Abstract
The conserved membrane-proximal external region (MPER) of HIV-1 envelope is a target for the rare broadly neutralizing 2F5, Z13, and 4E10 monoclonal antibodies (mAbs). One strategy to elicit such antibodies is to design an immunogen with increased exposure of the 2F5 and 4E10 mAb epitopes. In this study we characterize a single leucine to serine substitution at position 669 (L669S) in the gp41 Env MPER that confers >250-fold more neutralization sensitivity to 2F5 and 4E10 mAbs than does the wild-type gp41 sequence. On synthetic liposomes, increased solvent exposure of MPER tryptophan residues and stable docking of 2F5 and 4E10 mAbs to mutant MPER peptide liposomes indicate more favorable membrane orientation of MPER neutralizing epitopes with L669S substitution. The time during which virus is sensitive to 2F5 mAb-mediated neutralization is approximately 3-fold longer when the mutation is present. These data suggest that a major contribution to the L669S mutant virus phenotype of enhanced susceptibility to MPER mAbs is prolonged exposure of the MPER neutralizing epitope during viral entry.
Keywords: immunogen, broadly neutralizing antibodies
A major challenge for an effective HIV-1 vaccine is the inability of immunogens to induce broadly neutralizing antibodies (nAbs). One goal for antibody-based HIV-1 vaccine strategies is to elicit broadly neutralizing antibodies similar in breadth to the membrane-proximal external region (MPER) 2F5 and 4E10 monoclonal antibodies (mAbs) that neutralize a majority of transmitted viruses (1). MPER-specific broadly neutralizing antibodies are rarely made in HIV-1 infection (2–4), but recent studies from our group and others have shown that 2F5-like antibodies responsible for neutralization breadth can be found in ≈0.3% of HIV-1 infected subjects (5), whereas 4E10-like antibodies can be found in ~3% of HIV-1 positive subjects (6). Vaccination with antigenic envelope constructs expressing 2F5 and 4E10 epitopes has not induced high-titered neutralizing antibodies (7–10). One hypothesis for the failure of such vaccines to elicit broadly neutralizing antibodies is that the Env epitopes presented to host B cells are not in the correct envelope conformation; for the MPER, this conformation may be the transient, prehairpin gp41 intermediate (11, 12). Another hypothesis is that 2F5 and 4E10 mAbs, which are unusual in having long hydrophobic CDR3 loops, may be particularly effective in reaching epitopes near the virion lipid bilayer, but may be difficult to induce because of down-regulation of polyreactive B cell clones through B cell tolerance mechanisms (13–18).
Previous work has identified two amino acid substitutions (T569A, I675V) (19) and L669S (5) in the MPER that increased neutralization sensitivity by MPER antibodies; however, the mechanism by which neutralization sensitivity was increased was not determined (5, 19). We now report that the mechanism of enhanced neutralization sensitivity of a single amino acid substitution (leucine to serine substitution at position 669, L669S) in the heptad repeat-2 region of gp41 is due to prolonged exposure of the MPER neutralization determinant during virus entry, such that the neutralization potencies of MPER mAbs are dramatically enhanced.
Results
A Single L669S Mutation Accounts for the Enhanced Potency of 2F5 Neutralization with TND_669S Env Pseudotyped Virus.
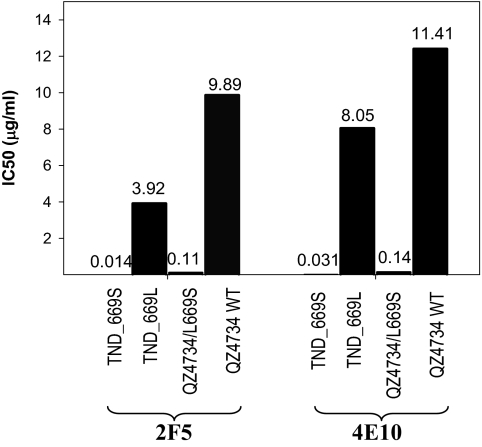
HIV-1 Env pseudotyped virus clones containing L669 alanine or serine mutations with enhanced sensitivity to MPER neutralizing antibodies have been previously reported (5, 20). For this study, we used env-pseudotyped viruses with the wild-type 669L (termed Env TND_669L pseudotyped virus) and the Env pseudotyped virus TND_669S with the 669S mutation (Fig. S1) to determine if that single mutation in the MPER was responsible for enhanced sensitivity to MPER mAb neutralization. To investigate whether the virus backbone could be involved in the phenotypic change in neutralization sensitivity, an L669S mutation was introduced into the envelope of a primary isolate, HIV-1 QZ4734 (21). The L669S mutation rendered the QZ4734 Env-pseudotyped virus more sensitive to neutralization by both 2F5 and 4E10 mAbs with similar magnitudes of increase in sensitivity (Fig. 1). The IC50 values for 2F5 mAb neutralization were 9.89 and 0.11 μg/mL for QZ4734 and QZ4734/L669S, respectively, and 11.41 and 0.14 μg/mL, respectively, for 4E10 mAb. Furthermore, Env-pseudotyped viruses made with two additional clones, 7534.2 and 7534.11, containing the L669S mutation in MPER also showed >100-fold lower IC50 for 2F5 mAb neutralization. In addition, when the mutation was reversed (an S669L mutation was introduced into the TND_669S envelope by site-directed mutagenesis), neutralization sensitivity to 2F5 mAb was reversed to that of wild-type TND_669L, confirming that the 669 leucine to serine change was solely responsible for the potency of 2F5 mAb with TND_669S pseudotyped virus. Thus, the L669S mutation by itself can dramatically increase the envelope sensitivity to MPER mAbs 2F5 and 4E10 neutralization in different virus backbones.
Fig. 1.
Neutralization of pseudotyped viruses containing the L669S mutation. IC50 values of 2F5 mAb and 4E10 mAb neutralization of a primary isolate, QZ4734 containing the L669S mutation (QZ4734/L669S) by site-directed mutagenesis are shown compared to TND_669S and TND_669L.
L669 and S669 Linear MPER Peptides Have Similar Binding to 2F5 or 4E10 mAb.
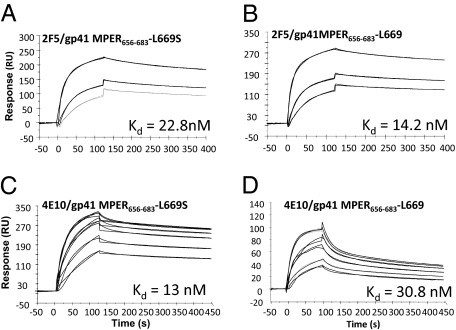
To investigate the mechanisms by which the L669S mutation increases Env pseudotyped virus neutralizing sensitivity to MPER mAbs, we used a BIAcore surface plasmon resonance (SPR) assay to study the kinetics of 2F5 mAb binding to linear MPER peptides containing either the 669 leucine (gp41MPER656–683) or the 669 serine (gp41 MPER656–683/L669S), as well as the scrambled version for each peptide. The dissociation constants (Kd) for 2F5 mAb binding to the gp41 MPER656–683 and gp41 MPER656–683/L669S peptides were 14.2 and 22.8 nM, respectively (Fig. 2), indicating that peptide gp41 MPER656–683 bound to 2F5 mAb with comparable affinity to gp41 MPER656–683/L669S. SPR with the shorter 2F5 mAb epitope (gp41 MPER652–671) (Fig. S2) also confirmed that there were no significant differences between the binding of 2F5 mAb to the wild-type and mutant two peptides. The Kd of 4E10 mAb was ~2-fold higher for the MPER peptide with the L669S substitution (Kd = 13 and 30.8 nM, respectively, for MPER656–683/L669S and MPER656–683 peptides, respectively, Fig. 2 C and D). The dissociation rate constants (koff) for the interaction of 4E10 mAb with WT and L669S peptides were 4.1 × 10−3 s−1 and 1.2 × 10−3 s−1, respectively. The 4-fold slower off rate for the mutant leads to a relatively higher affinity.
Fig. 2.
Binding of 2F5 and 4E10 mAbs to gp41 MPER peptide epitopes. Specific binding responses of 2F5 mAb (A and B: 41.6, 83.3, and 167 nM) and 4E10 mAb (C and D: 33.3, 67, 133, 200, 267, and 333 nM) to gp41 MPER656–683/L669S (A) and gp41MPER656–683 (B) peptides are shown. The Kd values shown were estimated by global curve fitting of the specific binding responses using a bivalent analyte model. Fitted curves are in black.
MPER/L669S Peptide–Lipid Conjugates Have More Favorable 2F5 and 4E10 mAb Binding Kinetics.
From spectroscopic analysis, other investigators have suggested that in peptide–lipid complexes, the side chain of L669 and several key residues of 2F5 mAb and 4E10 mAb, which include well conserved Trp residues (W666, W670, and W672), are not surface exposed but project into the membrane bilayer (22, 23). The lack of binding of MPER mAb 13H11, whose epitope includes the L669 residue, to MPER peptide liposomes also suggested that the L669 residue is not surface exposed (Table S1 and Figs. S3 and S4) and provides support to the above model (23). Therefore, a leucine (hydrophobic) to serine (hydrophilic) mutation at position 669 could result in structural rearrangements of the helical segments of gp41 MPER such that the key residues of 2F5 and 4E10 epitopes are surface exposed and more accessible to MPER mAb binding. Circular dichroism spectroscopic studies of the native and mutant peptides MPER656–683 in liposomes showed that in the longer peptide, the L669S mutation substantially enhanced helical content (Fig. S5). Such ready access of mAbs to MPER neutralizing epitopes could be one explanation for the hypersensitivity of L669 pseudotyped viruses to 2F5 and 4E10. To test this hypothesis, we compared the extent of solvent exposure of Trp residues and the binding kinetics of 2F5 and 4E10 mAbs to wild-type and L669S mutant MPER peptides conjugated to synthetic liposomes. The influence of the L669S mutation on tryptophan fluorescence emission spectra of MPER peptide liposomes was determined using the 2F5 mAb nominal epitope peptide (gp41 MPER652–671), which has two Trp residues (W666 and W670) modeled to be at the membrane interface of the lipid bilayer (22, 23). Differences in tryptophan fluorescence quenching (Ksv values) between wild-type and L669S mutant peptide–lipid conjugates indicated relatively greater solvent exposure of Trp residues in the peptide–lipid conjugates with L669S substitution (Fig. 3). The tryptophan fluorescence spectra of MPER652–671 and its L669S mutant in liposomes were further analyzed for spectral peak shift to a higher wavelength to explain enhanced exposure of tryptophans. There was a 2-nm shift to higher wavelength for the L669S mutant, indicating a slightly higher exposure of tryptophans in the mutant L669S (Fig. S6). This result was consistent with the observed favorable kinetics and lower Kd of 2F5 binding to liposome conjugates of this same peptide (gp41 MPER652–671/L669S) when compared to those of the WT (gp41 MPER652–671) (Table 1, Fig. S7). Similarly, the Kd of 4E10 mAb was also 10-fold lower for the gp41656–683/L669S peptide liposomes (Fig. 4, Table 1). For both 2F5 and 4E10 mAb binding to liposomes conjugated to MPER peptides with L669S substitution, the observed lower Kd was largely due to slower off rates of the docked complex (kd2). 2F5 mAb binding to the longer gp41656–683 gave similar Kd for both the wild-type and the L669S mutant peptide liposomes (Table 1). As described earlier (24), the observed difference in 2F5 mAb binding to the two peptides could be due to differences in the overall orientation of the MPER residues on the lipid surface. Taken together, the fluorescence quenching data and the binding kinetics data suggested that the L669S substitution may have altered the conformation of the MPER residues on the liposome surface and made the 2F5 and 4E10 mAb epitope core residues more accessible.
Fig. 3.
Effect of L669S mutation on 2F5 epitope exposure for membrane-anchored peptides. Tryptophan fluorescence emission spectra of MPER652–671 peptide liposomes (A) and MPER652–671/L669S peptide liposomes (B) at different added acrylamide concentrations are shown. Stern–Volmer plots of quenching experiments shown in A and B are shown in C and D, respectively. F0/F is the ratio between the intensities of tryptophan fluorescence in the absence of and at different concentrations of acrylamide. a.u, arbitrary unit.
Table 1.
Binding parameter of 2F5 and 4E10 mAbs to peptide liposome
| Peptide liposome | 2F5 binding |
4E10 binding |
||||||||
| ka1 | kd1 | ka2 | kd2 | Kd | ka1 | kd1 | ka2 | kd2 | Kd | |
| 105 M−1·s−1 | 10−2 s−1 | 10−2 s−1 | 10−4 s−1 | 10−9 M | 105 M−1·s−1 | 10−2 s−1 | 10−2 s−1 | 10−4 s−1 | 10−9 M | |
| MPER656–683 | 2.92 | 4.83 | 2.22 | 2.49 | 2.0 | 0.52 | 1.65 | 1.77 | 4.10 | 7.0 |
| MPER656–683/L669S | 1.00 | 3.32 | 2.22 | 1.69 | 2.0 | 1.32 | 6.48 | 3.02 | 0.41 | 0.6 |
| MPER652–671 | 9.31 | 0.69 | 1.81 | 3.70 | 0.2 | NA | NA | NA | NA | NA |
| MPER652–671/L669S | 4.98 | 0.12 | 0.54 | 0.20 | 0.009 | NA | NA | NA | NA | NA |
The calculated rate constants of association (ka1 and ka2) and dissociation (kd1 and kd2) phases in encounter and docking steps, respectively, of 2F5 and 4E10 mAb interactions with peptide liposomes are listed along with the apparent binding constant (Kd). Parameters were estimated by fitting the normalized binding responses in Fig. 4 A and B to a two-step encounter docking model. NA, not analyzed.
Fig. 4.
Binding of 2F5 and 4E10 mAbs and Fabs to peptide–liposome conjugates. Comparison of 2F5 and 4E10 mAb (A and B) and the respective Fab (C and D) binding to MPER656–683 peptide (red), MPER656–683/L669S peptide (blue), and peptide-free synthetic liposomes (black) is shown. RU, resonance unit.
L669S Mutation Prolongs the Temporal Window of 2F5 mAb Neutralization.
To assess the relevance of epitope exposure on HIV-1 neutralization, we compared the lifetime of 2F5 mAb neutralization of wild-type and L669S mutant pseudotyped viruses. The exposure during the fusion process of MPER neutralizing epitopes to MPER mAbs (2F5, 4E10) and gp41 entry inhibitor peptides has previously been estimated as 15–20 min (11, 25–28). We hypothesized that the L669S mutation could alter the MPER structure such that gp41 neutralizing epitopes are exposed for even longer. The extended window of opportunity for the MPER mAbs to sample the optimal conformation for binding could explain the observed hypersensitivity of L669S mutant pseudotyped virus. Using a virus–cell postattachment neutralization assay (25), viral infectivity as a function of time of addition of 2F5 mAb was measured for the WT Env pseudotyped virus (TND_669L) and L669S mutant Env pseudotyped virus (TND_669S). We observed that the lifetime of 2F5 neutralization was ~3-fold longer for the TND_669S pseudotyped virus (t1/2 = 45.1) when compared with TND_669L pseudotyped virus (t1/2 = 14.5 min) (Fig. 5 A and B). The inhibition kinetics of T20, a fusion inhibitor peptide that blocks six-helix bundle formation (11, 29, 30), showed that with TND_669L virus T20 was effective for 20 min postattachment. The T20 neutralization time was also prolonged (33.3 min) for the TND_669S mutant pseudotyped virus (Fig. 5 C and D). These results suggested that the L669S mutation resulted in the alteration of the time course of the presentation of MPER neutralizing epitopes, such that the time window for 2F5 mAb neutralization of HIV-1 was increased. This prolonged accessibility of MPER to 2F5 mAb provides an explanation of the hypersensitivity of L669S mutant viruses to 2F5 and 4E10 mAb neutralization.
Fig. 5.
Time course of 2F5 neutralization of TND L669 and TND 669S pseudoviruses. 2F5 mAb (A and B) or T20 peptide (C and D) at inhibitory concentrations (100 μg/mL for TND 669L and 5 μg/mL for TND 669S for 2F5 mAb and 20 μg/mL for both viruses for T20) was added at the indicated time to TZM-bl cells preincubated with either the wild-type TND 669L (A and C) or the mutant TND_669S (B and D) pseudotyped virus. The mean t1/2 values (±SE) of 2F5 and T20 neutralizations are as follows: 2F5 mAb, 14.5 ± 0.9 (WT TND_669L), 45.1 ± 2.1 (mutant TND_669S); T20, 20.2 ± 0.52 (WT TND_669L), 33.3 ± 3.2 (mutant TND_669S). t1/2 values were derived from a four-parameter sigmoid curve-fitting analysis (goodness of fit >0.98) and are means of three measurements. Three individual plots are overlaid for 2F5 mAb (A and B) and two for T20 peptide (C and D).
Neutralization Sensitivity of HIV Strains Containing L669S to Broadly Neutralizing mAbs.
The single L669S mutation that rendered the TND_669S highly sensitive to both 2F5 and 4E10 mAb neutralization was examined for sensitivity to other neutralizing monoclonal antibodies that target the HIV-1 envelope. As shown in Table 2 and Fig. S8, the IC50 of 2F5 and 4E10 mAbs against TND_669S Env pseudotyped virus was 279- and 275-fold lower, respectively, than that against TND_669L Env pseudotyped virus, whereas neutralizing sensitivities to CD4 binding site antibody 1b12, glycan-dependent antibody 2G12, and CD4i antibody 17b were not significantly affected. Interestingly, the 669S mutant was neutralized by the anti-gp120 V3 loop mAb 447–52D with an ~160-fold lower IC50 and was moderately sensitive to CCR5 binding site antibodies 1.7B and E51 and CD4i antibody 23E, whereas the TND_669L Env pseudotyped virus was resistant. Additionally, we examined whether Env expression on virus particles could be responsible for the differences in neutralization sensitivity. The relative levels of p24 and gp160 proteins were comparable between the wild-type virus and that with the L669S substitution (Fig. S9), indicating that the L669S mutation did not significantly alter Env expression on the virus. These data indicate that whereas the L669S amino acid change in the MPER did not affect HIV-1 Env sensitivity to neutralization globally or alter total Env expression, it may have resulted in conformational changes that exposed other neutralization targets in the HIV-1 envelope.
Table 2.
Neutralization of WT and L669S mutant pseudotyped viruses by different monoclonal antibodies
| mAb | TND_669S | TND_669L | Fold Δ |
| 2F5 | 0.014 | 3.915 | 279 |
| 4E10 | 0.031 | 8.054 | 275 |
| 1b12 | 0.53 | 2.06 | 3.9 |
| 2G12 | 12.5 | 16.68 | 1.3 |
| 17b | 9.73 | 19 | 2.0 |
| 1.7B | 27.5 | >50 | >1.8 |
| 23E | 24.5 | >50 | >2.0 |
| E51 | 7.6 | >50 | >6.6 |
| 447-52D | 0.31 | >50 | >161 |
The IC50 values are shown in μg/mL. Fold Δ is calculated by IC50 of WT/IC50 of L669S mutant. Neutralization curves for these tests are shown in Fig. S8. 2F5 and 4E10 are broadly neutralizing mAbs targeting MPER; 1b12 is a broadly neutralizing mAb targeting the CD4 binding site; 2G12 is a glycan-dependent mAb; 17b and 23E are CD4i mAbs; 1.7B and E51 are mAbs targeting the CCR5 binding site; 447-52D is a V3 loop mAb.
Discussion
In this study we demonstrate that the envelope gp41 MPER L669S mutation enhances Env pseudotyped virus sensitivity to 2F5 and 4E10 mAb neutralizing antibodies, but does not similarly enhance neutralization sensitivity to most other neutralizing antibodies. When introduced into peptides bound to synthetic liposomes, the mutation induces alteration in the exposure of lipid-associated, but not free MPER neutralizing epitopes, resulting in more stable association of gp41 MPER with mAb 2F5. Moreover, the period over which the virus is sensitive to 2F5 mAb and to T20 suggests that the L669S mutation also prolongs exposure of the gp41 neutralizing epitopes, thus allowing for a longer window of time during which MPER mAb binding and neutralization can occur.
Others have reported mutations in gp41 that enhanced neutralization sensitivity of the HIV-1 envelope (5, 19). Blish et al. (19) demonstrated that mutations in gp41 (T569A, I675V) resulted in enhanced neutralization sensitivity. Although the mechanism of the enhanced neutralization sensitivity was not determined in this study, Env infectivity, Env concentration, Env shedding, and fusion kinetics were found not to be responsible. Like Blish et al., we show that an amino acid substitution in the MPER can enhance neutralization sensitivity and that the differences in neutralization sensitivity are not due to differences in envelope expression on the virions. We further go on to show that an amino acid substitution in the MPER that is responsible for enhanced neutralization sensitivity indeed alters the conformation of the peptide in lipids in vitro, but, more importantly, also prolongs exposure of the MPER neutralization epitope during virus entry. Thus, our present study demonstrates the mechanism by which the MPER mutations affect the fusion kinetics of the virus. It also provides further evidence to support the notion that MPER-directed antibodies target a fusion intermediate (12).
There can be at least two contributions by L669S mutation to the increased accessibility of MPER to MPER neutralizing antibodies. The first is that at some stage following CD4/coreceptor triggering (probably at the extended intermediate stage), there might exist equilibrium between a lipid-interacting MPER (modeled by the MPER peptide tethered to a liposome) and a fully accessible MPER state. It is possible that the leucine to serine mutation shifts the equilibrium toward the state of greater MPER accessibility. A second contribution may also come from the way the serine mutant delays completion of the six-helix bundle formation. Once the gp41 postfusion six-helix bundle forms, the MPER is occluded, and thus completion of the six-helix bundle precludes 4E10 or 2F5 mAb binding. Thus if L669S creates an additional barrier to completion of the six-helix bundle, then we expect a longer lifetime for the intermediate (as observed) and hence a longer period of MPER availability. The data obtained with functional pseudotyped viruses clearly show that the L669S mutation prolongs exposure of MPER neutralizing epitopes. Whereas evidence that this mutation might also enable greater access and more stable binding of MPER neutralizing mAbs comes from work with peptides on model liposomes, a similar effect could also contribute in the context of intact Env on virions. Indeed, the enhancement of neutralization is substantially greater than the prolongation of exposure, suggesting that both factors could be relevant.
In a previous report (24), we described the observation that the docking dissociation rates of both 2F5 and 4E10 differ between the shorter nominal epitope peptide and the biepitope MPER656–683 peptide. We also showed that the MPER mAb binding rates were influenced by the immersion depth of Trp residues. Similar observations were also described by Huarte et al. (31), who reported that the free energy of partitioning from water into membrane interfaces was highest for the D664–K683 stretch and N terminus residue extension progressively reduced the overall free energy. Others reported (19, 32, 33) that amino acid changes in one region of the HIV-1 envelope can affect neutralization epitopes at distant sites, likely due to conformational changes that can unmask epitopes. In agreement with these reports, we also find that the L669S mutation results in increased sensitivity of TND_669S to the anti-V3 loop antibody 447–52D, consistent with the notion that conformational changes in the MPER could alter exposure of neutralization epitopes in other regions of the HIV-1 Env.
A critical need is for immunogens that are in preferred or native conformations of the targets of rare broadly neutralizing anti-HIV-1 Env antibodies (34). MPER mAbs 2F5 and 4E10 have long hydrophobic CDR3 regions, are polyreactive, and bind lipids (15, 35). The ability of these mAbs to bind lipids appears relevant to their mechanism of neutralization of HIV-1, in that the hydrophobic CDR3 loops are available for binding to virion lipids (13) and are required for broad neutralization (36). A structure with enhanced exposure of the MPER neutralizing epitopes might be a preferred immunogen (12). The L669S mutation in nominal gp41 MPER peptides has only minimal effects on binding of MPER neutralizing antibodies. In contrast, the L669S mutation in gp41 peptide–lipid complexes does improve 2F5 and 4E10 mAb binding, implying the requirement of lipid for enhanced somatically mutated 2F5 and 4E10 mAb binding. Moreover, the L669S mutation in HIV-1 virions leads to an ~300-fold increase in 2F5 mAb sensitivity of the mutant HIV strain compared to wild-type virions (5). Therefore, it will be of interest to determine if L669S peptide–lipid complexes and inactivated virions (37) containing the mutation will be able to more readily induce anti-MPER neutralizing antibodies.
In summary, the enhanced sensitivity of L669S mutant pseudotyped viruses to MPER mAbs is likely due to prolonged and favorable exposure of the MPER neutralizing epitopes. These data suggest that a virion bearing an envelope protein with a more antigenic membrane proximal epitope, such as in the membrane-proximal region with L669S, might have improved immunogenicity.
Materials and Methods
Sources of Peptides and Antibodies.
Peptides used in this study were synthesized by CPC Scientific and Primm Biotech and purified by reverse-phase HPLC. Purity of the peptides was assessed by HPLC to be >95% and confirmed by mass spectrometric analysis. Sequences of the peptides used are indicated in the appropriate subsections.
The following reagents were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health: HIV-1 gp41 monoclonal antibodies (2F5 and 4E10 mAbs) and HIV-1 gp120 monoclonal antibody (2G12), from Hermann Katinger; HIV-1 gp120 monoclonal antibodies (17b and E51 mAbs), from James E. Robinson; V3 monoclonal antibody (447-52D), from Susan Zolla-Pazner; and T-20 fusion inhibitor, from Division of AIDS, National Institute of Allergy and Infectious Diseases. Mabs 1.7b and 23E, CD4i-directed mAbs (38, 39), were obtained from James E. Robinson, Tulane University.
Origin of env Clones.
Mutant TND_669S, wild-type TND_669L, 7534.2, 7534.11, and QZ4734 (previously described in ref. 21) were generated using bulk PCR from plasma from clade B HIV-1+ infected subjects. For mutant TND_669S, subsequent single-genome amplification of the plasma indicated that the L669S mutation was likely not present in vivo; therefore, it could be the result of the cloning process. Alignment of 1,963 complete HIV-1 env sequences at http://HIV-1.lanl.gov revealed only 1 sequence (0.05%) containing this L669S mutation.
Molecular Cloning of Full-Length Envelopes, Production of Pseudotyped Viruses, and Neutralization Assay.
Cloning strategy of full-length gp160 has been described previously (40, 41). Production and titration of the env-pseudotyped viruses were conducted following procedures modified from methods previously described (40). The 50% tissue culture infectious dose (TCID50) of each virus preparation was determined (42). Neutralization assays with pseudotyped viruses were performed on TZM-bl cells on 96-well flat-bottom plates as previously described (40). The IC50 was determined as the concentration of Ab able to inhibit virus infection by 50% compared to the virus control (41).
Time Course of 2F5 Neutralization Assay.
The time course of neutralization of 2F5 mAb or T20 peptide was determined in a synchronized postattachment HIV-1 pseudotyped virus neutralization assay as described earlier (22). TZM-bl cells (104/well) were plated and allowed to adhere overnight. Each of the plates was then cooled and incubated on ice for 2 h following addition of cold Env pseudotyped viruses. To remove unbound viruses, plates were washed with fresh, cold medium. Warm medium (150 μL/well) was added to each well followed by 100 μL of inhibitory concentrations of either 2F5 mAb (5 or 20 μg/mL) or T20 peptide (20 μg/mL) at different time intervals (0, 10, 20, 40, 60, 120, 180, and 240 min) after infection. A32 mAb and scrambled T20 peptide were used as controls. Infectivity was measured by relative light units (RLUs) as described above for the standard neutralization assay.
SPR Assays.
All SPR binding assays were performed on a BIAcore 3000 instrument at 25 °C and data analyses were performed using the BIAevaluation 4.1 software (BIAcore) as previously described (35).
Kinetics and Affinity of 2F5 and 4E10 mAb Binding to Peptide Epitopes.
Biotinylated versions of peptides MPER657–671 (EQELLELDKWASLWN) and MPER657–671/L669S (EQELLELDKWASSWN), MPER656–683 and MPER656–683/L669S, and control peptides with scrambled sequences were individually anchored on a BIAcore SA sensor chip as described previously (35, 43). Each peptide was injected until 100–150 response units (RU) of binding to streptavidin were observed. Specific binding responses of mAb binding were obtained following subtraction of nonspecific binding on the scrambled peptide surfaces. Rate constants were measured using the bivalent analyte model (to account for the avidity of bivalent Ig molecules) and global curve fitting to binding curves obtained from 2F5 mAb titrations, which ranged from 0.01 to119 nM. 2F5 mAb was injected at 30 μL/min for 2–6 min and glycine-HCl (pH 2.0) and surfactant P20 (0.01%) were used as the regeneration buffer.
Kinetics and Affinity of 2F5 mAb and 4E10 mAb Binding to Peptide–Liposome Conjugates.
2F5 and 4E10 mAb binding to peptide–liposome conjugates was examined using a BIAcore L1 sensor chip as described previously (35). Peptide–liposome conjugates were made following an extrusion method as described earlier, using phospholipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine, 1,2-dimyristoyl-sn-glycero-3-phosphate, and cholesterol at a molar ratio of 45:25:20:1.33 and a peptide to lipid ratio of 1:420 (35). Peptide liposomes were made using MPER652–671-GTH1 (QQEKNEQELLELDKWASLWN-YKRWIILGLNKIV-RMYS), MPER652–671/L669S-GTH1 (EQELLELDKWASSWN-YKRWIILGLNKIVR-MYS), MPER656–683 (NEQELLELDKWASLWNWFNITNWLWYIK-YKRWIILGLNKIVRMYS), and MPER656–683/L669-GTH1 (NEQELLELDKWASSWNWFNITNWLWYIK-YKRWIILGLNKIVRMYS) peptides. The peptides used in constructing peptide–liposome conjugates contained either the consensus 2F5 epitope (gp41652–671–GTH1, QQEKNEQELLELDKWASLWN-YKRWIILGLNKIVRMYS) or the 2F5 epitope with the L669S mutation (gp41652–671/L669S–GTH1, EQELLELDKWASSWN-YKRWIILGLNKIVRMYS). Using BIAevaluation 4.1 software, the low levels of nonspecific binding to peptide-free control liposomes were subtracted to obtain the peptide specific binding of mAbs and were used to analyze binding of 2F5 and 4E10 mAb in a two-step encounter docking model as before (35).
Supplementary Material
Acknowledgments
We thank Shi-Mao Xia, Vicki Ashley, Judith Lucas, Kevin Saunders, Xiaozhi Lu, Regina Chustz, Kelli Greene, and Ming Li for expert technical assistance and Drs. Brandon Keele, Beatrice Hahn, and George Shaw for sequence analyses. These studies were supported by grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases [Center for HIV/AIDS Vaccine Immunology AI067854, Duke Center for AIDS Research (AI64518) Molecular Virology Core and Small Grant Award] and the Bill and Melinda Gates Foundation (Grants 38619, 38643, and 38617).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912381107/DCSupplemental.
References
- 1.Mehandru S, et al. Neutralization profiles of newly transmitted human immunodeficiency virus type 1 by monoclonal antibodies 2G12, 2F5, and 4E10. J Virol. 2004;78:14039–14042. doi: 10.1128/JVI.78.24.14039-14042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuste E, et al. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV-1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J Virol. 2006;80:3030–3041. doi: 10.1128/JVI.80.6.3030-3041.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhillon AK, et al. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from HIV-1 infected donors. J Virol. 2007;81:6548–6562. doi: 10.1128/JVI.02749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley JM, et al. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol. 2008;82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen X, et al. In vivo gp41 antibodies targeting the 2F5 monoclonal antibody epitope mediate human immunodeficiency virus type 1 neutralization breadth. J Virol. 2009;83:3617–3625. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sather DN, et al. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phogat S, et al. Analysis of the human immunodeficiency virus type 1 gp41 membrane proximal external region arrayed on hepatitis B surface antigen particles. Virology. 2008;373:72–84. doi: 10.1016/j.virol.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coëffier E, et al. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2000;19:684–693. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 9.Eckhart L, et al. Immunogenic presentation of a conserved gp41 epitope of human immunodeficiency virus type 1 on recombinant surface antigen of hepatitis B virus. J Gen Virol. 1996;77:2001–2008. doi: 10.1099/0022-1317-77-9-2001. [DOI] [PubMed] [Google Scholar]
- 10.Montero M, van Houten NE, Wang X, Scott JK. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: Dominant site of antibody neutralization and target for vaccine design. Microbiol Mol Biol Rev. 2008;72:54–84. doi: 10.1128/MMBR.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 12.Frey G, et al. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc Natl Acad Sci USA. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ofek G, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson JD, et al. An affinity-enhanced neutralizing antibody against the membrane-proximal external region of human immunodeficiency virus type 1 (HIV-1) gp41 recognizes an epitope between those of 2F5 and 4E10. J Virol. 2007;81:4033–4043. doi: 10.1128/JVI.02588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 16.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: A hypothesis. Hum Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 17.Alam SM, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: Antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkoczy L, et al. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci USA. 2009;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blish CA, Nguyen MA, Overbaugh J. Enhancing exposure of HIV-1 neutralization epitopes through mutations in gp41. PLoS Med. 2008;5:e9. doi: 10.1371/journal.pmed.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwick MB, et al. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J Virol. 2005;79:1252–1261. doi: 10.1128/JVI.79.2.1252-1261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomaras GD, et al. CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc Natl Acad Sci USA. 2000;97:3503–3508. doi: 10.1073/pnas.070521097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun ZY, et al. HIV-1 broadly neutralizing antibody extracts its epitope from a kinked gp41 ectodomain region on the viral membrane. Immunity. 2008;28:52–63. doi: 10.1016/j.immuni.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Song L, et al. Broadly neutralizing anti-HIV-1 antibodies disrupt a hinge-related function of gp41 at the membrane interface. Proc Natl Acad Sci USA. 2009;106:9057–9062. doi: 10.1073/pnas.0901474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennison SM, et al. Stable docking of neutralizing human immunodeficiency virus type 1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J Virol. 2009;83:10211–10223. doi: 10.1128/JVI.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gustchina E, Bewley CA, Clore GM. Sequestering of the prehairpin intermediate of gp41 by peptide N36Mut(e,g) potentiates the human immunodeficiency virus type 1 neutralizing activity of monoclonal antibodies directed against the N-terminal helical repeat of gp41. J Virol. 2008;82:10032–10041. doi: 10.1128/JVI.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimitrov AS, et al. Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry. 2007;46:1398–1401. doi: 10.1021/bi062245f. [DOI] [PubMed] [Google Scholar]
- 27.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 28.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: Correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 31.Huarte N, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 4E10 monoclonal antibody is better adapted to membrane-bound epitope recognition and blocking than 2F5. J Virol. 2008;82:8986–8996. doi: 10.1128/JVI.00846-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang PF, et al. A variable region 3 (V3) mutation determines a global neutralization phenotype and CD4-independent infectivity of a human immuno-deficiency virus type 1 envelope associated with a broadly cross-reactive, primary virus-neutralizing antibody response. J Virol. 2002;76:644–655. doi: 10.1128/JVI.76.2.644-655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins BA, et al. Resistance of human immunodeficiency virus type 1 to neutralization by natural antisera occurs through single amino acid substitutions that cause changes in antibody binding at multiple sites. J Virol. 1996;70:8431–8437. doi: 10.1128/jvi.70.12.8431-8437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson Hedestam GB, et al. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 35.Alam SM, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam SM. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci USA PNAS. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossio JL, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson JE, et al. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Methods. 1990;132:63–71. doi: 10.1016/0022-1759(90)90399-g. [DOI] [PubMed] [Google Scholar]
- 39.Xiang SH, Doka N, Choudhary RK, Sodroski J, Robinson JE. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res Hum Retroviruses. 2002;18:1207–1217. doi: 10.1089/08892220260387959. [DOI] [PubMed] [Google Scholar]
- 40.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im1211s64. Chap 12:Unit 12.11. [DOI] [PubMed] [Google Scholar]
- 42.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 43.Alam SM, et al. An inducible HIV type 1 gp41 HR-2 peptide-binding site on HIV type 1 envelope gp120. AIDS Res Hum Retroviruses. 2004;20:836–845. doi: 10.1089/0889222041725181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.