Abstract
Poly(ADP ribose) polymerase 1 (PARP1) is a nuclear protein that regulates chromatin remodeling and transcription as well as DNA repair and genome stability pathways. Recent studies have revealed a paradoxical dual role of PARP1 protein in transcription. Specifically, although PARP1 controls transcriptional activation of a subset of genes that are heat shock- or hormone-dependent, it also directly inactivates transcription, establishes heterochromatin domains, and silences retrotransposable elements. However, the domains required for these disparate functions are currently unknown. In this paper, we report the discovery of a previously undescribed mutation in the Drosophila Parp locus. We show that the mutants express a deletion mutant of PARP1 protein with an altered DNA binding domain that carries only the second Zn-finger. We demonstrate that this alteration specifically excludes PARP1 protein from heterochromatin and makes PARP1 unable to maintain repression of retrotransposable elements. By characterizing the biological activity of this unique PARP1 mutant protein isoform, we have uncoupled the transactivation and transrepression functions of this protein.
Keywords: chromatin, poly(ADP ribose) polymerase, transcription
Poly(ADP ribose) polymerase 1 (PARP1) protein has been known for decades as a nuclear protein that recognizes and binds nicks and ends of DNA and catalyses poly(ADP ribose) (pADPr) synthesis (1). The basic enzymatic reactions catalyzed by PARP1 involve transferring ADPr from nicotinamide–adenine dinucleotide to either a protein acceptor or an existing pADPr chain, the average length of which is 80 or more residues (2). PARP1 protein can modify numerous chromatin proteins in vivo and in vitro (3). A key role of PARP1 was shown in DNA repair and apoptosis (3), where PARP works as a trigger between the DNA repair (4) and apoptotic pathways (5). PARP1 enzymatic activity has also been shown to be required for normal assembly of higher order chromatin structures and for transcriptional activation (6). Moreover, it has been shown that PARP1 regulates the transcription of these genes by inducing chromatin loosening at targeted genetic loci (6, 7). Finally, PARP1 establishes silent chromatin domains and represses retrotransposable elements (8).
The characterization of deletion mutants of PARP that distinguish among the varied functions of this protein is essential to establish a more complete understanding of PARP1 protein biology. At present, however, we have identified neither the mechanism of PARP protein targeting to specific chromatin domains nor the mechanism of local PARP activation. Closing these gaps in our current knowledge is complicated because the presence of 18 paralogous PARP proteins (9) in mammals most likely results in corresponding functional redundancies. The Drosophila genome (8, 10, 11) encodes only a single nuclear PARP (PARP1), making this animal an invaluable model system for the study of PARP functions.
The PARP1 protein has three functionally defined domains conserved from human to Drosophila (12, 13): (i) an N-terminal DNA binding domain (DBD) encompassing two Zn-fingers, which recognizes DNA lesions; (ii) a centrally localized automodification domain (AD), which can be modified by ADPr and is also required for dimerization; and (iii) a C-terminal catalytic domain, which performs ADPr transfer (Fig. 1A). Zn-finger 2 of PARP1 was shown to be critical for the recognition and binding of DNA breaks in vitro (14), whereas a role in stabilization of established interaction was suggested for Zn-finger 1 (3). Recently, the DBD and, specifically, the Zn-fingers were suggested to control PARP1-dependent chromatin condensation (15). Here, we report the discovery of a unique C03256 pBac element-mediated mutation in the Drosophila Parp locus that leads to expression of a truncated mutant of PARP1 (PARPΔ300) (Fig. 1A). PARPΔ300 lacks the first Zn-finger of the DBD, enabling us to elucidate the in vivo function of Zn-finger 1 and the effect that this unique PARP1 deletion mutant has on chromatin and PARP1-dependent nuclear processes. We find that this mutation uncouples the transactivation and transcriptional repression functions of PARP.
Fig. 1.
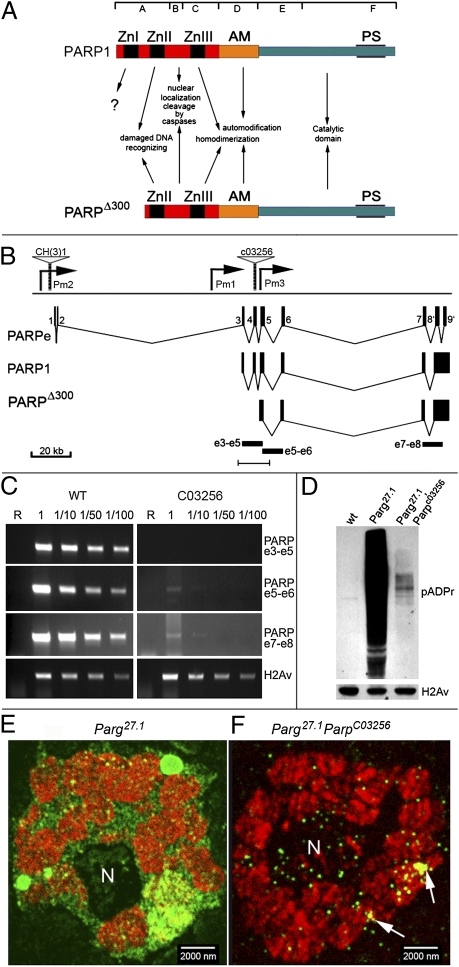
(A) Domain structure comparison for PARP1 protein and PARPΔ300 protein isoform. (Top) Subdivision on seven domains (A–F) is shown according to D'Amours et al. (3). (Middle) Known functions of domains are indicated. ZnI, ZnII, and ZnIII correspond to Zn-finger domains. AM, automodification domain; PS, PARP signature, evolutionarily conserved PARP catalytic site. (B–F) C03256 mutation disrupts PARP expression. (B) Structure of Drosophila Parp locus. A diagram summarizing the organization of the Parp genomic region as determined from this and a previous study (8) is shown. Gray triangles indicate mobile element insertions disrupting locus CH(3)1 (8) and C03256. Pm1 and Pm2 indicate promoters reported previously (8), and Pm3 indicates a presumptive promoter, as suggested from this study. The arrangement of the exons, as described in the article by Tulin et al. (8), encoding a naturally occurring catalytically inactive PARPe (Upper) and full-length catalytically active PARP1 (Lower) is shown. Black rectangles e3–e5, e5–e6, and e7–e8 indicate PCR fragments used to show disruption of PARP transcription in C. The bar at the bottom indicates the region that is magnified in Fig. S1B. (C) C03256 disrupts PARP transcription; RT-PCR analysis of WT and C03256 mutant flies. R, RNA; 1, 1/10, 1/50, 1/100, dilutions of RNA sample before cDNA synthesis. Specific primers to exons 3 and 5 (PARP e3–e5), exons 5 and 6 (PARP e5–e6), and exons 7 and 8 (PARP e7–e8) were used. Specific primers to H2Av transcript were used as a loading control. The C03256 disrupts transcription completely in the region of exons 3–5, but a low amount of mRNA specific to exons 5–8 is still detected, suggesting an additional start of transcription downstream from C03256. (D) Western blots of proteins isolated from third-instar larvae of WT (wt), Parg27.1 mutant, and Parg27.1, ParpC03256 double-mutant animals probed with anti-pADPr antibody. C03256 suppresses the accumulation of pADPr in Parg mutants. An antihistone H2Av antibody was used as a loading control. (E and F) Immunofluorescent detection of pADPr accumulation is shown in salivary gland nuclei of Parg27.1 mutant and Parg27.1, ParpC03256 double-mutant animals. Rabbit anti-pADPr antibody was used (green). To stain chromatin, mouse anti-1H histone antibody was used (red).
Results
parpC03256 Has Residual Poly(ADP Ribosyl)ation Activity in Chromatin.
Previously, we described a P element-mediated mutation, parpCH1, which impairs expression of a single Drosophila parp gene in embryos. Localization of the parpCH1 element in the remote upstream promoter region, which spans more than 100 kb of transposon-rich centromeric heterochromatin (Fig. 1B), causes developmental arrest in Drosophila at ecdysis II stage and lethality, which precludes the study of PARP protein function late in the development in this mutation. To find unique mutations in the parp gene, we analyzed the Exelexis collection of pBac element-induced mutations in this gene (16). Sequence identity was observed for the 3′-end flanking sequence of the C03256 insertion site and exon 4 of the PARP1 coding sequence (Fig. S1 A and B). To confirm the C03256 insertion site, we performed Southern blot analysis of genomic DNA from WT and C03256 homozygous flies using PARP1 cDNA as a probe. We found that insertion of the C03256 element changed the pattern of Parp-specific genomic DNA digestion, as expected, based on the computer-generated map (Fig. S1 B and C). To test whether C03256 disrupts transcription of the Parp locus, we performed RT-PCR analysis. We found that Parp expression is completely abolished in the region of exons 3–5, which encode the Zn-finger 1 of PARP1 protein (Fig. 1C). Surprisingly, we found that C03256 mutants accumulate a low but detectable amount of Parp RNA encoded by exons 5–8 (Fig. 1C). Taken together, these findings define C03256 as a previously undescribed hypomorphic mutation in the Drosophila Parp locus and suggest that transcription in homozygous C03256 starts from a cryptic promoter, which we denote as Pm3 (Fig. 1B).
To analyze this mutation in the Parp locus further, we characterized C03256 viability. Although 27% of C03256 mutant animals survived up to pupae, most homozygous C03256 animals died at the second and third larval stages (Table S1). Precise excision of the C03256 pBac element generated 100% viable stocks, arguing strongly that the C03256 element is responsible for the original lethality.
We next sought to verify that the C03256 mutation could be rescued by expression of a WT PARP cDNA transgene or by suppression of the activity of the PARP antagonist poly(ADP ribose) glycohydrolase (PARG), which is responsible for cleavage of pADPr (17, 18). For the first approach, we expressed previously verified (6, 18, 19) UAS::PARP1-DsRed and UAS::PARP embryonic (PARPe)-EGFP transgenic constructs with G1-Gal4 ubiquitous driver. Ectopic expression of full-length PARP1 protein (PARP1-DsRed) but not the catalytically inactive PARPe protein (PARPe-EGFP) rescues C03256 lethality completely (Table S2). Mutant Drosophila bearing a single copy of PARP1-DsRed and G1-Gal4 driver can be readily maintained as homozygous stock (Gal4G1, UAS::PARP1-DsRed/FM7i; parpC03256/parpC03256). The ability of PARP1-DsRed expression to rescue C03256 mutants completely demonstrates that the C03256 element directly disrupts the Parp gene. We will therefore call the C03256 allele parpC03256 in the following text. For the second approach, we found that reduction of PARG protein activity in the parg27.1/FM7i; parpC03256/parpC03256 animals also partially rescues C03256 lethality (Table S2). This finding supports the premise that parpC03256 is a nonnull (hypomorphic) mutation and that residual activity of PARP is sufficient for animals to survive if PARG function is partially suppressed.
The absence of antibodies against Drosophila PARP proteins complicates the quantification of PARP1 function reduction in parpC03256 animals. However, PARP protein is a pADP ribosylating enzyme, allowing us to immunoblot for pADPr to detect levels of PARP protein enzymatic activity in parpC03256 animals. We previously demonstrated significant reduction of the pADPr level in parpC03256 flies when compared with WT flies (20 [figure 5a], 21). Small levels of activity can be seen when PARG is reduced because it catalyzes the opposite reaction (Fig. 1D, second lane). Examination of parpC03256 in a PARG mutant background revealed enzymatic activity in the mutant (Fig. 1D, third lane). Thus, PARP1 protein enzymatic activity is certainly present in parpC03256 mutants, and the amount of this activity correlates with the amount of residual PARP RNA detected, as shown in Fig. 1C. To localize this activity in situ, thick sections of third-instar larvae salivary gland nuclei were immunostained with anti-pADPr antibodies. We compared nuclear pADPr in parg27.1 and parg27.1; parpC03256 using confocal microscopy. Despite a dramatic reduction of the pADPr signal (Fig. 1D) by immunoblot analysis, the residual pADPr had proper localization in specific domains of chromatin (Fig. 1 E and F and Fig. S1D), suggesting that the residual PARP protein in parpC03256 localizes to chromatin and nucleoli in a pattern that is similar to PARP1 protein in WT flies.
Fig. 5.
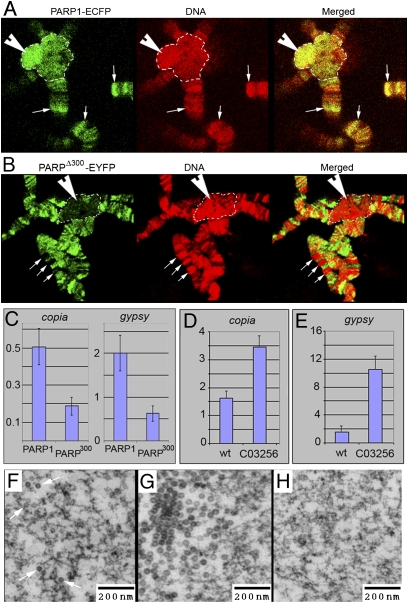
PARPΔ300 protein is excluded from the regions of heterochromatin. (A and B) Comparison of PARP1 and PARPΔ300 protein localization in salivary gland polytene chromosomes. Salivary glands were dissected from UAS::PARP1-ECFP, Arm::Gal4; ParpC03256/ParpC03256 (A) or UAS::PARPΔ300-EYFP, Arm::Gal4; ParpC03256/ParpC03256 (B) third-instar larvae, squashed on slides, and immunostained with anti-GFP antibody (green). DNA is shown in red. PARP1 protein shows significant accumulation in the regions of constitutive (arrowhead) and intercalary (arrows) heterochromatin (A), whereas PARPΔ300 is excluded from these regions (B). Constitutive heterochromatin is indicated with a dashed line. (C) ChIP assay demonstrates that chromatin of retrotransposons copia and gypsy accumulates significantly less PARPΔ300 protein than PARP1. (D–H) C03256 mutation disrupts silencing of retrotransposons. The quantitative RT-PCR assay using primers specific to copia (D) and gypsy (E) retrotransposons detects an elevated level of their RNA in ParpC03256 mutant larvae compared with heterozygous animals. EM microphotographs reveal the accumulation of retroviral particles in the nucleoplasm of ParpC03256 animals: individual particles (F) and clusters (G). (H) Silencing of retrotransposons could be restored by PARP1-DsRed expression.
parpC03256 Mutants Express a Short PARP1 Protein Isoform, PARPΔ300, Which Encodes PARP1 Protein Without the Zn-Finger 1 Domain.
To characterize the PARP1 protein isoform that is expressed in parpC03256, we cloned PARP cDNAs from parpC03256 homozygous larvae using an RT-PCR approach. A single cDNA for ParpΔ300 was identified (Fig. S2A). RT-PCR experimentation demonstrated that the start of transcription in parpC03256 occurs downstream from the pBac element 3′ end, inside of intron 4, and that ParpΔ300 cDNA includes PARP1 protein-encoding exons 5–8 (Fig. S2A). The analysis of the ParpΔ300 cDNA sequence reveals three putative ORFs that encode PARP (Fig. S2A). The longest ORF, PΔ300, starts in intron 4 and carries all domains of PARP1 except the Zn-finger 1 (Fig. S2 A and B). Another two putative ORFs (PΔ740 and PΔ1302) encode shorter polypeptides without nuclear localization signals. To validate the biological properties of the predicted PARP protein isoforms, we generated transgenic flies that express each ORF fused to EYFP (Fig. S2B and Materials and Methods). We studied the localization of those recombinant PARP proteins throughout many tissues in WT and parpC03256 flies (Fig. S2 C–E). Only PARPΔ300-EYFP protein localized to chromatin and nucleoli (Fig. S2C), whereas other recombinant proteins are excluded from chromatin (PARPΔ1302-EYFP) (Fig. S2D) or completely removed from nuclei (PARPΔ740-EYFP) (Fig. S2E). Ubiquitous expression of PARPΔ300-EYFP partially rescues parpC03256 viability (Table S2), although the other two proteins do not rescue parpC03256. This suggests that the main reason for parpC03256 lethality is an insufficient amount of PARP protein. Together, our results support the idea that PARPΔ300 protein is produced and functional in parpC03256 mutants.
PARPΔ300 Protein Is Activated by γ-Irradiation and Is Sufficient for the Genotoxic Stress Response.
The earliest known PARP1 protein function was to protect the genome from genotoxic stresses such as irradiation (3). PARP1 protein is known to be activated on the appearance of DNA damage (3), and the PARP1-dependent pADPr reaction has been shown to be critical for proper DNA repair (4) and genome stability (22). Thus, to examine the biological properties of PARPΔ300 protein and parpC03256 mutation, we analyzed the genotoxic stress response of parg27.1; parpC03256 double-mutant animals, which express only PARPΔ300-like protein. We compared genotoxic responses in parg27.1; parpC03256 mutants with those in WT flies and parg27.1 mutants alone. First, we found that on irradiation, both parg27.1; parpC03256 and parg27.1 were able to increase the amount of pADPr (Fig. S3A). Thus, full-length WT PARP1 protein and PARP1 lacking the first Zn-finger were activated by DNA damage. Second, we compared the viability of the parg27.1/FM7i; parpC03256/parpC03256 mutants with that of their WT siblings after 36-Gy irradiation at the third-instar larval stage (Fig S3B and Materials and Methods). No significant difference was observed. These data support our conclusion that PARPΔ300 protein is sufficient for viability after genotoxic stress and, as a consequence, Zn-finger 1 is not required for PARP1 protein activation on irradiation.
PARPΔ300 Protein Has Similar Nuclear Dynamics Compared with PARP1.
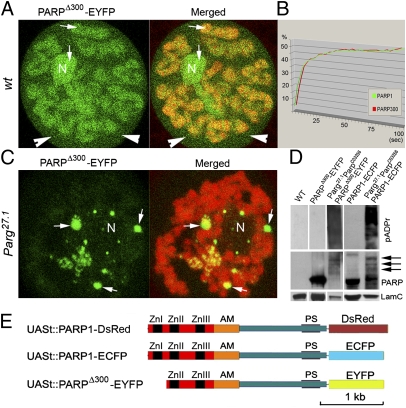
To study the nuclear dynamics of the PARPΔ300-EYFP protein isoform, we first analyzed the chromosomal localization of this protein when expressed in parpC03256. Immunostaining using anti-GFP antibody demonstrated nuclear localization and broad association with chromatin of diploid and polyploid nuclei. In vivo imaging showed that most PARPΔ300-EYFP protein remains bound to chromatin in interphase nuclei and accumulates in nucleoli (Fig. 2A, arrows). However, a significant pool of this protein is present in soluble nucleoplasm (Fig. 2A, arrowheads), suggesting that there is an equilibrium of PARPΔ300 protein binding to chromatin. To investigate the nuclear dynamics of this PARP isoform, we employed the fluorescence recovery after photobleaching (FRAP) approach (19). We compared the FRAP dynamics of PARPΔ300-EYFP protein with those of full-length previously validated (19, 23) PARP1-DsRed in Drosophila polyploid nuclei. We expressed both recombinant proteins separately in parpC03256 mutant animals using the Armadillo-Gal4 driver, which is expressed ubiquitously (24). The average FRAP rates of PARPΔ300-EYFP and PARP1-DsRed are not statistically different (Fig. 2B), suggesting that the Zn-finger 1 is not necessary for PARP protein dynamics in chromatin.
Fig. 2.
PARPΔ300 protein localization dynamics and enzymatic activity in vivo. (A) Life imaging shows ubiquitous distribution of PARPΔ300-EYFP protein (green) in chromatin (red) and nucleolus (N). Arrows show presence of PARPΔ300-EYFP protein in soluble nucleoplasm. (B) FRAP assay demonstrates similar average in vivo dynamics for PARPΔ300-EYFP and PARP1-ECFP proteins. (C) Life imaging shows relocalization of PARPΔ300-EYFP protein (green) from chromatin (red) and nucleolus (N) into nucleoplasmic bodies in Parg27.1 mutants. (D) Western blot demonstrates automodification of PARPΔ300-EYFP and PARP1-ECFP proteins in Parg27.1;ParpC03256 mutant animals. The following antibodies were used: mouse anti-pADPr, rabbit anti-GFP (to detect PARPΔ300-EYFP and PARP1-ECFP proteins), and mouse anti-lamin C (loading control). Positions of heavily modified forms of PARPΔ300-EYFP and PARP1-ECFP proteins are shown with arrows. (E) Structure of UAS/Gal4-inducible transgenic recombinant PARP1-DsRed, PARP1-ECFP, and PARPΔ300-EYFP proteins. The DNA-binding domain of PARP is indicated in red, the automodification (AM) domain is indicated in orange, and the catalytic domain in blue contains a PARP enzymatically active site (PS).
PARP1 protein is active as a dimer (25, 26). Covalent ADPr automodification of the PARP1 “Brca1 C-terminal” (BRCT) domain (AM) causes PARP1 dimers to dissociate from each other and from active chromatin and to lose activity (18). A cluster of 10–28 Glu residues located near the center of the PARP1 BRCT domain serves as the major acceptor of this ADPr in vivo (3). The negative feedback loop mediated by automodification limits the time during which PARP1 molecules can remain active. In Parg mutants, we previously showed that PARP1 protein is pADP-ribosylated and removed from chromatin into specific nucleoplasmic bodies (18, 23). To test the enzymatic activation of PARPΔ300and its ability to react to automodification, we expressed the PARPΔ300-EYFP transgene in parg27.1; parpC03256 double mutants and analyzed PARPΔ300-EYFP protein dynamics using in vivo imaging. We found that PARPΔ300 protein is completely relocalized into nucleoplasmic bodies at the third-instar larvae stage (Fig. 2C), which is a pattern identical to what we had previously shown for full-length PARP1 protein (23). Immunoblot analysis of proteins extracted from parg27.1; parpC03256 double-mutant animals expressing PARPΔ300-EYFP confirmed that the PARPΔ300 isoform is able to perform a pADPr reaction and could largely automodify itself (Fig. 2D). Taken together, our findings suggest that the Zn-finger 1 of PARP1 protein is not required for general interaction of PARP protein with chromatin or PARP enzymatic activation in vivo. Taking into account that expression of extra-PARPΔ300 is also sufficient to rescue the parpC03256 flies to viability in steady state and on irradiation, we conclude that PARPΔ300 performs essentially like full-length PARP1 protein.
Deletion of the Zn-Finger 1 of PARP1 Protein Alters Specificity of Chromatin Domains Recognized by PARP Protein.
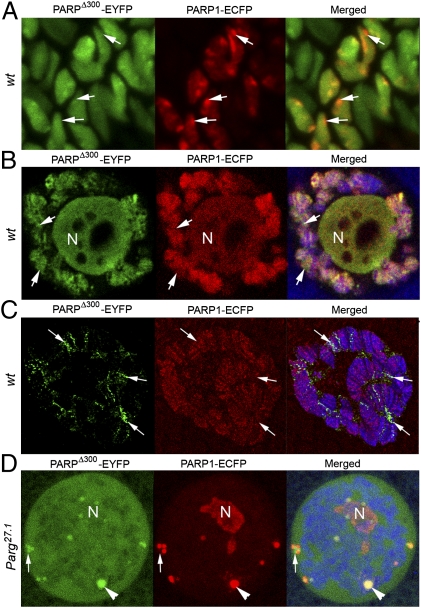
To investigate the roles of Zn-finger 1 for PARP1 protein functions further, we compared the localization of PARPΔ300 and PARP1 protein using high-resolution confocal microscopy of fixed whole-mount and thick-sectioned Drosophila tissues. For maximal elimination of artificial differences between PARPΔ300 and PARP1 recombinant proteins, we used the PARP1-ECFP transgene instead of PARP1-DsRed (Fig. 2E). We coexpressed PARPΔ300-EYFP and PARP1-ECFP in WT Drosophila. A significant difference in localization of those proteins was revealed (Fig. 3). Specifically, some specific domains of chromatin predominantly accumulate PARP1, but others have enriched PARPΔ300 (Fig. 3 A–C, arrows). In general, the PARP1 isoform has more widespread distribution in chromatin (Fig. 3C, compare green and red channels, respectively). Coexpression of PARPΔ300-EYFP and PARP1-ECFP in parg27.1 mutants also revealed a difference in the targeting of those proteins into nucleoplasmic bodies (Fig. 3D), confirming our suggestion about broader PARP1 localization. These observations, taken together, suggest that the Zn-finger 1 of PARP1 functions to govern the binding specificity of the protein.
Fig. 3.
PARPΔ300 and PARP1 proteins have a different localization in chromatin. High-resolution confocal microscopy of fixed tissues reveals differences in PARPΔ300-EYFP and PARP1-ECFP protein localization and dynamics in chromatin in WT whole-mount preparations (A and B) and thick-section samples (C) as well as in Parg27.1 mutant animals (D). The difference between PARP1-ECFP and PARPΔ300-EYFP localization in chromatin and Cajal bodies is maximal in the regions labeled with arrows. Larval imaginal disks (A) and salivary glands (B–D) were dissected from UAS::PARP1-ECFP, Arm::Gal4; ParpC03256/ParpC03256 and UAS::PARPΔ300-EYFP, Arm::Gal4; ParpC03256/ParpC03256 animals. (C) Fine-frozen sections of salivary gland were prepared and stained with anti-1H antibody to detect chromatin (blue). PARP1-ECFP (red) and PARPΔ300-EYFP (green) protein localization was detected by autofluorescence of ECFP and EYFP.
PARPΔ300 Protein Is Enriched in Regions of Euchromatin and Is Sufficient for Heat-Shock Activation of Genes.
To determine patterns of PARP1-ECFP and PARPΔ300-EYFP localization in chromatin, we compared the localization of these proteins on Drosophila salivary gland polytene chromosomes (Fig. 4 A and B). Both proteins show broad but distinct distribution along chromosomes. PARP1-ECFP protein shows significant accumulation in regions of inactive condensed chromatin with high DNA content (Fig. 4A, arrows). The PARPΔ300-EYFP protein, however, is excluded from “dense” chromatin and accumulates in decondensed loci (with low DNA content) (Fig. 4B, arrows). This observation suggests that PARP1 and PARPΔ300 proteins have different mechanisms of targeting to chromatin and that the Zn-finger 1 domain of PARP1 protein controls one of those.
Fig. 4.
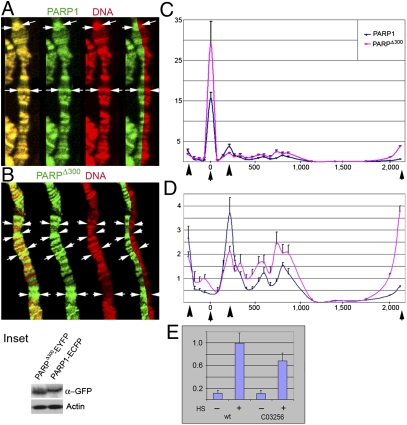
PARPΔ300 protein preferentially localizes into domains of euchromatin. A comparison of PARP1 and PARPΔ300 protein localization in salivary gland polytene chromosomes is shown. Salivary glands were dissected from UAS::PARP1-ECFP, Arm::Gal4; ParpC03256/ParpC03256 (A) or UAS::PARPΔ300-EYFP, Arm::Gal4; ParpC03256/ParpC03256 (B) third-instar larvae, squashed on slides, and immunostained with anti-GFP antibody (green). DNA is shown in red. PARP1 protein shows significant localization with DNA (condensed heterochromatic regions) (A), whereas PARPΔ300 localizes preferentially in regions of decondensed chromatin (euchromatin) (B). (Inset) Arrows indicate regions of PARP protein enrichment. The expression level of PARPΔ300-EYFP and PARP1-ECFP recombinant proteins is compared. (C and D) ChIP assay demonstrates that the hsp70 locus accumulates significantly more PARPΔ300 protein than PARP1. (C) Distribution of PARPΔ300 (red) and PARP1 (blue) proteins along the hsp70 gene. PARPΔ300 shows significant enrichment in the regions of transcriptional start and termination (black arrows). Arrowheads indicate positions of nucleosomes −1 and +1. (D) For better representation of PARP protein localization in the body of the hsp70 gene, we include the same ChIP experimental data with depleted point “0.” (E) C03256 mutation does not block hsp70 gene activation. The quantitative RT-PCR assay using primers specific to Hsp70 cDNA detects only 30% less Hsp70 mRNA in ParpC03256 mutants than in WT flies.
To examine the pattern of PARP1 and PARPΔ300 protein binding in active chromatin, we performed ChIP experiments. Previously, we demonstrated that PARP controls heat shock response and activation of hsp70 expression (6). Subsequently, binding of PARP and its enzymatic activation were shown to be critical for Pol2 polymerase-dependent transcription within the Hsp70 gene (7, 27). Here, we compared distribution of PARP1-ECFP and PARPΔ300-EYFP proteins along the hsp70 locus in parpC03256 mutants. To detect hsp70 DNA in ChIP experiments, we used 24 pairs of primers distributed along 3 kb of the hsp70 genomic sequence (base pairs −500 to +2,500 relative to transcriptional start) (Fig. 4 C and D). Although both PARP isoforms were enriched at the transcriptional start and termination as they distributed among nucleosomes at the 5′ end of the hsp70 sequence, they were depleted from the second half of the locus (Fig. 4 C and D). PARPΔ300, however, demonstrated much higher accumulation at transcriptional start and termination sites (Fig. 4 C and D, arrows). These data support the observation that deletion of Zn-finger 1 facilitates accumulation in active chromatin.
To explore the functional differences of PARP1 and PARPΔ300 proteins in transcription, we compared transcriptional activation between hsp70 genes in WT Drosophila larvae and parpC03256 mutants, which express only PARPΔ300 protein. We found that parpC03256 mutants could activate expression of hsp70 on 30 min of heat shock (Fig. 4E). The hsp70 mRNA production, which was reduced by 25%, could be explained by the reduced level of total PARP protein in the mutant (Fig. 1 C and D). Moreover, expression of the PARPΔ300-EYFP transgene rescues hsp70 transcription in parpC03256 mutants completely. Collectively, our results again support the conclusion that PARPΔ300 protein is fully functional PARP and that Zn-finger 1 is only required for fine-tuning of PARP protein localization in the genome.
Zn-Finger Domain 1 Is Necessary for the Silencing of Retrotransposable Elements.
We previously showed that PARP1 protein is required for silencing of heterochromatic repeated DNAs (8). This, along with our current observation that PARPΔ300 is absent from condensed chromatin domains, suggests that Zn-finger 1 may target PARP1 to loci that should be silent. We examined localization of PARP1-ECFP and PARPΔ300-EYFP in heterochromatin using immunostaining of Drosophila polytene chromosomes and ChIP approaches. Although PARP1 protein shows significant accumulation in both constitutive (Fig. 5A, arrowhead) and intercalary (Fig. 5A, arrows) heterochromatin, PARPΔ300 protein is excluded from both (Fig. 5B). At the same time, PARP1 protein is enriched on sequences of retrotransposable elements copia and gypsy (typical content of silent chromatin), whereas the amount of PARPΔ300 is diminished (Fig. 5C). Moreover, we found that parpC03256 mutants expressing PARPΔ300 but not PARP1 dramatically overproduce mRNA of both retrotransposons (Fig. 5 D and E) and accumulate retroviral particles in nucleoplasm (Fig. 5 F–H). These phenotypes could be rescued by PARP1 expression but not by PARPΔ300. Thus, we conclude that disruption of the Zn-finger 1 domain abolishes proper targeting of PARP1 to heterochromatin and leads to the desilencing of retrotransposable elements.
Discussion
PARP1 is the second most abundant nuclear nonhistonic protein. The distribution of PARP1 in chromatin is nonrandom, occurring in characteristic profiles specific for distinct cell types (28–31). At present, however, the molecular basis for PARP1 binding to chromatin remains poorly understood. Here, we described a Drosophila mutant, which expresses a short isoform of PARP1 protein without Zn-finger 1. We discovered that mutating Zn-finger 1 does not affect average mobility of PARP protein in chromatin but that it does abolish localization of PARP protein in heterochromatin and increase accumulation of PARP in euchromatic loci. This observation suggests that the N-terminal domain of PARP1 is necessary for suppressing the binding of PARP1 to some affinity chromatin sites specifically related to retrotransposable DNA.
The molecular basis for PARP1 binding to chromatin remains poorly understood. Zn-finger 1 may contribute to the direct recognition of DNA sequences specific for heterochromatin. Such a sequence could be part of the genome of retrotransposable elements. The ability to bind sequence TGTTG has been previously reported for mammalian PARP1 (32). Sequence TGTTG is known to be an evolutionarily conserved flanking sequence for many retroviral and retrotransposable elements with long terminal repeats, such as copia and gypsy in Drosophila. Therefore, Zn-finger 1 may control direct binding of PARP1 protein to these high-affinity sites, followed by redistribution into surrounding chromatin.
Alternatively, Zn-finger 1 may interact with a specific chromatin protein or with a specific modification of an abundant chromatin protein rather than directly with DNA. Considerable evidence sug-gests that PARP1 directly interacts with histones (3). For example, histones H1, H2A, and H2B are preferential targets for PARP1 binding in vitro (33), and they are enzymatically modified by PARP1 (34–36). However, Drosophila histone H1 was recently reported as an antagonist of PARP1 binding to chromatin (28). To date, of all the PARP1 interactors identified through in vitro experiments, none shows significant colocalization with PARP1 in chromatin. Therefore, it is clear that the chromatin components that are responsible for PARP1 targeting have not yet been identified.
Another possible explanation of the effects of Zn-finger 1 deletion on PARP interaction with chromatin could be that Zn-finger 1 interacts with other PARP1 domains and masks them from the recognition of high-affinity sites in chromatin. This suggestion is supported by our previous observation, wherein we demonstrated in vitro that the N-terminal domain of PARP1 suppresses interaction of PARP1 with histones H3 and H4 (19). This might explain the enrichment of PARPΔ300 in active open chromatin observed in vivo in the present work. Specifically, sites of chromatin with a high level of H3/H4 exposure in open chromatin (by nucleosome modifications) may titrate PARPΔ300 protein and bind it with high affinity, although the affinity of PARPΔ300 to the rest of chromatin is unchanged compared with PARP1 protein.
Collectively, our data show that the second zinc finger is sufficient for PARP1 activation, localization to active chromatin, and most PARP1 functions required for viability of the fly. Further, we demonstrate that the first zinc finger is required for PARP localization to heterochromatin, where it regulates transcriptional silencing. We can now separate PARP functions molecularly. This reagent will enable us to identify the PARP transcriptional targets required for viability.
Experimental Procedures
Drosophila Strains.
Flies were cultured on standard cornmeal-molasses-agar media at 22 °C unless otherwise indicated. The fly stocks were generated by standard genetic methods or obtained from the Bloomington Drosophila Stock Center and the Exelixis collection at the Harvard University Medical School. Detailed information is provided in SI Text.
Mononucleosome ChIP.
The chromatin and PARP protein complexes were immunoprecipitated using anti-GFP rabbit polyclonal antibody (Torrey Pines Biolabs). DNA from the elutes was measured by the real-time PCR assay. Detailed information is provided in SI Text.
Real-Time RT-PCR Assay.
RNA was reverse-transcribed, and the real-time PCR assays were performed using an ABI 7900 HT instrument (Applied Biosystems). Detailed information is provided in SI Text.
Online Supplemental Material.
Fig. S1 describes the C03256 mutation that disrupts the PARP locus. Fig. S2 illustrates the structure of the putative PARP protein isoforms that we discovered in C03256 mutants. Fig. S3 shows that the PARPΔ300 protein isoform is sufficient for Drosophila viability during the genotoxic stress response. SI Text includes supplemental experimental procedures and references.
Supplementary Material
Acknowledgments
We thank Drs. G. Cavalli, A. Veraksa, P. Fisher, R. Glaser, and R. Lewis for providing materials. We also thank the Exelexis mutagenesis project for the C03256 mutant Drosophila line. Drs. Maureen E. Murphy and Alana O'Reilly contributed valuable comments on the manuscript. We also thank Corinne Stobbe for assistance with irradia-tion experiments. Quantitative RT-PCR analysis was provided by Dr. Emmanuelle Nicolas of the Fox Chase Cancer Center Genomics Facility. The research was supported by grants from the National Institutes of Health (R01 GM077452) and the Ellison Medical Foundation (GM27875) (to A.V.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914152107/DCSupplemental.
References
- 1.De Murcia G, Shall S. From DNA Damage and Stress Signaling to Cell Death Poly (ADP-Ribosylation) Reactions. New York: Oxford Univ Press; 2000. [Google Scholar]
- 2.Rippmann JF, Damm K, Schnapp A. Functional characterization of the poly(ADP-ribose) polymerase activity of tankyrase 1, a potential regulator of telomere length. J Mol Biol. 2002;323:217–224. doi: 10.1016/s0022-2836(02)00946-4. [DOI] [PubMed] [Google Scholar]
- 3.D'Amours D, Desnoyers S, D'Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249–268. [PMC free article] [PubMed] [Google Scholar]
- 4.El-Khamisy SF, Masutani M, Suzuki H, Caldecott KW. A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 2003;31:5526–5533. doi: 10.1093/nar/gkg761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrabi SA, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tulin A, Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–562. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- 7.Petesch SJ, Lis JT. Rapid, transcription-independent loss of nucleosomes over a large chromatin domain at Hsp70 loci. Cell. 2008;134:74–84. doi: 10.1016/j.cell.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tulin A, Stewart D, Spradling AC. The Drosophila heterochromatic gene encoding poly(ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development. Genes Dev. 2002;16:2108–2119. doi: 10.1101/gad.1003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amé JC, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- 10.Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 11.Miwa M, Hanai S, Poltronieri P, Uchida M, Uchida K. Functional analysis of poly(ADP-ribose) polymerase in Drosophila melanogaster. Mol Cell Biochem. 1999;193:103–107. [PubMed] [Google Scholar]
- 12.Kameshita I, Matsuda Z, Taniguchi T, Shizuta Y. Poly (ADP-Ribose) synthetase. Separation and identification of three proteolytic fragments as the substrate-binding domain, the DNA-binding domain, and the automodification domain. J Biol Chem. 1984;259:4770–4776. [PubMed] [Google Scholar]
- 13.Kameshita I, Matsuda M, Nishikimi M, Ushiro H, Shizuta Y. Reconstitution and poly(ADP-ribosyl)ation of proteolytically fragmented poly(ADP-ribose) synthetase. J Biol Chem. 1986;261:3863–3868. [PubMed] [Google Scholar]
- 14.Gradwohl G, et al. The second zinc-finger domain of poly(ADP-ribose) polymerase determines specificity for single-stranded breaks in DNA. Proc Natl Acad Sci USA. 1990;87:2990–2994. doi: 10.1073/pnas.87.8.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wacker DA, et al. The DNA binding and catalytic domains of poly(ADP-ribose) polymerase 1 cooperate in the regulation of chromatin structure and transcription. Mol Cell Biol. 2007;27:7475–7485. doi: 10.1128/MCB.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artavanis-Tsakonas S. Accessing the Exelixis collection. Nat Genet. 2004;36:207. doi: 10.1038/ng1316. [DOI] [PubMed] [Google Scholar]
- 17.Hanai S, et al. Loss of poly(ADP-ribose) glycohydrolase causes progressive neurodegeneration in Drosophila melanogaster. Proc Natl Acad Sci USA. 2004;101:82–86. doi: 10.1073/pnas.2237114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tulin A, Naumova NM, Menon AK, Spradling AC. Drosophila poly(ADP-ribose) glycohydrolase mediates chromatin structure and SIR2-dependent silencing. Genetics. 2006;172:363–371. doi: 10.1534/genetics.105.049239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinnola A, Naumova N, Shah M, Tulin AV. Nucleosomal core histones mediate dynamic regulation of poly(ADP-ribose) polymerase 1 protein binding to chromatin and induction of its enzymatic activity. J Biol Chem. 2007;282:32511–32519. doi: 10.1074/jbc.M705989200. [DOI] [PubMed] [Google Scholar]
- 20.Ji Y, Tulin AV. Poly(ADP-ribosyl)ation of heterogeneous nuclear ribonucleopro-teins modulates splicing. Nucleic Acids Res. 2009;37:3501–3513. doi: 10.1093/nar/gkp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sala A, et al. The nucleosome-remodeling ATPase ISWI is regulated by poly-ADP-ribosylation. PLoS Biol. 2008;6:e252. doi: 10.1371/journal.pbio.0060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Murcia JM, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci USA. 1997;94:7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotova E, Jarnik M, Tulin AV. Poly (ADP-ribose) polymerase 1 is required for protein localization to Cajal body. PLoS Genet. 2009;5:e1000387. doi: 10.1371/journal.pgen.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza-Alvarez H, Alvarez-Gonzalez R. Poly(ADP-ribose) polymerase is a catalytic dimer and the automodification reaction is intermolecular. J Biol Chem. 1993;268:22575–22580. [PubMed] [Google Scholar]
- 26.Pion E, et al. DNA-induced dimerization of poly(ADP-ribose) polymerase-1 triggers its activation. Biochemistry. 2005;44:14670–14681. doi: 10.1021/bi050755o. [DOI] [PubMed] [Google Scholar]
- 27.Weake VM, Workman JL. Clearing the way for unpaused polymerases. Cell. 2008;134:16–18. doi: 10.1016/j.cell.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Kim MY, Mauro S, Gévry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Molinete M, et al. Overproduction of the poly(ADP-ribose) polymerase DNA-binding domain blocks alkylation-induced DNA repair synthesis in mammalian cells. EMBO J. 1993;12:2109–2117. doi: 10.1002/j.1460-2075.1993.tb05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dantzer F, Nasheuer HP, Vonesch JL, de Murcia G, Ménissier-de Murcia J. Functional association of poly(ADP-ribose) polymerase with DNA polymerase alpha-primase complex: A link between DNA strand break detection and DNA replication. Nucleic Acids Res. 1998;26:1891–1898. doi: 10.1093/nar/26.8.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnakumar R, et al. Reciprocal binding of PARP-1 and histone H1 at promoters specifies transcriptional outcomes. Science. 2008;319:819–821. doi: 10.1126/science.1149250. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, et al. Analysis of nucleotide sequence-dependent DNA binding of poly(ADP-ribose) polymerase in a purified system. Biochemistry. 2004;43:217–223. doi: 10.1021/bi0301800. [DOI] [PubMed] [Google Scholar]
- 33.Buki KG, Bauer PI, Hakam A, Kun E. Identification of domains of poly(ADP-ribose) polymerase for protein binding and self-association. J Biol Chem. 1995;270:3370–3377. doi: 10.1074/jbc.270.7.3370. [DOI] [PubMed] [Google Scholar]
- 34.Poirier GG, de Murcia G, Jongstra-Bilen J, Niedergang C, Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci USA. 1982;79:3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aubin RJ, et al. Correlation between endogenous nucleosomal hyper(ADP-ribosyl)ation of histone H1 and the induction of chromatin relaxation. EMBO J. 1983;2:1685–1693. doi: 10.1002/j.1460-2075.1983.tb01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krupitza G, Cerutti P. Poly(ADP-ribosylation) of histones in intact human keratino-cytes. Biochemistry. 1989;28:4054–4060. doi: 10.1021/bi00435a063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.