Abstract
The cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in many segments of the mammalian nephron, where it may interact with and modulate the activity of a variety of apical membrane proteins, including the renal outer medullary potassium (ROMK) K+ channel. However, the expression of CFTR in apical cell membranes or its function as a Cl− channel in native renal epithelia has not been demonstrated. Here, we establish that CFTR forms protein kinase A (PKA)-activated Cl− channels in the apical membrane of principal cells from the cortical collecting duct obtained from mice. These Cl− channels were observed in cell-attached apical patches of principal cells after stimulation by forskolin/3-isobutyl-1-methylxanthine. Quiescent Cl− channels were present in patches excised from untreated tubules because they could be activated after exposure to Mg-ATP and the catalytic subunit of PKA. The single-channel conductance, kinetics, and anion selectivity of these Cl− channels were the same as those of recombinant mouse CFTR channels expressed in Xenopus laevis oocytes. The CFTR-specific closed-channel blocker CFTRinh-172 abolished apical Cl− channel activity in excised patches. Moreover, apical Cl− channel activity was completely absent in principal cells from transgenic mice expressing the ΔF508 CFTR mutation but was present and unaltered in ROMK-null mice. We discuss the physiologic implications of open CFTR Cl− channels on salt handling by the collecting duct and on the functional CFTR–ROMK interactions in modulating the metabolic ATP-sensing of ROMK.
The kidney functions in volume, osmotic, and ionic homeostasis by regulating and coordinating activities of ion, water, and solute transport proteins that are axially distributed along the kidney nephron. The cystic fibrosis transmembrane conductance regulator (CFTR) protein is highly expressed in many segments of the mammalian nephron (1–3), where it can function in these homeostatic processes as a regulator of other transport proteins (4, 5). We (6–8) and others (9) have identified CFTR as an important regulator of the 30 pS inward rectifier potassium channel ROMK (Kir1.1) that mediates potassium secretion by distal nephron segments (10). The expression of CFTR in apical plasma membranes is required for the gating of ROMK by cytosolic ATP, providing a link between cell metabolism and potassium secretory activity (8, 9). Accordingly, the sensitivity of ROMK to ATP is lost in CFTR knockout and CFTR-ΔF508 transgenic mice (8). Interaction of ROMK with CFTR is also required for partial inhibition of ROMK channel activity by the sulfonylurea compound glibenclamide (6–8).
CFTR also functions as a cAMP-dependent, protein kinase A (PKA)-regulated Cl− channel in many epithelia (4, 11). Several studies have found forskolin (FSK)- or 1-desamino-8-D-arginine vasopressin (dDAVP)-stimulated electrogenic Cl− secretion in apical membranes of mouse principal cells in primary culture (12–14) and a whole-cell Cl− conductance in the M1 principal cell line (15). The PKA-stimulated currents have been suggested to be due to CFTR, on the basis of expression of CFTR mRNA and/or protein in these cultured cells, the absence of Cl− currents in cells cultured from CFTR null mice (14), and an anion permeability sequence compatible with CFTR in M1 cells (15). A small apical Cl− conductance has also been found in primary cultures of rabbit principal cells (16, 17), and single Cl− channel activity exhibited some of the characteristics of human CFTR. Although these studies suggest that cultured renal principal cells may express CFTR, the presence of CFTR Cl− single-channel activity in native renal tubule epithelial cells and the role of any such channel activity in kidney function have not been established (2).
PKA-regulated CFTR Cl− channel activity may have relevance to its functional interaction with the ROMK channel because metabolic regulation of ROMK by CFTR is modifiable by increasing the activity of PKA in thick ascending limb and cortical collecting duct (CCD) principal cells (7, 8, 18). For example, preexposing CCDs from wild-type mice to FSK and 3-isobutyl-1-methylxanthine (IBMX) results in the complete loss of the inhibitory effect of cytosolic ATP on ROMK channels (8). Thus, we explore here whether mouse principal cells from freshly isolated CCDs exhibit PKA-activated CFTR Cl− channels on their apical membranes. Comparing Cl− channel activity and characteristics in principal cells of wild-type mice, in principal cells from ΔF508-CFTR transgenic mice, and in Xenopus laevis oocytes expressing mouse CFTR provides definitive evidence for CFTR functioning as an apical Cl− channel in the mammalian kidney.
Results
PKA-Activated Cl− Channels in Apical Membranes of Principal Cells from Wild-Type Mice.
We determined whether Cl− channels were present in apical membranes of principal cells in cell-attached patches and whether these channels could be activated by cAMP/PKA.
Fig. 1A shows a representative cell-attached patch recording from the apical membrane of a CCD segment isolated from a wild-type mouse using a patch pipette solution optimized for detecting Cl− currents (i.e., containing no permanent cations as well as containing barium and amiloride). Little current activity was observed on initiating the cell-attached patch. Within 1 min after exposure of the cell to 10 μM FSK + 1 mM IBMX, channel activity increased, consistent with the activation or insertion of CFTR Cl− channels.
Fig. 1.
Single-channel recordings showing Cl− channel activity in a cell-attached patch held at 40 mV (–Vp) (A) or in an excised inside-out patch held at 80 mV (B) from apical membranes of mouse principal cells. Recording in A was made before and after stimulation by 10 μM FSK and 1 mM IBMX. Recording in B was made before and after stimulation by Mg-ATP and PKA. C, closed level. O1-Oi, open state levels.
Because FSK/IBMX can enhance insertion of CFTR into apical membranes as well as activate preexisting quiescent Cl− channels, we assessed the effect of Mg-ATP and PKA on Cl− channel activity in excised inside-out patches of the principal cell apical membrane. A representative current tracing is shown in Fig. 1B before and after the sequential addition of Mg-ATP and the catalytic subunit of PKA to the bath solution. After excision of the patch into a bath containing 145 mM N-methyl-d-glucamine (NMDG)-Cl that was identical to the pipette solution, a few brief channel openings were observed at a holding potential of +80 mV. Addition of 0.5 mM Mg-ATP had little effect on channel activity in this patch. This concentration of Mg-ATP should have been sufficient to activate maximally any mouse CFTR channels that were already phosphorylated in the patch (19). Subsequent addition of 50 nM PKA rapidly led to the appearance of three Cl− channel current levels, demonstrating activation of quiescent Cl− channels by PKA in a patch. Thus, apical membranes of principal cells from the CCD contain Cl− channels in both the quiescent and active states.
Fig. 2A shows representative channel characteristics of apical Cl− channels in an inside-out excised patch from a principal cell at membrane potentials (−Vp) of 40 and 80 mV using symmetrical 145 mM NMDG-Cl bath and pipette solutions with addition of 0.5 mM Mg-ATP and 50 nM PKA in the bath. Fig. 2B shows the representative current–voltage relationships of single Cl− channels activated by FSK/IBMX in cell-attached and excised inside-out patches. In cell-attached patches the reversal potential was −22 mV, and the mean conductance between −80 mV and 0 mV was 7.9 pS. In excised patches, the reversal potential in symmetrical Cl− was 0 mV, and the mean slope conductance was 6.59 pS (Table 1).
Fig. 2.
Single-channel recordings showing Cl− channel activity after stimulation by 10 μM FSK and 1 mM IBMX in apical membranes of mouse principal cells (A) and in X. laevis oocytes expressing mouse CFTR (C) held at the indicated potentials. Current–voltage plots in mouse principal cells (B) and in X. laevis oocytes expressing mouse CFTR (D) after stimulation by FSK and IBMX. The reversal potential of -Vp for cell-attached patches in B is −22 mV. Assuming an intracellular [Cl] of 30 mM and a chloride equilibrium potential of −41.4 mV, the apical membrane potential in the cell-attached configuration would be −19.4 mV. C, closed level; O1, O2, open state levels.
Table 1.
Single-channel properties of Cl− channels
| Cell | G (pS) | Po* | to (ms)* | tc (ms)* |
| Mouse CCD† (n = 3) | 6.59 ± 0.03 | 0.06 ± 0.02 | 6.81 ± 2.49 | 5.66 ± 1.37 128 ± 1.36 |
| X. laevis oocyte‡ (n = 5) | 5.62 ± 0.12 | 0.08 ± 0.04 | 4.12 ± 1.21 | 1.81 ± 1.01 30.49 ± 2.97 |
All data are from inside-out patches. G, slope conductance.
*Patches held at −Vp = 40 mV.
†Apical membrane from principal cells of wild-type mouse CCD.
‡X. laevis oocytes expressing mouse CFTR.
Channels from excised patches exhibited a low open probability (Po) of 0.06 with one open time (to) of 6.81 ms and two closed times (tc) of 5.66 and 128 ms (Table 1). These single-channel properties are similar to those of cloned mouse CFTR expressed in CHO cells but quite distinct from those of the human CFTR that exhibits a longer to and a higher Po (19). Indeed, there is only 78% amino acid identity between the human and mouse CFTR (20).
Characteristics of Mouse CFTR Cl− Channels Expressed in X. laevis Oocytes.
Given the distinct single-channel properties of CFTR channels from different species, we wanted to verify that the Cl− channels observed in mouse principal cells were CFTR. Thus, we compared the properties of the PKA-activated apical Cl− channel observed in principal cells with those of the cloned mouse CFTR expressed in oocytes. The X. laevis oocyte is an expression system that has been used by us and many other laboratories to study the regulation of ROMK channels and interactions of ROMK and human CFTR (7, 9, 21, 22).
Fig. 2C shows representative single-channel characteristics of CFTR channels in an inside-out excised patch from an oocyte treated with FSK/IBMX at holding potentials (-Vp) of 40 and 80 mV using symmetrical bath and pipette solutions containing 100 mM NMDG-Cl. The corresponding current–voltage relationship is shown in Fig. 2D. Table 1 compares the single-channel properties of the Cl− channels observed in the apical membrane of mouse principal cells and CFTR channels expressed in X. laevis oocytes. CFTR channels expressed in oocytes had a mean slope conductance of 5.6 pS and a Po of 0.08, with one to of 4.1 ms and two tc of 1.8 and 30.5 ms. Thus, the single-channel properties of mouse CFTR are similar to the single-channel properties observed in apical membranes of mouse principal cells.
Fig. 3 shows representative examples of whole-cell currents from water-injected (Fig. 3 A and B) and CFTR cRNA-injected (Fig. 3 C and D) oocytes in a bath containing 105 mM Cl−. No currents were observed in water-injected oocytes before or after exposure to 10 μM FSK and 1 mM IBMX (n = 12). In contrast, in oocytes injected with mouse CFTR cRNA, FSK/IBMX increased the current at +80 mV from 0.8 ± 0.2 to 11.6 ± 0.4 μA (Fig. 3D). FSK/IBMX-activated current exhibited a reversal potential of −32.5 ± 6.4 mV (n = 15; Fig. 3D), consistent with activation of CFTR Cl− channels.
Fig. 3.
Whole-cell currents recorded in X. laevis oocytes. No Cl− currents were observed water-injected oocytes before (A) or after (B) stimulation with 10 μM FSK and 1 mM IBMX. In oocytes expressing mouse CFTR, FSK and IBMX stimulates Cl− current (C and D) that exhibits a reversal potential ≈32 mV (D).
Inhibitors of CFTR Block Cl− Channels in Mouse Principal Cells.
The sulfonylurea compound glibenclamide is an open-channel inhibitor of CFTR (23–25), and CFTR is required for inhibition of ROMK by glibenclamide (7, 8, 21). However, increasing cAMP-dependent PKA activity abolishes the sensitivity of ROMK channels to glibenclamide (7). Thus we examined the effect of 1 mM glibenclamide on whole-cell currents from mouse CFTR expressed in oocytes stimulated by FSK/IBMX. As shown in Fig. 4A, current at +80 mV increased from 1.1 ± 0.3 μA to 14.9 ± 0.8 μA (n = 5) with exposure of CFTR-injected oocytes to FSK/IBMX. Glibenclamide reduced whole-cell current at +80 mV by ≈48% (14.9 ± 0.8 μA to 7.2 ± 0.7 μA; n = 5) without changing the reversal potential (Fig. 4A). In inside-out giant patches using symmetrical 100-mM NMDG-Cl solutions, FSK/IBMX increased patch current at +50 mV from 3.8 ± 0.3 pA to 20.4 ± 0.8 pA (n = 6; P < 0.5; Fig. 4B), and this FSK/IBMX-stimulated current was reduced to 12.2 ± 0.4 pA by 1 mM glibenclamide.
Fig. 4.
Mouse CFTR Cl− channels expressed in the X. laevis oocytes are sensitive to 1 mM glibenclamide. (A) Glibenclamide inhibits ≈45% of whole-cell Cl− current stimulated by 10 μM FSK and 1 mM IBMX. (B) Glibenclamide inhibits ≈50% of Cl− current in single giant inside-out patch after stimulation by FSK and IBMX.
We also assessed the effects of glibenclamide on ATP+PKA-activated CFTR channels in inside-out apical patches of principal cells from wild-type mice. Glibenclamide (1 mM) blocked ≈50% of CFTR Cl− channel activity (NPo = 0.09 ± 0.04; control NPo = 0.18 ± 0.08; n = 5; Fig. 5A). Thus, the fractional inhibition by glibenclamide was similar in principal cells and mouse CFTR expressed in X. laevis oocytes (Fig. 4).
Fig. 5.
Single-channel recordings of Cl− channel activity in excised inside-out patches held at 80 mV (−Vp) after stimulation by Mg-ATP and PKA showing the effect of 1 mM glibenclamide (A) or 5 μM CFTRinh-172 (B). C, closed level; O1-Oi, open state levels.
CFTRinh-172 is a potent inhibitor of human CFTR, functioning as a closed-channel blocker (26, 27). Thus we examined the ability of CFTRinh-172 to inhibit Cl− channel activity in mouse principal cells. As shown in Fig. 5B, 5 μM CFTRinh-172 virtually completely blocked Cl− channel activity in ≈5 min (NPo = 0.02 ± 0.01; control NPo = 0.23 ± 0.09; n = 6).
Comparison of Anion Selectivity of Mouse CFTR and Cl− Channels Expressed in Principal Cells.
Although the anion selectivity of mouse CFTR channels has not been reported, human CFTR channels exhibit a characteristic anion selectivity (28–32) that differs from either human ClC-1 type Cl− channels (33) or X. laevis Ca2+-activated Cl− channels (34). Therefore, we assessed the anion selectivity of the Cl− currents observed in X. laevis oocytes expressing the mouse cloned CFTR to discriminate CFTR currents from currents due to other Xenopus native Cl− channels. Permeability ratios were obtained from bionic reversal potentials measured in excised giant patches of FSK/IBMX-treated oocytes expressing mouse CFTR. Patch pipettes contained 140 mM NaCl and baths contained equal molar concentrations of NaCl, Na-aspartate, Na-iodide, Na-bromide, or Na-fluoride. Current–voltage plots for the different bionic conditions were obtained using voltage ramps (Fig. 6A). The permeability sequence obtained from the reversal potential was I- > Br- > Cl− > F- > Asp- (Table 2). The slope conductance and conductance ratios (Gx/GCl) showed the sequence of I− = Br− > Cl− > F- = Asp− (Table 2). These results are in agreement with the selectivity patterns previously reported for the human CFTR that are consistent with the “lyotropic” or Hofmeister series (28–32).
Fig. 6.
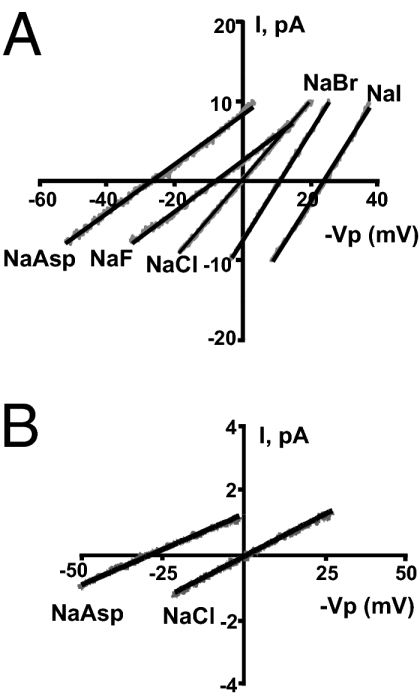
Anion selectivity of mouse CFTR expressed in X. laevis oocytes (A) and apical Cl− channels in mouse principal cells (B). Traces represent currents obtained from voltage ramps applied to inside-out giant patches (A) or standard patches (B). Bath 140 mM Cl− was replaced by 140 mM of different anions. Oocytes and principal cells were stimulated by application of 10 μM FSK and 1 mM IBMX.
Table 2.
Anion permeability and conductance sequences
| Anion (x) | Erev (mV) | G (pS) | Gx/GCl |
| Oocyte | |||
| Asp- | −30 ± 5.1 | 297 ± 40 | 0.59 ± 0.04 |
| F− | −9 ± 3.2 | 320 ± 56 | 0.61 ± 0.07 |
| Cl− | 0 ± 0.4 | 496 ± 53 | 1 ± 0 |
| Br− | 11 ± 1.5 | 684 ± 61 | 1.47 ± 0.15 |
| I− | 23 ± 2.8 | 689 ± 72 | 1.41 ± 0.11 |
| Mouse | |||
| Asp− | −29 ± 8.04 | 44 ± 0.08 | 0.84 ± 0.12 |
| Cl− | 0.7 ± 0.89 | 52 ± 0.15 | 1 ± 0 |
Erev, reversal potential; G, slope conductance.
Giant patches cannot be obtained from apical membranes of mouse principal cells, limiting our ability to perform a full anion permeability sequence. Therefore, we compared the currents of the least-permeable anion, aspartate, with that of chloride using standard excised patches from principal calls. Current–voltage plots for the two conditions were obtained using voltage ramps (Fig. 6B). The reversal potentials shown in Table 2 indicate, as expected for CFTR, that the permeability of aspartate for the mouse principal cell Cl− channel is much less than that of chloride. In addition, the shift in reversal potential with aspartate was identical in mouse CFTR expressed in oocytes and the Cl− channel in principal cells (Table 2). Although the slope conductance was reduced in principal cells for the Cl−Asp bionic condition compared with symmetrical Cl−, this reduction was much less than that observed with mouse CFTR expressed in oocytes. A change in the total number of open Cl− channels between the two conditions in the small patches obtained from principal cells could have obscured a larger change in GAsp/GCl. In other words, a change in the opening of a few channels would make a large difference in the total current of the small patches from principal cells but would have little effect in the giant patches containing an order of magnitude greater number of open channels (e.g., compare total current at 0 mV for NaAsp in Fig. 6A vs. Fig. 6B). Thus, the reversal potential is a more accurate measure of the permeability difference in the principal cell patches.
Apical Cl− Channels Are Absent in Principal Cells of ΔF508-CFTR Mice.
Although comparisons of the single-channel characteristics of apical Cl− channels in principal cells with those from mouse CFTR expressed in oocytes strongly support that the apical Cl− channels in principal cells are CFTR channels, we used ΔF508-CFTR mice to confirm this notion. In these transgenic mice, the mutant CFTR is retained in the ER. We used 0.5 mM Mg-ATP and 50 nM PKA to activate any CFTR Cl− channels resident in inside-out patches from wild-type or ΔF508-CFTR mice. In wild-type mice, CFTR Cl− channel activity was detected in 15 patches out of a total of 37 successful patches (i.e., 40.5%; Table 3). The average channel activity (NPo) in the 15 patches from wild-type mice was 0.24 ± 0.02 (Table 3). However, in ΔF508-CFTR mice, no CFTR Cl− channels were observed in inside-out patches (n = 13; Table 3). These results demonstrate that mutant CFTR channels in ΔF508-CFTR mice do not reach the apical membrane of principal cells and support the notion that the channels observed in wild-type mice are CFTR.
Table 3.
Apical Cl− channels in principal cells from wild-type and transgenic mice
| Parameter | Wild-type | ΔF508-CFTR | ROMK−/− |
| No. of patches* | 37 | 13 | 19 |
| No. of patches with Cl− channels (%) | 15 (40.5) | 0 | 9 (47.4) |
| Cl− channel NPo, mean ± SEM | 0.24 ± 0.02 | 0 | 0.27 ± 0.03 |
*Inside-out patches using symmetrical bath and pipette solutions containing 145 mM NMDG-Cl and held at -Vp = 60 mV.
Apical Cl− Channels in Principal Cells of ROMK−/− Mice.
We previously reported that in mCFTR−/− mice the NPo for ROMK in cell-attached patches of TAL cells was increased 2- to 3-fold compared with wild-type mice (8). The latter is consistent with the requirement of CFTR expression in apical membranes for the reduction in ROMK activity in the presence of cytosolic ATP concentrations (i.e., the ATP sensitivity of ROMK). Given that CFTR can influence the gating behavior of ROMK, does the lack of ROMK expression influence the functional expression of CFTR? To examine this possibility, we compared Cl− channel NPo in cell-attached patches of principal cells from wild-type and ROMK knockout mice (ROMK−/−). In ROMK−/− mice, CFTR Cl− channel activity was detected in 9 of a total of 19 successful patches (i.e., 47.4% of patches; Table 3). The average Cl− channel activity (NPo) in the 9 patches from ROMK−/− mice was 0.27 ± 0.03 (Table 3), which is unchanged from the NPo observed in wild-type mice. Thus, the functional interaction of CFTR and ROMK seems to be unidirectional: the absence of CFTR alters the functional expression of ROMK, but the absence of ROMK does not affect the functional expression of CFTR.
Discussion
Although both CFTR mRNA and protein are widely expressed along the nephron of the mammalian kidney, the localization of CFTR in the apical membrane, its function as a Cl− channel, and its role in renal Cl− handling or regulation of other renal transport mechanisms have been unclear. In this report, we establish that CFTR forms PKA-activated Cl− channels in the apical membrane of principal cells from the intact CCD obtained from mice. This conclusion is based on several findings. First, Cl− channels were observed in apical patches of mouse principal cells after stimulation by FSK/IBMX. Second, apical Cl− channels in mouse principal cells have single-channel properties similar to those of mouse CFTR expressed in CHO cells (19) or in oocytes expressing the cloned mouse CFTR. Third, the specific CFTR closed-channel blocker CFTRinh-172 inhibited the activity of Cl− channels. Fourth, these apical Cl− channels are not observed in principal cells from the ΔF508-CFTR mouse even after stimulation by FSK/IBMX.
What are the potential physiologic roles of apical CFTR Cl− channels in principal cells? There are at least two possibilities that are not mutually exclusive. First, CFTR channels could carry significant Cl− current that could function to short-circuit the difference between Na+ and K+ currents across apical membranes, thereby altering the ratio of apical Na+ to K+ fluxes. A second possible role of CFTR could be that phosphorylation of CFTR functions as a switch modulating ROMK activity. For instance, a low cAMP-PKA state in water diuresis would reduce CFTR phosphorylation, allowing functional interaction between CFTR and ROMK channels so that cytoplasmic ATP levels inhibit ROMK. Inhibition of ROMK would impede excessive K secretion and thereby prevent kaliuresis during water diuresis (35). In contrast, during the high PKA phosphorylation condition associated with antidiuresis, the interaction of ROMK with CFTR is altered such that ROMK is no longer sensitive to ATP, and therefore ROMK would be open and support K+ secretion. This regulatory role for CFTR does not necessarily require a large (or any) Cl− current but may depend on PKA phosphorylation events, including phosphorylation of CFTR.
Regarding the first possible role of CFTR channels in principal cells, what effect could apical Cl− currents have on Na+ absorption and K+ secretion? Sodium absorption through ENaC depolarizes, whereas K+ secretion through potassium channels like ROMK hyperpolarizes the principal cell apical membrane. However, the magnitudes of these cation currents may not be equivalent. In the prevailing model, the extent of this difference in cation current generates a lumen negative transepithelial potential that, in turn, drives Cl− absorption through the paracellular pathway that matches the difference in cation currents. During antidiuresis when cAMP-dependent PKA activity is high and CFTR Cl− channels are open, any Cl− current from cell to lumen (Cl− secretion) or from lumen to cell (Cl− absorption) would alter the electrical coupling between Na+ and K+, thereby altering the ratio of Na+ absorption to K+ secretion. The extent of this effect of open CFTR channels depends on the magnitude of the Cl− current. In apical patches from mice, we observed a small number of current levels that are quite small compared with K+ currents. Observing multiple current levels depends on the simultaneous opening of more than one channel, and the probability of this occurrence is reduced in CFTR with the very short to and long tc of mouse CFTR channels. As a consequence, the time-averaged Cl− current across the principal cell apical membrane would be very small in comparison with Na+ or K+ currents. Thus, the apical CFTR Cl− current would be expected to have little effect on the Na-to-K coupling in mouse principal cells. However, the apical Cl− current could be higher in different species, thereby affecting the Na-to-K coupling to a greater extent than in mouse. For example, compared with the mouse CFTR, the human CFTR channel exhibits a longer to and shorter tc, which results in a higher Po (here and ref. 19).
Regarding the second possible role of apical CFTR Cl− channels, could the phosphorylation state of CFTR be important in modulating the Mg-ATP sensitivity of ROMK K+ channels? We previously showed that expression of CFTR is required for the sensitivity of ROMK to either cytosolic Mg-ATP or glibenclamide and that increasing the PKA phosphorylation state of the cell abrogates the inhibitory effects of these agents (7, 8). Furthermore, we suggested that the effect of CFTR on ROMK Mg-ATP sensitivity could be due either to increased surface delivery of CFTR or to altered phosphorylation states of ROMK, CFTR, and/or other associated proteins, or both (8). Direct phosphorylation of ROMK could contribute to ROMK activation separately from CFTR-mediated effects. This additional mechanism could involve a shift of the ROMK-pH dependence favoring the open state (36).
Several observations in this report further define the mechanism for the PKA-regulated functional switch that determines the distribution of open and ATP-inhibited K channels in principal cell apical membranes. First, excised apical patches from principal cells contain inactive CFTR Cl− channels that can be opened by exposure to PKA and Mg-ATP, showing that increased trafficking of CFTR to the apical membrane is not required for an increase in CFTR Cl− channel activity in the apical membrane. This suggests a model whereby the functional interaction between CFTR and ROMK occurs entirely within the apical membrane, as opposed to distinct pools of ROMK and/or CFTR trafficking into or out of the membrane. Second, the CFTR–ROMK interaction in modulating channel activity is unidirectional. We previously showed that the absence of apical CFTR expression (ΔF508-CFTR mouse) increases ROMK activity (NPo) by 2- to 3-fold in cell-attached patches where this K+ channels is exposed to cytosolic concentrations of ATP (8). In the present study, we show that deletion of ROMK (ROMK−/− mouse) has no effect on the NPo for CFTR Cl− channels in cell-attached apical patches under maximal stimulation (FSK/IBMX). Thus, in the intact principal cell, CFTR modulates ROMK activity, whereas ROMK does not alter CFTR activity. Third, the condition required to open CFTR Cl− channels, namely increasing cAMP-dependent PKA phosphorylation in principal cells, is the same that abolishes the sensitivity of ROMK channels to 2 mM Mg-ATP (8) or glibenclamide (7). This finding, together with the second observation, suggests that the PKA phosphorylation state of CFTR is the major factor in determining the Mg-ATP and glibenclamide sensitivities of ROMK. Whether the phosphorylation of the CFTR regulatory domain, the conformational change in CFTR associated with channel opening, or the flow of Cl− ions through the CFTR pore is the molecular effector altering the gating behavior of ROMK is yet to be determined.
Materials and Methods
Animals.
C57BL/6 wild-type mice (25–30 g) were purchased from Charles River Laboratories and maintained on a normal diet for 7–10 days before being killed. The ΔF508-CFTR transgenic mice (8) were fed a Harlan Teklad 9F diet and drinking water that was supplemented with 17.5 g/250 mL of Colyte (Schwarz Pharma) to improve survival. ROMK-deficient transgenic mice (ROMK−/−) were maintained on a normal diet and generated as described previously (37). Mice were maintained at the Yale Animal Resources Center and genotyped using standard protocols. Freshly isolated ovaries from X. laevis frogs were obtained from Nasco. Stage V–VI oocytes were defolliculated in a collagenase (2 mg/mL type 1A; Sigma-Aldrich) containing Ca2+-free solution (in mM): 96 NaCl, 2 KCl, 2 MgCl2, and 5 Hepes titrated to pH 7.4. All procedures were performed in compliance with relevant laws and institutional guidelines and were approved by the Yale University Institutional Animal Care and Use Committee.
Patch Clamping of Mouse Principal Cells.
Patch-clamping was performed as described previously (8). Pipette solutions were optimized for detecting Cl− or K+ currents. In single-channel patches, pipette resistance was 8–9 MΩ when filled with (in mM): 145 NMDG-Cl, 1.8 MgCl2, 10 TES, 1 BaCl2, and 20 μM amiloride titrated to pH 7.4. In inside-out patch experiments, the bath solution was the same as the pipette solution. In cell-attached patches, the bath solution contained (in mM): 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 Hepes titrated to pH 7.4. Glibenclamide, Mg-ATP, FSK, and IBMX were purchased from Sigma-Aldrich. CFTRinh-172 and the catalytic subunit of PKA were purchased from Calbiochem; CFTRinh-172 was dissolved in DMSO as a stock solution. The concentration of DMSO in the experimental solution is less than 1/1,000, which has no effect on channel activity. Signals were digitized at a sampling rate 4 kHz (DigiData 1322A; Molecular Devices), and patch data were analyzed using Clampfit 9.2 (Molecular Devices) at a digital filter frequency of 250 Hz. Channel activity was calculated during sampling periods of 10–30 s as: NPο = Σ (t1 + t2 + … tn), where N is the number of observed current levels in the patch, Pο is the single-channel open probability, and tn is the fractional open time spent at each of the current levels. Channel conductance was calculated by using GraphPad Prism (GraphPad Software) from linear regression analysis of single-channel current–voltage curves. Voltage (Vp) applied to the pipette was referenced to the bath potential and reported as -Vp. Data are shown as mean ± SEM, and a paired Student t test was used to calculate the significance between the control and experimental groups. Statistical significance was taken as P < 0.05.
X. laevis Oocyte Electrophysiologic Protocols.
Mouse CFTR cDNA (a kind gift from A. P. Naren, University of Tennessee, with permission of B. J. Wainwright, University of Queensland, Brisbane, Australia and D. N. Sheppard, University of Bristol, United Kingdom) was stripped of introns and subcloned into the pGEMHE vector. CFTR cDNA was linearized with Not-1 restriction endonuclease to generate a full-length cRNA. Oocytes were injected with 50 nL of CFTR cRNA (5 ng) or water using an INJECT + MATIC. Oocytes were then incubated at 18 °C for 48 h in ND-96 solution (in mM): 96 NaCl, 2 KCl, 1 MgCl2, and 1.8 CaCl2 titrated to pH 7.4, containing 500 U/mL penicillin and 500 μg/mL streptomycin (Invitrogen). For single-channel and giant patches, the oocyte vitelline membrane was manually removed with forceps after immersion in a hyperosmotic solution for 1–2 min (in mM): 200 N-methyl-D-glucamine, 2 KCl, 1 MgCl2, and 10 EGTA at pH 7.4. Electrodes were pulled from borosilicate glass capillaries on a Narishige PP-83 puller and polished on a MF-83 microforge. Pipette solutions were optimized for detecting Cl− currents. Whole-cell currents were measured by two-electrode voltage clamp using an oocyte clamp (OC725C; Warner Instruments) in a modified ND96 (in mM): 96 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, and 10 Hepes titrated to pH 7.4. Voltage and current microelectrodes had resistances of 1–1.2 MΩ when filled with 3 M KCl. The oocyte membrane potentials were held at −40 mV and whole-cell currents measured at 20 mV steps from −160 to +80 mV. Current–voltage plots were generated using pCLAMP software (Molecular Devices). In single-channel patches, pipette resistance was 8–9 MΩ when filled with (in mM): 100 NMDG-Cl, 1 MgCl2, 10 TES, and 1 BaCl2 titrated to pH 7.4. Inside-out giant current patches were used for ion selectivity measurements in which bath solutions (in mM): 1 MgCl2, 10 TES, and 140 NaCl or 140 Na-iodide or 140 Na-bromide or 140 Na-fluoride or 140 Na-aspartate, at pH 7.4. Giant patch electrodes had resistances of 0.5–1 MΩ when filled with (in mM): 140 NMDG-Cl, 1 MgCl2, 10 TES, and 1 BaCl2 titrated to pH 7.4. Bath solutions were exchanged using a multibarrel quick-exchange solution system (model SF-77B; Warner Instruments). Macroscopic currents were recorded by using Axopatch 200B and digitized by Digidata 1322A with pCLAMP 9.2 during a 300-ms voltage ramp ranging from (−Vp) 80 mV to –100 mV. Data were analyzed using Clampfit 9.2 (Molecular Devices).
Acknowledgments
This work was supported by National Institutes of Health Grant P01 DK17433 (to S.C.H. and E.L.B.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Crawford I, et al. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci USA. 1991;88:9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devuyst O, Guggino WB. Chloride channels in the kidney: Lessons learned from knockout animals. Am J Physiol Renal Physiol. 2002;283:F1176–F1191. doi: 10.1152/ajprenal.00184.2002. [DOI] [PubMed] [Google Scholar]
- 3.Morales MM, et al. Both the wild type and a functional isoform of CFTR are expressed in kidney. Am J Physiol. 1996;270:F1038–F1048. doi: 10.1152/ajprenal.1996.270.6.F1038. [DOI] [PubMed] [Google Scholar]
- 4.Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79(1 Suppl):S145–S166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- 5.Kunzelmann K. CFTR: Interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Wang WH, Klein-Robbenhaar G, Giebisch G. Effects of glyburide on renal tubule transport and potassium-channel activity. Ren Physiol Biochem. 1995;18:169–182. doi: 10.1159/000173914. [DOI] [PubMed] [Google Scholar]
- 7.McNicholas CM, et al. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by co-expression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, et al. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest. 2006;116:797–807. doi: 10.1172/JCI26961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruknudin A, Schulze DH, Sullivan SK, Lederer WJ, Welling PA. Novel subunit composition of a renal epithelial KATP channel. J Biol Chem. 1998;273:14165–14171. doi: 10.1074/jbc.273.23.14165. [DOI] [PubMed] [Google Scholar]
- 10.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kartner N, et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991;64:681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- 12.Chang CT, et al. Vasopressin-stimulated CFTR Cl− currents are increased in the renal collecting duct cells of a mouse model of Liddle’s syndrome. J Physiol. 2005;562:271–284. doi: 10.1113/jphysiol.2004.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Huyen JP, Bens M, Teulon J, Vandewalle A. Vasopressin-stimulated chloride transport in transimmortalized mouse cell lines derived from the distal convoluted tubule and cortical and inner medullary collecting ducts. Nephrol Dial Transplant. 2001;16:238–245. doi: 10.1093/ndt/16.2.238. [DOI] [PubMed] [Google Scholar]
- 14.Bens M, Van Huyen JP, Cluzeaud F, Teulon J, Vandewalle A. CFTR disruption impairs cAMP-dependent Cl(-) secretion in primary cultures of mouse cortical collecting ducts. Am J Physiol Renal Physiol. 2001;281:F434–F442. doi: 10.1152/ajprenal.2001.281.3.F434. [DOI] [PubMed] [Google Scholar]
- 15.Letz B, Korbmacher C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. Am J Physiol. 1997;272:C657–C666. doi: 10.1152/ajpcell.1997.272.2.C657. [DOI] [PubMed] [Google Scholar]
- 16.Gross P, Minuth WW, Ketteler M, Frömter E. Ionic conductances of cultured principal cell epithelium of renal collecting duct. Pflugers Arch. 1988;412:434–441. doi: 10.1007/BF01907564. [DOI] [PubMed] [Google Scholar]
- 17.Ling BN, Kokko KE, Eaton DC. Prostaglandin E2 activates clusters of apical Cl− channels in principal cells via a cyclic adenosine monophosphate-dependent pathway. J Clin Invest. 1994;93:829–837. doi: 10.1172/JCI117037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansdell KA, et al. Comparison of the gating behaviour of human and murine cystic fibrosis transmembrane conductance regulator Cl− channels expressed in mammalian cells. J Physiol. 1998;508:379–392. doi: 10.1111/j.1469-7793.1998.379bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tata F, et al. Cloning the mouse homolog of the human cystic fibrosis transmembrane conductance regulator gene. Genomics. 1991;10:301–307. doi: 10.1016/0888-7543(91)90312-3. [DOI] [PubMed] [Google Scholar]
- 21.McNicholas CM, et al. A functional CFTR-NBF1 is required for ROMK2-CFTR interaction. Am J Physiol. 1997;273:F843–F848. doi: 10.1152/ajprenal.1997.273.5.F843. [DOI] [PubMed] [Google Scholar]
- 22.McNicholas CM, Yang Y, Giebisch G, Hebert SC. Molecular site for nucleotide binding on an ATP-sensitive renal K+ channel (ROMK2) Am J Physiol. 1996;271:F275–F285. doi: 10.1152/ajprenal.1996.271.2.F275. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheppard DN, Robinson KA. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz BD, et al. Glibenclamide blockade of CFTR chloride channels. Am J Physiol. 1996;271:L192–L200. doi: 10.1152/ajplung.1996.271.2.L192. [DOI] [PubMed] [Google Scholar]
- 26.Taddei A, et al. Altered channel gating mechanism for CFTR inhibition by a high-affinity thiazolidinone blocker. FEBS Lett. 2004;558:52–56. doi: 10.1016/S0014-5793(04)00011-0. [DOI] [PubMed] [Google Scholar]
- 27.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabcharani JA, Linsdell P, Hanrahan JW. Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J Gen Physiol. 1997;110:341–354. doi: 10.1085/jgp.110.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsdell P, et al. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linsdell P, Tabcharani JA, Hanrahan JW. Multi-Ion mechanism for ion permeation and block in the cystic fibrosis transmembrane conductance regulator chloride channel. J Gen Physiol. 1997;110:365–377. doi: 10.1085/jgp.110.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansoura MK, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) anion binding as a probe of the pore. Biophys J. 1998;74:1320–1332. doi: 10.1016/S0006-3495(98)77845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson MP, et al. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- 33.Fahlke C, Yu HT, Beck CL, Rhodes TH, George AL., Jr Pore-forming segments in voltage-gated chloride channels. Nature. 1997;390:529–532. doi: 10.1038/37391. [DOI] [PubMed] [Google Scholar]
- 34.Qu Z, Hartzell HC. Anion permeation in Ca(2+)-activated Cl(-) channels. J Gen Physiol. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassola AC, Giebisch G, Wang W. Vasopressin increases density of apical low-conductance K+ channels in rat CCD. Am J Physiol. 1993;264:F502–F509. doi: 10.1152/ajprenal.1993.264.3.F502. [DOI] [PubMed] [Google Scholar]
- 36.Leipziger J, et al. PKA site mutations of ROMK2 channels shift the pH dependence to more alkaline values. Am J Physiol Renal Physiol. 2000;279:F919–F926. doi: 10.1152/ajprenal.2000.279.5.F919. [DOI] [PubMed] [Google Scholar]
- 37.Lu M, et al. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter’s) knockout mice. J Biol Chem. 2002;277:37881–37887. doi: 10.1074/jbc.M206644200. [DOI] [PMC free article] [PubMed] [Google Scholar]







