Abstract
Bacteriophage Φ2954 contains three dsRNA genomic segments, designated L, M, and S. The RNA is located inside a core particle composed of multiple copies of a major structural protein, an RNA-dependent RNA polymerase, a hexameric NTPase, and an auxiliary protein. The core particle is covered by a shell of protein P8, and this structure is enclosed within a lipid-containing membrane. We have found that normal infection of the host Pseudomonas syringae is dependent on the action of a host protein, glutaredoxin 3 (GrxC). GrxC removes the P8 shell from the infecting particle and binds to the inner core. Removal of P8 activates the transcription of segments S and M, whereas binding of GrxC to the core particle activates the transcription of segment L. The differences in transcription behavior are due to the preference of the polymerase for G as the first base of the transcript. Transcripts of segments S and M begin with GCAA, whereas those of segment L begin with ACAA. The binding of GrxC to the particle results in changes in polymerase activity. Mutations resulting in independence of GrxC are found in the gene for protein P1, the major structural protein of the inner core particle.
Keywords: dsRNA, GFP fusion, temporal control
Bacteriophage Φ2954 is a member of the Cystoviridae, a family of bacteriophages that have genomes of three double-stranded RNA molecules, L, M, and S, packaged inside a polyhedral capsid structure covered by a lipid-containing membrane (1). Φ6 was the first member of this family to be discovered (2). A number of relatives of Φ6 were isolated in 1999 (3). Some of these relatives were closely related to Φ6, whereas others were more distantly related and attached to host cells through rough LPS rather than the type IV pili used by Φ6. Φ2954 is rather similar to Φ12, a member of the latter group, but it attaches to host cells by means of the type IV pilus. Cystoviridae infect Gram-negative bacteria, primarily Pseudomonas syringae and its relatives. Φ6 has been the primary subject of studies of the Cystoviridae.
Φ6 infects by fusing its membranes with the outer membrane of the host cells, breaching the cell wall through the action of muramidases, and finally having the nucleocapsid of the virion enter the host cell (4). The nucleocapsid loses its outer shell of protein P8 to expose the viral core. The core contains an RNA-dependent RNA polymerase that synthesizes transcripts that are released from the core to program the synthesis of phage proteins (5). How the P8 shell of Φ6 is removed in vivo is unclear; however, P8 can be removed in vitro by incubation with calcium-chelating agents, such as EGTA. In that case, transcription of segments S and M is activated. Transcription of segment L does not occur, because the phage polymerase prefers G as the second nucleotide in the transcript, and segment L begins with GUAA, whereas segments S and M begin with GGAA. A host protein, YajQ, is required for the activation of segment L transcription of Φ6 (6). The binding of YajQ to protein P1 of the core capsid apparently activates the polymerase P2 located inside the particle. Φ2954 is not dependent on YajQ. In the case of Φ2954, EGTA treatment does not result in the removal of the P8 shell. We have found that a host protein, glutaredoxin 3 (GrxC), is responsible for this function and for the activation of the transcription of segment L.
Results
Transposon Knockout of GrxC.
In previous work, we showed that a host protein, YajQ, is required for normal infection of bacteriophage Φ6 (6). Distantly related phages of the same family are independent of YajQ, however. We undertook a search for the host proteins required by phage Φ2954. The gene for kanamycin resistance was inserted at random into the genome of P. syringae using the transposon preparation EZ-Tn5 (Epicentre Biotechnologies) (7). A collection of several thousand kanamycin-resistant colonies was screened for resistance to bacteriophages Φ6 and Φ2954. Because both phages adsorb to host cells via the type IV pilus, we pursued only those colonies that were resistant to Φ2954 but sensitive to Φ6. We found two types of insertions, located in the genes mdoG and grxC. The mdoG insertions are only partially inhibitory to Φ2954, whereas the grxC insertions are severely inhibitory to Φ2954. mdoG codes for periplasmic glucan biosynthesis (GenBank ID AAZ33878.1), and grxC codes for GrxC (GenBank ID AAZ37758.1). Oligonucleotide primers were prepared for the cloning of the intact gene of grxC to enable production of the protein GrxC with and without n-terminal his tags. The genes were placed in shuttle plasmid pLM254, which propagates in both Escherichia coli and P. syringae (8). Both the WT and the his-tag proteins complemented the transposon knockout. The sequence CPYC in GrxC is important for its function in oxidation-reduction reactions (9). We prepared a construct of GrxC with the sequence SPYT and found that it was able to complement the knockout strain. Plaques appeared on the knockout strain at a frequency of about 10−5 with respect to the plating on normal strains. Mutations were found in gene 1, which codes for the major structural protein of the virus core. Two independent mutants had a gene 1 mutation at C5345T in segment L, resulting in A354V in protein P1 (Φ3015), or at A5351T in segment L, resulting in D356V in protein P1 (Φ3016).
Viral RNA Synthesis in the grxC Knockout Strain.
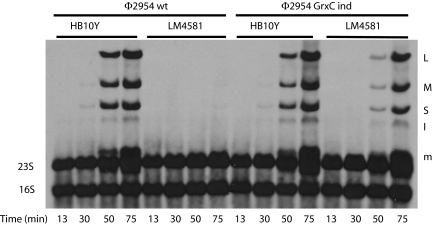
Cultures of strains HB10Y and LM4581, the grxC knockout strain, were infected with WT and GrxC independent mutants of Φ2954. The cells were labeled with tritiated uracil. The patterns of RNA synthesis are shown in Fig. 1. Ethidium labeling of the gels revealed infection in all lanes, but with very poor viral RNA synthesis for WT virus in the knockout strain. The independent mutant exhibited greater RNA synthesis compared with WT virus in the knockout strain, but much less than that in the WT host cells.
Fig. 1.
Autoradiogram of a polyacrylamide gel displaying H3-labeled RNA of Φ2954-infected cells. Phage is WT Φ2954 or Φ2954 mutant Φ3015 independent (ind) of GrxC. Cells are WT P. syringae pv. phaseolicola HB10Y or grxC knockout strain LM4581. The transcript of S is obscured by 23S RNA. L, M, and S refer to dsRNA genomic segments; lowercase letters refer to transcripts.
Interaction of GrxC and Nucleocapsids.
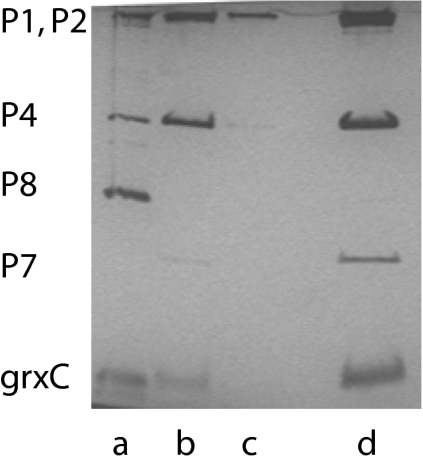
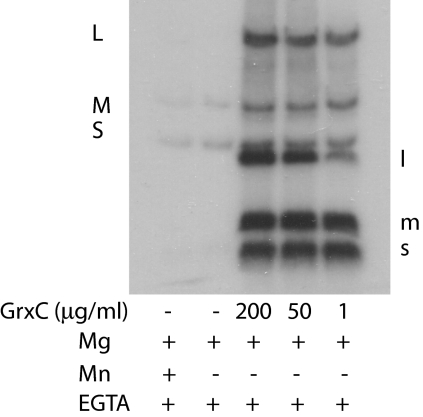
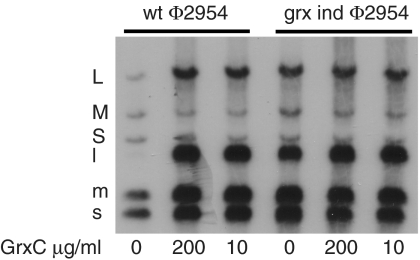
P. syringae GrxC with an N-terminal his tag was produced in E. coli. The protein was purified on nickel-containing agarose columns. Nucleocapsids of Φ6 that have had the protein P8 shell removed with EGTA to form cores carry out transcription in vitro to produce transcripts of segments S and M in the presence of magnesium ions. If manganese ions are present, then transcription of segment L is seen as well (10). Adding host protein YajQ to cores incubated in magnesium buffers results in transcription of the L segment (6). EGTA does not remove P8 from Φ2954 nucleocapsids, and in vitro transcription activity is low; however, applying GrxC to nucleocapsids of Φ2954 results in the removal of P8 and binding of GrxC to core particles (Fig. 2). These core particles show enhanced transcription of all three genomic segments without the addition of more GrxC. Adding GrxC to nucleocapsids treated with Triton X-100 results in activation of transcription (Fig. 3). P8 is removed from the entering nucleocapsid of Φ6 and digested early in infection (4). We confirmed this observation for Φ6, but found that parental P8 of Φ2954 was not digested during the course of infection. The mutants of Φ2954 that were independent of GrxC in vivo showed independent transcriptase activity in vitro for all three genomic segments (Fig. 4).
Fig. 2.
Silver-stained PAGE gel showing the loss of P8 from Φ2954 nucleocapsids on treatment with GrxC. Virions of Φ2954 were treated with 2% Triton to remove the membrane envelope. GrxC was then applied, and the sample was sedimented in a 10–30% sucrose gradient. Lane d is the major band and corresponds to the inner core of the virion with proteins P1, P2, P4, and P7. GrxC is in this fraction as well. Lane a is the pellet in the gradient; protein P8 can be seen. Lane b is the fraction below the major band, and lane c is the fraction above the major band.
Fig. 3.
In vitro transcription with nucleocapsids in magnesium buffers with and without GrxC. Samples were untreated or treated with GrxC or manganese ions. Labeling was done with [α-32P]UTP.
Fig. 4.
In vitro transcription of WT virus and GrxC-independent virus Φ3015 with and without GrxC.
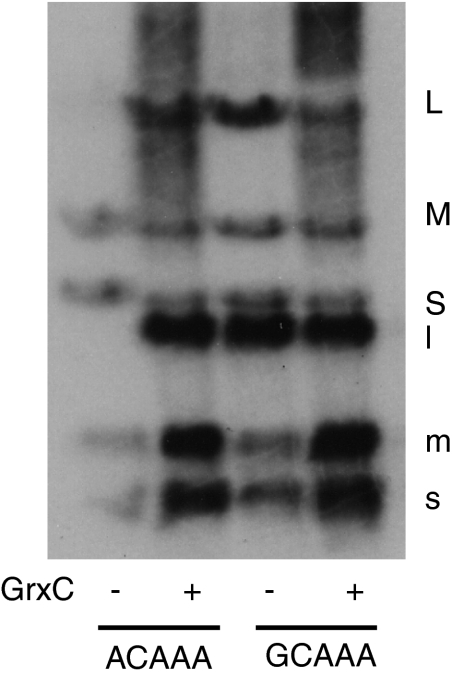
The base sequence at the 5′ end of the L transcript differs from that of S and M transcripts. The L transcript is ACAAA, whereas the S and M transcripts are GCAAA. In most members of Cystoviridae, the difference in sequence in L as opposed to S and M results in different behavior in transcription. In the case of Φ6, where the L transcript starts with GU, as opposed to GG in the S and M transcripts, transcription of L can be activated by changing the sequence to GG. This change also causes Φ6 to be independent of host protein YajQ for the transcription of L (6). We have found that changing the sequence of Φ2954 L from ACAAA to GCAAA causes the phage to be independent of GrxC. In vitro transcription behavior also is modified by this change (Fig. 5). However, there is a difference in vitro transcription behavior between the GrxC-independent mutant that has changes in protein P1 and the mutant that has a change in the 5′ base sequence of segment L. In the first case, the transcriptase activity for all three segments is high in the absence of GrxC (Fig. 4). In the second case, the transcriptase activity for the three segments is equal in the absence of GrxC, but the activity is low (Fig. 5). Adding GrxC increases the activity of all three segments.
Fig. 5.
In vitro transcription of WT nucleocapsids with the sequence ACAAA at the 5′ end of the L transcript and mutant nucleocapsids with the sequence GCAAA at the 5′ end of the L transcript. Samples were incubated with and without GrxC.
We interpret these findings as suggesting that GrxC removes P8 from the nucleocapsid, which stimulates transcriptase activity of S and M, but the binding of GrxC to the core activates transcription of L. The mutant in gene 1 causes both a loosening of the P8 bond with the core and a conformational change in P1 that activates P2 for transcription of L. The mutant in the 5′ sequence of L still must deal with the binding of P8 to the core and thus benefits from the interaction with GrxC. We also prepared phage with a single-segment genome by linking the transcripts of S, M, and L together with the S sequence at the 5′ end (1). This phage also is independent of GrxC.
Abundance of GrxC in the Host Cell.
We prepared antibody against the purified his-tag protein for use in Western blot analysis. We found a very low level of GrxC in strain HB10Y that did not change appreciably during phage infection or after induction with hydrogen peroxide. GrxC production in E. coli is known to be controlled by oxyR and to increase under peroxide stress (11). Western blot analysis confirmed the absence of GrxC in the knockout strain and its complementation by the plasmid carrying the grxC gene. We calculated the concentration of GrxC in the host cells as about 200 molecules per cell.
Visualization of in Vivo Interaction of GrxC with Φ2954.
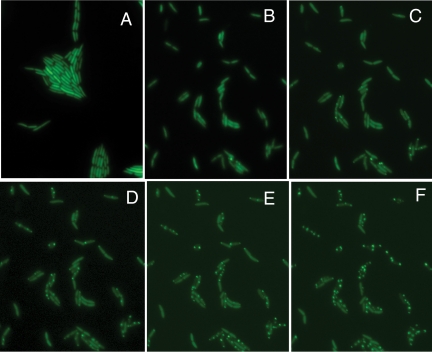
Gfp fusions of grxC were prepared in plasmid pLM350, a derivative of pLM254. Cells of LM2489 carrying this plasmid exhibited uniform fluorescence, even when infected with phages distantly related to Φ2954. But when infected with Φ2954, the cells exhibited punctate fluorescence (Fig. 6). The fluorescent spots appear slowly and increase with time, but there are rarely more than two spots per cell, which is the same number of virus particles that enter piliated cells (4). The spots do not move much and persist to the end of the infection cycle. Apparently the infecting particles are covered by GrxC and are responsible for transcription of the L segment. A small number of newly formed particles also might be covered by GrxC. Some of the newly formed particles that have not yet been covered by P8 are the source of additional S and M transcripts.
Fig. 6.
Φ2954-infected cells. LM4624 producing GFP alone (A) and LM4628 producing fusion protein of GFP and GrxC at 30 min postinfection (B), 45 min postinfection (C), 60 min postinfection (D), 75 min postinfection (E), and 120 min postinfection (F).
Discussion
The participation of host proteins in viral functions is widespread. The functions of GroE in lambda prohead assembly (12) and the incorporation of proteins S1 and EF-Tu in leviviridae polymerase (13) are well known. Reovirus infection involves the action of host proteins, including Hsc70, in the removal of delta protein from the virus core so as to activate transcription (14). In the case of Φ6, an unkown mechanism is responsible for the removal of the P8 shell; however, a host protein YajQ is responsible for the activation of transcription of segment L.
The cores of the Cystoviridae, Reoviridae, and Totiviridae are unique in their structure and modes of transcription. All have 120 molecules of the major structural protein of the core particle. All have polymerase molecules inside the cores, with the polymerases under the control of the core structure. In the case of rotavirus, core transcription is activated only when an additional shell composed of viral protein VP6 is applied (15). This is similar to the case in Φ6, where transcription of the L segment is activated by the binding of host protein YajQ to protein P1 of the core (6). Reovirus transcription is activated by the removal of delta protein by host protein Hsc70 (14), whereas Φ2954 transcription is activated by the removal of the P8 shell by host protein GrxC, as demonstrated in the present study. Totivirus translation is dependent on host proteins (16).
It is noteworthy that Φ6 and Φ2954 use different host proteins. Phages Φ12 and Φ13 are independent of YajQ and GrxC, but whether they are dependent on other host proteins or carry mutations that allow them to be independent of host proteins is unclear. Bacteriophage Φ8 uses the host ribonuclease R for control of message decay (17), rather different from the host proteins that act on the internal cores of these viruses.
Most members of the Cystoviridae regulate transcription by means of a different sequence at the 5′ end of segment L compared with segments S and M. In the case of Φ6, the terminal sequence of S and M is GG, whereas that of L is GU. Structural studies of the Φ6 polymerase show that the second base is challenged before the first, and that pairing with G is preferred over pairing with U (18). Because of this, transcription of L is minimal compared with transcription of S and M. Changing the second nucleotide to G results in an equal level of transcription for L relative to S and M. In the case of Φ2954, the difference in sequence involves the first nucleotide, which is A in the case of L and G in the case of S and M. It seems likely that the polymerase of Φ2954 prefers G over A. In fact, changing the first base in the L transcript to G raises the level of transcription to that of the S and M transcripts.
Both YajQ and GrxC apparently act on the transcriptase activity of segment L by binding to protein P1, the major structural protein of the inner core. This binding to P1 seems to alter the activity of the polymerase P2 such that it sees the L segment as a proper template. GrxC also acts to remove the P8 shell from the nucleocapsid of Φ2954. This increases the transcription activity of S and M, but not of L. The activity of the Φ6 polymerase is known to be dependent on the conformation of the capsid structure. Isolated P2 shows activity on both single-stranded and double-stranded RNA, but P2 in the core particle does not catalyze minus-strand synthesis until the three genomic segment transcripts are packaged, and plus-strand synthesis does not begin until minus-strand synthesis of all segments is complete (19, 20). How the conformation of the capsid structure acts to control the polymerase is unknown.
Materials and Methods
Bacterial Strains and Phage.
The host for Φ2954 was P. syringae pv. phaseolicola HB10Y (2). Plasmid transformation into E. coli used strains JM109 del(lac proA,B), thi, gyrA96, recA, str-s endA1, hsdR17, relA1, supE44, or (rk−, mk+) lam-[F′ traD36, proAB, laciqZdelM15] or Stratagene XL1-Blue super-competent cells (recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)]. The sequences of Φ2954 genomic segments L, M, and S are available in GenBank as (GenBank ID FJ608823, FJ608824, and FJ608825).
Media.
(LC) and M8 media were used (21). Ampicillin plates contained 200 μg/mL of ampicillin in LC agar. Kanamycin was used at 40 μg/mL.
Knockout of P. syringae grxC.
EZ-Tn5 (Epicentre Biotechnologies), a complex of transposase and transposon (7), was electroporated into cells of HB10Y, which were then plated on LB agar containing kanamycin. A collection of several thousand kanamycin resistant colonies was screened for resistance to bacteriophages Φ6 and Φ2954. Because both phages adsorb to host cells via the type IV pilus, we investigated only those colonies that were resistant to Φ2954 but sensitive to Φ6. Candidates that demonstrated resistance to Φ2954 were tested further by plating dilutions of phage on lawns. The sites of insertions were determined by preparing EcoRI restriction digests of chromosomal DNA, ligating to form circles, and then amplifying inserts by PCR using forward and reverse primers (Epicentre Biotechnologies. The PCR products were then sequenced using the forward primer, and the sequences were compared with those in the National Institutes of Health databank using the blast search facility. All insert sequences demonstrated high identity to sequences of P. syringae pv. phaseolicola 1448A (GenBank ID CP000058).
Cloning of grxC.
The grxC gene of P. syringae was cloned with a hexameric his tag at the N terminus using olm1244 and olm1245 as primers for PCR. The primer sequences were TTTATCTAGAGGAGAACATACATGCACCACCATCATCACCACGCTCAAGTCATCGTCTATTCC and AAACTCGAGTTAGGCCAGCAGCGCATCAAGCTTGCC, respectively. The PCR product was inserted into plasmid pET28a (Novagen) XbaI and XhoI sites to form pLM3648. It also was inserted without an n-terminal his tag into pLM254 BamHI and PstI sites to form pLM3634 using primers olm1242 and olm1243, with respective sequences AAAGGATCCGGTGGAGTGAACATGGCTCAAGTCATCG and GGGCTGCAGTTTAAGGGGAATCAGGCCAGCAGCGCATCAAGC. PLM254 is a shuttle vector that propagates in both E. coli and P. syringae. GrxC also was cloned with primers olm1246 and olm1243 and inserted into pLM254 to produce GrxC with an n-terminal his tag. This plasmid is pLM3649.
Labeling of RNA During Phage Infection.
Host cells were diluted from an overnight culture in synthetic M8 medium and grown to 5 × 108 cells per mL. Then 1-mL aliquots were placed on ice, and phage was added at a multiplicity of 10. The cells were left on ice for 30 min, then transferred to 28 °C, with 10 μCi of 3H-5,6-uracil added at various times. The cultures were centrifuged at 6,000 g 10 min later, after which the cells were resuspended in 200 μL of lysis buffer [20 mM Tris-HCl (pH 8), 1% SDS, and 5 mM EDTA] with 2 M sodium acetate (pH 5.4). The resulting mixture was frozen at −20 °C for 20 min and then centrifuged at 8,000 g for 10 min at room temperature. The supernatant liquid was extracted twice with phenol-chloroform and precipitated with ethanol. The precipitate was resuspended in 20 μL of DNA buffer and analyzed on 0.8% SeaKem GTG agarose (FMC BioProducts) in Tris borate EDTA buffer with 2 μg/mL of ethidium bromide. The bands were visualized under UV light and subjected to autoradiography with sodium salicylate enhancement.
In Vitro Transcription with Nucleocapsids.
Nucleocapsids of Φ2954 were prepared from purified virions stripped of their lipid-containing membranes by treatment with 2% Triton X-100 (22). Transcription was performed in magnesium buffers with and without added manganese ions (10, 23), EGTA (24), and GrxC. Nucleocapsids also were purified by zone sedimentation in 10%–30% sucrose solution after treatment with Triton X-100 and GrxC. The nucleocapsid concentration in transcription assays was ∼300 μg/mL.
Western Blot Analysis of GrxC.
Antibody production was induced in rabbits injected with purified n-terminal his tag GrxC (Lampire Biological Laboratories). Samples were fractionated on polyacrylamide gels, transferred to PVDF sheets, and developed with a 1–1,000 dilution of antibody and protein A linked to alkaline phosphatase. The amount of GrxC in cells was determined by comparing the intensity of bands in cell extracts with that of dilutions of purified GrxC, whose concentration was determined by optical density at 280 nm.
Construction of the gfp/grxC Fusion.
The gfp gene was copied from plasmid ED430 (25) using oligonucleotides 588 and 1255 with respective sequences CCCGGATCCTAAGAAGGAGATATACATATGAGTAAAGGAGAAGAACTTTTCAC and CCCCCTGCAGTTTGTATAGTTCATC. The PCR product was inserted into plasmid pLM350 with a 5′ BamHI site and a 3′ PstI site to form plasmid pLM3653. Plasmid pLM350 is a shuttle vector for E. coli and pseudomonads derived from plasmid pLM254 . The grxC gene was copied from plasmid pLM3648 using oligonucleotides 1256 and 1261, which placed a PstI site and a his-tag sequence at the 5′ end and a SacII site at the 3′ end. The sequences were CCCCCTGCAGCACCACCATCATCACCAC for 1256 and CCCCCCGCGGTTAGGCCAGCAGCGCATCAAGCTTGCC for 1261. The resulting PCR product was inserted into pLM3653 using a 5′ PstI site and a 3′ SacII site to form plasmid pLM3657. Plasmid pLM3657 was transferred to strain LM4581, which is P. syringae strain HB10Y with a knockout of gene grxC. The resulting strain was LM4628. Plasmid pLM3653, which codes for GFP without the fusion to YajQ, was transferred to strain LM2489 to make strain LM4624.
Microscopy.
Cells were incubated with phage at 0 °C for 30 min before being washed and deposited on 1.2% agarose pads containing LB medium. Images were collected with a Nikon 90i microscope using a TIRF Plan Neo-Fluor 100× oil immersion objective (numerical aperture, 1.45) and a BrightLine GFP-3035B-NTE-ZERO filter set (Semrock Optical). Version 4 of the Volocity application (Improvision) was used to acquire and quantify fluorescence intensities.
Stability of Protein P8.
Cells of P. syringae HB10Y were infected with bacteriophages Φ6 and Φ2954 in media containing 35S methionine. Radioactive phage was purified by zone centrifugation and used to infect cells in nonradioactive media. Samples were obtained at specified intervals and fractionated on polyacrylamide gels. The gels were analyzed by autoradiography to determine the fate of protein P8 during the infection period.
Acknowledgments
This work was supported by National Institutes of Health Grant GM34352.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Qiao X, Sun Y, Qiao J, Di Sanzo F, Mindich L. Characterization of phi2954, a newly isolated bacteriophage containing three dsRNA genomic segments. BMC Microbiol. 2010;10:55. doi: 10.1186/1471-2180-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vidaver AK, Koski RK, Van Etten JL. Bacteriophage phi 6: A lipid-containing virus of Pseudomonas phaseolicola. J Virol. 1973;11:799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mindich L, et al. Isolation of additional bacteriophages with genomes of segmented double-stranded RNA. J Bacteriol. 1999;181:4505–4508. doi: 10.1128/jb.181.15.4505-4508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romantschuk M, Olkkonen VM, Bamford DH. The nucleocapsid of bacteriophage phi 6 penetrates the host cytoplasmic membrane. EMBO J. 1988;7:1821–1829. doi: 10.1002/j.1460-2075.1988.tb03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Etten JL, Vidaver AK, Koski RK, Semancik JS. RNA polymerase activity associated with bacteriophage phi 6. J Virol. 1973;12:464–471. doi: 10.1128/jvi.12.3.464-471.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao X, Sun Y, Qiao J, Mindich L. The role of host protein YajQ in the temporal control of transcription in bacteriophage Phi6. Proc Natl Acad Sci USA. 2008;105:15956–15960. doi: 10.1073/pnas.0807489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reznikoff WS. Transposon Tn5. Annu Rev Genet. 2008;42:269–286. doi: 10.1146/annurev.genet.42.110807.091656. [DOI] [PubMed] [Google Scholar]
- 8.Mindich L, et al. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985;162:992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslund F, et al. Glutaredoxin-3 from Escherichia coli: Amino acid sequence, 1H and 15N NMR assignments, and structural analysis. J Biol Chem. 1996;271:6736–6745. doi: 10.1074/jbc.271.12.6736. [DOI] [PubMed] [Google Scholar]
- 10.Emori Y, Iba H, Okada Y. Transcriptional regulation of three double-stranded RNA segments of bacteriophage phi 6 in vitro. J Virol. 1983;46:196–203. doi: 10.1128/jvi.46.1.196-203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao K. oxyR-dependent induction of Escherichia coli grx gene expression by peroxide stress. J Bacteriol. 1997;179:5967–5970. doi: 10.1128/jb.179.18.5967-5970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgopoulos CP, Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product) Proc Natl Acad Sci USA. 1978;75:131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown S, Blumenthal T. Reconstitution of Qbeta RNA replicase from a covalently bonded elongation factor Tu–Ts complex. Proc Natl Acad Sci USA. 1976;73:1131–1135. doi: 10.1073/pnas.73.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanovic T, Agosto MA, Chandran K, Nibert ML. A role for molecular chaperone Hsc70 in reovirus outer capsid disassembly. J Biol Chem. 2007;282:12210–12219. doi: 10.1074/jbc.M610258200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charpilienne A, Lepault J, Rey F, Cohen J. Identification of rotavirus VP6 residues located at the interface with VP2 that are essential for capsid assembly and transcriptase activity. J Virol. 2002;76:7822–7831. doi: 10.1128/JVI.76.15.7822-7831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickner RB. Double-stranded RNA viruses of Saccharomyces cerevisiae. Microbiol Rev. 1996;60:250–265. doi: 10.1128/mr.60.1.250-265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao X, Sun Y, Qiao J, Mindich L. Temporal control of message stability in the life cycle of double-stranded RNA bacteriophage phi8. J Virol. 2009;83:633–639. doi: 10.1128/JVI.01766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher SJ, Grimes JM, Makeyev EV, Bamford DH, Stuart DI. A mechanism for initiating RNA-dependent RNA polymerization. Nature. 2001;410:235–240. doi: 10.1038/35065653. [DOI] [PubMed] [Google Scholar]
- 19.Mindich L. Precise packaging of the three genomic segments of the double-stranded RNA bacteriophage phi6. Microbiol Mol Biol Rev. 1999;63:149–160. doi: 10.1128/mmbr.63.1.149-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frilander M, Gottlieb P, Strassman J, Bamford DH, Mindich L. Dependence of minus strand synthesis on complete genomic packaging in the double-stranded RNA bacteriophage phi 6. J Virol. 1992;66:5013–5017. doi: 10.1128/jvi.66.8.5013-5017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair JF, Cohen J, Mindich L. The isolation of suppressible nonsense mutants of bacteriophage Φ6. Virology. 1976;75:198–208. [PubMed] [Google Scholar]
- 22.Van Etten JL, Lane L, Gonzalez C, Partridge J, Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976;18:652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlieb P, Strassman J, Qiao XY, Frucht A, Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: Studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990;172:5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olkkonen VM, Ojala PM, Bamford DH. Generation of infectious nucleocapsids by in vitro assembly of the shell protein on to the polymerase complex of the dsRNA bacteriophage phi 6. J Mol Biol. 1991;218:569–581. doi: 10.1016/0022-2836(91)90702-8. [DOI] [PubMed] [Google Scholar]
- 25.Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]