Abstract
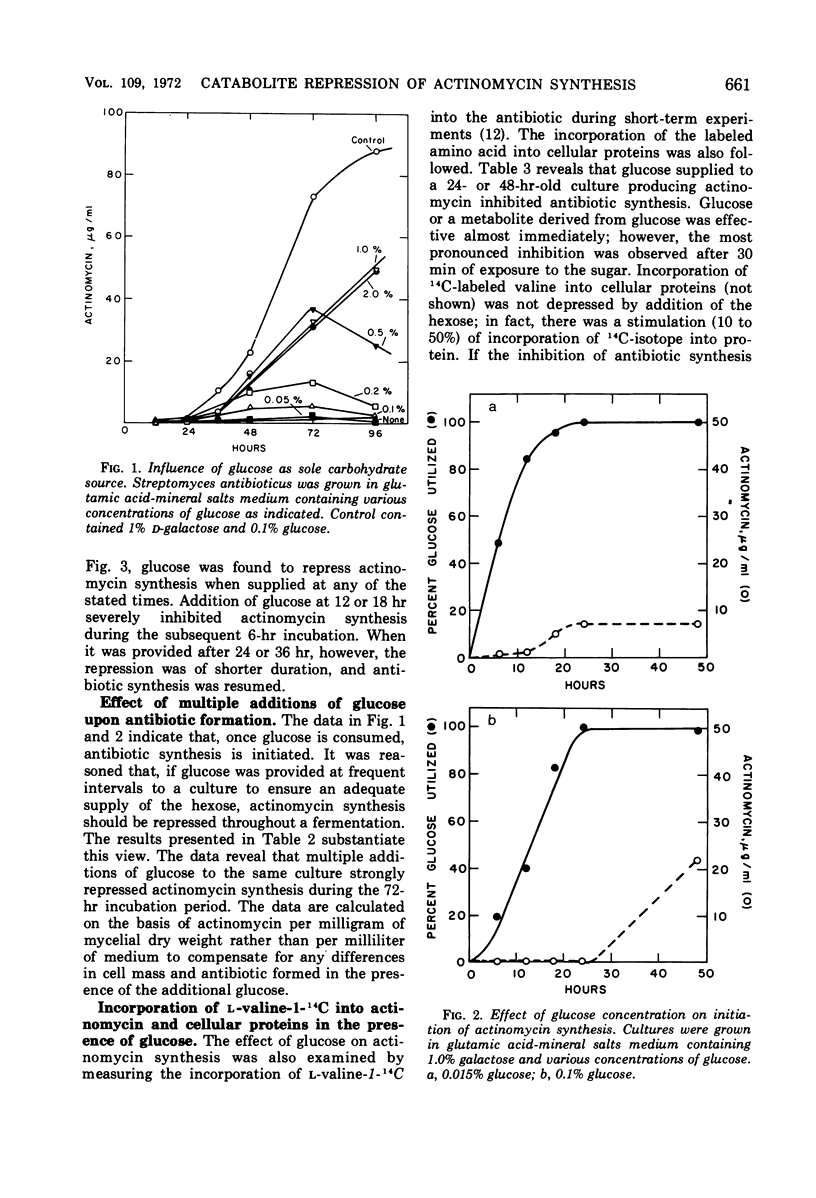
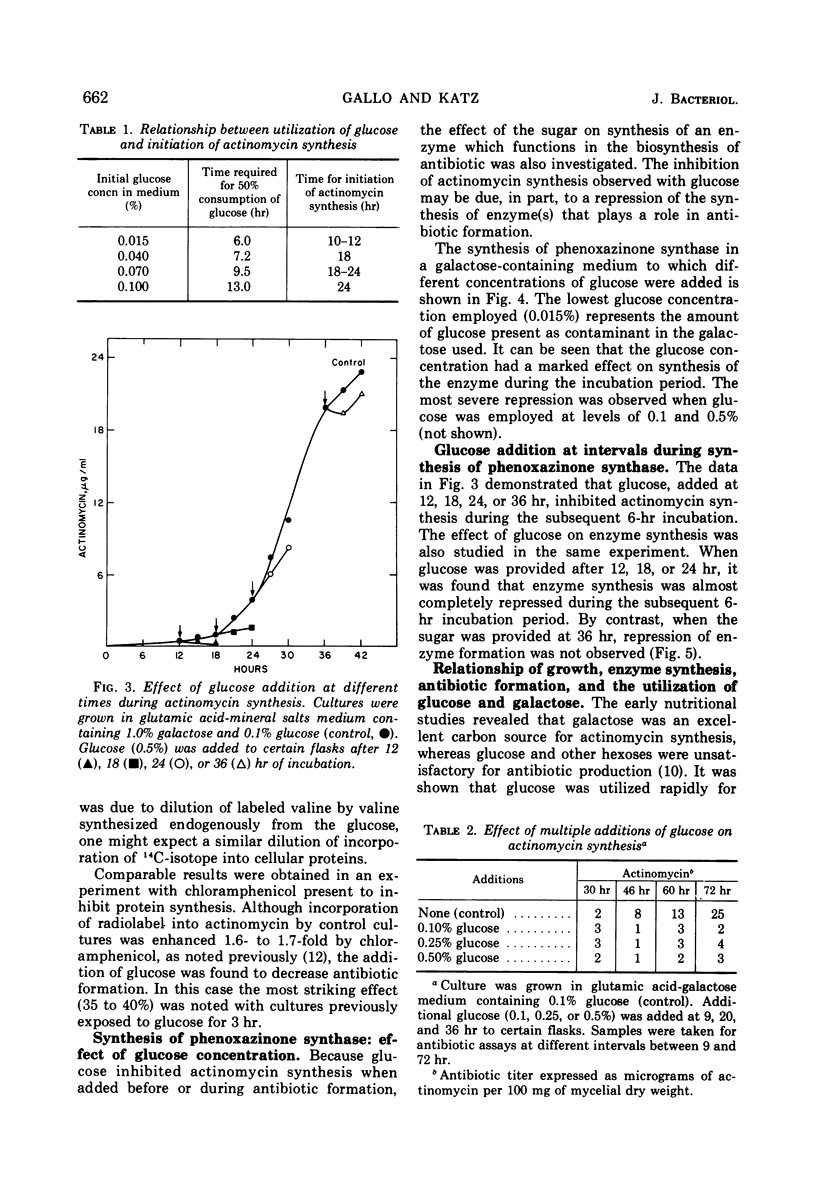
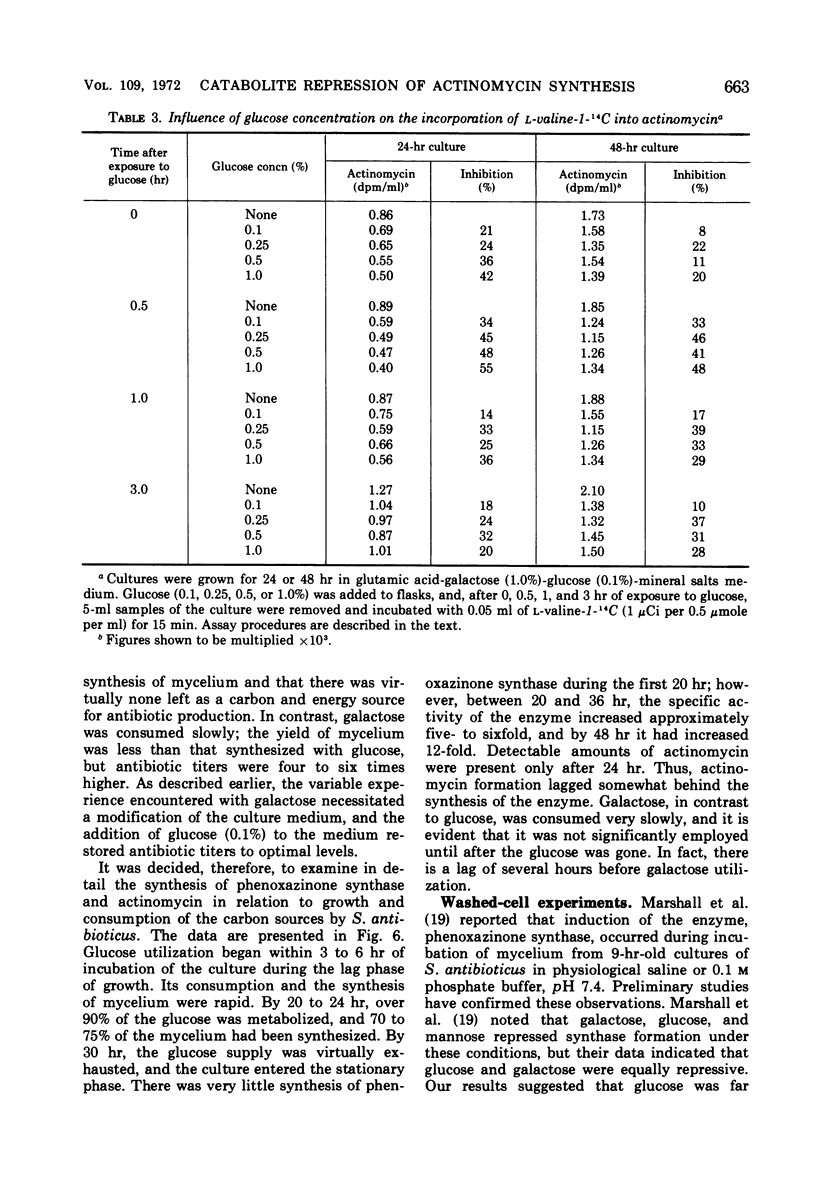
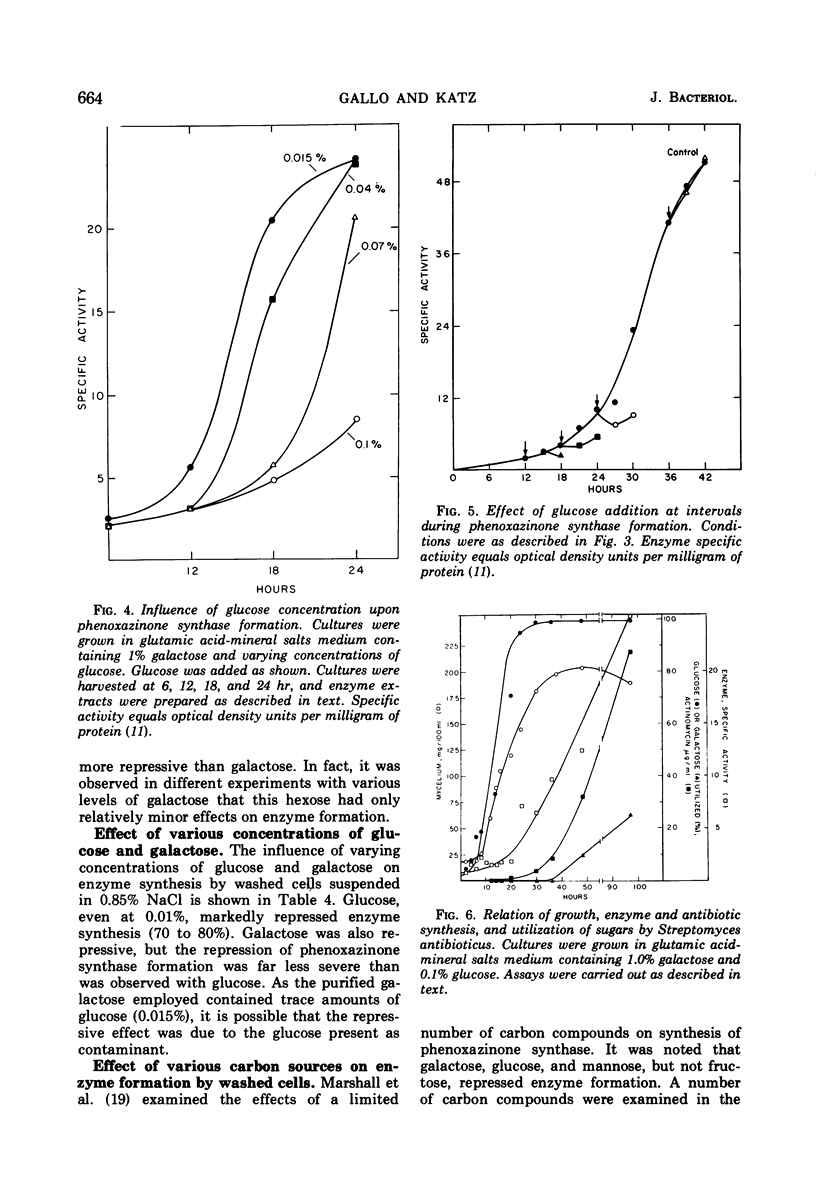
Synthesis of the secondary metabolite, actinomycin, and the enzyme, phenoxazinone synthase, involved in the biosynthesis of the antibiotic, were shown to be under severe catabolite repression by glucose. Of a variety of hexoses and carbon compounds examined, glucose, and to a lesser extent, mannose, proved to be the most repressive for enzyme synthesis. The repression by glucose was most evident before production of the antibiotic. In a chemically defined medium suitable for actinomycin production, synthesis of phenoxazinone synthase began at the time the glucose (0.1%) supply was depleted. Soon after, antibiotic synthesis was initiated. Galactose, the major carbon source for growth and antibiotic synthesis, was not utilized until the glucose was consumed. Generally, carbon compounds which supported a rapid rate of growth were most effective in producing catabolite repression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAD G., AMARAL D., ASENSIO C., HORECKER B. L. The D-galactose oxidase of Polyporus circinatus. J Biol Chem. 1962 Sep;237:2736–2743. [PubMed] [Google Scholar]
- Demain A. L., Inamine E. Biochemistry and regulation of streptomycin and mannosidostreptomycinase (alpha-D-mannosidase) formation. Bacteriol Rev. 1970 Mar;34(1):1–19. doi: 10.1128/br.34.1.1-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. J. Recent advances in penicillin fermentation. Bull World Health Organ. 1952;6(1-2):99–121. [PMC free article] [PubMed] [Google Scholar]
- KATZ E., GOSS W. A. Controlled biosynthesis of actinomycin with sarcosine. Biochem J. 1959 Nov;73:458–465. doi: 10.1042/bj0730458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E., PIENTA P., SIVAK A. The role of nutrition in the synthesis of actinomycin. Appl Microbiol. 1958 Jul;6(4):236–241. doi: 10.1128/am.6.4.236-241.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E., WEISSBACH H. Biosynthesis of the actinomycin chromophore; enzymatic conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin. J Biol Chem. 1962 Mar;237:882–886. [PubMed] [Google Scholar]
- KATZ E., WEISSBACH H. Incorporation of C14-labeled amino acids into actinomycin and protein by Streptomyces antibioticus. J Biol Chem. 1963 Feb;238:666–675. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. Inhibitory effect of glucose on enzyme formation. Nature. 1956 Oct 13;178(4537):801–802. doi: 10.1038/178801b0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The repression of constitutive beta-galactosidase in Escherichia coli by glucose and other carbon sources. Biochem J. 1962 Mar;82:489–493. doi: 10.1042/bj0820489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R., Redfield B., Katz E., Weissbach H. Changes in phenoxazinone synthetase activity during the growth cycle of Streptomyces antibioticus. Arch Biochem Biophys. 1968 Feb;123(2):317–323. doi: 10.1016/0003-9861(68)90141-0. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Effect of mixtures of substrates on the biosynthesis of inducible enzymes in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):260–263. doi: 10.1128/jb.73.2.260-263.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C. Mutant of Aerobacter aerogenes lacking glucose repression. J Bacteriol. 1960 Oct;80:536–543. doi: 10.1128/jb.80.4.536-543.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- SOLS A., DE LA FUENTE G. Hexokinase and other enzymes of sugar metabolism in the intestine. Methods Med Res. 1961;9:302–309. [PubMed] [Google Scholar]



