nematode physiology. Although some hypersusceptibility was seen between benzimidazole-resistant nematodes and tribendimidine, the extent was less robust and did not extend to all measures of activity or to levamisole.
Mutual hypersusceptibility has, to our knowledge, not been previously identified with any anthelmintic combinations and has profound implications for anthelmintic chemotherapy. Hypersusceptibility is a key characteristic of one of the most successful antiinfective therapies today, HAART. In the HIV field, hypersusceptibility is often associated with the nucleoside reverse transcriptase inhibitors (NRTIs) and the nonnucleoside reverse transcriptase inhibitors (NNRTI) combinations (52). Evolution of viral resistance to one class results in hypersusceptibility to the second class. HS combinations are associated with improved clinical outcome and virological response (52–54) and with improved resistance outcomes, such as delaying/suppressing resistance, reducing the risk of cross-resistance, and even reversing an existing resistance phenotype (53–57). The experience with HAART suggests that the combination of L-subtype nAChR agonists such as tribendimidine with Cry proteins should result in significantly improved clinical outcomes while simultaneously working to prevent the emergence of resistance to either drug.
Our HS studies were complemented with measurements of synergy between L-subtype nAChR agonists and Cry5B. To our knowledge, quantitation of the synergy between any anthelmintics has yet to be reported. These data demonstrate that Cry5B and L-subtype nAChR agonists show strong synergy when combined, and greatly exceed that predicted from simple additive effects. Synergy is highly desirable. When the drugs used in the combination therapy are synergistic, the therapeutic efficacy of each component is greatly increased. As a result, much smaller doses of each drug can be used in the combination therapy than would otherwise be required if each drug were used individually. This results in reductions in drug side effects and in cost. With regards to resistance, there is precedent for synergistic interactions precluding the development of resistance, such as in the case of synergy between Cry and the cytolytic proteins found in mosquitocidal Bt strains (58–60)
The implications of our findings are profound. Due to the threat of resistance to individual anthelmintics and the fact that we have no single drug that has high efficacy against all of the parasites, therapeutic agents with novel modes of action and, more importantly, intelligently designed combination therapies are urgently needed for the treatment of these diseases (9, 18, 22). The excellent combination of Cry proteins and L-subtype nAChR agonists therefore potentially represent an evolution in anthelmintic chemotherapy, as this combination displays two different characteristics—hypersusceptibility and synergy. Such a combination is predicted to be highly valuable in MDA treatments for intestinal nematodes in which tens to hundreds of millions of children and pregnant women are targeted for single-dose treatment. In such circumstances, having a powerful combination therapy is likely to maximize therapeutic outcome in a single dose while delaying/preventing resistance that may be inevitable with massive use of single anthelmintics.
In summary, we demonstrate that Cry proteins and nAChR agonist anthelmintics possess a powerful set of characteristics defined by mutual hypersusceptibility and strong synergy. These data define a potentially unique combination therapy for treating intestinal nematode infections that is predicted to be a very potent and recalcitrant to the development of parasite resistance. These studies also demonstrate the unique utility of C. elegans in the study of anthelmintic therapy, and open the door to similar characterizations of other anthelmintic combinations and therapies.
Materials and Methods
C. elegans Strains and Reagents.
C. elegans strains were cultured using standard techniques including the use of Escherichia coli strain OP50 as standard food source (61). Different strains were allowed to grow for different amounts of time at 20 ° from the L1 to L4 stage before testing on drugs to reflect slight differences in their growth rates relative to N2 wild type: unc-63(ye492), lev-8(ye493) unc-50(ye494), bre-2 (ye31), and bre-5(ye17) were allowed to develop for 45 h. N2 wild-type animals and ben-1 (e1880) were used at 44 h. The preparation of Cry5B, tribendimidine, levamisole, pyrantel, Cry21A, and all worm plates and buffers were as described elsewhere (17, 29, 38, 40, 62).
Intoxication Assays.
Mortality assays with nAChR agonists and purified Cry5B were as described for 6 days at 25° (17, 40). Assays with Cry21A spore-crystal lysates (SCLs) were carried out in the presence of 15 μg/mL tetracycline to prevent Bt from infecting and at 20° for 3 days (62). The reason for the lower temperature and reduced time with Cry21A assays was to keep the dose–response range similar to that for other drugs, as the Bt spores present in Cry21A SCLs enhance mortality. Each experiment was performed with ~20 L4 nematodes per well in triplicate wells and then repeated in three independent repeats [~100–240 nematodes/data point; except for the pyrantel and bre-5(ye17) assay, which was carried out in duplicate]. All liquid assays were carried out in special S medium.
Developmental inhibition assays for tribendimidine were as previously described at 20 ° (17). Triplicate wells and three independent assays were carried out as described above. To measure developmental inhibition by Cry5B, 20 L1 nematodes were seeded on ENG-IC plates with various percentages of Cry5B-expressing E. coli diluted with non–Cry5B-expressing E. coli as described elsewhere (40) and incubated at 20 ° for 60 h. The number of nematodes that did/did not reach gravid adulthood was tallied for each plate. The experiment was independently repeated three times.
To calculate brood sizes, individual L4 worms were placed with an eyelash in a 48-well plate containing 40 μL OP50 (OD600 = 3.0) and tribendimidine or purified Cry5B in a total volume of 200 μL (five nematodes/treatment/assay; three independent assays). The plates were incubated for 64 h at 25 °. The progeny were pipetted onto an empty NG agar plate for counting.
Effect of Cry21A on H. bakeri Infections in Mice.
Swiss Webster female mice (6–8 weeks of age) were orally infected with 150 H. bakeri third-stage larvae. Animals were split into two groups, and on days 15, 16, and 17 postinfection were given either 99 nM/kg of Cry21A SCL in 0.1 mL H2O or 0.1 mL spore lysates of the Bt host strain 4D22. At day 22, infected mice were euthanized and their small intestines removed and dissected. Adult H. bakeri present in the small intestine were then counted.
Statistical and Synergy Analyses.
LC50 values and 95% confidence limits were calculated by combining all data from the three independent experiments and using PROBIT (from XLSTAT add-on to EXCEL, Addinsoft). All other data analyses and plots were carried out with Prism 5 (GraphPad Software). For brood size data, pair-wise comparisons between groups were carried out via one-way ANOVA and Tukey's HSD test. For developmental and mortality data, pair-wise comparisons among groups and doses was carried out via two-way ANOVA and Bonferroni post tests. Cry21A in vivo treatment results were analyzed by Student's t test (unpaired, one-tailed). Drug combination curves were plotted using Prism 5. Results of combination studies (CI and DRI values, isobologram plots) were processed using the CompuSyn software package (CompuSyn), with the modification that we manually added on the correction for mutually nonexclusive drugs, which is not incorporated in the CompuSyn program. This modification is appropriate, as we believe that the two classes of drugs have totally independent modes of action and, in any event, result in more conservative (i.e., higher) CI values. The formula of Chou and Talalay used for calculating CI values is given in SI Text. For the combination studies in which Cry5B and tribendimidine were to be added at a 1:1 ratio based on LC50 values, we performed pilot dose–response experiments with tribendimidine and Cry5B just before the actual experiments. Based on these pilot data, we then selected 4 μg of Cry5B and 102 μg of tribendimidine as the 1:1 LC50 ratio dose (and 2-fold dilutions up and down) and performed three independent synergy experiments (including Cry5B, tribendimidine, and Cry5B+tribendimidine dose–response curves). In the actual experiments, upon analyses of the completed data, the LC50 of Cry5B and tribendimidine alone were found to be 4.6 μg/mL and 78 μg/mL respectively. Thus, the actual ratio present in this experiment was 1 × LC50 Cry5B: 1.5 × LC50 tribendimidine.
Supplementary Material
Acknowledgments
We are grateful to the members of the Aroian Laboratory for discussions. We are grateful to Drs. Margaret Wirth and Bruce Tabashnik for discussions. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) National Center for Research Resources. This work was funded by NIH AI056189 (to R.V.A.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912327107/DCSupplemental.
References
- 1.Bethony J, et al. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Hall A, Hewitt G, Tuffrey V, de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern Child Nutr. 2008;4(Suppl 1):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, et al. New technologies for the control of human hookworm infection. Trends Parasitol. 2006;22:327–331. doi: 10.1016/j.pt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ. Forgotten people, Forgotten diseases: The neglected tropical diseases and their impact on global health and development. Washington, DC: ASM Press; 2008. pp. 13–27. [Google Scholar]
- 5.Larocque R, Gyorkos TW. Should deworming be included in antenatal packages in hookworm-endemic areas of developing countries? Can J Public Health. 2006;97:222–224. doi: 10.1007/BF03405590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin MG, Humphreys ME. Social consequence of disease in the American South, 1900-World War II. South Med J. 2006;99:862–864. doi: 10.1097/01.smj.0000231265.03256.1f. [DOI] [PubMed] [Google Scholar]
- 7.Watkins WE, Pollitt E. “Stupidity or worms”: Do intestinal worms impair mental performance? Psychol Bull. 1997;121:171–191. doi: 10.1037/0033-2909.121.2.171. [DOI] [PubMed] [Google Scholar]
- 8.Alexander PE, De P. HIV-1 and intestinal helminth review update: Updating a Cochrane Review and building the case for treatment and has the time come to test and treat? Parasite Immunol. 2009;31:283–286. doi: 10.1111/j.1365-3024.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 9.Brooker S, et al. Epidemiology of plasmodium-helminth co-infection in Africa: Populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77(6, Suppl):88–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper PJ, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper PJ, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 13.Harris JB, et al. Immunologic responses to Vibrio cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl Trop Dis. 2009;3:e403. doi: 10.1371/journal.pntd.0000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walson JL, et al. Albendazole treatment of HIV-1 and helminth co-infection: A randomized, double-blind, placebo-controlled trial. AIDS. 2008;22:1601–1609. doi: 10.1097/QAD.0b013e32830a502e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotez PJ, et al. Helminth infections: The great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao SH, Hui-Ming W, Tanner M, Utzinger J, Chong W. Tribendimidine: A promising, safe and broad-spectrum anthelmintic agent from China. Acta Trop. 2005;94:1–14. doi: 10.1016/j.actatropica.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Xiao SH, Aroian RV. The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis. 2009;3:e499. doi: 10.1371/journal.pntd.0000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 19.Steinmann P, et al. Tribendimidine and albendazole for treating soil-transmitted helminths, Strongyloides stercoralis and Taenia spp.: Open-label randomized trial. PLoS Negl Trop Dis. 2008;2:e322. doi: 10.1371/journal.pntd.0000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits HL. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev Anti Infect Ther. 2009;7:37–56. doi: 10.1586/14787210.7.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: A pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Flohr C, et al. Low efficacy of mebendazole against hookworm in Vietnam: Two randomized controlled trials. Am J Trop Med Hyg. 2007;76:732–736. [PubMed] [Google Scholar]
- 23.Gunawardena NK, Amarasekera ND, Pathmeswaran A, de Silva NR. Effect of repeated mass chemotherapy for filariasis control on soil-transmitted helminth infections in Sri Lanka. Ceylon Med J. 2008;53:13–16. doi: 10.4038/cmj.v53i1.220. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan RM. Drug resistance in nematodes of veterinary importance: A status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Stepek G, Buttle DJ, Duce IR, Behnke JM. Human gastrointestinal nematode infections: Are new control methods required? Int J Exp Pathol. 2006;87:325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100:415–431. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]
- 27.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: A pooled analysis of six randomized trials. Malar J. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portsmouth S, Stebbing J, Gazzard B. Current treatment of HIV infection. Curr Top Med Chem. 2003;3:1458–1466. doi: 10.2174/1568026033451808. [DOI] [PubMed] [Google Scholar]
- 29.Cappello M, et al. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc Natl Acad Sci USA. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffitts JS, et al. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- 31.Holden-Dye L, Walker RJ. Anthelmintic drugs. WormBook. 2007 doi: 10.1895/wormbook.1.143.1. ed The C. elegans Re- search Community, doi/10.1895/wormbook.1.143.1. Available at http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones AK, Buckingham SD, Sattelle DB. Chemistry-to-gene screens in Caenorhabditis elegans. Nat Rev Drug Discov. 2005;4:321–330. doi: 10.1038/nrd1692. [DOI] [PubMed] [Google Scholar]
- 33.Kaminsky R, et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- 34.Roh JY, Choi JY, Li MS, Jin BR, Je YH. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J Microbiol Biotechnol. 2007;17:547–559. [PubMed] [Google Scholar]
- 35.Betz FS, Hammond BG, Fuchs RL. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul Toxicol Pharmacol. 2000;32:156–173. doi: 10.1006/rtph.2000.1426. [DOI] [PubMed] [Google Scholar]
- 36.Li XQ, et al. Expression of Cry5B protein from Bacillus thuringiensis in plant roots confers resistance to root-knot nematode. Biological Control. 2008;47:97–102. [Google Scholar]
- 37.Li XQ, Wei JZ, Tan A, Aroian RV. Resistance to root-knot nematode in tomato roots expressing a nematicidal Bacillus thuringiensis crystal protein. Plant Biotechnol J. 2007;5:455–464. doi: 10.1111/j.1467-7652.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 38.Wei JZ, et al. Bacillus thuringiensis crystal proteins that target nematodes. Proc Natl Acad Sci USA. 2003;100:2760–2765. doi: 10.1073/pnas.0538072100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffitts JS, Whitacre JL, Stevens DE, Aroian RV. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science. 2001;293:860–864. doi: 10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- 40.Bischof LJ, Huffman DL, Aroian RV. Assays for toxicity studies in C. elegans with Bt crystal proteins. Methods Mol Biol. 2006;351:139–154. doi: 10.1385/1-59745-151-7:139. [DOI] [PubMed] [Google Scholar]
- 41.Monroy FG, Enriquez FJ. Heligmosomoides polygyrus: A model for chronic gastrointestinal helminthiasis. Parasitol Today. 1992;8:49–54. doi: 10.1016/0169-4758(92)90084-f. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, et al. Sensitivity of breast cancer cell lines to recombinant thiaminase I. Cancer Chemother Pharmacol. 2009 doi: 10.1007/s00280-009-1148-9. 10.1007/s00280-009-1148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M, Osman I, Orlow SJ. Antifolate activity of pyrimethamine enhances temozolomide-induced cytotoxicity in melanoma cells. Mol Cancer Res. 2009;7:703–712. doi: 10.1158/1541-7786.MCR-08-0263. [DOI] [PubMed] [Google Scholar]
- 45.Falà F, et al. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor (2S)-1-(1H-Indol-3-yl)-3-[5-(3-methyl-2H-indazol-5-yl)pyridin-3-yl]oxypropan2-amine (A443654) in T-cell acute lymphoblastic leukemia. Mol Pharmacol. 2008;74:884–895. doi: 10.1124/mol.108.047639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajabalian S. Methanolic extract of Teucrium polium L. potentiates the cytotoxic and apoptotic effects of anticancer drugs of vincristine, vinblastine and doxorubicin against a panel of cancerous cell lines. Exp Oncol. 2008;30:133–138. [PubMed] [Google Scholar]
- 47.Bassit L, Grier J, Bennett M, Schinazi RF. Combinations of 2′-C-methylcytidine analogues with interferon-alpha2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antivir Chem Chemother. 2008;19:25–31. doi: 10.1177/095632020801900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulgheroni E, et al. Analysis of protease inhibitor combinations in vitro: Activity of lopinavir, amprenavir and tipranavir against HIV type 1 wild-type and drug-resistant isolates. J Antimicrob Chemother. 2004;53:464–468. doi: 10.1093/jac/dkh103. [DOI] [PubMed] [Google Scholar]
- 49.Bonnet J, Corbel V, Darriet F, Chandre F, Hougard JM. Topical applications of pyrethroid and organophosphate mixtures revealed positive interactions against pyrethroid-resistant Anopheles gambiae. J Am Mosq Control Assoc. 2004;20:438–443. [PubMed] [Google Scholar]
- 50.Darriet F, Corbel V. Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J Med Entomol. 2006;43:1190–1194. doi: 10.1603/0022-2585(2006)43[1190:leopas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Hideshima T, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haubrich RH. Resistance and replication capacity assays: Clinical utility and interpretation. Top HIV Med. 2004;12:52–56. [PubMed] [Google Scholar]
- 53.Kim R, Baxter JD. Protease inhibitor resistance update: Where are we now? AIDS Patient Care STDS. 2008;22:267–277. doi: 10.1089/apc.2007.0099. [DOI] [PubMed] [Google Scholar]
- 54.Tozzi V, et al. Mutations in HIV-1 reverse transcriptase potentially associated with hypersusceptibility to nonnucleoside reverse-transcriptase inhibitors: Effect on response to efavirenz-based therapy in an urban observational cohort. J Infect Dis. 2004;189:1688–1695. doi: 10.1086/382960. [DOI] [PubMed] [Google Scholar]
- 55.Frankel FA, Marchand B, Turner D, Götte M, Wainberg MA. Impaired rescue of chain-terminated DNA synthesis associated with the L74V mutation in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 2005;49:2657–2664. doi: 10.1128/AAC.49.7.2657-2664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geretti AM. Clinical implications of HIV drug resistance to nucleoside and nucleotide reverse transcriptase inhibitors. AIDS Rev. 2006;8:210–220. [PubMed] [Google Scholar]
- 57.Shulman N, et al. Phenotypic hypersusceptibility to non-nucleoside reverse transcriptase inhibitors in treatment-experienced HIV-infected patients: Impact on virological response to efavirenz-based therapy. AIDS. 2001;15:1125–1132. doi: 10.1097/00002030-200106150-00007. [DOI] [PubMed] [Google Scholar]
- 58.Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Georghiou GP, Wirth MC. Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae) Appl Environ Microbiol. 1997;63:1095–1101. doi: 10.1128/aem.63.3.1095-1101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soberón M, Fernández LE, Pérez C, Gill SS, Bravo A. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon. 2007;49:597–600. doi: 10.1016/j.toxicon.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellier A, Chen CS, Kao CY, Cinar HN, Aroian RV. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLoS Pathog. 2009;5:e1000689. doi: 10.1371/journal.ppat.1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.