Abstract
c-Jun N-terminal kinase (JNK) 1-dependent signaling plays a crucial role in the development of obesity-associated insulin resistance. Here we demonstrate that JNK activation not only occurs in peripheral tissues, but also in the hypothalamus and pituitary of obese mice. To resolve the importance of JNK1 signaling in the hypothalamic/pituitary circuitry, we have generated mice with a conditional inactivation of JNK1 in nestin-expressing cells (JNK1ΔNES mice). JNK1ΔNES mice exhibit improved insulin sensitivity both in the CNS and in peripheral tissues, improved glucose metabolism, as well as protection from hepatic steatosis and adipose tissue dysfunction upon high-fat feeding. Moreover, JNK1ΔNES mice also show reduced somatic growth in the presence of reduced circulating growth hormone (GH) and insulin-like growth factor 1 (IGF1) concentrations, as well as increased thyroid axis activity. Collectively, these experiments reveal an unexpected, critical role for hypothalamic/pituitary JNK1 signaling in the coordination of metabolic/endocrine homeostasis.
Keywords: brain, diabetes, obesity, inflammation, insulin resistance
Obesity causes increased circulating concentrations of cytokines such as tumor necrosis factor (TNF) α and interleukin (IL) 6 in animal models as well as humans (1, 2). These proinflammatory mediators act on peripheral tissues, causing peripheral insulin resistance via inflammatory and stress signaling cascades including activation of c-Jun N-terminal kinase (JNK) signaling (3). Thus, cytokine and fatty acid-induced activation of JNK1 causes insulin resistance in vitro and in vivo (4, 5). JNKs are able to regulate transcription, survival, apoptosis, and other cellular events in response to diverse stimuli such as UV irradiation or cytokine stimulation (6). Interestingly, JNK1 plays an important role in obesity-associated pathologies, because conventional JNK1 but not JNK2 knockout mice are protected from obesity-induced hyperglycemia, hyperinsulinemia, and insulin resistance (4, 7). Moreover, fat cell-specific disruption of JNK1 provided evidence for an important role of adipose tissue JNK1 in glucose metabolism (8).
Glucose homeostasis and body weight are under control of hypothalamic circuits regulating food intake, energy expenditure, and hepatic glucose production (9). The hypothalamus has been identified as one of the main targets both for insulin and leptin (10). Consistently, insulin and leptin action on hypothalamic neuron subpopulations is necessary for normal body weight control and glucose homeostasis (11–15).
Recent findings indicate that obesity leads to activation of stress signaling cascades not only in peripheral, classical insulin target tissues such as skeletal muscle, liver, and adipose tissue, but also in the central nervous system thereby causing neuronal insulin and leptin resistance (16). Similar to findings in peripheral tissues, obesity causes an increase in NFκB activity in the hypothalamus, and neuronal inactivation or pharmacological inhibition of inhibitor of NFκB kinase (IKK) 2 signaling protects from insulin and leptin resistance (17, 18), in line with the notion that proinflammatory cytokines, fatty acids, or both activate the NFĸB pathway in neurons (18–20).
However, the role of central JNK1 signaling in insulin and leptin resistance is less clear. In rats, it has been shown that high-fat diet (HFD) feeding increases total hypothalamic JNK activity, and intracerebroventricular (ICV) application of a general JNK inhibitor ameliorated hyperphagia and obesity (21). Moreover, conventional JNK1 knockout mice showed reduced adiposity, potentially indicating improved central leptin and/or insulin action in these animals (4). In light of these findings, we ablated JNK1 specifically in the CNS and pituitary cells. We report an unexpected role of neuronal and pituitary JNK1 as a regulator of hypothalamic and peripheral insulin sensitivity as well as somatic growth.
Results
High-Fat Feeding Causes Neuronal and Pituitary JNK Activation.
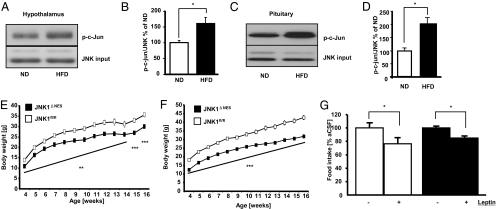
To address the role of central JNK1 signaling in regulation of energy homeostasis, we analyzed the activation of the JNK signaling cascade in hypothalami of diet-induced obese mice. Hypothalamic JNK activity, as assessed by the ability of immunoprecipitated JNK to phosphorylate c-Jun in vitro, was significantly increased upon exposure to HFD (Fig. 1 A and B). Obesity is associated with numerous endocrine abnormalities affecting the somatotrophic, thyroid, and glucocorticoid axes (22–24). Thus, we asked whether obesity not only promotes JNK activation in the CNS, but also in the pituitary. Indeed, we detected significantly increased JNK activity in pituitaries of HFD-fed mice, indicating that JNK-dependent signaling may play a role in pituitary regulation of peripheral metabolism (Fig. 1 C and D).
Fig. 1.
JNK1ΔNES mice show reduced body weight but unchanged leptin sensitivity. (A) High-fat diet (HFD) feeding increases hypothalamic JNK activation. C57bl/6 mice were fed either ND or HFD for 8 weeks, and hypothalami were microdissected. Total JNK activity in this tissue was measured by performing JNK kinase assays, and phosphorylation of the JNK target c-Jun was detected by immunoblot. JNK1/3 loading was used as input control. A representative immunoblot from one ND and one HFD animal is shown. (B) Quantification of HFD-induced JNK activation in the hypothalamus. The ratio of p-c-Jun to JNK1/3 input was quantified in Western blots of hypothalami from 8 ND and 8 HFD-fed animals as shown in A. (C) High-fat diet (HFD) feeding increases pituitary JNK activation. C57bl/6 mice were fed either ND or HFD for 8 weeks, and pituitaries were isolated. Total JNK activity in this tissue was measured by performing JNK kinase assays, and phosphorylation of the JNK target c-Jun was detected by immunoblot. JNK1/3 loading was used as input control. A representative immunoblot from one ND and one HFD animal is shown. (D) Quantification of HFD-induced JNK activation in the pituitary. The ratio of p-c-Jun to JNK1/3 input was quantified in Western blots of pituitaries from 4 ND and 4 HFD-fed animals as shown in C. (E) Average body weight of JNK1fl/fl (▫) and JNK1ΔNES (■) mice on normal diet (n = 12 per group). (F) Average body weight of JNK1fl/fl (▫) and JNK1ΔNES (■) mice on high-fat diet (n = 12 per group). (G) Twenty-four-hour food intake after intracerebroventricular leptin treatment in JNK1fl/fl (▫) (n = 5) and JNK1ΔNES (■) mice (n = 4) mice on normal diet at the age of 12 weeks. Mice were injected with either vehicle or 2 μg leptin immediately before onset of dark phase, and food intake was measured 24 h later. Displayed values are means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Generation of JNK1ΔNES Mice.
To unravel the functional role of JNK1 in the CNS and pituitary, we generated mice with targeted ablation of JNK1. It has recently been demonstrated that in addition to neurons, the Nestin gene is also expressed in pituitary stem cells, allowing deletion of target genes in all cell types of the adult pituitary using Nestin-Cre transgenic mice (25). Hence, after generation of mice with a loxP-flanked JNK1 allele (Fig. S1C), we crossed these animals with mice expressing the Cre recombinase under control of the Nestin promoter to subsequently generate mice homozygous for the loxP-flanked JNK1 allele and positive for the Nestin-Cre transgene (genotype JNK1fl/fl, Nestin-Cre+, i.e., JNK1ΔNES mice). Littermates negative for Cre were used as controls (JNK1fl/fl, called controls henceforth). As noted in similar studies, we assured that neither presence of the Nestin-Cre transgene nor loxP sites per se impaired control of body weight (Fig. S2A) (19).
Immunoblot analyses of JNK protein expression revealed unchanged JNK1 expression in peripheral tissues, whereas JNK1 expression was largely reduced in the brain of JNK1ΔNES mice compared to controls (Fig. S2B). Thus, hypothalamic expression of JNK1 was decreased by more than 95% in JNK1ΔNES mice, whereas expression of JNK2 or JNK3 remained unaltered (Fig. S2C). Importantly, we also detected pituitary JNK1 deletion in JNK1ΔNES mice using PCR-based analysis (Fig. S2D). Notably, CNS JNK1 ablation did not affect anxiety, motor control, or memory retrieval in behavioral tests (Fig. S3 A–F).
Reduced Body Weight but Unchanged Adiposity in JNK1ΔNES Mice.
To investigate the potential role of neuronal/pituitary JNK1 in control of energy homeostasis and metabolism, we analyzed male control and JNK1ΔNES mice exposed to normal diet (ND) and high-fat diet (HFD). Beginning at the age of 4 weeks, body weight of JNK1ΔNES mice on either diet was decreased compared to control animals (Fig. 1 E and F). Next, we measured epigonadal fat pad weight and body composition of control and JNK1ΔNES mice. Surprisingly, body composition was unchanged in JNK1ΔNES mice compared to control mice (Fig. S4A). Accordingly, although absolute weight of epigonadal fat pads was decreased in JNK1ΔNES mice (Fig. S4B), the relation of fat pad to body weight was unchanged between genotypes (Fig. S4C). Consistent with unaltered fat mass in JNK1ΔNES mice, we could not detect significant alterations in food intake (Fig. S4D), physical activity (Fig. S4E), or respiratory quotient (Fig. S4E) in JNK1ΔNES mice. We have previously demonstrated that JNK1ΔNES mice are not protected from obesity-induced leptin resistance (20). In line with unchanged leptin sensitivity also under ND conditions, intracerebroventricular (icv) injection of leptin reduced body weight and food intake to the same extent in lean control and JNK1ΔNES mice, indicating that homeostatic control by leptin is not changed upon central JNK1 ablation (Fig. 1G and Fig. S4F). Taken together, central JNK1 ablation affects body weight, but does not affect body composition or leptin action in mice.
Impaired Somatic Growth in JNK1ΔNES Mice.
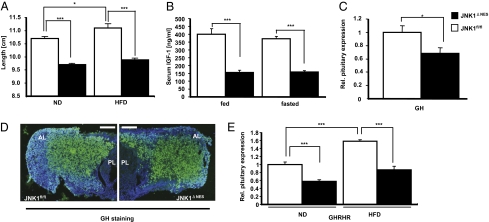
As body composition was unchanged, but body weight was significantly decreased in JNK1ΔNES mice compared to control mice, we sought to determine whether somatic growth differed between these two groups of mice. Importantly, somatic growth and insulin sensitivity are interlinked, as reduced somatic growth induced either genetically or by caloric restriction (CR) improves glucose homeostasis and insulin sensitivity in mice, rats, apes, and humans, whereas caloric overabundance will lead to increased somatic growth (26–30). Strikingly, JNK1ΔNES mice showed significantly decreased naso-anal length on both diets (Fig. 2A). Although high-fat diet caused a significant increase in length (increase of 0.4 cm, P = 0.01) in control animals compared to control animals on normal diet, this diet-induced elongation was blunted in JNK1ΔNES mice (increase of 0.2 cm, not significant) (Fig. 2A).
Fig. 2.
JNK1ΔNES mice show decreased activation of the somatotrophic axis. (A) Naso-anal length of JNK1fl/fl (▫) (n = 9 vs. 9) and JNK1ΔNES (■) (n = 9 vs. 9) on normal or high-fat diet at the age of 16 weeks. (B) Serum IGF1 concentrations of JNK1fl/fl (▫) (n = 10) and JNK1ΔNES (■) (n = 10) on normal diet either random fed or fasted at the age of 10 weeks. (C) Pituitary expression of GH of JNK1fl/fl (▫) (n = 6) and JNK1ΔNES (■) mice (n = 6) at the age of 16 weeks as measured by real-time–PCR. (D) Immunohistochemistry for GH from pituitary sections of JNK1fl/fl (▫) and JNK1ΔNES (■) mice at the age of 16 weeks (green, GH; blue, DAPI). At least 3 mice of each genotype were analyzed. (Original magnification, ×100.) (Scale bar, 80 μm.) PL, posterior lobe; AL, anterior lobe. (E) Pituitary expression of GHRHR of JNK1fl/fl (▫) (n = 6) and JNK1ΔNES (■) mice (n = 6) fed either normal chow diet or high-fat diet at the age of 16 weeks as measured by real-time PCR. Displayed values are means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
In light of these findings, we examined somatotrophic regulation in both groups of mice. Somatic growth is controlled via growth hormone releasing hormone (GHRH), which is secreted by hypothalamic neurons, and acts on somatotrophs in the pituitary to produce and release growth hormone (GH). GH in turn acts on GH receptors on the liver, inducing insulin-like growth factor 1 (IGF1) transcription and release, which acts on chondrocytes to increase longitudinal growth (for review see ref. 31). Strikingly, serum IGF1 levels were decreased by 50% in JNK1ΔNES mice (Fig. 2B), as was hepatic IGF1 mRNA expression (Fig. S4G). Despite considerable variation of GH concentrations in control mice, presumably due to the pulsatile secretion pattern of GH (32), JNK1ΔNES mice showed decreased circulating GH concentrations (Fig. S4H).
However, hypothalamic mRNA expression of both GHRH and somatostatin, which can inhibit GH release, was unchanged, indicating that JNK1-mediated regulation of the somatotrophic axis does not occur in the hypothalamus (Fig. S4I). Thus, we assessed expression of GH mRNA directly in the pituitary and found decreased GH mRNA expression in pituitaries of JNK1ΔNES mice (Fig. 2C). However, immunohistochemical analysis of GH expression revealed unaltered pituitary structure, indicating that cell loss does not account for the decrease in GH mRNA (Fig. 2D).
On the other hand—consistent with unchanged GHRH expression but decreased GH production—GHRH receptor (GHRHR) expression was decreased in pituitaries from JNK1ΔNES mice compared to control mice, indicating that decreased GHRHR signaling may account for the decreases in circulating GH and subsequently IGF1 (Fig. 2E). Taken together, JNK1ΔNES mice show decreased pituitary GHRHR expression, and subsequently reduced GH and IGF1 serum levels, and ultimately impaired somatic growth, revealing an unexpected critical role for pituitary JNK1 signaling in control of somatic growth and body length.
JNK1ΔNES Mice Show Increased Central Insulin Sensitivity.
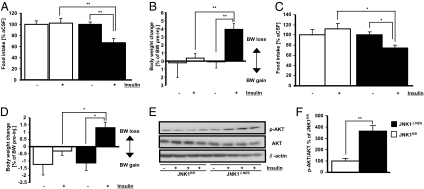
We next asked whether central insulin action may be altered in JNK1ΔNES mice (18). Intracerebroventricular administration of insulin at a dosage that had no effect on food intake and body weight in control animals reduced food intake and triggered significant weight loss in JNK1ΔNES mice fed ND (Fig. 3 A and B). Insulin’s anorexigenic effect was also retained under HFD conditions in JNK1ΔNES mice (Fig. 3 C and D), indicating that central JNK1 ablation improves central insulin sensitivity under ND and HFD conditions. Importantly, improved hypothalamic insulin action in the absence of JNK1 was additionally confirmed by increased AKT activation (phosphorylation) upon icv. insulin treatment in JNK1ΔNES mice (Fig. 3 E and F).
Fig. 3.
JNK1ΔNES mice show increased hypothalamic insulin sensitivity. (A) Twenty-four-hour food intake after intracerebroventricular insulin treatment in JNK1fl/fl (▫) (n = 9) and JNK1ΔNES (■) (n = 8) mice on normal diet at the age of 12 weeks. Mice were injected with either vehicle or 2 mU insulin immediately before onset of dark phase, and food intake was measured 24 h later. (B) Body weight loss 24 h after intracerebroventricular insulin treatment in JNK1fl/fl (▫) (n = 9) and JNK1ΔNES (■) (n = 8) mice on normal diet at the age of 12 weeks. Mice were injected with either vehicle or 2 mU insulin immediately before onset of dark phase, and body weight was measured 24 h later. Shown is percent change of body weight after insulin injection compared to vehicle injection. (C) Twenty-four-hour food intake after intracerebroventricular insulin treatment in JNK1fl/fl (▫) (n = 5) and JNK1ΔNES (■) (n = 5) mice on high-fat diet at the age of 10 weeks. Mice were injected with either vehicle or 4 mU insulin immediately before onset of dark phase, and food intake was measured 24 h later. (D) Body weight change 24 h after intracerebroventricular insulin treatment in JNK1fl/fl (▫) (n = 5) and JNK1ΔNES (■) (n = 5) mice on high-fat diet at the age of 10 weeks. Mice were injected with either vehicle or 4 mU insulin immediately before onset of dark phase, and body weight was measured 24 h later. Shown is percent change of body weight after insulin injection compared to ACSF injection. (E) Hypothalamic AKT activation upon icv insulin treatment is improved in JNK1ΔNES mice. JNK1fl/fl and JNK1ΔNES mice on high-fat diet at the age of 10 weeks were fasted for 48 h, injected with ACSF or 4 mU insulin, and killed 20 min later. Immunoblot was performed for phosphorylated (activated) AKT, total AKT, and β-actin protein content. (F) Quantification of insulin-induced AKT phosphorylation compared to total AKT content shown in Fig. 3E. N = 3 per group. Displayed values are means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
JNK1ΔNES Mice Are Protected from Diet-Induced Glucose Intolerance and Insulin Resistance.
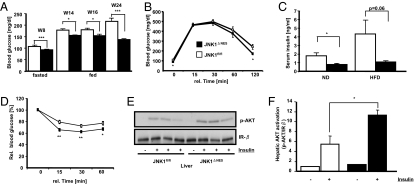
We next addressed whether elevated CNS insulin sensitivity as well as reduced GH levels altered peripheral glucose homeostasis and insulin sensitivity. Indeed, random fed and fasted blood glucose concentrations were significantly reduced in lean (Fig. S5A) and obese JNK1ΔNES mice compared to controls (Fig. 4A). Concomitantly, these mice performed significantly better in glucose tolerance tests (Fig. 4B), exhibited decreased serum insulin concentrations (Fig. 4C) and a significantly improved performance in insulin tolerance tests (ITTs) (Fig. S5B and Fig. 4D).
Fig. 4.
JNK1ΔNES mice show improved glucose homeostasis and elevated insulin sensitivity. (A) Fasted and fed blood glucose concentration of JNK1fl/fl (▫) and JNK1ΔNES (■) mice on high-fat diet at the indicated ages (n = 10–25 per group). (B) Intraperitoneal glucose tolerance test in JNK1fl/fl (▫) and JNK1ΔNES (■) mice on high-fat diet at the age of 14 weeks (n = 10 per group). (C) Serum insulin concentrations in JNK1fl/fl (▫) and JNK1ΔNES (■) mice on normal and high-fat diet at the age of 8–10 weeks (n = 8 per group). (D) Intraperitoneal insulin tolerance test in JNK1fl/fl (▫) and JNK1ΔNES (■) mice on high-fat diet at the age of 15 weeks (n = 9–10 per group). (E) Insulin-stimulated hepatic AKT-phosphorylation in JNK1fl/fl and JNK1ΔNES mice on high-fat diet at the age of 12 weeks (n = 3 per group). (F) Quantification of insulin-stimulated hepatic AKT phosphorylation as shown in Fig. 4E. Displayed values are means ± SEM. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
To identify the tissues responsible for the improved glucose homeostasis, we directly determined liver insulin sensitivity, because hepatic glucose production is under both direct (hepatic) and indirect (CNS) control of insulin action (9, 11). Intraperitoneal insulin injections led to enhanced AKT phosphorylation in obese JNK1ΔNES mice (Fig. 4 E and F), indicating that JNK1ΔNES mice are protected from diet-induced hepatic insulin resistance. Moreover, in line with previous reports that CNS insulin action up-regulates hepatic IL6 expression to reduce gluconeogenesis, we detected 1.6-fold elevated IL6 expression in livers of JNK1ΔNES mice compared to controls (Fig. S5C) (11). Besides enhanced insulin sensitivity, we also noted reduced hepatic glucose production as measured by pyruvate tolerance test, possibly due to reduced GH stimulation (Fig. S5D). Collectively, central JNK1 ablation enhances hypothalamic and hepatic insulin sensitivity.
JNK1ΔNES Mice Show Enhanced Thyroid Activation.
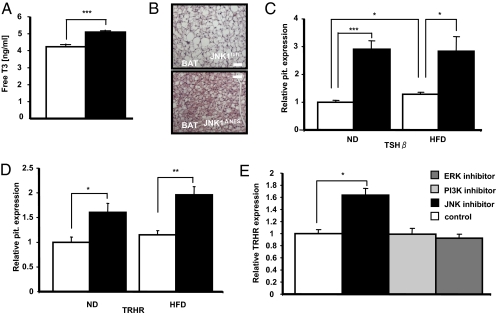
We examined whether other pituitary functions besides the somatrophic axis were affected in JNK1ΔNES mice. Although corticosterone concentrations were unchanged between control and JNK1ΔNES mice (Fig. S6A), serum T3 levels were surprisingly increased in the latter (Fig. 5A). In line with increased T3 levels, we detected increased O2 consumption in JNK1ΔNES mice (Fig. S6B), and additionally lipid load of brown adipocytes in interscapular brown adipose tissue (BAT) of JNK1ΔNES mice appeared less (Fig. 5B) and UCP1 mRNA expression tended to be increased (Fig. S6C).
Fig. 5.
JNK1ΔNES mice show increased activation of the thyrotropic axis. (A) Serum-free T3 concentration of JNK1fl/fl (▫) (n = 8) and JNK1ΔNES (■) mice (n = 8) on normal diet at the age of 10 weeks. ***, P ≤ 0.001. (B) Representative H&E staining of brown adipose tissue of control and JNK1ΔNES mice on high-fat diet at the age of 16 weeks. BAT sections of 3 mice per genotype were analyzed. (C) Pituitary expression of TSHβ of JNK1fl/fl (▫) (n = 6) and JNK1ΔNES (■) mice (n = 6) on normal chow or high-fat diet at the age of 16 weeks as measured by real-time PCR. *, P ≤ 0.05; ***, P ≤ 0.001. (D) Pituitary expression of TRHR of JNK1fl/fl (▫) (n = 6) and JNK1ΔNES (■) mice (n = 6) on normal chow or high-fat diet at the age of 16 weeks as measured by real-time PCR. *, P ≤ 0.05; **, P ≤ 0.01. (E) Expression of TRHR in the rat pituitary cell line GH4C1. Expression of TRHR was measured after 16 h incubation with either 0.1% DMSO (control), SP600125 (JNK inhibitor), LY294002 (PI3K inhibitor), or PD98059 (ERK inhibitor). For each sample within an experiment, triplicate values were averaged and then the means of the real-time–PCR results from three independent experiments were compared. *, P ≤ 0.05.
We next asked whether hypothalamic mRNA expression of thyrotropin-releasing hormone (TRH) was also increased. Real-time analysis revealed no consistent change in TRH mRNA expression on either diet, again pointing to a pituitary autonomous dysregulation of thyroid control in these mice (Fig. S6D). In line with the finding of increased circulating T3 concentrations, we observed a more than 3-fold increase in thyroid-stimulating hormone β (TSHβ) mRNA expression in JNK1ΔNES mice (Fig. 5C). Also TRH receptor (TRHR) expression was increased in pituitaries of JNK1ΔNES mice (Fig. 5D). Immunohistological analysis of TSHβ positive cells did not show significant structural alterations in pituitaries of JNK1ΔNES mice, indicating that TRHR expression, but not a change in TSHβ cell number, underlies the increased expression of TSHβ mRNA (Fig. S6E).
To further study the pituitary cell-autonomous regulation of TRHR expression, we employed a rat pituitary cell line (GH4C1), which has been previously used to study TRHR expression (33). Although incubation with either a PI3K or an ERK inhibitor did not have an effect on TRHR expression, incubation with a JNK inhibitor significantly increased TRHR expression by 70% (Fig. 5E). Thus, in line with the increase in pituitary TRHR expression of JNK1ΔNES mice, this finding highlights the importance of JNK-dependent signaling in pituitary, cell-autonomous regulation of the thyroid axis. Taken together, JNK1ΔNES mice show increased thyroid action caused by an increase in pituitary TSHβ and TRHR expression.
Discussion
Our study identifies JNK1 as a specific inhibitor of hypothalamic insulin action and thus together with earlier findings in adipose tissue-specific JNK1-deficient mice partially explains the improved glucose homeostasis and systemic insulin sensitivity in conventional JNK1 knockout mice (4, 8). Moreover, JNK1ΔNES mice are protected from obesity-associated hepatic steatosis and adipose tissue inflammation (Fig. S7), further underlining the comprehensive protection from obesity-associated pathologies.
Moreover, the current study reveals unique important insights into the role of JNK1 in pituitary control of somatic growth and thyroid function. We show that similar to the hypothalamus and peripheral tissues, JNK activation in the pituitary is increased upon HFD feeding. This finding has profound effects on somatic growth, energy expenditure, and glucose metabolism, as demonstrated in JNK1ΔNES mice. Here, we show that JNK1 deficiency in somatotroph pituitary cells reduces GHRH receptor and subsequently GH mRNA expression, translating into significantly decreased circulating GH and IGF1 serum concentrations ultimately resulting in mildly reduced somatic growth.
As indicated in multiple studies of dwarfism and caloric restriction, mice with reduced GH and IGF1 levels are insulin sensitive, demonstrate decreased serum glucose concentrations, and interestingly, show increased life span and decreased aging-associated pathologies (26–29). Notably, caloric restriction does not further improve insulin sensitivity (and life span) in growth hormone receptor-deficient mice, indicating that caloric restriction elicits its beneficial effects by dampening of the somatotrophic axis (34). It is conceivable that JNK activation in times of nutritional surplus evokes hormonal changes resulting in somatic growth, i.e., increased GH expression. In line with this model, overfeeding and/or obesity, induced by mutations in genes essential for weight control, increases and accelerates somatic growth in rodents and humans, and we show that in control animals, HFD feeding significantly increases pituitary expression of GHRHR, offering a unique mechanism for obesity-associated overgrowth (24, 30, 35). Taken together, pituitary JNK1 deficiency in control of somatotrophic function can cooperate with the insulin-sensitizing effect of JNK1 deficiency in the CNS to improve peripheral glucose metabolism (Fig. 5G).
Furthermore, our study reveals a critical role for JNK1 in pituitary control of thyroid function, as demonstrated by increased T3 serum concentrations, pituitary TSHβ expression, and energy expenditure in JNK1ΔNES mice especially under HFD conditions. Although corrected for lean mass, the energy expenditure in obese JNK1ΔNES mice may be confounded by decreased body length and mass. However, the well-documented hyperthyroidism along with altered BAT morphology indicate a true increase in energy expenditure in these mice. Additionally, we show that JNK inhibition increased TRHR expression in vitro, further demonstrating the pituitary-autonomous role of JNK1 signaling in control of thyroid regulation. Because control of TRHR and GHRHR transcription is only partly understood, and at this point we cannot exclude an additional role for altered hypothalamic TRH/GHRH release, future work must be aimed at identifying the mechanisms by which JNK1 controls regulation of both.
Collectively, the pleiotropic effects of CNS and pituitary JNK1 deficiency result in a phenotype that has previously been connected to healthy aging (increased insulin sensitivity accompanied by reduced glucose, insulin, and GH levels). Thus, further analysis of JNK1ΔNES mice with respect to control of life span may allow new insights into the connections between metabolism, growth, and aging.
Methods
Intracerebroventricular Leptin and Insulin Stimulation.
Cannulas were implanted as previously described (36), except that the lateral ventricle was targeted using coordinates located using a Brain Atlas (coordinates were bregma 1.0 mm lateral, 0.2 mm caudal, and 2.0 mm ventral). Mice were allowed to recover for 1 week after surgery. For baseline measurements, mice were injected with 2 μL artificial cerebrospinal fluid (ACSF) immediately before onset of dark phase. Food intake was measured 24 h later. After a 1-day break, ACSF injection was repeated. After an additional 1-day break, either 2 μg mouse leptin (Sigma-Aldrich), 2 mU (ND), or 4 mU (HFD) porcine insulin (Sigma-Aldrich) were injected and food intake measured 24 h later. Leptin and insulin were dissolved according to manufacturer’s instructions to generate stock solutions, and fresh aliquots were dissolved in ACSF immediately before injection. Injected volume was always 2 μL.
Western Blotting and JNK Assay.
Indicated tissues were dissected and homogenized in homogenization buffer with a polytron homogenizer (IKA Werke), and Western blot analyses were performed by standard methods with antibodies raised against insulin receptor subunit β (Santa Cruz, sc-711), β-actin (Sigma, no. 4970), JNK1/3 (Santa Cruz, sc-474), phospho-S473-AKT, and pan-AKT (Cell Signaling) (37). SAPK/JNK assays were performed following the manufacturer’s guidelines (no. 9810; Cell Signaling).
Body Composition.
Body fat content was measured in vivo by NMR using a minispec mq7.5 (Bruker Optik) as previously described (38).
Glucose, Insulin, and Pyruvate Tolerance Tests.
Glucose, insulin, and pyruvate tolerance tests were performed as previously described (39).
Intraperitoneal Insulin Stimulation.
Insulin stimulated hepatic AKT phosphorylation was determined as previously described (8).
Immunohistochemistry.
For GH stainings, pituitaries were extracted, put rapidly in tissue freezing medium, and sectioned on a cryostat. Stainings were performed as previously reported (12). The GH antibody (A0570) was purchased from DAKO.
Statistical Methods.
Data were analyzed for statistical significance by two-tailed unpaired Student’s t test unless indicated otherwise.
Supplementary Material
Acknowledgments
We thank G. Schmall and Tanja Rayle for excellent secretarial assistance and Pia Scholl, Sonja Becker, and Jens Alber for outstanding technical assistance. This work was supported by grants from the Center of Molecular Medicine Cologne (TV2) and the European Union (LSHM-CT-2003-503041) to J.C.B., the Fritz Thyssen Stiftung (Az. 10.04.1.153/Az. 10.06.2.175) to J.C.B., the European Foundation for the Study of Diabetes/Lilly European Diabetes Research Programme to J.C.B., and the German Research Foundation (Deutsche Forschungsgemeinschaft) (Br. 1492/7-1) to J.C.B., and research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013), acronym “TOBI,” under grant agreement no. 201608 (to J.C.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001796107/DCSupplemental.
References
- 1.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 2.Vgontzas AN, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 4.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MT, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 6.Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal kinases (JNKs): Differences revealed by gene targeting. Bioessays. 2006;28:923–934. doi: 10.1002/bies.20458. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (2005) 2008;32(Suppl 7):S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signaling in POMC and AgRP neurons. J Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz MW. Neuronal pathways regulating food intake and body adiposity. Ann Endocrinol (Paris) 2002;63:117–120. [PubMed] [Google Scholar]
- 11.Könner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Belgardt BF, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura T, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 15.Gropp E, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- 16.Velloso LA, Araújo EP, de Souza CT. Diet-induced inflammation of the hypothalamus in obesity. Neuroimmunomodulation. 2008;15:189–193. doi: 10.1159/000153423. [DOI] [PubMed] [Google Scholar]
- 17.Posey KA, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Kleinridders A, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 22.Jung R. Endocrinological aspects of obesity. Clin Endocrinol Metab. 1984;13:597–612. doi: 10.1016/s0300-595x(84)80040-7. [DOI] [PubMed] [Google Scholar]
- 23.Salehi M, Ferenczi A, Zumoff B. Obesity and cortisol status. Horm Metab Res. 2005;37:193–197. doi: 10.1055/s-2005-861374. [DOI] [PubMed] [Google Scholar]
- 24.Vignolo M, Naselli A, Di Battista E, Mostert M, Aicardi G. Growth and development in simple obesity. Eur J Pediatr. 1988;147:242–244. doi: 10.1007/BF00442687. [DOI] [PubMed] [Google Scholar]
- 25.Gleiberman AS, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6:620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Coschigano KT, et al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 28.Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 29.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kappeler L, et al. Early postnatal nutrition determines somatotropic function in mice. Endocrinology. 2009;150:314–323. doi: 10.1210/en.2008-0981. [DOI] [PubMed] [Google Scholar]
- 31.Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson JO, Edén S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 33.Høvring PI, Matre V, Fjeldheim AK, Loseth OP, Gautvik KM. Transcription of the human thyrotropin-releasing hormone receptor gene-analysis of basal promoter elements and glucocorticoid response elements. Biochem Biophys Res Commun. 1999;257:829–834. doi: 10.1006/bbrc.1999.0545. [DOI] [PubMed] [Google Scholar]
- 34.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin NM, et al. Abnormalities of the somatotrophic axis in the obese agouti mouse. Int J Obes (2005) 2006;30:430–438. doi: 10.1038/sj.ijo.0803076. [DOI] [PubMed] [Google Scholar]
- 36.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Brüning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 38.Mesaros A, et al. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Brüning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.