Abstract
Previous work by us, and others, has shown that the formation of amino acids on prebiotic earth with the geometric arrangement called the L configuration can be understood. Some meteorites of the carbonaceous chondritic type deliver unusual amino acids, with alpha-methyl groups, which have an excess of the L isomers. We previously showed that in decarboxylative transamination reactions under credible prebiotic conditions they produce normal amino acids that also have a preference for the L isomer, as is found in our proteins. We, and others, showed that as little as a 1% excess of the L isomers could be amplified up to a 95/5 ratio of L over D on simple evaporation of a solution, so life could start with such a solution in which the dominant L isomers would be selectively chosen. We now find that the geometry of sugars referred to D, as in D-ribose or D-glucose, is not an independent mystery. D-glyceraldehyde, the simplest sugar with a D center, is the basic unit on which other sugars are built. We find that the synthesis of glyceraldehyde by reaction of formaldehyde with glycolaldehyde is catalyzed under prebiotic conditions to D/L ratios greater than 1, to as much as 60/40, by a representative group of L-amino acids (with the exception of L-proline). The D/L glyceraldehyde ratio in water solution is amplified to 92/8 using simple selective solubilities of the D and the DL forms. This D center would then be carried into the prebiotic syntheses of larger sugars.
Keywords: aldol reaction, formaldehyde, formose reaction, meteorites
For life to start, it was necessary that the amino acids that are building blocks for proteins, and the sugars that play many biological roles and are part of both RNA and DNA, have a single handedness, called chirality. The amino acids could have been all in the handedness referred to as L, analogous to the left hand, or they could have been all D, analogous to the right hand, but on our planet the L geometry was preferred. After many years of speculation about why this is so, evidence has now accumulated that the L handedness of amino acids was derived from a special group of amino acids, with an extra methyl group that prevented loss of their small excess of the L form by a process that can scramble the handedness of ordinary amino acids on prolonged heating. These special L-amino acids with the extra methyl group were isolated from a meteorite that fell in Murchison Australia in 1969 (1, 2). We showed that they could generate normal amino acids (without the methyl group) under credible prebiotic conditions, and those also had an excess of the L isomer, along with a smaller amount of the D amino acids (3).
Once there was a small excess of the L-amino acid, we showed that it could be amplified in solution, because the DL 1∶1 mixture was less soluble in water (4). By evaporating a water solution with only a 1% excess of L-phenylalanine, for instance, we obtained a solution with a 95/5 L/D ratio; equal amounts of the D and L components precipitated from the solution as a less soluble crystal in which the D and L components were bound to each other. At the same time, Klussman et al. published the first of a series of papers on such selective concentration of the L-amino acids in water (5) and Hayashi et al. published the amplification of proline (6). We were all preceded by Morowitz, who described the theory of such processes and some experimental exploration of it (7).
With a dominance of the L-amino acids in solution, where life might have started, it is reasonable that the life form that used the dominant L-amino acids would win out over any life form based on the minor amount of D amino acid still in solution.
Although there are still questions about why the special amino acids that came in on meteorites had a small excess of the L form over the D form, one idea is that they were formed in space as an equal mixture, but then some of the D form was destroyed by high-energy light that has right circular polarization and that is known to be in this sector of the universe. Such selective destruction of D amino acids by right circularly polarized light has been demonstrated experimentally (8). The excess of light with right, rather than left, circular polarization is often ascribed to a synchrotron or cyclotron process from a nearby neutron star. If so it is an accident, and in other parts of the universe there could be a preference for D amino acids if those regions have an excess of left circularly polarized light. There should be no preference for one or the other handedness of their amino acids if they do not have any excess of one or the other polarized light. Such a preference is important for life to start, because on prebiotic earth equal mixtures of an amino acid and its mirror image would not easily form biological molecules, such as proteins, with well-defined structures.
Results and Discussion
Sugars can also have either L or D handedness, but the situation here is a bit more complex. D sugars are named that way for the handedness of the carbon next to the end of the sugar chain, but there are other places where chirality (handedness) also occurs in sugars.
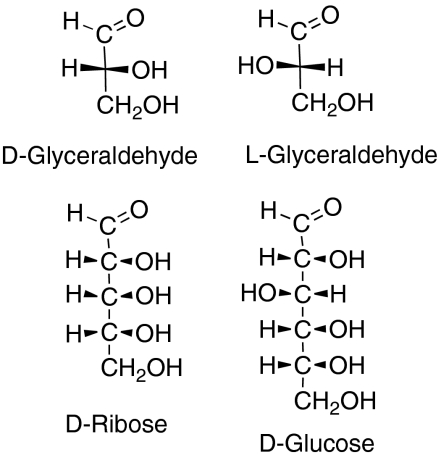
In D-ribose (Fig. 1) all three chiral carbons have the handedness defined as D, which in modern chemical terminology is R, but in other sugars the other carbons can have R or S handedness and they are still called D sugars if the next to bottom carbon has the D (R) handedness. For example, in D-glucose (Fig. 1) the next to bottom carbon still has this R configuration, but one other carbon nearer the top of the molecule has the S configuration.
Fig. 1.
The two mirror-image optical isomers of glyceraldehyde, the simplest sugar, and the structures of D-ribose and D-glucose.
All the D sugars were probably initially derived from D-glyceraldehyde, the simple three-carbon sugar (Fig. 1). This has only one carbon atom at which the arrangement of the groups can describe either D-glyceraldehyde (Fig. 1) or its mirror-image L-glyceraldehyde (Fig. 1). In the D sugars such as D-ribose or D-glucose or D-fructose other pieces are added to D-glyceraldehyde: a two-carbon piece to make D-ribose (9), a three-carbon piece to make D-glucose, etc. Those natural sugars are all in the D family, suggesting that they were derived prebiotically from D-glyceraldehyde by adding such pieces.
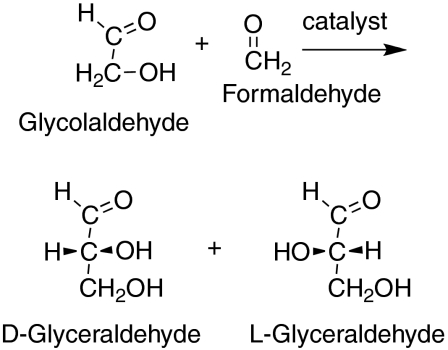
On prebiotic earth, sugars are believed to have formed from formaldehyde. In a process called the formose reaction, formaldehyde can indeed be induced to form a series of different sugars by a process (10) whose early step was shown to involve the reaction of formaldehyde with glycolaldehyde leading to glyceraldehyde (Fig. 2). Without any special catalysis this process forms an equal mixture of D- and L-glyceraldehyde. Thus we have now investigated whether adding L-amino acids (Fig. 3) to the reaction mixture would catalyze the process and lead to a preference in the product, and if so what the preferred product would be.
Fig. 2.
The aldol reaction between glycolaldehyde and formaldehyde can lead to both D-glyceraldehyde and L-glyceraldehyde, but with a preference for the D isomer when an L-amino acid is the catalyst.
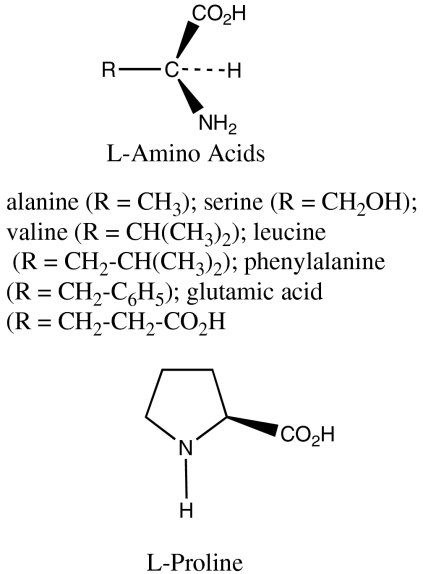
Fig. 3.
The amino acids used as catalysts in the synthesis of glyceraldehyde.
If the L-amino acids had led to preferential formation of the L-glyceraldehyde, it would be necessary to think of a completely new reason for the dominance of D sugars in our biology. In fact, all the L-amino acids we examined (except L-proline) catalyzed the preferential formation of D-glyceraldehyde, as shown in Table 1, although the enantiomeric excesses with L-alanine and with L-serine were very small. The conditions of the reactions were certainly credibly prebiotic: we simply held a solution of formaldehyde, glycolaldehyde, and various L-amino acids at 20 °C, and then isolated and purified the glyceraldehyde formed as its dinitrophenylhydrazone. The D/L ratio was then determined by using chiral high-pressure liquid chromatography.
Table 1.
Ratio of D to L glyceraldehydes synthesized from glycolaldehyde and formaldehyde in the presence of amino acids
| Amino acid | Ratio, D/L |
| L-serine | 50.3/49.7 |
| L-alanine | 50.8/49.2 |
| L-phenylalanine | 52.2/47.8 |
| L-valine | 52.2/47.8 |
| L-leucine | 54.4/45.6 |
| L-glutamic acid | 60.7/39.3 |
| L-proline | 28.9/71.1 |
The amino acids used are normal L-amino acids that can be formed from the meteoritic alpha-methyl amino acids by heating with simple components. Proline is not formed by transamination of keto acids, as were used in our decarboxylative transaminations using the meteoritic amino acids, and is unlikely to have been present early on prebiotic earth. It can be formed by subsequent nontrivial modifications of glutamic acid.
As the table shows, these reactions will lead to small or medium excesses of the D sugars relative to the L sugars that are formed from glyceraldehyde, but not to their dominance. For this a process of amplification in water solution is needed, as was used to amplify small excesses of L-amino acids to dominance in water. We have now shown that this is possible.
D-Glyceraldehyde is a syrupy liquid at room temperature, but DL-glyceraldehyde is a solid melting at 145 °C. As an x-ray structure determination shows (11), one L and one D enantiomer form a six-membered ring, a substituted 1,4-dioxane in which the substituents are equatorial and crystal packing is favorable. By contrast, the dioxane structure from D-glyceraldehyde alone would have an axial hydroxymethyl substituent and crystal packing would be less favorable. Thus selective solubility can amplify the solution concentration of D-glyceraldehyde.
Starting with the 61/39 D/L ratio produced by catalysis with glutamic acid, we find that the solution ratio is raised to 81/19 by equilibration with small amounts of water. When we started with a solution that was 81/19 D/L in water, and slowly evaporated the water, a small volume left after much of the DL compound had precipitated was 92/8 D/L in solution. This is probably not the upper limit of amplification. Also, we have shown with amino acids that kinetic processes, such as would happen if rain fell on the mixture and ran off before equilibrium was reached, led to solution ratios that can be even higher than those reached at equilibrium (3). We find that exposing the 61/39 D/L glyceraldehyde mixture to water for only a few seconds raises the solution ratio to 88/12.
We have also examined amplification recently (12, 13), focusing on ribose, and found that ribose itself did not form a less soluble DL complex that would permit its amplification. However, when the ribose was incorporated in the nucleosides uridine, adenosine, or cytidine there were large differences in solubility between the D crystals and the less soluble DL crystals, so that very small excesses of the D components were amplified to solutions with as much as a 199/1 ratio of D to L. Thus again the solutions would have allowed life to start with a preference for the dominant D handedness in the components of RNA. After such amplification D-ribose itself could have been formed by hydrolysis of the nucleosides.
Conclusions
Of course, all such scenarios about how our prebiotic world could have established dominant handedness in both amino acids and sugars do not prove that this is the way it actually occurred. However, the finding that the defining chiral center in D sugars can be formed using L-amino acids as catalysts under really simple credible prebiotic conditions does simplify the requirements for all proposals. It shows that the origin of the D sugars in life today is not a mystery if indeed the small excesses of L alpha-methyl amino acids were the seeds from space that started the preference for the L isomers in normal amino acids. The L-amino acids would then cause the preference for D sugars (see ref. 14 for a different proposal and ref. 15 for a previous attempt at our glyceraldehyde synthesis).
Materials and Methods
Materials.
Glycolaldehyde (dimer), glyceraldehyde, formaldehyde (37% solution in water), dihydroxyacetone, 2,4-dinitrophenylhydrazine, and amino acids were purchased from Aldrich. HPLC grade ethanol and hexanes were obtained from Fisher Scientific.
Reaction and Derivatization Procedures.
60 mg of glycolaldehyde (1.0 mmol), and formaldehyde solution (3.0 mL, 37% H2O solution, 40.0 mmol) were dissolved in 17.0 mL deionized H2O. An amino acid (1.0 mmol) was added to the resulting solution. The mixture was stirred at 20 °C for 3–5 days (monitored by thin layer chromatography). Then 2,4-dinitrophenylhydrazine (with 30% H2O, 11.6 g, 1.0 eq to aldehyde ) was added to the reaction solution to form a mixture of dinitrophenyhydrazones. The glyceraldehyde dinitrophenylhydrazone was isolated by flash chromatography on a silica gel column, and shown to be pure by NMR spectroscopy (the spectrum was identical with that of the synthetically prepared pure compound). It was then subjected to HPLC analysis and showed no significant impurity peaks.
HPLC Analysis.
HPLC was run on a Waters 600 HPLC system with a Waters 996 photodiode array spectrophotometer. The dinitrophenylhydrazones of D and L glyceraldehyde were monitored by their absorbance at 350 nm. A Chiralpak AD column was used for chiral analysis, with a mobile phase using gradient hexane/EtOH. Under our conditions, the retention times for the dinitrophenylhydrazones of L-glyceraldehyde and D-glyceraldehyde were 82 and 110 min, respectively.
Acknowledgments.
This work was supported by the US National Science Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cronin J, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951–955. doi: 10.1126/science.275.5302.951. [DOI] [PubMed] [Google Scholar]
- 2.Pizzarello S. The chemistry of life’s origin: A carbonaceous meteorite perspective. Acc Chem Res. 2006;39:231–235. doi: 10.1021/ar050049f. [DOI] [PubMed] [Google Scholar]
- 3.Levine M, Kenesky C, Mazori D, Breslow R. Enantioselective synthesis and enantiomeric amplification of amino acids under prebiotic conditions. Org Lett. 2008;10:2433–2436. doi: 10.1021/ol8007099. [DOI] [PubMed] [Google Scholar]
- 4.Breslow R, Levine M. Amplification of enantiomeric concentrations under credible prebiotic conditions. Proc Natl Acad Sci USA. 2006;103:12979–12980. doi: 10.1073/pnas.0605863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klussman M, et al. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature. 2006;441:621–623. doi: 10.1038/nature04780. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi Y, et al. Large nonlinear effect observed in the enantiomeric excess of proline in solution and that in the solid state. Angew Chem. 2006;45:4593–4597. doi: 10.1002/anie.200601506. [DOI] [PubMed] [Google Scholar]
- 7.Morowitz H. Mechanism for the amplification of fluctuations in racemic mixtures. J Theor Biol. 1969;25:491–494. doi: 10.1016/s0022-5193(69)80035-4. [DOI] [PubMed] [Google Scholar]
- 8.Flores J, Bonner W, Massey G. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J Am Chem Soc. 1977;99:3622–3625. doi: 10.1021/ja00453a018. [DOI] [PubMed] [Google Scholar]
- 9.Pizzarello S, Weber A. Stereoselective syntheses of pentose sugars under realistic prebiotic conditions. Orig Life Evol B. 2010;40:3–10. doi: 10.1007/s11084-009-9178-1. [DOI] [PubMed] [Google Scholar]
- 10.Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;21:22–26. [Google Scholar]
- 11.Senma M, Taira Z, Osaki K, Tagas T. Glyceraldehyde: An x-ray study of dl-glyceraldehyde dimer. J Chem Soc. 1973:880–881. [Google Scholar]
- 12.Breslow R, Cheng Z-L. On the origin of terrestrial homochirality for nucleosides and amino acids. Proc Natl Acad Sci USA. 2009;106:9144–9146. doi: 10.1073/pnas.0904350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breslow R, Levine M, Cheng Z-L. Imitating prebiotic homochirality on earth. Origins Life Evol B. 2010;40:11–26. doi: 10.1007/s11084-009-9179-0. [DOI] [PubMed] [Google Scholar]
- 14.Powner M, Gerland B, Sutherland J. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Pizzarello S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc Natl Acad Sci USA. 2006;103:12713–12717. doi: 10.1073/pnas.0602320103. [DOI] [PMC free article] [PubMed] [Google Scholar]