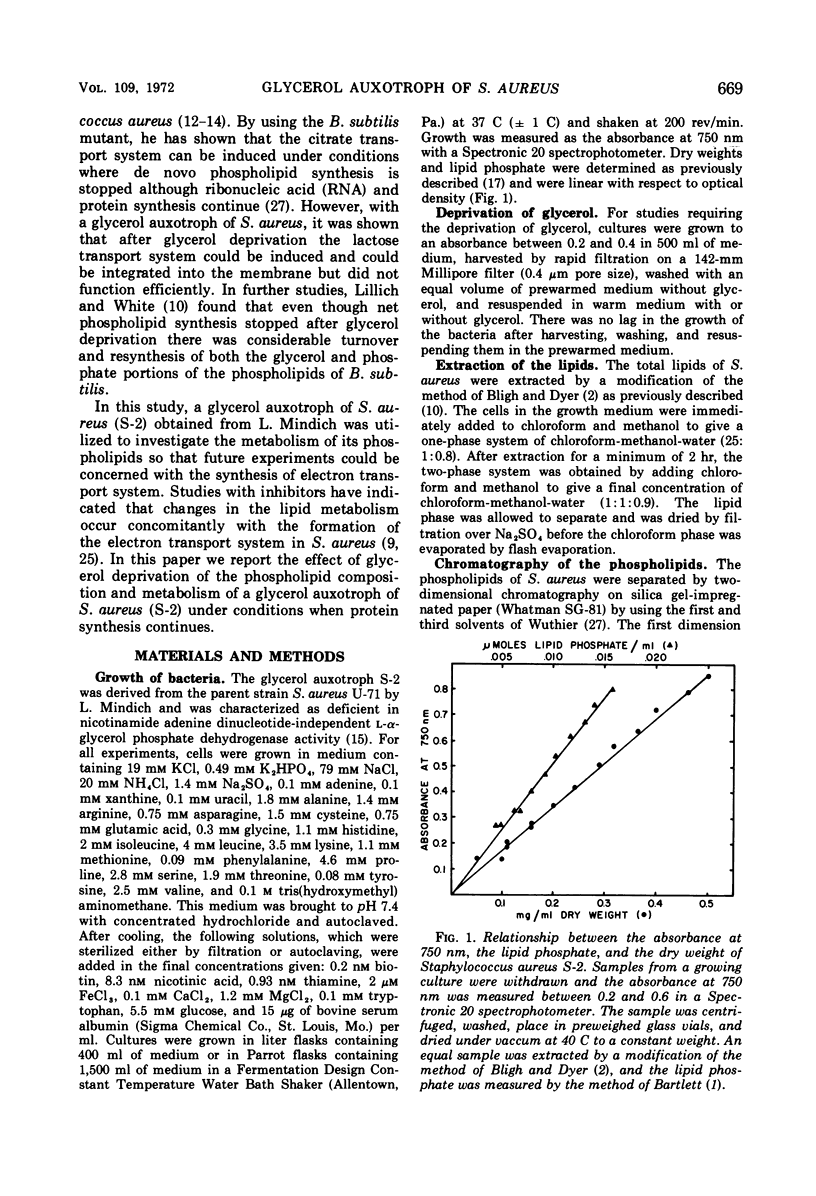
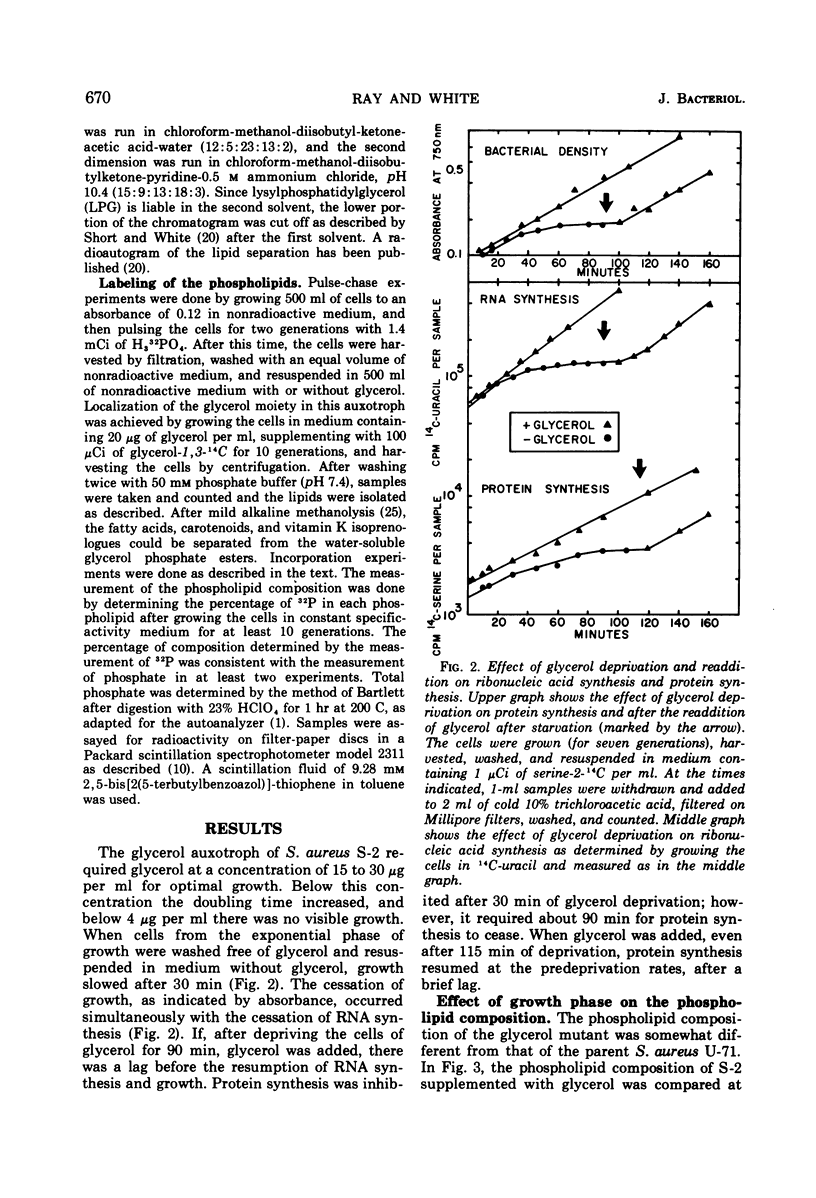
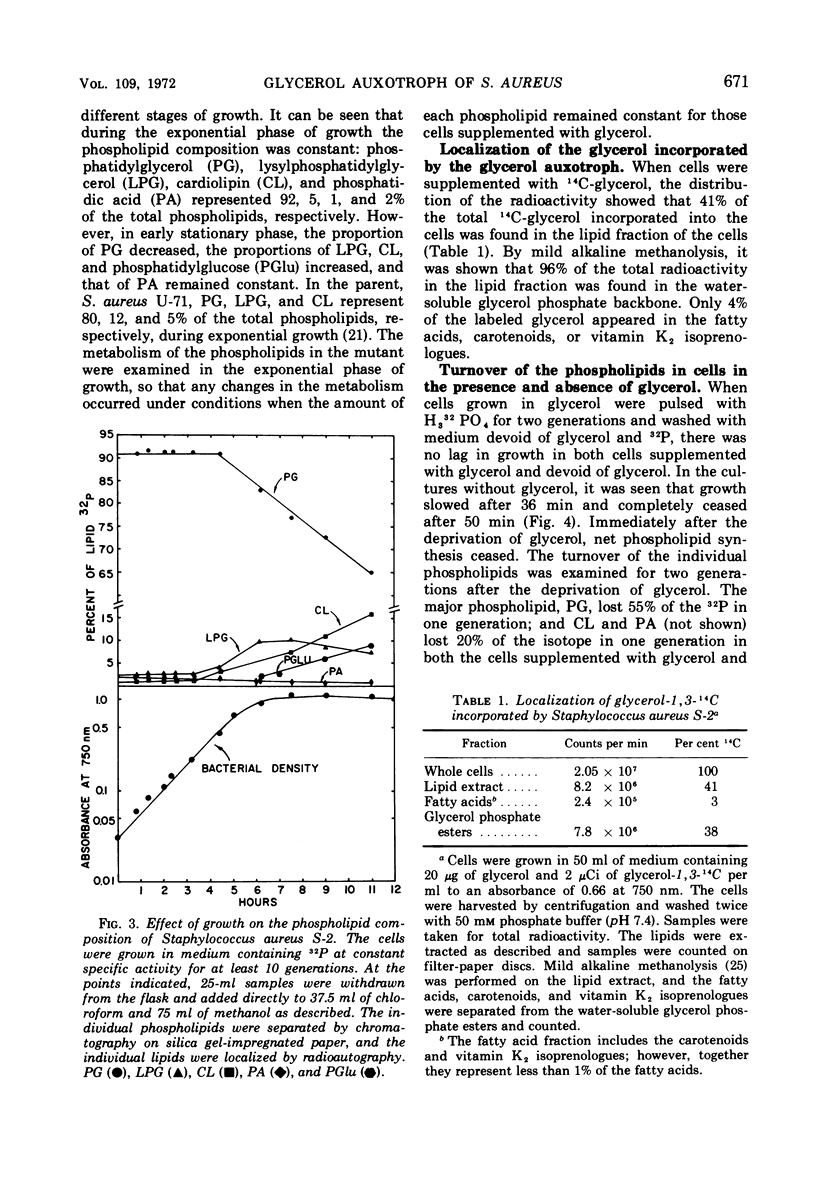
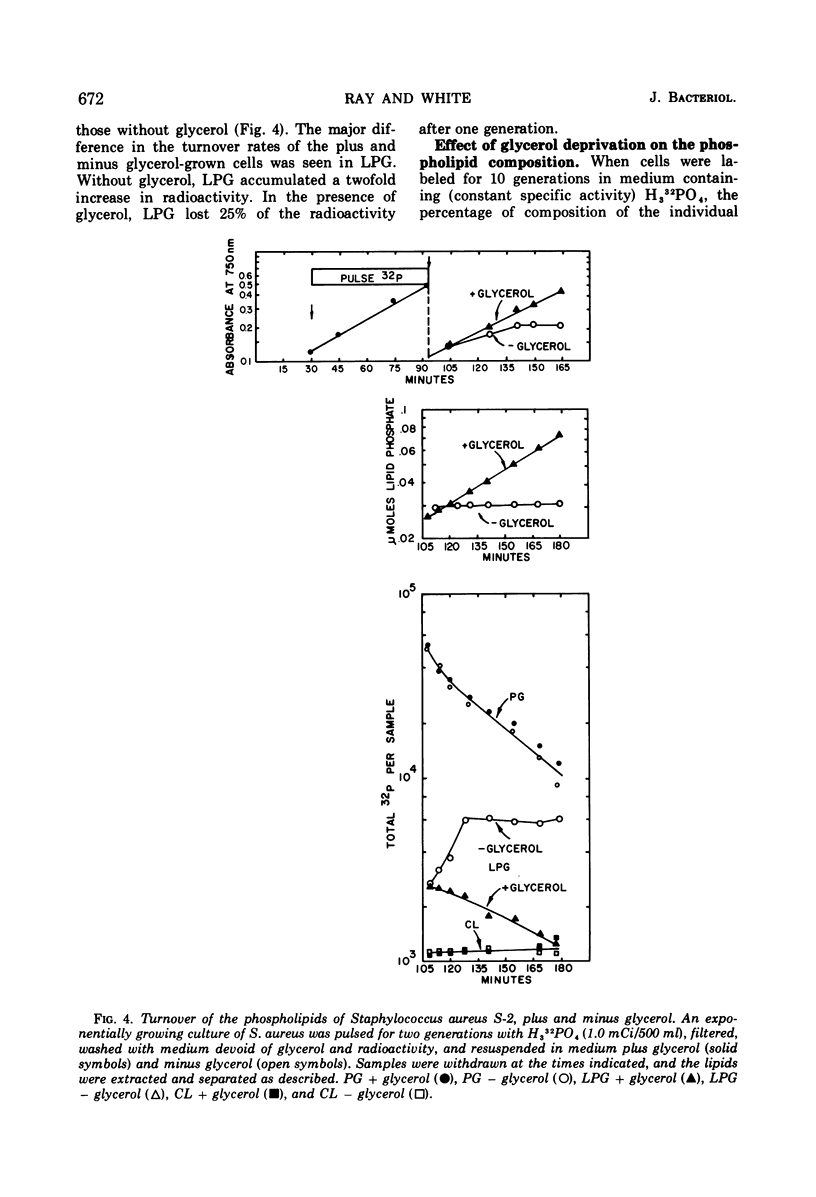
Abstract
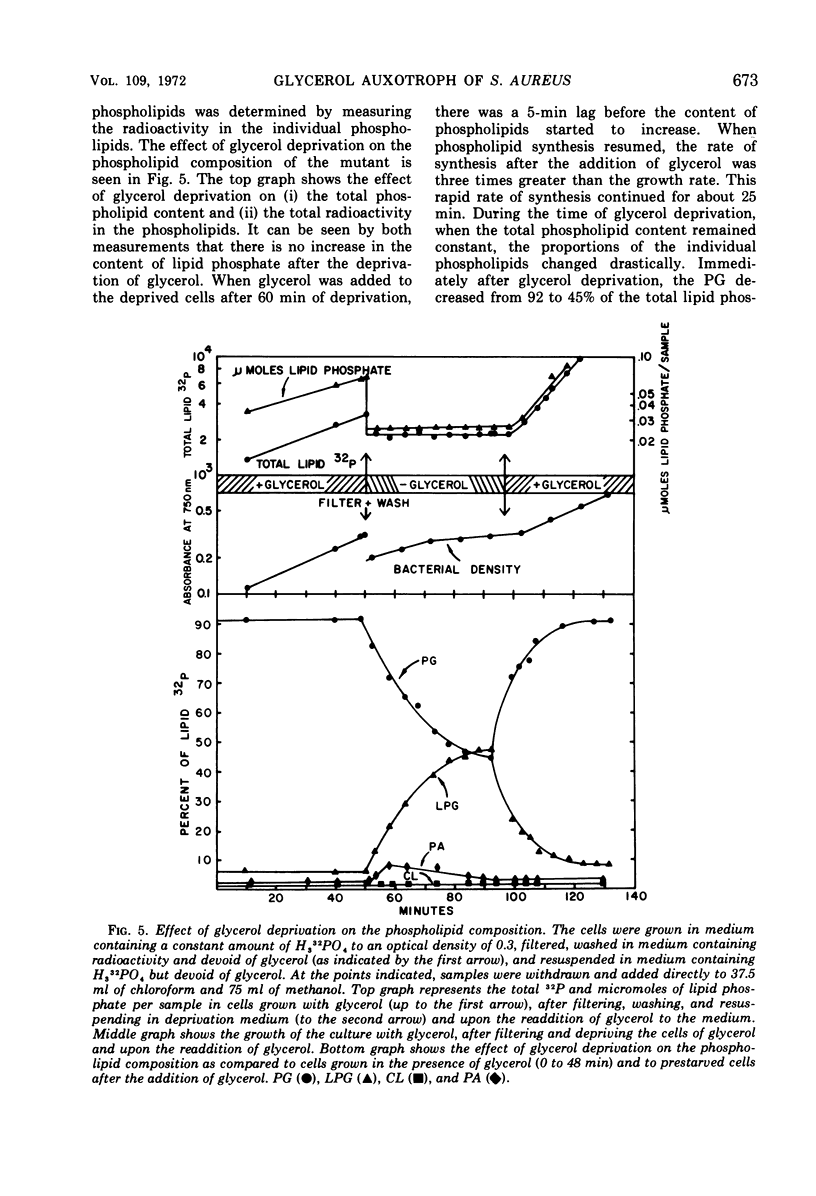
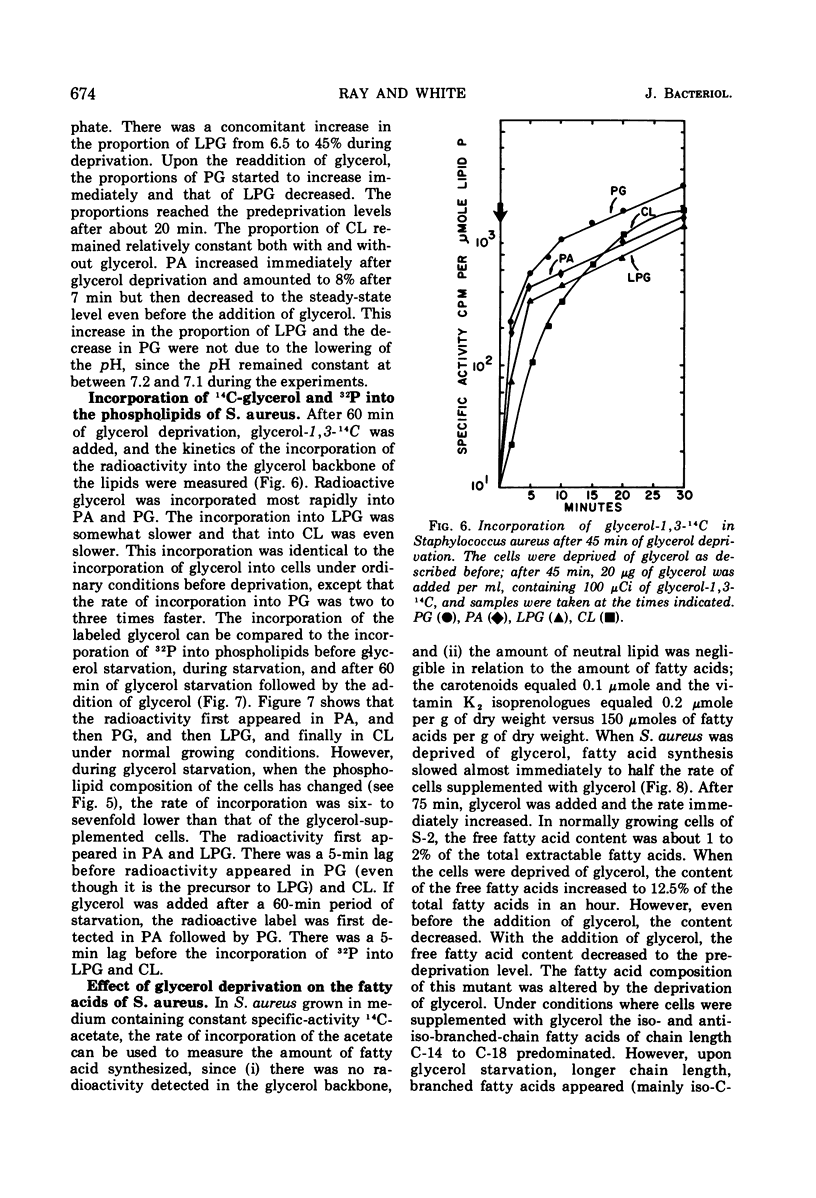
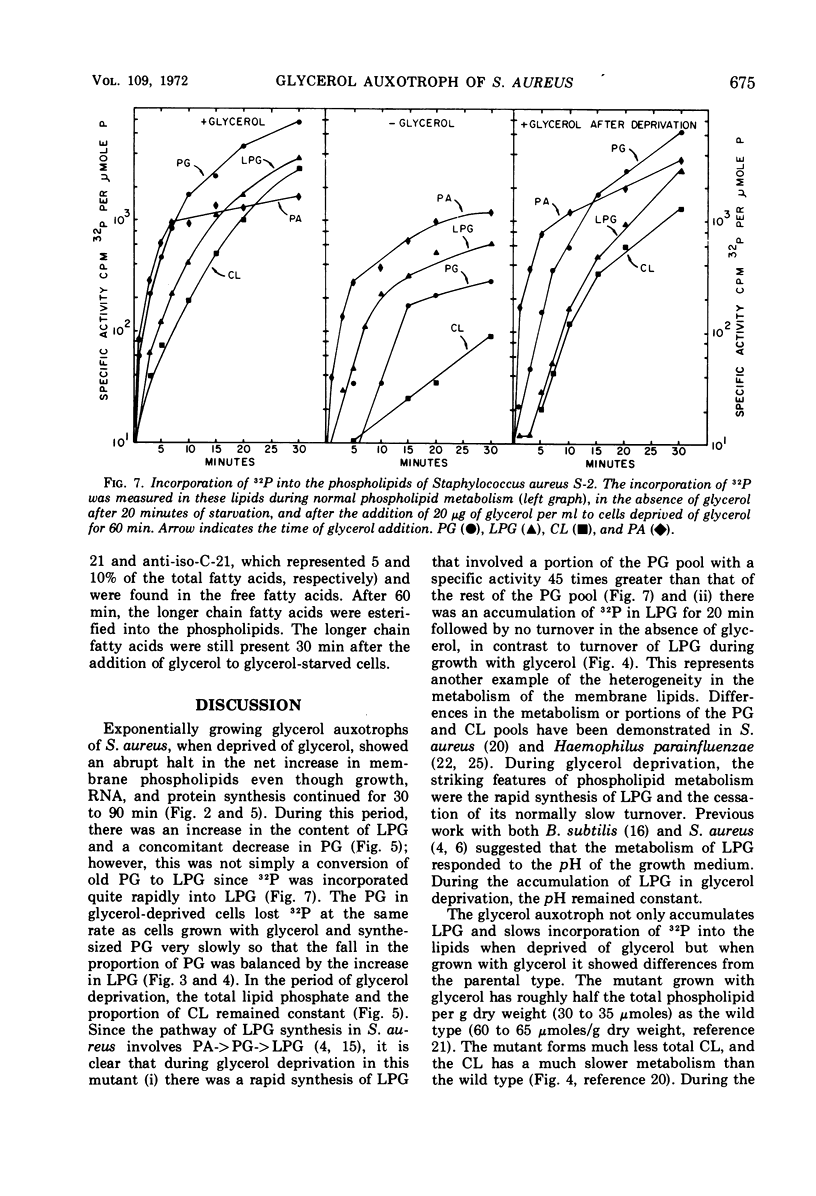
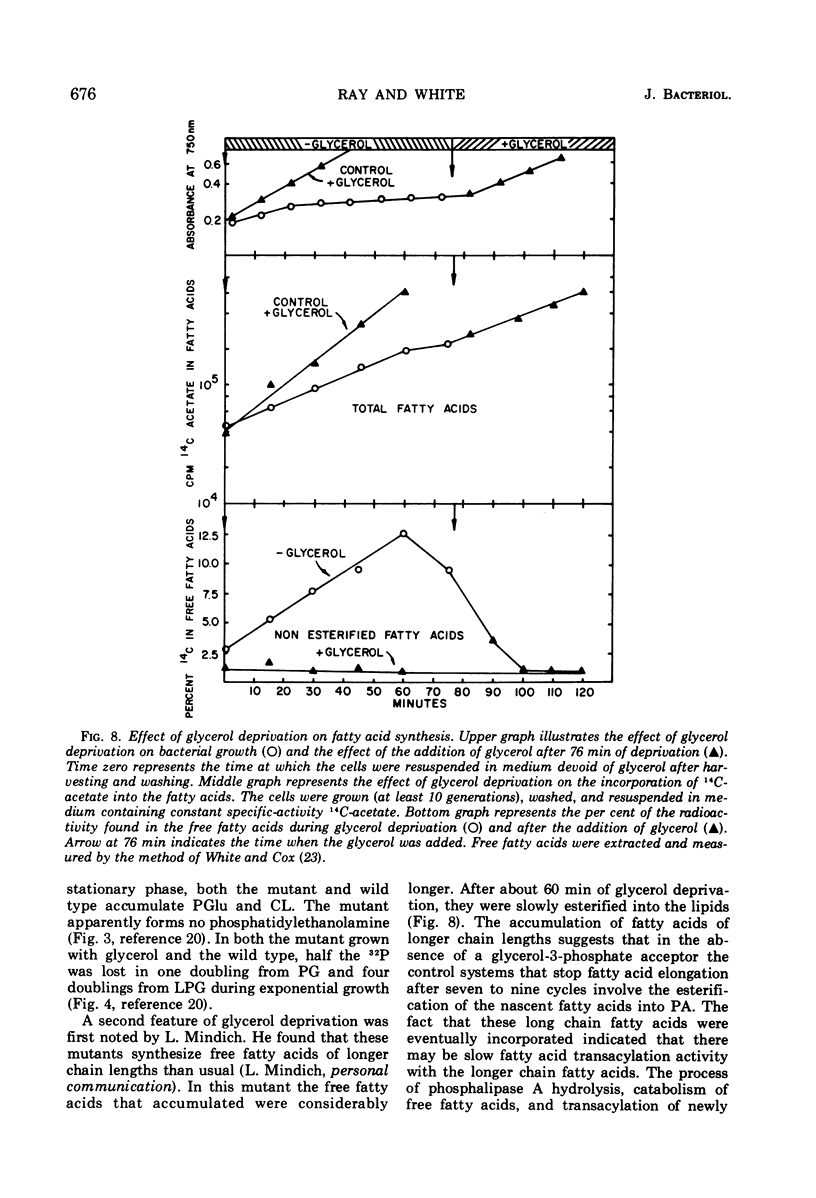
A study of the effects of glycerol deprivation on the content and metabolism of the phospholipids of a glycerol auxotroph of Staphylococcus aureus showed that (i) there was an increase in the proportions of lysylphosphatidylglycerol (LPB) and a concomitant decrease in the proportion of phosphatidylglycerol. The total phospholipid content per sample and the proportion of cardiolipin did not change, but the phosphatidic acid increased transiently and then fell to pretreatment levels. (ii) The loss of 32P from the lipids during the chase in a pulse-chase experiment was essentially the same in phosphatidylglycerol, cardiolipin, and phosphatidic acid during glycerol deprivation or growth in the presence of glycerol. LPG lost half the radioactivity in slightly more than two doubling times when grown with glycerol. In the absence of glycerol, 32P accumulated in LPG for about 20 min and then stopped, after which time there was no apparent turnover. (iii) During glycerol deprivation, the initial 32P incorporation decreased sixfold compared to that of the control with glycerol. The initial incorporation into LPG decreased only 2.5-fold, whereas that of PG decreased 45-fold. (iv) During glycerol deprivation, the free fatty acid content increased from 1.2 to 12.5% of the total extractable fatty acids and then slowly decreased. The increase was largely iso- and anti-iso-branched 21-carbon-atom fatty acids. In glycerol-supplemented cultures, the major fatty acids were branched 14- to 18-carbon fatty acids. The decrease in longer chain free fatty acids after 60 min represented their esterification into lipids. (v) During glycerol deprivation ribonucleic acid synthesis and cell growth continued for 40 min and protein synthesis continued for 90 min. Then synthesis and growth stopped. (vi) After the addition of glycerol to glycerol-deprived cells, 32P and 14C-glycerol were incorporated into the phospholipids without lag; ribonucleic acid, protein synthesis, and cell growth began after a 5- to 10-min lag at the pretreatment rate. The initial rate of lipid synthesis after the addition of glycerol was three times greater than the growth rate. This rapid rate continued for about 25 min until the lipid content and proportions of LPG and phosphatidylglycerol were restored.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. M., Lennarz W. J. Metabolism of Phosphatidylglycerol and Lysyl Phosphatidylglycerol in Staphylococcus aureus. J Bacteriol. 1970 Dec;104(3):1135–1144. doi: 10.1128/jb.104.3.1135-1144.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. H., Hammond R. K., White D. C. Changes in membrane lipid composition in exponentially growing Staphylococcus aureus during the shift from 37 to 25 C. J Bacteriol. 1970 Oct;104(1):323–330. doi: 10.1128/jb.104.1.323-330.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- Lillich T. T., White D. C. Phospholipid metabolism in the absence of net phospholipid synthesis in a glycerol-requiring mutant of Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):790–797. doi: 10.1128/jb.107.3.790-797.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner L. S., Kaback H. R. The role of phosphatidylglycerol in the vectorial phosphorylation of sugar by isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Mar;65(3):683–690. doi: 10.1073/pnas.65.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Induction of Staphylococcus aureus Lactose Permease in the Absence of Glycerolipid Synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):420–424. doi: 10.1073/pnas.68.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3088–3095. [PubMed] [Google Scholar]
- Ray P. H., White D. C., Brock T. D. Effect of temperature on the fatty acid composition of Thermus aquaticus. J Bacteriol. 1971 Apr;106(1):25–30. doi: 10.1128/jb.106.1.25-30.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Finkelstein A. Membrane biochemistry. Annu Rev Biochem. 1968;37:463–496. doi: 10.1146/annurev.bi.37.070168.002335. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Overath P. Lipids containing trans-unsaturated fatty acids change the temperature characteristic of thiomethylgalactoside accumulation in Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):209–214. doi: 10.1016/0022-2836(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol. 1971 Oct;108(1):219–226. doi: 10.1128/jb.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C. Metabolism of the glycosyl diglycerides and phosphatidylglucose of Staphylococcus aureus. J Bacteriol. 1970 Oct;104(1):126–132. doi: 10.1128/jb.104.1.126-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Detection of a rapidly metabolizing portion of the membrane cardiolipin in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1058–1064. doi: 10.1128/jb.108.3.1058-1064.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- Willecke K., Mindich L. Induction of citrate transport in Bacillus subtilis during the absence of phospholipid synthesis. J Bacteriol. 1971 May;106(2):514–518. doi: 10.1128/jb.106.2.514-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]
- den Kamp J. A., Redai I., van Deenen L. L. Phospholipid composition of Bacillus subtilis. J Bacteriol. 1969 Jul;99(1):298–303. doi: 10.1128/jb.99.1.298-303.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



