Abstract
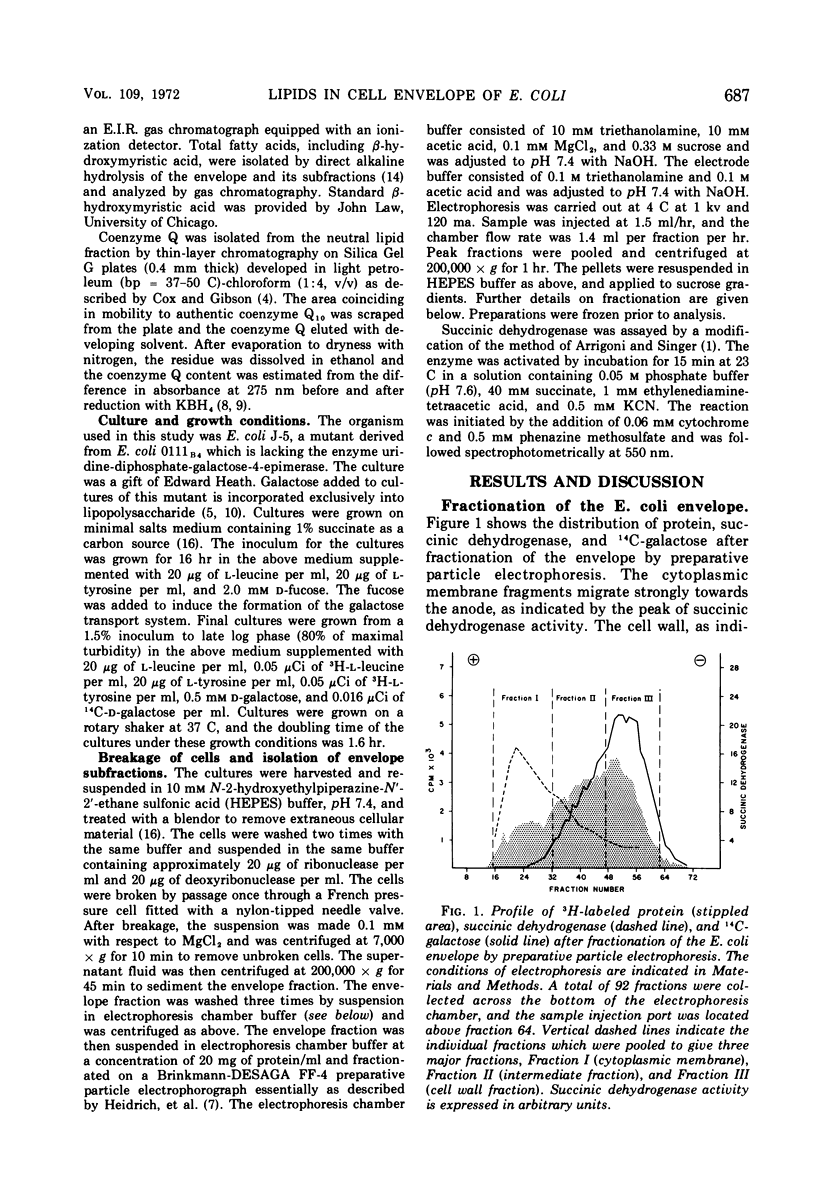
Cell wall and membrane subfractions of the cell envelope of Escherichia coli have been isolated by a procedure involving particle electrophoresis and sucrose gradient density centrifugation. The lipid content of each fraction has been investigated. The individual phospholipids of both fractions are quantitatively similar except that the proportion of lysophosphatidylethanolamine is greater in the wall than in the membrane. Fatty acid analysis of the phospholipids of each fraction revealed that the wall phospholipids contain a greater proportion of palmitic acid. Coenzyme Q is almost exclusively localized in the cell membrane.
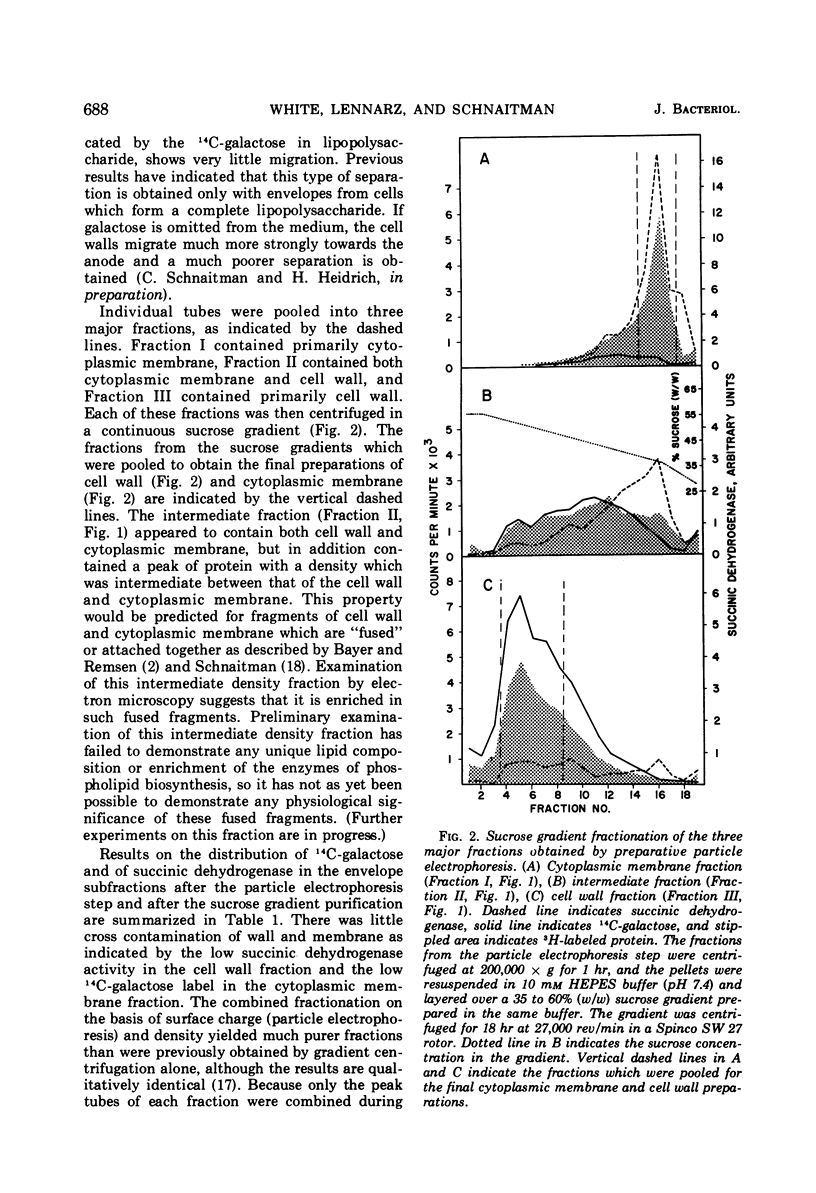
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Remsen C. C. Structure of Escherichia coli after freeze-etching. J Bacteriol. 1970 Jan;101(1):304–313. doi: 10.1128/jb.101.1.304-313.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. The role of shikimic acid in the biosynthesis of vitamin K2. Biochem J. 1966 Jul;100(1):1–6. doi: 10.1042/bj1000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- GARBUS J., DELUCA H. F., LOOMANS M. E., STRONG F. M. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1963 Jan;238:59–63. [PubMed] [Google Scholar]
- Heidrich H. G., Stahn R., Hannig K. The surface charge of rat liver mitochondria and their membranes. Clarification of some controversies concerning mitochondrial structure. J Cell Biol. 1970 Jul;46(1):137–150. doi: 10.1083/jcb.46.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- LESTER R. L., HATEFI Y., WIDMER C., CRANE F. L. Studies on the electron transport system. XX. Chemical and physical properties of the coenzyme Q family of compounds. Biochim Biophys Acta. 1959 May;33(1):169–185. doi: 10.1016/0006-3002(59)90511-6. [DOI] [PubMed] [Google Scholar]
- LONG C., PENNY I. F. The structure of the naturally occurring phosphoglycerides. III. Action of moccasin-venom phospholipase A on ovolecithin and related substances. Biochem J. 1957 Feb;65(2):382–389. doi: 10.1042/bj0650382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- NESBITT J. A., 3rd, LENNARZ W. J. COMPARISON OF LIPIDS AND LIPOPOLYSACCHARIDE FROM THE BACILLARY AND L FORMS OF PROTEUS P18. J Bacteriol. 1965 Apr;89:1020–1025. doi: 10.1128/jb.89.4.1020-1025.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Hawthorne J. N. Zymogen secretion and phospholipid metabolism in the pancreas. Phospholipids of the zymogen granule. Biochem J. 1970 Dec;120(3):533–538. doi: 10.1042/bj1200533. [DOI] [PMC free article] [PubMed] [Google Scholar]



