Abstract
Among the myriad complications involved in the current food crisis, the relationship between agriculture and the rest of nature is one of the most important yet remains only incompletely analyzed. Particularly in tropical areas, agriculture is frequently seen as the antithesis of the natural world, where the problem is framed as one of minimizing land devoted to agriculture so as to devote more to conservation of biodiversity and other ecosystem services. In particular, the “forest transition model” projects an overly optimistic vision of a future where increased agricultural intensification (to produce more per hectare) and/or increased rural-to-urban migration (to reduce the rural population that cuts forest for agriculture) suggests a near future of much tropical aforestation and higher agricultural production. Reviewing recent developments in ecological theory (showing the importance of migration between fragments and local extinction rates) coupled with empirical evidence, we argue that there is little to suggest that the forest transition model is useful for tropical areas, at least under current sociopolitical structures. A model that incorporates the agricultural matrix as an integral component of conservation programs is proposed. Furthermore, we suggest that this model will be most successful within a framework of small-scale agroecological production.
Keywords: food crisis, biodiversity, fragmented landscapes, matrix quality, small-scale farmers
The current food crisis calls attention to the need for construction of sustainable ecosystems more generally. As Robert Watson, the cochair of the International Assessment of Agricultural Knowledge, Science and Technology for Development (IAASTD) stated in a press conference when the report was released in 2008, “Business as usual is not an option.” Although the particulars are variable, the underlying sense is clear—the longue durée of economic, social, and political development in which environmental variables are regarded as externalities has come to a close. Within this awakening, the loss of biodiversity is regarded as one of the more important environmental issues related to both sustainability and food production. With extinction rates currently at greater levels than natural background, some have suggested that we are in the midst of another mass extinction comparable to the one that occurred at the end of the Cretaceous (1), except this time it is driven by humans rather than a natural catastrophic event, and the major human activity involved is agriculture, which clearly links the biodiversity crisis with the current food crisis.
In this article, we focus on one aspect of these crises—the debate about the application of the traditional forest transition (FT) model to the tropics in general, a debate that has subtle but important relations with the world food system. We contrast this model with what we refer to as the “matrix quality” model, in which agriculture is seen as an intimate and inextricable component of the biodiversity conservation agenda.
The Forest Transition Model
The European colonization of eastern North America began with massive deforestation that accompanied the expansion of agriculture. But then, through industrialization and the urbanization that accompanied it, agriculture declined and forests returned (2). The dynamics that drove this process are evident at a broad qualitative level—wealth from agriculture drives local industrialization that, in turn, acts as a magnet for labor, which depopulates the countryside, leaving natural succession to take over. Although this general view has many complications that drive local ecological and sociopolitical dynamics, as an overview of eastern North American forest history it seems historically accurate, and has been referred to as the “forest transition model” (3–5). Similar processes have been described for some European countries (5), the rural U.S. South (6), and, most importantly given its tropical location, Puerto Rico (7–10). Based on this and other examples, some have proposed that the FT model could be a framework for understanding tropical landscape dynamics in general and even be used for promoting a conservation agenda (8, 9, 11).
Although the argument is usually made in an informal qualitative sense, there is an underlying quantitative logic that drives the conclusions. Understanding that logic is helpful for understanding exactly where the argument is wrong.
Consider a defined land area of total size T divided into one portion that is agricultural (a) and another set aside for conservation (c); p represents the units of production (in energy per unit area), NL is the local (rural) population density, and e is the energy requirements of a single person. Clearly, at equilibrium,
which suggests that we can minimize a* by minimizing NL and/or maximizing p (assuming e will always remain constant). At its most simplistic level, this is the land-sparing argument (12).
The argument is elementary, based on simple accounting, suggesting that there are basically two sociopolitical-ecological forces in operation: first, a spatial concentration and intensification of agricultural production and, second, an exodus of the rural population to industrializing urban centers. Taken together, these forces reduce the demand for cropland, thus freeing marginal farmlands and leading to recovery of forests. This idea has become common and is sometimes taken as a self-evident process, worthy of paradigmatic status for conservation (e.g., refs. 11–13).
Obvious complications arise with only a slightly larger view of the population that must be serviced by agriculture. Consider, for example, that the total population, NT, consists of the sum of the rural population, NL, and the urban population (i.e., the population not involved in agricultural production but needing the products of agriculture), NU; in other words, NL + NU = NT. Modifying Eq. 1, we have a* = e(NL + NU)/p. Presuming each person works (w) land units to maintain and produce in the agricultural system, we have, at equilibrium,
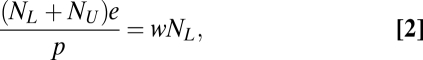 |
where the left-hand side is the amount of agricultural land needed to support the relevant population (NL + NU) and the right-hand side is the amount of agricultural land maintainable with NL workers working at a rate w. If the agricultural land needed is greater than the agricultural land maintainable, we see (from Eq. 2)
and the local experience will be one of a labor shortage (because the agricultural land needed to sustain the population is greater than the available labor can sustain). Making the reasonable assumption that equilibrium will be a social goal, the FT model proposes that we can equilibrate 3 by increasing either w or p, which could be done with labor-saving technology or higher units of production. However, with this formulation it is evident that increasing w or p are not the only ways of equilibrating 3. An alternative would be to increase the local rural population (contrary to the FT model). Ironically, as rural-to-urban migration proceeds, the inequality in 3 becomes more accentuated and the need to increase rural population consequently increases yet further.
Consider the reverse situation, where the agricultural land needed is less than the agricultural land maintainable (i.e., the inequality in 3 is reversed). Here the local experience is overproduction. Equilibrating the equation can be done by decreasing w (which is easily accomplished by taking land out of production), decreasing NL, or decreasing p, the last of which is clearly contrary to the basic ideas of the FT model.
Human response to the experience of either labor shortage (relation 3) or overproduction (relation 3 with the sign reversed) has always been complicated, with strong dependence on the way the society is organized. For example, in early barter and exchange societies where most agricultural production was for the use of the agricultural family itself, the response to overproduction is likely to be simply reducing w, that is, to take land out of production (no need to produce what you will not need). However, in more market-oriented societies, overproduction may lead to lowered market prices and the tendency by individual producers to increase production further to increase total farm revenue, or a shift to another commodity which may require more land (for example, extensive cattle pasture). In both cases, the result is the reverse of what would be expected from the simple FT model. Additionally, if production planning is keyed to current price conditions, simple nonlinearities may lead to chaotic price and production trajectories over the long haul, making it, in principle, impossible to say whether w will increase or decrease (14). Clearly, the social context makes an enormous difference.
Ultimately, the FT model rests on two quantitative assumptions and a seemingly logical conclusion. The two assumptions are, first, a given population density requires a certain land base to enable productive activities adequate to survival of the whole population (the “sustainable” population) and, second, the amount of food required to support that population, divided by current per-area productivity, equals the land area necessary for agricultural production (the rural population density required to support that production is the “necessary” population). The logical conclusion is that the total land area minus the area necessary for production is what is available for conservation.
The “rural-to-urban migration” part of the FT model focuses on the first assumption and notes that, with the reduction in rural population, more land will be available for conservation (fewer rural people, less use of land for agriculture, and thus natural regeneration of forest or other natural habitat). The “productivity” argument focuses on the second assumption and argues that if per-unit production could be increased, the required land base would be reduced, and consequently more land would be available for conservation (the same number of people needing food but higher productivity, thus less land for agriculture and more land for conservation). Referring again to 3, it is certainly possible for the FT model to operate, but our point is that it is not in any way quantitatively assured that it actually will. Theoretically, the issue is indeterminate. It thus makes sense to ask to what extent do real-world data suggest that recent tropical situations replay the experience of the previous examples that had given conservationists such hope (e.g., Puerto Rico or New England).
Angelsen and Kaimowitz (15) report on detailed studies that, as might be expected from the argument presented above, sometimes support the FT model, sometimes fail to support it. Their study notes an underlying contradiction in the basic ideas of the FT model. First, “the belief that technological progress in agriculture reduces pressure on forests by allowing farmers to produce the same amount of food in a smaller area has become almost an article of faith in development and environmental circles.” Second, “basic economic theory suggests that technological progress makes agriculture more profitable and gives farmers an incentive to expand production onto additional land,” suggesting that whether the predictions of the FT model are true or not depends to a great extent on specific sociopolitical and ecological circumstances. Examining 17 case studies from Latin America, Africa, and Asia (16), these authors conclude that the issue of intensification of agriculture and its relationship to deforestation is complex and, effectively, that agricultural policy could be modified in such a way as to promote forest-preservative policies rather than policies that, however unintentionally, actually promote more deforestation with “improved” agricultural technologies. Below, in the context of our matrix quality model, we discuss the qualitative nature of the sorts of agricultural development models that might be expected to restrain deforestation.
To be sure, a few studies show support for the forest transition model (9, 17–19) whereas others describe more complex situations (20, 21), but the great majority of the studies show no effect or increased deforestation with either agricultural intensification or rural population decline (15, 16). Other studies reflect similar complexity:
In the Sarapiqui region of Costa Rica (22), in spite of all of the conditions appropriate for the FT model (agricultural intensification, a national shift to an industrial and service economy that attracts people from rural to urban areas) in addition to changes in attitude of landowners in favor of forests (in part due to an increase in ecotourism), forest recovery has been prevented and forest fragmentation has continued due to the concentration of land into absentee-owned cattle ranches, producing what has been called “hollow frontiers” (22–24).
In El Salvador, through analysis of satellite images, it was found that local rural population density was uncorrelated with forest recovery, whereas remittances from family members living abroad correlated positively with forest recovery (25).
In a review of the evidence surrounding the claim that population drives deforestation in Panama (11), Sloan (26) concludes that where institutional, economic, or contextual factors are considered, population-deforestation correlations are found to be “spurious or even counter-intuitive.”
In Missiones, Argentina, Izquierdo et al. (27) note that although the population growth rate is slowing and the rural population is declining, forest cover continues to decline. They further note that, especially when soil and other physical conditions are not limiting, rural-to-urban migration does little to prevent further agricultural penetration into natural habitat, as has been happening in the Atlantic forest of Brazil.
A recent review of 17 studies of rural population dynamics in Mexico (28) found little evidence that either intensification (in the form of eliminating peasant agriculture) or rural outmigration has had the result expected from the forest transition paradigm. Of the 17 studies, 16 exhibited net deforestation even though the background conditions correspond to the requisites for the FT model to be applicable.
In sum, social context makes a difference in the direction as well as the degree of impact of agricultural intensification on deforestation, what Schmink calls the “socioeconomic matrix of deforestation” (29). These and other studies reject the simplifying assumptions of the forest transition model and echo the call of Angelsen and Kaimowitz (16) for careful examination of the social-political forces operative in land-use planning so as to develop programs that indeed will function to reduce deforestation. It is clear that the optimistic projections of a simple forest transition model, taking from the experience of some regions (e.g., Eastern United States, Europe) and applying wholesale to tropical regions in today's political climate, could be misleading.
In a break with such simplifying assumptions, Hecht (30) proposes the addition of a new conceptual framework specifically tuned to the contemporary situation (and most evidently applicable to Latin America). This new conceptual framework is called the “new rurality,” and categorizes rural landscapes into four broad and overlapping categories: environmental, socioenvironmental, agroindustrial, and peasant. Such a categorization would not have made a great deal of sense either before the Cold War or during the heydays of neoliberalism after the Cold War, but, argues Hecht, it is a framework that strongly aids our understanding of rural dynamics in the contemporary world as it has been unfolding since the end of the Cold War. Analysts concerned with rural landscapes tend to fall into one of these categories, and their analysis is consequently driven by the vision they bring to the table. Environmentalists seek to preserve native habitats, socioenvironmentalists seek to incorporate indigenous and local communities in their conservation plan, and agroindustrialists see tremendous opportunity in the expansion of industrial agriculture, which sometimes includes, sometimes excludes, the peasant element. Those who see the rural areas still populated with peasants (small family farms) see them acting in a variety of complex ways, sometimes with strong economic and sociocultural links to cities. These complicated actions and linkages ultimately will determine the fate of rural landscapes, according to this point of view.
The Matrix Quality Model
Aligning ourselves effectively in Hecht's description of those who see rural areas still populated with peasants and small-size family farms, and focusing on the past few decades of development in the science of ecology, we argue that data and theory suggest that conservation should be viewed from a larger landscape perspective and that, with that perspective, moving agriculture toward a sustainability priority rather than a productivist priority has more potential to affect biodiversity conservation positively. Furthermore, there is at least circumstantial evidence that such a model would help, indirectly, to solve several aspects of the world food crisis.
The Ecological Component, a Mean-Field Approach.
Reflecting older arguments in ecology, the standard preservationist attitude is effectively a “local carrying capacity” attitude, focusing on the size of a natural area, noting, correctly, that a minimum area is required for the long-term persistence of target species but failing to acknowledge up front that the larger landscape is sometimes more important for species survival than the size of a particular patch of natural habitat. This preservationist attitude has been criticized mostly from a social, moral, and ethical point of view (31, 32). More recently, the criticism has been enriched with ecological theory that supports what might be called an “interfragment migration” approach, deriving mainly from recent ecological research on metapopulations (33, 34). This new approach emphasizes the matrix within which fragments are located, and frames the argument as the “quality” of that matrix. This framing can be formalized through the use of metapopulation theory (35–37). To this end, an extension of the Levins model has been employed (38–40), namely, letting p be the proportion of potential habitats occupied by the species in question, m be the migration rate, and e be the extinction rate,
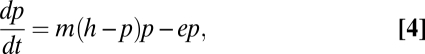 |
where h is the amount of appropriate habitat still available (h = 1 is an unperturbed habitat). Thus, the equilibrium situation will be p* = h – e/m, and the critical habitat loss that results in regional extinction would then be h = e/m (38, 41).
This approach carries with it the critical assumption that as habitats are lost, the migration coefficient will remain constant. This assumption is not likely to be satisfied in many cases in nature. Consider, for example, a set of n very small habitat patches arranged in a one-dimensional space of length N. The average distance between patches is N/n. If the fraction of suitable habitat remaining is changed from 1 to h (where 0 < h < 1), the number of habitats will be hn, and the distance between patches will be approximately N/hn. Given an organism that is capable of migrating at some fixed rate, the effective patch-to-patch rate will be proportional to N/hn, which is to say the migration rate will be a function of h, the fraction of remaining suitable habitats. Thus, in Eq. 4 the migration coefficient should be replaced by a function of h.
As a first approximation, take the function to be a simple proportion (that is, the migration coefficient multiplied by the fraction of suitable habitat remaining = m1h), which gives
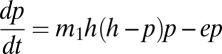 |
with an equilibrium value of p* = h − e/m1h, whence we can calculate that the metapopulation will persist (i.e., p* will be greater than zero) as long as
 |
And because e/m1 < 1 for persistence even without habitat destruction, we note that
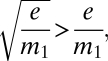 |
which means that the original notion that h must be greater than the extinction-to-migration ratio for persistence is optimistic. Because of the common, if not inevitable, reduction in overall migration rate with the reduction in fragment numbers, the critical habitat loss is scaled to the square root of that ratio, not the ratio itself.
From the point of view of our matrix quality model, an additional point about h is essential. In the real world it is only rarely the case that habitats are “completely” destroyed. Furthermore, a great deal of conservation biology now concerns itself with the quality of the matrix, partially because of the significant amounts of biodiversity that may be contained therein but especially because interfragment migration is necessary for metapopulation survival (33, 42–50). In previous work (44), the limited nature of the classic metapopulation approach has been noted, especially with respect to its assumption that the matrix in which subpopulations are situated is homogeneous, showing one way in which that assumption could be relaxed—that is, by allowing the quality of the matrix to enter the basic equation as a linear input to the migration rate. The framework presented here expands on that relaxation by focusing on h, a focus explicitly relevant to anthropogenic landscapes but retaining the heuristic convenience of the mean-field approach. If h is the amount of original habitat left, suppose the rest is divided between q1 and q2, good-quality matrix and poor-quality matrix, respectively. Suppose the good-quality matrix (q1) in fact does permit the same migration coefficient as when h = 1 but there is a significant reduction in the poor-quality matrix (q2). Thus, assuming q2 = 0, we have
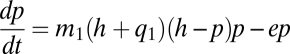 |
with an equilibrium value of p* = h − e/m1(h + q1), whence we calculate that the metapopulation will persist as long as
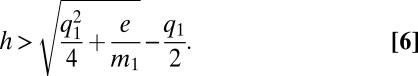 |
And, comparing this value with the original criterion on h, we find persistence always enhanced by matrix quality, not surprisingly. Relating the critical habitat with zero matrix quality to the critical habitat with q1 matrix quality, we can formulate the benefit of improving matrix quality as the ratio of those two critical habitats, which is
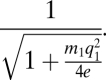 |
Note the somewhat surprising result that an improvement in matrix quality can outweigh the negative effects of habitat loss at values of h > 1 − q1, a fact that could have important practical consequences and clearly relates to the question of what is being done in the matrix habitat. It is worth noting also that, as in the standard metapopulation model, when p is very small it is especially sensitive to changes in migration and extinction rates.
This approach, using the simple mean-field metapopulation model, only relates to the question of persistence or extinction of a particular species, and is, effectively, an extension of previous approaches (40, 44). Scaling up to the community level is in the realm of metacommunity theory (51). If a metacommunity is thought of as only a collection of metapopulations (not the only possible definition), then our argument extends in an elementary fashion. Furthermore, we acknowledge the obvious fact that the direct biodiversity conservation value of agriculture varies greatly, with some forms of agriculture well-known to contain within their associated biodiversity almost as many species of some taxa as the natural habitat from which they were carved (42). Finally, we note that matrix quality will vary for different species, and in particular with the type of natural habitat that agriculture replaces.
Extinctions in Fragments and Migrations Through the Matrix.
Much of spatial ecological theory depends on extinction as one of the major processes driving patterns, including patterns of biodiversity (52–57). Although the fact of local extinctions is well-established it does not occur randomly, and certainly deserves more study (58–60). Nevertheless, there is little doubt that amid many complications, populations living in isolated fragments of natural vegetation can expect to experience extinctions, if enough time passes. If conservation is to be a long-term goal, this elementary and undeniable fact must be incorporated into planning.
A further complication may result from spatial self-organization. Consider, for example, plant communities in which the constituent species tend to expand in space through seed dispersal but are attacked by natural enemies in a density-dependent fashion according to the Janzen/Connell effect (61–63). It can be shown that such an arrangement will result in the clumping of organisms even in a uniform environment (64). Because of the dynamic interplay of seed dispersal and density-dependent control, any given clump is expected to go locally extinct over the long run. In such a situation, fragmenting the continuous habitat does not change much about the local extinct rates, which are a consequence of density-dependent operation of natural enemy dynamics. However, normal migration (i.e., seed dispersal) will be reduced.
Unfortunately, long-term studies that uncover such patterns of extinctions in continuous habitat are not common in the literature. Rooney et al. (65) demonstrated dramatic changes in species composition in plots embedded in natural forest communities in the northern Great Lakes region of the United States. Environmental drivers in this case included forces such as deer hunting and invasive species, but one of their key results is that, even in this unfragmented forest, species loss at a local level was dramatic. In a 20-year study of the amphibians occupying small ponds in a forested matrix, ≈30 local extinction events were observed (66). In this case, the researchers were able to demonstrate that “reinvasions,” which is to say, migration events, completely balanced these local extinctions (66). In summary, both ecological theory and empirical studies strongly suggest a three-part conclusion. First, local extinctions are normal and occur even in areas of continuous natural habitats. Second, migrations throughout the matrix can balance those extinctions and maintain a metapopulation structure that will prevent regional extinction. Third, the quality of the matrix matters; high-quality matrices are those that promote migration, thus maintaining metapopulation structures that obviate regional extinction.
Convergence of Food Production with Nature Conservation
The matrix quality model challenges the assumption that agriculture is the enemy of conservation. It is the kind of agriculture, not the simple fact of its existence, that matters. Whether looked at from the point of view of the simple mean-field model (Eqs. 4–6) or from the more qualitative empirical fact that some habitats promote more migration than others, the agricultural matrix is perhaps the most important habitat on which conservation efforts must focus. But this brings us face to face with one of the multiple functions of agriculture: to produce food, fiber, drugs, and energy for human use.
Regarding the productivity of agriculture, we face what seems at first to be a dilemma. The sort of high-energy-demanding, chemically intensive agriculture associated with modernity generates a very low quality matrix, whereas alternative agriculture (organic, agroecological, natural-systems agriculture, etc.) would seem to be precisely the forms that would produce a high-quality matrix. Yet it is just such agricultural types that are normally assumed to be less productive. A simple accounting from this assumption is precisely what generates the land-sparing model, the forest transition model, and the optimistic assessments that rural-urban migration, as it decreases the number of “peasant” producers (automatically presumed to be inefficient), will result in equivalent, or even higher, production on less land, generating more forest recovery. However, what evidence supports this fundamental assumption?
Anecdotes can easily support the assumption, especially when highly subsidized farmers from the United States and other industrialized regions are compared with small-scale farmers of the Global South, and the measure of productivity is yield of the main commercial crop or net profit. However, if the measure of productivity is simply total output per area, relevant data do not seem to support the basic assumption. For example, analyzing data relating farm size to productivity (output per unit area), Cornia (67) found that in all cases the trend was decreasing productivity as farm size increased. Indeed, the “productivity-size inverse relationship” is a well-known fact among agricultural economists, and was first pointed out by Nobel laureate Amartya Sen in the 1960s (68, 69). It seems that small owner-operated farms tend to be more efficient in that the farmer knows the land and its ecology well, and plants crops with that knowledge, usually using a multicropping strategy to take advantage of local peculiarities such as, for example, the Kayapó’s management of their Amazonian landscape where the patches of the matrix are an entangled mosaic that takes advantage of microclimatic and soil differences to produce and promote hundreds of species of plants and animals (70). Many other examples could be cited. Contrarily, large, highly capitalized farms seek economies of scale in which those local ecological peculiarities are purposefully ignored. Ironically, the recent enthusiasm for so-called precision farming (71) acknowledges precisely this underlying ecological structure, but proposes to resolve it with a high-tech strategy of sensors and delivery systems. As one of our students reviewing the literature on precision farming quipped, “small-scale farmers already do precision farming.” Thus, both the logic and the data (67) suggest that small-scale agriculture can be more productive, on a per unit-area basis, than large-scale agriculture.
The assumption that large-scale intensive monocultures are more productive than agroecological and organic systems is likewise debatable. In a recent review of almost 300 studies comparing yields of organic/agroecological and conventional agriculture throughout the world, it was found that, on average, organic and agroecological systems produce as much, if not more, than conventional systems (72), corroborating many other studies (73–77). Furthermore, it has now been well-established that energy efficiency in traditional and many organic systems is higher than in high-industrial/conventional agriculture (78–81).
In summary, contrary to the conventional wisdom that industrial agriculture is needed to produce enough food to feed the world, the empirical evidence suggests that peasant and small-scale family farm operations adopting agroecological methods can be as (or more) productive than industrial agriculture. Given that most of the world’s poor live in rural areas or are urban poor recently displaced from rural areas, an agricultural matrix composed of small-scale sustainable farms can thus create a win-win situation that addresses both the current food crisis and the biodiversity crisis.
However, there exists a very complicated irony that is rarely addressed. The search for more productivity, part and parcel of the research agenda of most agricultural researchers, is not necessarily a rational project. In many cases (and here coffee and maize would be excellent recent examples), the major agricultural problem is “overproduction” and consequent low prices. The recent (and temporary) increase in food prices notwithstanding, it is often the case that farmers receive inadequate compensation for their efforts largely because markets become saturated. If unregulated markets must be the rule, an assumption that itself might be questioned, overproduction and low prices will continue to plague farmers, not continuously, but on a boom-and-bust cycle. Indeed, the IAASTD, an intergovernmental assessment process that involved 3 years of research and 400 experts from all over the world, concluded that conventional/industrial agriculture is not a rational option for alleviating poverty and ending hunger and malnutrition nor for sustainable development, further noting that more equality is needed for alleviating hunger and malnutrition (82). This equality is more likely to be achieved through a land reform that redistributes land that is in the hands of big agrobusiness and planted in commercial monocultures and puts it in the hands of small- and medium-size family farmers who are more likely to construct a landscape mosaic that promotes biodiversity and produces more food.
Discussion
In this paper, we present a framework for analyzing the relationship between agriculture and conservation, what we refer to as the matrix quality approach, intended to be an alternative to some other approaches such as the forest transition model. Our analysis does not aim to prove that the predictions of the forest transition model cannot be true, but rather seeks to frame the problem in such a way as to first see that its predictions are weak from a theoretical point of view and do not inevitably play out as expected in the real world. On the other hand, the realities of the current tropical world, which is mainly in a state of extreme fragmentation, coupled with the growing consensus among ecologists that metapopulations, metacommunities, and landscape processes are important determinants of biodiversity, suggest that the matrix framing has a better chance of capturing reality than the alternatives. Given this model, the practical consequences suggest that promotion of small-scale sustainable agriculture, as an integral part of tropical landscapes, is more likely to preserve biodiversity in the long term. Furthermore, it is the small-scale agriculturalists who are more likely to adopt sustainable agricultural technologies because they use few or no external inputs, use locally and naturally available materials, and generate agroecosystems that are more diverse and resistant to stress than capital-intensive technologies (77, 82).
In the end, it appears that the real needs of people for a diet that is sufficient in quantity and quality is the same as the need of the landscape for a high-quality matrix within which fragments of high-diversity native vegetation can persist along with biodiversity-friendly agroecosystems to form an integrated landscape. Indeed, recent international documents that evaluate the role of agriculture in alleviating hunger and promoting sustainable development (including the IAASTD) coincide with the conclusion that small-scale sustainable farming systems are the best option for achieving both of these goals (77, 82, 83).
In a world where people go hungry amid an abundance of food and where the great majority of the poor live in rural areas or are forced, by economic necessity, to abandon their rural livelihood, models of agricultural intensification that continue this trend are bound to fail. A new rurality based on the matrix quality approach is more likely to lead to situations in which biodiversity is conserved at the same time that more food is available to those who need it the most.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.L. is a guest editor invited by the Editorial Board.
See Commentary article on page 5697.
References
- 1.Chen J. Legal mythmaking in a time of mass extinctions: Reconciling stories of origins with human destiny. Harv Environ Law Rev. 2005;29:279–319. [Google Scholar]
- 2.Pfaff ASP. In: World Forests from Deforestation to Transition? Palo M, Vanhanen H, editors. Dordrecht, The Netherlands: Kluwer Academic; 2000. pp. 67–82. [Google Scholar]
- 3.Mather AS. The forest transition. Area. 1992;24:367–379. [Google Scholar]
- 4.Mather AS, Needle CL. The forest transition: A theoretical basis. Area. 1998;30:117–124. [Google Scholar]
- 5.Mather AS. Forest transition theory and the reforesting of Scotland. Scott Geogr J. 2008;120:83–98. [Google Scholar]
- 6.Rudel TK. Is there a forest transition? Deforestation, reforestation and development. Rural Sociol. 1998;63:533–552. [Google Scholar]
- 7.Thomlison JR, Serrano MI, Lopez TM, Aide TM, Zimmerman JK. Land-use dynamics in a post-agricultural Puerto Rican landscape (1936–1988) Biotropica. 1996;28:525–536. [Google Scholar]
- 8.Grau HR, et al. The ecological consequences of socioeconomic and land-use changes in post-agriculture Puerto Rico. Bioscience. 2003;53:1159–1168. [Google Scholar]
- 9.Aide TM, Grau HR. Globalization, migration, and Latin American ecosystems. Science. 2004;305:1915–1916. doi: 10.1126/science.1103179. [DOI] [PubMed] [Google Scholar]
- 10.Mercano-Vega H, Aide TM, Báez B. Forest regeneration in abandoned coffee plantations and pastures in the Cordillera Central of Puerto Rico. Plant Ecol. 2002;161:75–87. [Google Scholar]
- 11.Wright JS, Muller-Landau HC. The future of tropical forest species. Biotropica. 2006;38:287–301. [Google Scholar]
- 12.Green RE, Cornell SJ, Scharlemann JPW, Balmford A. Farming and the fate of wild nature. Science. 2005;307:550–555. doi: 10.1126/science.1106049. [DOI] [PubMed] [Google Scholar]
- 13.Borlaug NE. Ending world hunger. Plant Physiol. 2000;124:487–490. doi: 10.1104/pp.124.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandermeer J. Notes on agroecosystem complexity: Chaotic price and production trajectories deducible from simple one-dimensional maps. Biol Agric Hortic. 1990;6:293–304. [Google Scholar]
- 15.Angelsen A, Kaimowitz D. In: Tradeoffs or Synergies? Agricultural Intensification, Economic Development and the Environment. Lee DR, Barrett CB, editors. Wallingford, UK: CABI; 2001. pp. 89–114. [Google Scholar]
- 16.Angelsen A, Kaimowitz D. In: Agricultural Technologies and Tropical Deforestation. Angelsen A, Kaimowitz D, editors. Wallingford, UK: CABI; 2001. pp. 383–402. [Google Scholar]
- 17.Kleinn C, Corrales L, Morales D. Forest area in Costa Rica: A comparative case study of tropical forest cover estimates over time. Environ Monit Assess. 2002;73:17–40. doi: 10.1023/a:1012659129083. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez JM. Final Report: Forest, Payment for Environmental Services and Forest Industries (Informe Final: Bosque, Pago de Servicios Ambientales e Industria Foresta) San José, Costa Rica: Comisión Nacional de Rectores and Defensoria de los Habitantes; 2004. [Google Scholar]
- 19.Klooster D. Forest transitions in Mexico: Institutions and forests in a globalized countryside. Prof Geogr. 2003;55:227–237. [Google Scholar]
- 20.Roebeling P, Ruben R. In: Agricultural Technologies and Tropical Deforestation. Angelsen A, Kaimowitz D, editors. Wallingford, UK: CABI; 2001. pp. 135–154. [Google Scholar]
- 21.Holden S. In: Agricultural Technologies and Tropical Deforestation. Angelsen A, Kaimowitz D, editors. Wallingford, UK: CABI; 2001. pp. 251–270. [Google Scholar]
- 22.Schelhas J, Sánchez-Azofeifa GA. Post-frontier change adjacent to Braulio Carrillo National Park, Costa Rica. Hum Ecol. 2006;34:407–431. [Google Scholar]
- 23.Bilsborrow ER, Carr DL. In: Tradeoffs or Synergies? Agricultural Intensification, Economic Development and the Environment. Angelsen A, Kaimowitz D, editors. Wallingford, UK: CABI; 2001. pp. 35–55. [Google Scholar]
- 24.Rudel TK, Bates D, Machinguishi R. A tropical forest transition? Agricultural change, out-migration, and secondary forests in the Ecuadorian Amazon. Ann Assoc Am Geogr. 2002;92:87–102. [Google Scholar]
- 25.Hecht SB, Saatchi SS. Globalization and forest resurgence: Changes in forest cover in El Salvador. Bioscience. 2007;57:663–672. [Google Scholar]
- 26.Sloan S. Fewer people may not mean more forest for Latin American forest frontiers. Biotropica. 2007;39:443–446. [Google Scholar]
- 27.Izquierdo AE, De Angelo CD, Aide MT. Thirty years of human demography and land-use change in the Atlantic forest of Missiones, Argentina: An evaluation of the forest transition model. Ecol Soc. 2008;13:3. [Google Scholar]
- 28.Garcia-Barrios L, et al. Neotropical forest conservation, agricultural intensification, and rural out-migration: The Mexican experience. Bioscience. 2009;59:863–873. [Google Scholar]
- 29.Schmink M. In: Population and Environment: Rethinking the Debate. Arizpe L, Stone MP, Major DC, editors. Bolder, CO: Westview; 1994. pp. 253–275. [Google Scholar]
- 30.Hecht S. The new rurality: Globalization, peasants and the paradoxes of landscapes. Land Use Policy. 2010;27:161–169. [Google Scholar]
- 31.Brockington D. Fortress Conservation: The Preservation of the Mkomazi Game Reserve. Oxford: James Currey; 2001. [Google Scholar]
- 32.Brechin S, Wilshusen P, Fortwangler C, West P, editors. Contested Nature: Promoting International Biodiversity with Social Justice in the Twenty-First Century. Albany: State University Press of New York; 2003. [Google Scholar]
- 33.Perfecto I, Vandermeer J. Biodiversity conservation in tropical agroecosystems: A new conservation paradigm. Ann N Y Acad Sci. 2008;1134:173–200. doi: 10.1196/annals.1439.011. [DOI] [PubMed] [Google Scholar]
- 34.Perfecto I, Vandermeer J. Nature's Matrix: Conservation, Agriculture and Food Sovereignty. London: Earthscan; 2009. [Google Scholar]
- 35.Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am. 1969;15:237–240. [Google Scholar]
- 36.Nee S. How populations persist. Nature. 1994;367:123–124. [Google Scholar]
- 37.Nee S, May RM, Hassell MP. In: Metapopulation Biology: Ecology, Genetics and Evolution. Hanski I, Gilpin M, editors. London: Academic; 1996. pp. 123–146. [Google Scholar]
- 38.Kareiva P, Wennergren U. Connecting landscape patterns to ecosystem and population processes. Nature. 1995;373:299–302. [Google Scholar]
- 39.Hanski I, Moilanen A, Gyllenberg M. Minimum viable metapopulation size. Am Nat. 1996;147:527–541. [Google Scholar]
- 40.Amarasekare P. Allee effects in metapopulation dynamics. Am Nat. 1998;152:298–302. doi: 10.1086/286169. [DOI] [PubMed] [Google Scholar]
- 41.Lawton JH, Nee S, Letcher AJ, Harvey PJ. In: Large-Scale Ecology and Conservation Biology. Edwards PJ, May RM, Webb NR, editors. Oxford: Blackwell; 1994. pp. 41–58. [Google Scholar]
- 42.Perfecto I, Rice R, Greenberg R, Van der Voolt M. Shade coffee as refuge of biodiversity. Bioscience. 1996;46:589–608. [Google Scholar]
- 43.Ricketts TH. The matrix matters: Effective isolation in fragmented landscapes. Am Nat. 2001;158:87–99. doi: 10.1086/320863. [DOI] [PubMed] [Google Scholar]
- 44.Vandermeer J, Carvajal R. Metapopulation dynamics and the quality of the matrix. Am Nat. 2001;158:211–220. doi: 10.1086/321318. [DOI] [PubMed] [Google Scholar]
- 45.Perfecto I, Vandermeer J. The quality of the agroecological matrix in a tropical montane landscape: Ants in coffee plantations in southern Mexico. Conserv Biol. 2002;16:174–182. doi: 10.1046/j.1523-1739.2002.99536.x. [DOI] [PubMed] [Google Scholar]
- 46.Luck GW, Daily GC. Tropical countryside bird assemblages: Richness, composition, and foraging differ by landscape context. Ecol Appl. 2003;13:235–247. [Google Scholar]
- 47.Dauber J, et al. Landscape structure as an indicator of biodiversity: Matrix effects on species richness. Agric Ecosyst Environ. 2003;98:321–329. [Google Scholar]
- 48.Donald PF, Evans AD. Habitat connectivity and matrix restoration: The wider implications of agri-environment schemes. J Appl Ecol. 2006;43:209–218. [Google Scholar]
- 49.Jha S, Dick CW. Shade coffee farms promote genetic diversity of native trees. Curr Biol. 2008;18:1126–1128. doi: 10.1016/j.cub.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Vandermeer J, Perfecto I, Philpott SM, Chappell MJ. In: Evaluation and Conservation of Biological Diversity in Fragmented Landscapes of Mesoamerica (Evaluación y Conservación de la Biodiversidad en Paisajes Fragmentados en Mesoamerica) Saenz J, Harvey C, editors. Heredia: Editorial de la Universidad Nacional Autónoma de Costa Rica; 2008. pp. 75–104. [Google Scholar]
- 51.Leibold MA, Miller TE. In: Ecology, Genetics and Evolution of Metapopulations. Hanski I, Gaggiotti OE, editors. Amsterdam: Elsevier/Academic Press; 2004. pp. 133–150. [Google Scholar]
- 52.Fischer M, Stöcklin J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950–1985. Conserv Biol. 1997;11:727–737. [Google Scholar]
- 53.Kéry M. Extinction rate estimates for plant populations in revisitation studies: Importance of detectability. Conserv Biol. 2004;18:570–574. [Google Scholar]
- 54.Matthies D, Bräuer I, Maibom W, Tscharntke T. Population size and the risk of local extinction: Empirical evidence from rare plants. Oikos. 2004;105:481–488. [Google Scholar]
- 55.Wilsey BJ, Martin LM, Polley HW. Predicting plant extinction based on species-area curves in prairie fragments with high β richness. Conserv Biol. 2005;19:1835–1841. [Google Scholar]
- 56.Williams NSG, Morgan JW, McDonnell MJ, McCarthy MA. Plant traits and local extinctions in natural grasslands along an urban-rural gradient. J Ecol. 2005;93:1203–1213. [Google Scholar]
- 57.Foufopoulos J, Ives AR. Reptile extinctions on land-bridge islands: Life-history attributes and vulnerability to extinction. Am Nat. 1999;153:1–25. doi: 10.1086/303149. [DOI] [PubMed] [Google Scholar]
- 58.Bolger DT, Alberts AC, Soulé ME. Occurrence patterns of bird species in habitat fragments: Sampling, extinction, and nested species subsets. Am Nat. 1991;137:155–166. [Google Scholar]
- 59.Helm A, Hanski I, Partel M. Slow response of plant species richness to habitat loss and fragmentation. Ecol Lett. 2006;9:72–77. doi: 10.1111/j.1461-0248.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 60.Brooks TM, Pimm SL, Oyugi JO. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv Biol. 1999;13:1140–1150. [Google Scholar]
- 61.Janzen DH. Herbivores and the number of tree species in tropical forests. Am Nat. 1970;104:501–528. [Google Scholar]
- 62.Connell JH. In: Dynamic Populations. den Boer PJ, Gradwell GR, editors. Wageningen, The Netherlands: Center for Agricultural Publishing and Documentation; 1971. pp. 298–312. [Google Scholar]
- 63.Hyatt LA, et al. The distance dependence prediction of the Janzen-Connell hypothesis: A meta-analysis. Oikos. 2003;503:590–602. [Google Scholar]
- 64.Vandermeer J, Perfecto I, Philpott SM. Clusters of ant colonies and robust criticality in a tropical agroecosystem. Nature. 2008;451:457–459. doi: 10.1038/nature06477. [DOI] [PubMed] [Google Scholar]
- 65.Rooney TP, Wiegmann SM, Rogers DA, Waller DM. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv Biol. 2004;18:787–798. [Google Scholar]
- 66.Werner EE, Yurewicz KL, Skelly DK, Relyea RA. Turnover in an amphibian metacommunity: The role of local and regional factors. Oikos. 2007;116:1713–1725. [Google Scholar]
- 67.Cornia GA. Farm size, land yields and the agricultural production function: An analysis for fifteen developing countries. World Dev. 1985;13:131–145. [Google Scholar]
- 68.Sen A. Economic Weekly. 1962. An aspect of Indian agriculture; pp. 243–246. February. [Google Scholar]
- 69.Carter MR. Identification of the inverse relationship between farm size and productivity: An empirical analysis of peasant agricultural production. Oxf Econ Pap. 1984;36:131–145. [Google Scholar]
- 70.Posey DA. Indigenous management of tropical forest ecosystems: The case of the Kayapó Indians of the Brazilian Amazon. Agrofor Syst. 1985;3:139–158. [Google Scholar]
- 71.McBratney A, Whelan B, Ancev T, Bouma J. Future directions of precision agriculture. Precis Agric. 2005;6:7–23. [Google Scholar]
- 72.Badgley C, et al. Organic agriculture and the global food supply. Renew Agric Food Syst. 2007;22:86–108. [Google Scholar]
- 73.Stanhill G. The comparative productivity of organic agriculture. Agric Ecosyst Environ. 1990;30:1–26. [Google Scholar]
- 74.Uphoff N. Higher yields with fewer external inputs? The system of rice intensification and potential contributions to agricultural sustainability. Int J Agric Sustain. 2003;1:38–50. [Google Scholar]
- 75.Pimentel D, Hepperly P, Hanson J, Douds D, Seidel R. Environmental, energetic and economic comparisons of organic and conventional farming systems. Bioscience. 2005;55:573–582. [Google Scholar]
- 76.Naerstad A. Africa Can Feed Itself. Oslo: The Development Fund; 2007. [Google Scholar]
- 77.UNCTAD-UNEP . Organic Agriculture and Food Security in Africa. UNEP-UNCTAD Capacity-Building Taskforce on Trade, Environment and Development. New York and Geneva: United Nations; 2008. [Google Scholar]
- 78.Pimentel D. Handbook of Energy Utilization in Agriculture. Boca Raton, FL: CRC; 1980. [Google Scholar]
- 79.Altieri MA. Applying agroecology to enhance the productivity of peasant farming systems in Latin America. Environ Dev Sustain. 1999;1:197–217. [Google Scholar]
- 80.Dalgaard T, Helberg N, Porter JN. A model for fossil energy use in Danish agriculture used to compare organic and conventional farming. Agric Ecosyst Environ. 2001;87:51–65. [Google Scholar]
- 81.Reganold JP, Glover JD, Andrews PK, Hinman HR. Sustainability of three apple production systems. Nature. 2001;410:926–930. doi: 10.1038/35073574. [DOI] [PubMed] [Google Scholar]
- 82.International Assessment of Agricultural Knowledge, Science and Technology for Development . In: International Assessment of Agricultural Knowledge, Science and Technology for Development: The Synthesis Report. McIntyre BD, Herren HR, Wakhungu J, Watson RT, editors. Washington, DC: Island; 2009. [Google Scholar]
- 83.Food and Agriculture Organization Organic Agriculture and Food Availability. 2007 International Conference on Organic Agriculture and Food Security, May 3–5, 2007, Rome. [Google Scholar]


