Abstract
Stopping an action in response to an unexpected event requires both that the event is attended to, and that the action is inhibited. Previous neuroimaging investigations of stopping have failed to adequately separate these cognitive elements. Here we used a version of the widely used Stop Signal Task that controls for the attentional capture of stop signals. This allowed us to fractionate the contributions of frontal regions, including the right inferior frontal gyrus and medial frontal cortex, to attentional capture, response inhibition, and error processing. A ventral attentional system, including the right inferior frontal gyrus, has been shown to respond to unexpected stimuli. In line with this evidence, we reasoned that lateral frontal regions support attentional capture, whereas medial frontal regions, including the presupplementary motor area (pre-SMA), actually inhibit the ongoing action. We tested this hypothesis by contrasting the brain networks associated with the presentation of unexpected stimuli against those associated with outright stopping. Functional MRI images were obtained in 26 healthy volunteers. Successful stopping was associated with activation of the right inferior frontal gyrus, as well as the pre-SMA. However, only activation of the pre-SMA differentiated stopping from a high-level baseline that controlled for attentional capture. As expected, unsuccessful attempts at stopping activated the anterior cingulate cortex. In keeping with work in nonhuman primates these findings demonstrate that successful motor inhibition is specifically associated with pre-SMA activation.
Keywords: attention, functional MRI, presupplementary motor area, stop signal task, stopping
The control of voluntary action depends critically upon the ability to inhibit unwanted responses. This process has been extensively studied using the Stop Signal Task (SST) (1). Previous work with this task provides evidence that both medial frontal regions, including the presupplementary motor area (pre-SMA), and more lateral regions, including the right inferior frontal gyrus (IFG; rIFG) and insula (Ins), are involved in stopping. However, the specific contributions of these regions to motor control are unresolved (2–4). Many functional imaging studies have demonstrated activation of right inferior frontal regions during stopping (2, 5–8) and individual differences in response inhibition correlate with the magnitude of the IFG/Ins activation during the SST (5). Activation of medial prefrontal regions are also observed during stopping (2, 5). Pre-SMA activation is correlated with the efficiency of inhibitory processing (2), and work in nonhuman primates supports a role for the medial prefrontal regions in behavioral inhibition (9, 10). Neuropsychological studies provide discrepant results, with correlations between the extent of damage and impairments of inhibitory function reported for both the right lateral and medial frontal regions (3, 4).
A limitation of much of the previous neuroimaging literature is that “stop trials” conflate processing associated with attentional capture of the perceptual cue and response inhibition. Stopping in response to a stop signal requires a subject to attend to a cue, appreciate its significance, and engage response inhibition (2, 11, 12). This is important because the attentional processing of an unexpected perceptual event is associated with significant brain activation, without necessarily signifying the presence of inhibitory processing. The attentional capture of a singleton stimulus activates the frontal cortex (13) and a right lateralized ventral attentional system that includes the right IFG/Ins has been described, which is thought to support the attentional capture of salient stimuli (14).
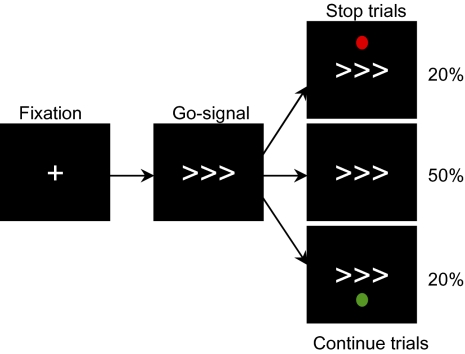
We propose that much of the network that is activated during stopping, including the right IFG/Ins, is the result of attentionally processing the stop signal. In contrast, we predict that the pre-SMA is critically involved in response inhibition. We test this hypothesis by manipulating the standard version of the SST to separate attentional processing of the cue to stop, from response inhibition that actually stops an initiated response. This is achieved by adding a high-level control condition that involves the presentation of an unexpected continue signal (Fig. 1). This cue is attentionally processed as an unexpected event, but requires no change in behavior. In addition, to limit the strategic slowing of task performance that can occur during performance of the SST (12), we provided negative feedback for response trials with slow reaction times. Crucially the comparison of stop and continue trials allows the separation of brain regions involved in attentional capture from those involved in response inhibition.
Fig. 1.
Stop signal paradigm. Interstimulus interval was 1,750 ms. A fixation cross was presented initially for 350 ms followed by the go stimulus for 1,400 ms (a right- or left-pointing arrow). For both the original and controlled versions of the SST, 20% of the trials involve an unpredictable stop signal (red dot) presented at a variable delay following the go signal (the stop signal delay). See SI Materials and Methods for more information about the staircase procedure. During the controlled version of the SST, a further 20% of trials involve a continue signal (green dot) presented with the same stop signal delay as the previous stop signal.
Results
Behavior.
Performance for both the original and controlled versions of the SST was similar to that observed in previous studies (7, 8, 15). The average accuracy for all runs was close to 50% (see SI Results for further information on individual performance). The addition of continue trials to the controlled SST had no significant effect on mean go reaction time (RT), median go RT, or stop signal reaction time (SSRT), i.e., these measures were similar in the original and controlled versions of the task. This suggests that adding the continue trials did not lead to a major strategic change in task performance. Go trial RT during the SST was significantly longer than the mean go RT for the simple choice reaction time (CRT) task [T(25) > 2.74, P < 0.05 for all four SST runs]. Error rates for continue and go trials were less than 4%. Across all subjects, RT for continue trials was approximately 40 ms slower than RT for go trials in the controlled version of the SST [T(25) = 10.43, P < 0.001; Table 1].
Table 1.
Behavioral results for the CRT task and SST (original and controlled)
| SST original |
SST controlled |
||||
| Trial type | CRT | Run 1 | Run 2 | Run 1 | Run 2 |
| Median Go RT (ms) | 411 ± 16 | 447 ± 14 | 448 ± 14 | 462 ± 17 | 453 ± 13 |
| SD Go RT (within subjects) | 69 ± 8 | 87 ± 5 | 83 ± 5 | 100 ± 8 | 90 ± 9 |
| Median Continue RT (ms) | NA | NA | NA | 502 ± 17 | 486 ± 17 |
| Accuracy on Go trials, % | 97.7 ± 0.4 | 98.3 ± 0.4 | 98.7 ± 0.4 | 98.2 ± 0.5 | 98.5 ± 0.3 |
| Accuracy on Continue trials, % | NA | NA | NA | 97.0 ± 0.9 | 98.2 ± 0.6 |
| Accuracy on Stop trials, % | NA | 48.3 ± 0.7 | 50.1 ± 0.7 | 50.9 ± 0.7 | 50.9 ± 0.7 |
| SSRT (ms) | NA | 231 ± 11 | 227 ± 11 | 222 ± 13 | 220 ± 11 |
Average of the median speed of response to Go trials (Go RT), median speed of response to continue trials (Continue RT), SSRT, and accuracy on Go, Continue, and Stop trials are reported (±SEM). Percentage accuracy is estimated by dividing the number of correct trials (Go, Continue, or Stop) by the total number of each trial type. The within-subject SD is also reported for Go RT. SSRT was calculated for each subject and each run by subtracting the critical SSD from the median Go RT. NA, not applicable.
Neuroimaging Results.
Overall network for stopping is consistent with the previous literature.
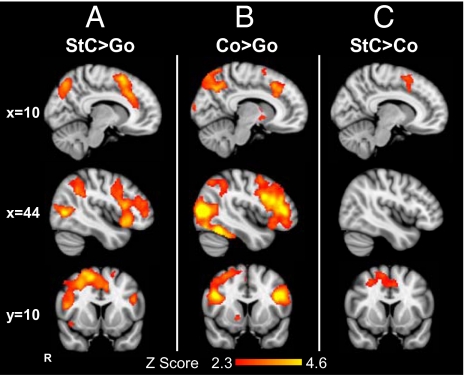
The stop network can be defined by contrasting trials wherein a stop signal occurred and a response was successfully inhibited with a go trial (i.e., stop vs. go). This network includes brain regions involved in the attentional processing of a perceptual cue, as well as those involved in response inhibition. In our main analyses of the original and controlled versions of the SST, we combined both runs of each version in two separate mixed effects analyses. In both versions of the task, we observed a stopping network that was similar to that previously reported, e.g., in refs. 6 and 7. Correcting for multiple comparisons, activation was observed within the right anterior insular cortex and middle frontal gyrus, as well as bilaterally within the inferior, middle, and superior frontal gyri. Activation was also observed within the right supramarginal gyrus, the left intraparietal sulcus and the lateral occipital cortices (Table S1 and Fig. 2A illustrating the controlled SST). We found no significant difference between the stop activation (i.e., stop vs. go trials) in the two versions of the SST, indicating that activation in the stop network was unaffected by the presence of continue trials.
Fig. 2.
Frontal activation during response inhibition. Rendered images show brain regions activated in the controlled SST for (A) correct stop (StC) more than correct go trials (StC > Go); (B) correct continue trials more than go trials (Co > Go); and (C) correct stop trials more than continue trials (StC > Co). Results are superimposed on the MNI 152 T1 2 mm brain template. For the whole-brain analysis, a Z-statistic threshold of 2.3 was employed, combined with a corrected cluster significance threshold probability of P < 0.05.
Continuing activates a similar brain network to stopping.
In the controlled version of the SST, we added a trial type consisting of unpredictable but behaviorally irrelevant stimuli (continue trials). These occurred with the same frequency and timing as stop signals, but required no change to the initiated motor response. The contrast between correctly continuing after an unexpected event (continue trials) and making a response when no unexpected event occurred (go trials) showed activation within the IFG/Ins, the frontal pole, and the middle frontal gyri, as well as within the lateral occipital lobes (Table S2 and Fig. 2B). Extensive activation was observed within bilateral IFG, including both the pars opercularis and pars triangularis. Activation also extended into the right anterior cingulate and paracingulate cortices, and a small amount of activation was observed within the superior frontal gyrus (pre-SMA). In posterior brain regions, activation was present in the fusiform gyrus and the parietal lobes bilaterally, as well as within subcortical regions including the right caudate, putamen, and pallidum.
Pre-SMA is specifically activated during response inhibition.
Brain regions specifically activated by the outright stopping of a motor response were identified by the contrast of correct stop with correct continue trials. Critically, the latter trial type provides a high-level baseline that controls for the attentional capture of an unexpected event, and so allows the isolation of brain regions that support response inhibition. This contrast demonstrated medial frontal activation, with peaks of activation within medial and lateral parts of the pre-SMA and the paracingulate cortex (Table S3 and Fig. 2C). Activation also extended from the pre-SMA laterally into the right middle frontal gyrus. No significant activation of the right IFG/Ins was observed, suggesting that this region was not specifically supporting response inhibition.
Distinct medial frontal regions for error processing, response conflict, and response inhibition.
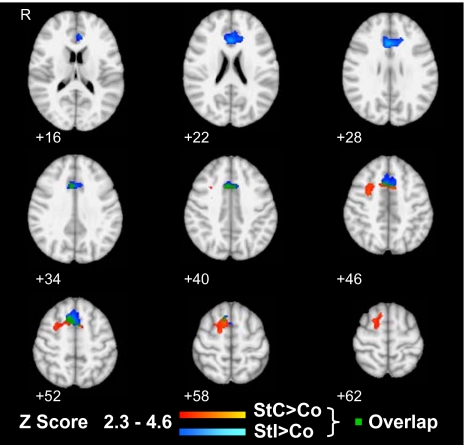
Failure to inhibit a response after a stop signal results in an erroneous button press. In keeping with previous reports of midline frontal activation for errors on the task (2, 6), incorrect stop trials in both the original and controlled versions of the SST were associated with greater ACC activation than successfully inhibited stop trials. This activation was not related to the appearance of an unexpected stimulus, as in the controlled SST a similar activation in the ACC was observed when continue trials were used as a baseline. This error-related activation was distinct from the lateral/caudal pre-SMA activation seen when responses were successfully inhibited. More medially within the pre-SMA, the patterns of activation for successfully and unsuccessfully inhibiting a motor response overlapped, with similar peaks of activation for the contrasts of both correct and incorrect stop with continue trials (Table S4 and Fig. 3).
Fig. 3.
Distinct medial frontal activations for successful response inhibition and error processing. Regions showing greater activation during the controlled SST in light to dark blue for incorrect stop trials (StI) and in yellow to red for correct stop trials (StC) compared with correct continue trials (Co). Overlap between these contrasts is shown in green. Activations are superimposed on axial sections from z = 16 to z = 62. Thresholding and overlay are as in Fig. 2.
Pre-SMA supports response slowing as well as stopping.
On average subjects slowed their responses slightly on continue trials (approximately 40 ms). This raised the possibility that incomplete response inhibition may have occurred, without the response actually being stopped. “Horse race” models of SST performance assume competition between excitatory motor processes triggered by the go signal and inhibitory processes triggered by the stop signal (1). We reasoned that slowing on continue trials could result from inhibitory processing triggered by the appearance of an unexpected event. In most situations this would be insufficient to cancel the motor response, but might produce response slowing. If the pre-SMA influences response slowing as well as outright stopping through a common inhibitory mechanism, then increased pre-SMA activation would be observed when continue trials showed high slowing. This relationship might then explain the small amount of pre-SMA activation observed for the overall continue versus go contrast.
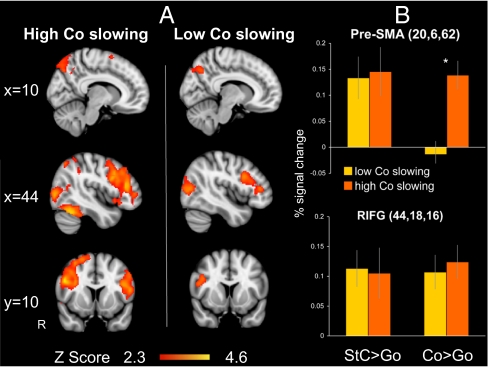
To investigate this hypothesis, we compared subjects in whom the continue trials showed a high degree of slowing against those with low slowing. Response speed on continue trials was defined by the difference between median RT for continue and go trials. As we had two runs per subject, run-to-run variability in continue slowing could be examined. Comparing the first and second runs, seven subjects changed from high to low slowing and seven subjects changed from low to high slowing (see SI Results for further information). As a result of this variability we analyzed the first and second runs separately. For the first run, a whole-brain analysis demonstrated common activation for both groups within the rIFG and the lateral occipital cortex. In contrast, activation of the pre-SMA was observed for only the high slowing group. In addition, a more extensive network of activation was observed for the high-slowing group, including activation in the right insular cortices and the left IFG, as well as bilaterally within the middle frontal gyrus, supramarginal gyrus, and fusiform gyri (Fig. 4A). Similar results were observed for the second run of the controlled SST.
Fig. 4.
Increased pre-SMA activation during response slowing. (A) Rendered images for the first run of the controlled SST showing brain regions activated for the contrast Co > Go when subjects slow their response to the Continue signal (high Co slowing) or respond as quickly as in Go trials (low Co slowing). Thresholding and overlay as in Fig. 2. (B) Percentage signal change within the right caudal/lateral pre-SMA and right inferior frontal gyrus (RIFG) for the two contrasts stop correct (StC) > go and continue correct (Co) against go (±SEM). *P < 0.05 in activation between the two groups in pre-SMA activation for continue versus go trials.
To test specifically whether the same region of the pre-SMA supports response slowing and outright stopping we used a region of interest (ROI) analysis. A pre-SMA ROI was constructed based on the coordinates of the peak of activation from the stopping contrast, along with another ROI from the rIFG (see SI Materials and Methods for further details of ROI definition). By using the contrast of stop against continue trials to generate the ROI, we were able to sample activation on the continue trials in an unbiased way, as the region was defined on the basis of increased activation during stopping relative to continuing overall. For the first run of the controlled SST, correcting for multiple comparisons, high slowing on continue trials was associated with significant activation of the pre-SMA [Run1: T(12) = 4.106, P = 0.008], but low slowing did not activate the pre-SMA. Directly comparing high and low slowing confirmed that the pre-SMA was more active when subjects slowed their responses [Run1: T(24) = −3.067, P = 0.02]. In contrast, there was no difference in right IFG activation for continue trials with high and low slowing (Fig. 4B). The same pattern was observed in the second run of the controlled SST: significantly greater pre-SMA activation for high slowing [T(24) = −2.797, P = 0.04], but no difference in rIFG activation. There was no evidence for a major strategic change in the way that individuals with high or low slowing on continue trials were performing the task, as there were no group differences on other behavioral variables (SI Results). In addition, the specificity of the slowing result for the continue trials is supported by fact the there was no group difference in the network activated by stop versus go trials, as demonstrated by whole-brain analysis of subjects with high and low slowing.
Relationship Between Brain Activation and SSRT.
We also examined the relationship between SSRT and neural activity within the pre-SMA and right IFG using predefined ROIs. Discrepant results have been reported previously for this relationship (2, 5). Mean SSRT values were calculated first across the two runs of the controlled SST. A median split was then employed to compare subjects with high versus low SSRT. No difference in the activation of either region was observed between groups with high or low SSRT (P > 0.2). There was also no significant correlation between neural activation and SSRT in these regions (P > 0.2).
Discussion
In this study we have successfully delineated the relative contributions of nodes within the frontal cortex that contribute to stopping an initiated motor action. We have achieved this by the use of a version of the SST that controls for the attentional capture of an unexpected stimulus. The SST has been adopted widely in the assessment of response inhibition, and has been applied frequently in work with clinical populations. However, previous neuroimaging studies have failed to separate the distinct cognitive processes that are involved in stopping, such as response inhibition itself, the attentional capture of an unexpected event, and error processing when stopping fails. We resolve this issue by experimentally controlling for attentional capture in a new version of the SST, and separately modeling correct and incorrect responses. In line with our hypotheses, the pre-SMA, but not the right IFG, showed a profile of activation in keeping with response inhibition. Similar activation was observed during both stopping and “continuing” within the rIFG. Errors on the task, i.e., failures of inhibition, were associated with activation of the medial frontal lobe, including the rostral ACC, which was distinct from the caudal/lateral part of the pre-SMA we observed to be specifically involved in response inhibition.
Our controlled version of the SST involved the presentation of an unexpected but behaviorally irrelevant event, with the same timing and frequency as the stop signal. These continue trials controlled for the attentional capture of an unexpected event, providing a high-level baseline with which to contrast stop trials. Caudal/lateral pre-SMA was robustly activated in the critical comparison of stop and continue trials, providing evidence for a specific role for this region in response inhibition. The introduction of continue trials did not notably alter task performance significantly as behavioral performance and brain activation patterns were similar for the preserved aspects of the new controlled and original versions of the task. The comparison of continue trials with go trials demonstrated brain regions engaged with processing an unexpected but behaviorally irrelevant cue. This revealed an extensive network that largely overlapped with that activated by stopping. In contrast to the selective activation of the pre-SMA, similar activation was observed within the right IFG/Ins region for both continuing and stopping. This profile is more in keeping with a role in attentional processing of unexpected events, regardless of whether the event signals a need to change task performance, and argues against a specific role for the right IFG/Ins in response inhibition.
The introduction of continue trials also allowed us to demonstrate that the same part of the pre-SMA is involved in both slowing a motor response, as well as in outright stopping. Reaction times for the new continue trials were, on average, approximately 40 ms longer than the go trials. This is in keeping with the unexpected continue cue triggering a degree of inhibitory processing. Activation of the pre-SMA was significantly greater when continue trials were slow, a result that was replicated across both runs of the controlled SST. This differential activation was not observed for the rIFG. This result suggests that inhibitory processing supported by the pre-SMA may result in outright stopping, if it is sufficient to overcome excitatory motor processing, or may slow a produced response by interacting with ongoing excitatory processing.
Our results are consistent with previous studies demonstrating that the medial PFC is necessary for motor inhibition (4, 9, 16, 17). In monkeys, microstimulation of the supplementary eye field within the medial prefrontal cortex improved performance on an oculomotor version of the SST by delaying saccade inhibition (9), and stimulation of the pre-SMA inhibited automatic unwanted actions while facilitating a desired alternative (10). In humans, direct cortical microstimulation of the medial PFC can produce motor inhibition (16, 17), and lesions in the medial frontal lobe impair inhibitory processing on the SST (4). Paired-pulse transcutaneous magnetic stimulation has demonstrated that the pre-SMA provides rapid context dependent modulation of motor cortical activity (18), and transcutaneous magnetic stimulation applied to the pre-SMA impairs inhibitory control during the SST (19). In addition, tasks involving either response selection or the inhibition of certain elements of a movement activate the SMA/pre-SMA (20, 21). Taken together this work delineates a role for the pre-SMA in the rapid selection of motor responses, including choosing to withhold a response and delaying a response.
Our observed pre-SMA activation is not likely to be a result of error processing, as the critical contrast of stop and continue trials involved only successful trials. In keeping with previous results (2, 6), unsuccessful stop trials were associated with midline activation within the rostral ACC. This activation is unlikely to be the result of incomplete inhibitory processing or nonspecific attentional factors, as similar activation was observed irrespective of whether successful stop or continue trials were used as a baseline. This rostral ACC activation is consistent with performance monitoring processes engaged following errors and is thought to arise from the ACC (22, 23). Previous functional MRI and PET studies (24, 25) show that activation of the ACC is associated with error processing and that selective damage to the rostral ACC impairs error processing (26).
We also observed an area of overlapping activation within the rostral pre-SMA for successful and unsuccessful inhibition. This was distinct from the more caudal/lateral pre-SMA activation seen during successful inhibition and superior to the ACC seen during failed stops (Fig. 3). Therefore, this result cannot be explained by either successful response inhibition or error processing. Instead it may relate to response conflict generated during both successful and unsuccessful stop trials from the temporal juxtaposition of go and stop stimuli. A previous study demonstrated greater activation within rostral pre-SMA for conflict trials that required the direction of eye movements to be switched compared with trials in which a preplanned eye movement was continued (27). Together these results suggest a specific role for the rostral pre-SMA in processing motor conflict that generalizes across motor outputs and includes conflict generated by competing motor plans or by the introduction of response inhibition.
A large amount of work provides evidence that the right inferior frontal region is important for cancelling or restraining a motor response, and that lesions to this area are associated with impairments of inhibitory processing (3, 5, 6, 28). However, it is not clear whether this region is critically involved in response inhibition during the SST (29). An alternative possibility is that activation within the right IFG/Ins is secondary to engagement of the ventral attentional system following the appearance of the stop signal (30). This right lateralized system includes the temporoparietal junction and right IFG/Ins, and it is engaged by the detection of unexpected stimuli. In our study, both stop and continue trials involve the unexpected (or low-frequency) appearance of a stimulus that has varying behavioral significance. Similar activation within the right IFG/Ins is observed for both trial types, suggesting that the region is involved in the attentional capture and subsequent decision-making associated with the cues.
Alternatively, it is possible that subregions within the right inferior frontal region may support distinct cognitive processes during behavioral inhibition. A recent study using a go/no-go task provides some evidence for this (28). In this study two types of go trials were used, with infrequent go stimuli controlling for the additional attentional demands of the unexpected no-go stimuli. Two ROIs were defined based on the contrast of no-go trials and frequent go trials. A region within the posterior inferior frontal gyrus showed greater response on no-go trials relative to infrequent go trials, in keeping with a specific role in response inhibition, whereas part of the right inferior frontal junction showed a profile in keeping with attentional capture. In contrast, our own results do not provide evidence for a functional dissociation within the right IFG/Ins on the SST, as we observed extensive activation of the right IFG/Ins for both stopping and continuing.
Differences between the go/no-go task and SST could be responsible for the discrepancy between our results and those of Chikazoe and colleagues (31, 32). Although the go/no-go task and SST are often thought of as interchangeable ways of assessing behavioral inhibition, they probe distinct aspects of action “restraint” and “cancellation” (32). In the go/no-go task, subjects decide whether to go before the initiation of the motor response, thus selecting a response strategy at the start of each trial (31). In contrast, the original form of the SST is specifically designed to minimize any decision-making, as the cancellation of an already initiated motor response occurs after the response strategy has been initiated. Engagement of the right IFG/Ins during behavioral inhibition may thus depend on both attentional capture and the initiation of a response strategy. This could explain the extensive activation we observed within this region during continue trials, as the introduction of an additional cue increases decision-making demands relative to the original version of the SST.
Along similar lines, distinct involvement of the pre-SMA and right IFG/Ins during behavioral inhibition may depend on the time frame over which motor inhibition is engaged (4). Strategic changes in task performance on the SST are known to affect inhibitory processing (12, 33, 34). For example, if subjects slow down on go trials in an attempt to improve “accuracy,” performance on stop trials may be changed, as subjects delay the initiation of a response strategy. In a similar way to a previous study (4), we counteracted this tendency by providing performance feedback about response speed adaptively during the experiment. Both studies reduced the amount of strategic slowing and together provide evidence that the medial PFC is critical for rapid motor inhibition.
Our findings support the results of Li and colleagues (2), who demonstrated that individuals with better motor inhibition as measured by the SSRT showed greater activation in the region of the pre-SMA. However, this is not a consistent finding in the literature (5), and the use of this type of individual difference approach has its limitations, as it assumes that the behavioral measure used to separate individuals is an accurate and reliable measure of the underlying cognitive process and that there is significant individual variability. Our study extends this previous work by experimentally controlling for attentional confounds in the baseline task and by limiting strategic changes in task performance to allow a more precise analysis of the motor inhibition, emphasizing the role of the pre-SMA.
Successful behavioral inhibition involves the interaction between the right IFG/Ins and the pre-SMA, as well as subcortical regions. Recent work has begun to investigate structural and functional connections within this network. White matter tracts directly connect the right IFG to both the pre-SMA and the subthalamic nucleus, providing a putative circuit for their interaction during motor control (5). Functional connectivity has been shown to increase between the right IFG/Ins and the pre-SMA during successful stopping, and Grainger causality analysis of the SST provides evidence that the right IFG/Ins influences motor response through its interaction with the pre-SMA (29). Thus, damage to the IFG/Ins could be expected to impair stopping secondary to a downstream effect, rather than through the disruption of neurons directly coding response inhibition.
In summary, we propose that distinct parts of the frontal lobe are engaged differentially during attempts to stop an action that has already been initiated. The attentional capture of low-frequency unexpected environmental stimuli requires the right IFG/Ins, which is engaged as part of the ventral attentional system. Recognition of the stop signal creates response conflict, which engages the rostral pre-SMA regardless of the outcome of inhibitory processing. The failure of response inhibition leads to an erroneous response, which engages an error processing system within the rostral ACC. In contrast, both successful inhibition and the slowing of an already initiated response are dependent on the caudal/lateral pre-SMA, a region able to rapidly influence motor cortical activity.
Materials and Methods
Participants.
Twenty-six right-handed healthy adult volunteers (nine women) were recruited, with a mean age of 34 y (range, 23–59 y). All participants gave written consent. The experiment was approved by the Hammersmith and Queen Charlotte’s and Chelsea Research ethics committee. Subjects had normal or corrected-to-normal vision and had no neurological, major medical, or psychiatric disorders.
SST.
The SST is a two-choice CRT task in which participants are required to respond to one or more go stimuli. At irregular intervals and unpredictably for the participants, a stop signal is presented (1). Following this stop signal, participants are required to attempt to inhibit their response to the go signal. We used two versions of the SST. The first (original SST) was similar to previous versions of the task used in neuroimaging studies. A second version (controlled SST) was designed to control for the attentional confound inherent in the original version. In both versions, subjects were presented with an initial fixation cross for 350 ms. This was followed by a go signal lasting 1,400 ms in the form of a left- or right-pointing arrow in the direction of the required response (Fig. 1). Finger presses were made with the index finger of each hand. Unpredictably, on 20% of the trials, a red circle (the stop signal) appeared above the location of the go stimulus. This stop signal indicated the need to attempt to inhibit the button press. The delay between the presentation of the go and stop signals is termed the stop signal delay (SSD). The ability to stop a response is a function of the length of the SSD. The longer the SSD, the more difficult it is to stop. The SSD was varied from trial to trial using a staircase procedure that converged subjects toward an overall performance of 50% for each run (see SI Materials and Methods for further details). In our controlled version of the SST, we introduced a continue signal in the form of a green circle presented below the go signal (Fig. 1). Subjects were instructed that, on some trials, this continue signal would appear unpredictably, but this should not alter their response, i.e., the initiated response to the go signal should be completed. The continue signal occurred on the same number of trials (20%) as the stop signal and with the same timing as the previous stop signal. The duration of both stop and continue cues was 1,400 msec minus the current SSD. Continue trials thus provide a control condition for the attentional processing associated with the presentation of an unexpected perceptual event in a context in which response inhibition is not required. We also introduced a further modification of the SST to limit any strategic slowing on the task by providing negative feedback when subjects slowed on the task (see SI Materials and Methods for a justification and further details).
MR Scanning Procedure.
Participants performed two runs of the original and controlled SST, each with 184 trials and an interstimulus interval of 1.75 s. The order of the runs was counterbalanced across subjects. The original SST consisted of 20% stop and 70% go trials, with 10% randomly interspersed “rest” trials consisting of a visual fixation cross. The controlled SST consisted of 20% stop, 20% continue, 50% go, and 10% rest trials. Stimulus order was randomized within a run (see SI Materials and Methods for more information). Before the SST, subjects performed a simple CRT task. This was identical to the original SST except that only go trials were presented. MRI data were obtained using a Philips Intera 3.0-T MRI scanner (see SI Materials and Methods for further information).
Functional MRI Analysis.
Imaging analysis was performed using FEAT (FMRI Expert Analysis Tool) version 5.98, a part of FSL version 4.1.2 [FMRIB Software Library (35)]. Image preprocessing involved realignment of EPI images to remove the effects of motion between scans, spatial smoothing using a 8-mm full-width half-maximum Gaussian kernel, prewhitening using FILM, and temporal high-pass filtering using a cutoff frequency of 1/50 Hz to correct for baseline drifts in the signal. The FMRIB Linear Image Registration Tool was used to register echoplanar imaging functional datasets into standard Montreal Neurological Institute space using the participant's individual high resolution anatomical images (36). Functional MRI data were analyzed using voxel-wise time series analysis within the framework of the General Linear model (see SI Materials and Methods for further information). Mixed-effects analysis of session and group effects was carried out by using the FMRIB Local Analysis of Mixed Effects. Final statistical images were thresholded using Gaussian random field–based cluster inference with a height threshold of Z > 2.3 and a cluster significance threshold of P < 0.05.
Supplementary Material
Acknowledgments
This work was supported by The Medical Research Council (United Kingdom) and The Hammersmith Hospitals Trustees’ Research Committee.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/1000175107/DCSupplemental.
References
- 1.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 2.Li CS, Huang C, Constable RT, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci. 2006;26:186–192. doi: 10.1523/JNEUROSCI.3741-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 4.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 5.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 7.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevrier AD, Noseworthy MD, Schachar R. Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp. 2007;28:1347–1358. doi: 10.1002/hbm.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 10.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 11.Logan GD. On the ability to inhibit thought or action: a user's guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic Press; 1994. pp. 189–239. [Google Scholar]
- 12.Liddle EB, et al. Looking before you leap: a theory of motivated control of action. Cognition. 2009;112:141–158. doi: 10.1016/j.cognition.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Fockert J, Rees G, Frith C, Lavie N. Neural correlates of attentional capture in visual search. J Cogn Neurosci. 2004;16:751–759. doi: 10.1162/089892904970762. [DOI] [PubMed] [Google Scholar]
- 14.Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- 15.Li CS, et al. Neural correlates of post-error slowing during a stop signal task: a functional magnetic resonance imaging study. J Cogn Neurosci. 2008;20:1021–1029. doi: 10.1162/jocn.2008.20071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried I, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luders H, et al. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988;29(suppl 2):S56–S65. doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- 18.Mars RB, et al. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. J Neurosci. 2009;29:6926–6931. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2008;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 21.Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci. 2009;21:1193–1203. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- 22.Gehring W, Goss B, Coles M. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 23.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychiatry. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 24.Sharp DJ, Scott SK, Mehta MA, Wise RJ. The neural correlates of declining performance with age: evidence for age-related changes in cognitive control. Cereb Cortex. 2006;16:1739–1749. doi: 10.1093/cercor/bhj109. [DOI] [PubMed] [Google Scholar]
- 25.Debener S, et al. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci. 2005;25:11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swick D, Turken AU. Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA. 2002;99:16354–16359. doi: 10.1073/pnas.252521499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachev P, Rees G, Parton A, Kennard C, Husain M. Volition and conflict in human medial frontal cortex. Curr Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikazoe J, et al. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 29.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 31.Eagle DM, Bari A, Robbins TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 32.Schachar R, et al. Restraint and cancellation: multiple inhibition deficits in attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2007;35:229–238. doi: 10.1007/s10802-006-9075-2. [DOI] [PubMed] [Google Scholar]
- 33.Alderson RM, Rapport MD, Sarver DE, Kofler MJ. ADHD and behavioral inhibition: a re-examination of the stop-signal task. J Abnorm Child Psychol. 2008;36:989–998. doi: 10.1007/s10802-008-9230-z. [DOI] [PubMed] [Google Scholar]
- 34.de Zeeuw P, et al. Inhibitory performance, response speed, intraindividual variability, and response accuracy in ADHD. J Am Acad Child Adolesc Psychiatry. 2008;47:808–816. doi: 10.1097/CHI.0b013e318172eee9. [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 36.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.