Abstract
Our previous analyses showed that allopregnanolone (APα) significantly increased proliferation of rodent and human neural progenitor cells in vitro. In this study, we investigated the efficacy of APα to promote neurogenesis in the hippocampal subgranular zone (SGZ), to reverse learning and memory deficits in 3-month-old male triple transgenic mouse model of Alzheimer's (3xTgAD) and the correlation between APα-induced neural progenitor cell survival and memory function in 3xTgAD mice. Neural progenitor cell proliferation was determined by unbiased stereological analysis of BrdU incorporation and survival determined by FACS for BrdU+ cells. Learning and memory function was assessed using the hippocampal-dependent trace eye-blink conditioning paradigm. At 3 months, basal level of BrdU+ cells in the SGZ of 3xTgAD mice was significantly lower relative to non-Tg mice, despite the lack of evident AD pathology. APα significantly increased, in a dose-dependent manner, BrdU+ cells in SGZ in 3xTgAD mice and restored SGZ proliferation to normal magnitude. As with the deficit in proliferation, 3xTgAD mice exhibited deficits in learning and memory. APα reversed the cognitive deficits to restore learning and memory performance to the level of normal non-Tg mice. In 3xTgAD mice, APα-induced survival of neural progenitors was significantly correlated with APα-induced memory performance. These findings suggest that early neurogenic deficits, which were evident before immunodetectable Aβ, may contribute to the cognitive phenotype of AD, and that APα could serve as a regenerative therapeutic to prevent or delay neurogenic and cognitive deficits associated with mild cognitive impairment and Alzheimer's disease.
Keywords: adult neurogenesis, subgranular zone, trace eyeblink conditioning, Alzheimer’s therapeutics, translational neuroscience
Allopregnanolone (APα, 3α-hydroxy-5α-pregnan-20-one), a me-tabolite of progesterone, is synthesized de novo in the embry-onic and adult CNS (1–4) and in pluripotential progenitor cells (5). Previously we showed that APα significantly increased proliferation of rodent and human neural progenitor cells in vitro via a GABAA receptor and L-type Ca2+ channel-dependent mechanism (1). In this study, we investigated the efficacy of APα as a neurogenic agent in vivo and its effects on learning and memory using the triple-transgenic Alzheimer's disease mouse (3xTgAD).
The adult brain has two stable regions of mitotic activity, the subventricular zone (SVZ) of the lateral ventricle in the frontal cortex and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (6, 7). Though the regenerative potential of the mammalian brain is sustained throughout the life span, proliferative capacity of neural progenitors declines with age and diseases, such Alzheimer's disease (AD) (8). Parallel to the diminution in neurogenesis, is the decline of neurosteroids in the aging and AD brain (9–13). Consistent with a decline in growth factors (10, 12, 13), APα content is significantly lower in aged subjects (9) and in brains of AD patients compared with age-matched controls (10, 11). In contrast, during fetal development when neural progenitor proliferation is maximal, APα is synthesized throughout the embryonic period and in pluripotential progenitor cells (5, 14).
To test the hypothesis that APα can function as a regenerative factor with an impact on cognitive function in AD, we investigated the efficacy of APα as a neurogenic agent in vivo using the triple transgenic Alzheimer's disease mouse (3xTgAD) and the nontransgenic control mouse (non-Tg). The 3xTgAD mouse carries mutations in two human familial AD genes (APPSwe, PS1M146V) and one frontal temporal dementia-linked tau mutation (tauP301L), and manifests age-dependent neuropathology of both β-amyloid plaques and neurofibrillary tangles (15). In addition to expressing neuropathological markers of AD, the 3xTgAD mouse exhibits early learning and memory deficits (16). Using this AD model, we characterized APα concentration within brain and serum, basal level of neurogenesis, and cognitive function at 3 months of age. These analyses were conducted in parallel to an investigation of the impact of APα on both neurogenic and cognitive function using unbiased stereology, immuncytochemistry (IHC), FACS, real-time RT-PCR, Western blot, and a hippocampal-dependent associative learning task, trace eye-blink conditioning (17, 18).
Results
Neural Progenitor Proliferation in Subgranular Zone of the Dentate Gyrus (SGZ) Is Deficient in 3-Month-Old Male 3xTgAD Mice Before Onset of Visible AD Pathology.
To determine development of amyloid β-deposition in 3xTgAD male mice, brain sections were immunolabeled with antiamyloid β-antibody (6E10) (Fig. 1A). At 3 months, intra- or extracellular Aβ immunoreactivity (IR) was not detectable in either hippocampus or cerebral cortex, whereas intraneuronal Aβ IR intensity was apparent at 6, 9, and 12 months and increased with age, consistent with that reported by Oddo and LaFerla (15). Extraneuronal Aβ IR was rarely observed in 9-month-old 3xTgAD hippocampi but was consistently present in the hippocampus of 12-month-old 3xTgAD mice (15).
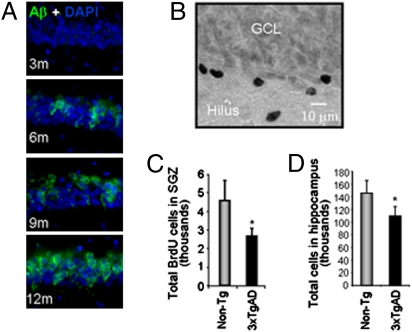
Fig. 1.
Neural progenitor proliferation is deficient in 3-month-old male 3xTgAD mice. (A) Aβ immunoreactivity (IR; 6E10 labeling appears as green) was not observed in 3-month-old 3xTgAD mouse brain, whereas intraneuronal Aβ IR within hippocampal CA1 region was evident at 6, 9, and 12 months of age. Extracellular Aβ IR occurred rarely in 9-month-old mice. (B) Representative image of BrdU-positive cells in a 3xTgAD mouse hippocampal subgranular zone (SGZ) of mice treated with a single injection of BrdU (100 mg/kg) and killed 24 h later. GCL, granular cell layer. (C) Unbiased stereology indicated a significant decrease in BrdU-positive cells in the 3xTgAD mouse SGZ relative to non-Tg mouse before detectable Aβ accumulation. Data are presented as average ± SEM (n = 4/group), *P < 0.05 as compared with non-Tg. (D) Data from fluorescence activated cell sorting of propidium iodide-positive nuclei indicate that 3xTgAD mice have significantly lower total hippocampal cell number relative to non-Tg mice. Data are presented as average ± SEM, *P < 0.05 compared with non-Tg.
To determine basal level of proliferation, a comparative analysis of BrdU incorporation was conducted in the SGZ of 3xTgAD and non-Tg mice. BrdU IHC was performed in adjacent sections immunolabeled for Aβ. The majority of the BrdU-positive cells were observed in the SGZ (Fig. 1B). The distribution of the newly formed cells within the 3xTgAD and non-Tg mice was consistent with that observed in both rat and mouse dentate gyrus (8). Results of unbiased quantitative stereological analyses indicated that 3-month-old nontransgenic mice generated 4,568 ± 1,089 BrdU-positive cells, which is consistent with that reported in C57BL/6 and SJL × C57BL/6 (19) (Fig. 1C). Basal proliferation in the 3xTgAD dentate gyrus was significantly lower (2,625 ± 426) compared with the non-Tg mouse (4,568 ± 1,089) dentate gyrus (P < 0.01; Fig. 1C) and is consistent with that reported for 3xTgAD mouse dentate (20). The 42% decrease in basal proliferation in the 3xTgAD dentate (Fig. 1 B and C) was evident before the appearance of markers of AD pathology (Fig. 1A) and consistent with that 3xTgAD mice exhibited a lower basal level of APα in the cerebral cortex than that in the non-Tg mice (Fig. S1). These data suggest a preexisting deficit in basal neurogenesis in 3xTgAD mice SGZ that is evident before development of overt AD pathology.
APα Reverses the Neurogenic Deficit of 3xTgAD Mouse.
To determine the impact of APα on neural progenitor cell proliferation, BrdU IHC and unbiased quantitative stereology were conducted. BrdU-positive cells were observed in clusters located in the SGZ of both 3xTgAD and non-Tg mouse hippocampus (Fig. 2 A and B). APα treatment was associated with a dose-dependent rise in BrdU incorporation in both non-Tg and 3xTgAD mice (Fig. 2C). In the non-Tg SGZ, in which the basal level of BrdU-positive cells was high (4,568 ± 1,089), APα (10 mg/kg) exerted a modest but nonsignificant increase in progenitor cell proliferation (5,616 ± 614, P < 0.36). In contrast, in 3xTgAD SGZ, APα induced a significant increase in progenitor cell proliferation with the greatest increase occurring at 10 mg/kg APα (55 + 18%, F(3, 9) = 3.86 P < 0.05 Neuman-Keuls, P < 0.05, n = 4/group; Fig. 2C). These findings were supported by APα-induced expression of two proliferation markers, proliferating cell nuclear antigen (PCNA) and cyclin dependent kinase 1 (CDK1/cdc2), in the hippocampus of 3xTgAD and non-Tg mice (Fig. S2).
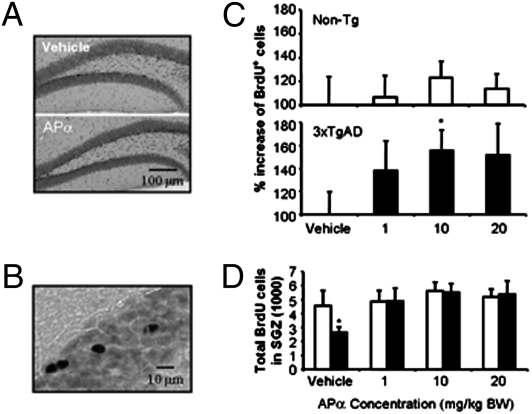
Fig. 2.
APα reversed the neurogenic deficits in dentate gyrus of 3-month-old 3xTgAD mice. Stereological estimates of the total number of BrdU-labeled cells in the dentate gyrus of non-Tg and 3xTgAD male mice treated with 1, 10, or 20 mg/kg APα or vehicle alone. (A) Representative immunolabeled BrdU-positive cell images of hippocampal dentate gyrus in APα- or vehicle-treated 3xTgAD mice. (B) A representative image showing magnification at which BrdU-positive cells were analyzed. (C) Summary of dose-dependent effects of APα on BrdU incorporation in non-Tg (Upper) and 3xTgAD (Lower) mice SGZ. Note that 10 mg/kg of APα exerted greatest and statistically significant efficacy. (D) APα reversed the neurogenic deficits within SGZ of the 3xTgAD mouse to restore 3xTgAD mouse proliferation to comparable levels of normal non-Tg mice. Bars represent mean ± SEM, n ≥ 4 in each group, *P < 0.05 vs. vehicle control of the same genotype group.
To ascertain whether APα increased proliferation beyond normal or restored neurogenesis to normal, the total number of BrdU-positive cells was determined for non-Tg and 3xTgAD at the three doses tested (Fig. 2D). APα reversed the neurogenic deficit of 3xTgAD (from 2,625 ± 426 to 5,520 ± 633, P < 0.01) such that proliferation within the SGZ of 3xTgAD was comparable to BrdU incorporation of non-Tg SGZ (4,568 ± 1,089, P < 0.25). These data indicate that APα treatment reversed the proliferative deficit of 3xTgAD mice and restored NPC proliferation to that of the normal non-Tg SGZ (Fig. 2D).
Newly Formed NPCs in APα-Treated 3xTgAD Mice Express Neuronal Cell Markers in Hippocampal Dentate Gyrus.
To verify the phenotype of the newly formed BrdU-positive cells in vivo, triple immunolabeling was conducted in sections of mouse hippocampi adjacent to those that were stereologically analyzed and derived from 3-month-old 3xTgAD mice treated with a single injection of 10 mg/kg APα. The following phenotypic markers were assessed: doublecortin (DCX), to label young immature neurons; NeuN, to label mature neurons; and glial fibrillary acidic protein (GFAP), to label astrocytes. Confocal microscopy identified BrdU-positive cells colocalized with DCX alone (arrowhead) or together with NeuN (arrow; Fig. 3 A and C). To confirm IR colocalization and subcellular distribution, IR fluorescent intensity was digitally recorded across two BrdU-and DCX-positive cells to generate a histogram of fluorescent intensity (Fig. 3B). The fluorescent intensity distribution profile showed an overlap in DCX- (red) and BrdU-(green) positive cells. These findings are consistent with the report by Kempermann et al. (19) that 24 h after BrdU injection, a subset of the BrdU-positive cells are NeuN positive, indicating a neuronal lineage of these newly formed cells. This observation was further confirmed by immunolabeling coronal sections derived from APα (10 mg/kg)-treated 3xTgAD mouse dentate 22 days post-APα treatment and behavioral analyses (Fig. 3D; see Fig. 4A for behavioral paradigm). BrdU-positive cells were located in the middle of granular cell layer, indicating the migration of newly formed cells from the SGZ to the GCL. Collectively, these data indicate that newly formed cells, generated following APα treatment, express a neuronal phenotype.
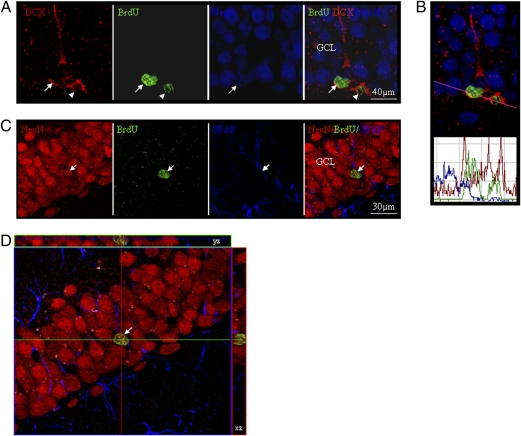
Fig. 3.
Phenotypic characterization of the BrdU-positive cells in mouse dentate gyrus. (A) An illustrative BrdU+ cell within the dentate gyrus (DG) is labeled with DCX and NeuN (arrowheads), indicating transition from a young to mature neuron and migration toward the granular cell layer (GCL) 24 h after APα and BrdU injections. (B) Confocal laser scanning histogram of IR fluorescent intensity profile verification of colocalization. DCX red fluorescent signal in the cytoplasm and BrdU green fluorescent signal in the nucleus overlapped with NeuN blue fluorescent signal (a nuclear neuronal marker). (C) Further, triple immunostaining was conducted in mouse brain sections 21 days following APα and BrdU injection. A fully mature BrdU-positive cell located in the middle of the GCL was positive for NeuN, whereas colocalization with the glial cell marker GFAP was not observed. (D) A 3D reconstruction of z-series images of neuron shown in C.
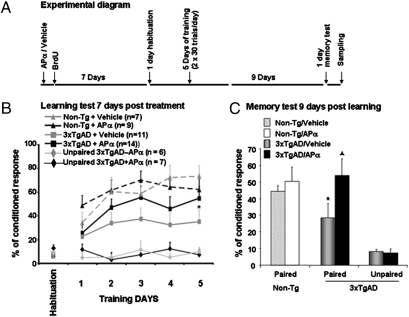
Fig. 4.
APα reversed the learning and memory deficits of 3xTgAD mice. (A) Experimental behavioral paradigm. (B) APα significantly reversed the conditioned response/associative learning deficits of 3xTgAD mice to a level comparable to non-Tg mice. (C) APα significantly increased memory performance of 3xTgAD to a level of conditioned response comparable to that of non-Tg mice. APα-induced increase in learning and memory performance was specific to associative paired trace conditioning as APα had no effect on performance of 3xTgAD in the unpaired trace conditioning on either learning (B) or memory (C). Two-way ANOVA analysis indicated significant differences in learning for genotype (P < 0.0002) and days of training (P < 0.000001).
APα Reverses Learning and Memory Deficits of 3xTgAD Mice to Performance Level of Normal Non-Tg Mouse.
To determine whether APα-induced neurogenesis was associated with a functional behavioral consequence, we assessed the impact of APα on a hippocampal-dependent associative learning and memory task, trace eye-blink conditioning (18), which has been shown to be dependent upon the generation of new neurons in the dentate gyrus (17, 21). Three-month-old male 3xTgAD and non-Tg mice were prepared for behavioral testing and received a single s.c. injection of APα (10 mg/kg) or vehicle 7 days before start of the learning trial (Fig. 4A). The rationale for the 7-day interval between exposure to APα and the start of the behavioral experiment was to allow time for the proliferation, migration, and integration of newly generated neurons into the dentate gyrus (22). Following the learning trial, mice were transferred back to their home cage for a period of 9 days with memory function determined on day 22 of the experiment (Fig. 4A).
Results of behavioral analyses indicate that at 3 months, 3xTgAD mice exhibited a learning deficit relative to the performance of normal non-Tg mice at the end of training (Fig. 4B; F(1, 19) = 8.177, P < 0.01). APα significantly increased the learning performance of 3xTgAD mice (Fig. 4B; F(1, 21) = 4.477, P < 0.04) to a level comparable to non-Tg mice such that the performance of APα-treated 3xTgAD mice was not statistically different from normal non-Tg mouse (Fig. 4B; F(1, 20)= 0.563, P < 0.5). Consistent with its modest effect on neurogenesis in normal non-Tg mice, APα did not augment their learning performance (Fig. 4B; F(1, 21) = 0.676, P < 0.5).
Following a 9-day period of no training, mice were tested for memory of the learned association. Vehicle-treated 3xTgAD mice exhibited significantly impaired memory performance relative to vehicle-treated non-Tg performance (Fig. 4C; F(1, 14)= 12.206, P < 0.01). APα-treated 3xTgAD mice exhibited a significant increase in memory performance (F(1, 19) = 11.204, P < 0.01) compared with vehicle-treated 3xTgAD mice. Memory performance of APα-treated 3xTgAD mice was restored to a level comparable with the normal non-Tg mice (Fig. 4C; F(1, 17) = 0.279, P < 0.5). As in the learning trial, APα did not significantly augment memory performance of non-Tg mice (Fig. 4C; F(1, 14) = 0.083, P < 0.8). A two-way ANOVA analysis indicated significant differences in learning by genotype (F(1, 126) = 19.787, P < 0.0001) and days of training (F(5, 126) = 8.729, P < 0.00001). No interaction was observed between days of training and genotype (F(5, 126) = 1.671, P < 0.2). Relative to vehicle-treated 3xTgAD mice, APα-treated 3xTgAD mice exhibited a significantly higher learning and memory performance (F(1, 138) = 11.086, P < 0.01; Fig. 4 B and C).
To determine the specificity of APα on associative learning, 3xTgAD mice underwent either paired or unpaired trace eye-blink conditioning. Results of these behavioral analyses indicated that APα significantly increased learning and memory performance of 3xTgAD mice that received paired trace eye-blink conditioning while exerting no effect upon performance in the unpaired condition (P = 0.48; Fig. 4 B and C).
To evaluate the contribution of behavioral training versus APα on generation of BrdU+ cells, a comparative analysis was conducted in which 3xTgAD mice received either no training ± APα or training ± APα. APα induced a significant increase in the number of BrdU-positive cells in the SGZ of mice under both the no-training (1,228 ± 280) and training (1,132 ± 210) conditions (P < 0.05), whereas no statistically significant change occurred in the absence of APα regardless of training condition (Fig. S3). Collectively, these findings indicate that APα directly reversed the deficits in neuroprogenitor cell proliferation and associative learning and memory of 3xTgAD mice.
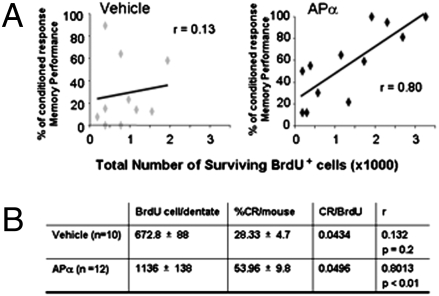
A correlational analysis was performed to determine the relationship between increased neurogenesis and memory enhancement for each 3xTgAD mouse. The number of BrdU-positive cells per dentate in the 3xTgAD vehicle-treated group was 672.8 ± 88, whereas in 3xTgAD APα-treated group the number of BrdU+ cells was 1,136 ± 138, and the average percent increase in conditioned response (CRs) was 28.33 ± 4.7 and 53.96 ± 9.8, respectively. Pearson correlation analysis indicated a significant correlation between BrdU-positive cells and increased memory performance in APα-treated group (r = 0.80, P < 0.01), which was not apparent in the vehicle-treated group where number of BrdU+ cells were not correlated with memory performance (r = 0.13, P < 0.3; Fig. 5). These data indicate that memory performance in the vehicle-treated group did not require survival of newly generated cells, whereas memory performance in the APα-treated 3xTgAD was correlated with and likely required the survival of APα-induced generated neurons.
Fig. 5.
APα reversal of memory deficit is correlated with neurogenesis in 3xTgAD mice. (A) Correlation (r value) between number of surviving BrdU-positive cells and memory performance in vehicle (r = 0.13) and APα-treated 3xTgAD mice (r = 0.80). (B) Pearson correlation analysis indicated a highly significant correlation (P < 0.01) between the number of surviving BrdU-positive cells and memory performance in APα-treated 3xTgAD mice. Performance in vehicle treated 3xTgAD mice was not significantly correlated (P < 0.2) with BrdU-labeled cells.
Discussion
APα reversed the neurogenic and cognitive deficits that typify the male 3xTgAD mouse before the onset of overt Alzheimer's pathology. APα-induced neural progenitor proliferation in vivo is consistent with earlier in vitro analyses in which APα significantly increased proliferation of human and rat neural progenitors in a dose-dependent and steroid-specific manner via a GABAA receptor and L-type Ca2+ channel mechanism unique to neural progenitor cells (1). In vivo, a single EC100 neurogenic dose of APα was functionally relevant and reversed hippocampal-dependent associative learning and memory deficits. The significant increase in associative memory induced by APα was highly correlated with APα-induced neurogenesis.
Neurogenic and Cognitive Deficit of 3xTgAD Mouse.
The 3xTgAD mouse, carrying homozygous mutant genes (APPSwe, PS1M146V, and tauP301L), mimics multiple aspects of AD neuropathology in AD-relevant brain regions (15). Results of our analyses showed a significant neurogenic deficit as early as 3 months in the 3xTgAD mouse SGZ. Consistent with our findings, several studies indicate a SGZ neurogenic deficit in transgenic and knock-in mice that express mutated human APP (23, 24) or PSEN1 and APP (25, 26). In the 3xTgAD SGZ, a neurogenic deficit was detected at 9 months (20), whereas our data indicate a significant deficit in the SGZ by 3 months. In our study, neurogenic deficit was detected using unbiased quantitative stereology, which was confirmed by analyses of cell-cycle gene expression, whereas Rodriguez et al. (20) quantified phosphorylated histone 3. However, a substantial decrement in phosphorylated Histone 3 positive cells was evident at 3 months but failed to reach statistical significance due to high variability (20). Our results confirm these findings but more importantly, indicate that the deficit in neurogenesis can occur before the development of overt AD pathology.
Emerging evidence indicates that neurogenic deficits are paralleled by cognitive deficits, and that associative learning and memory require temporal encoding that is dependent upon the generation of new neurons in the dentate gyrus (17, 21, 22, 27). The deficiency in hippocampus-dependent associative learning in the 3-month-old 3xTgAD mice is consistent with other cognitive deficits in these animals, including dysfunction in synaptic plasticity, deficits in LTP and paired-pulse facilitation, and early Alzheimer's disease-related cognitive deficits (15, 16). The coincidence of neurogenic and cognitive deficiency before overt evidence AD pathology suggests a close link between a deficit in neurogenesis and deficits in cognitive function. The neurogenic deficit of the 3xTgAD mouse is not unique to the subgranular zone of the dentate gyrus. We have detected a comparable deficit in proliferative capacity of the 3xTgAD subventricular zone (28). To date, we have no evidence of a neurogenic deficit in the cerebellum that contributes to the eye-blink conditioning associative learning and memory. However, analyses are currently underway to investigate this issue.
APα in Aging and Alzheimer's Disease.
Proliferation of neural progenitor cells (NSCs) is markedly diminished in the aged and AD brain (10, 12). Though underlying mechanisms that mediate the age and AD-associated decline in regenerative potential of neural progenitor cells remains to be fully determined, peptide growth factors and neurosteroids have been shown to play a key role. In middle-aged rat hippocampus, the average concentration of several peptide growth factors, FGF-2, IGF1, and VEGF are substantially lower relative to that in young rat hippocampus (12). Consistent with a decline in growth factors, a significant decrease in APα was evident in aged and AD brains (9–11). Interestingly, APα in the 3xTgAD mouse plasma and cortex was consistently lower in the 3xTgAD mouse compared with the non-Tg. As both non-Tg and 3xTgAD mice were treated with the same dose of APα under exactly the same conditions, the decrease in APα level must be attributable to either decreased absorption or increased metabolism. Our working hypothesis is that the decrease in APα is due to metabolism via increased expression of 17β-hydroxysteroid dehydrogenase (17βHSD, aka ERAB and ABAD) (4, 29, 30). This enzyme is significantly elevated in APP transgenic AD mice but not in normal controls (29, 31). Elevation of 17βHSD/ABAD in both liver and brain would be consistent with increased metabolic conversion of APα to 5alpha-dihydrotestosterone leading to lower levels of APα in both plasma and brain. Together, these data suggest that the changes in the local biochemical milieu of neurosteroids and peptide growth factors could contribute to neurogenic deficits in early stages of AD (32, 33).
Cell-cycle gene expression in neural progenitor cells is an obligatory requirement for neurogenesis and ultimately regeneration. However, aberrant entry into the cell cycle has been reported to precede neuronal death in the cortex and CA3 regions at all stages of AD, from MCI to late stage AD and within AD mouse models (34). Further, expression of the ectopic cell-cycle proteins ultimately predicts the demise of these neurons (35). These findings are especially challenging for therapeutics targeting the regenerative potential of endogenous neural stem/progenitor populations, as an unintended side effect may be to promote ectopic entry of neurons into the cell cycle and thereby exacerbate neuron demise. Herrup and coworkers (36) recently demonstrated that aberrant entry into the cell cycle is triggered by Aβ oligomers, which first appear in the frontal cortex layers II/III. In contrast, LaFerla and co-workers (37) recently reported that neuronal cell-cycle reactivation is not induced by Aβ or tau pathology, but rather appears to be triggered by acute neuronal loss. As the 3xTgAD mouse does not exhibit Aβ accumulation or neuronal loss at 3 months of age, APα-induced cell-cycle gene and protein expression, concomitant with markers of proliferation and survival, is unlikely due to aberrant entry into the cell cycle. An extensive series of analyses are currently underway to determine the impact of APα on neural progenitor proliferation and cell-cycle gene expression during the development of AD pathology in the 3xTgAD mouse brain.
Mechanism of APα-Induced Neurogenesis.
Earlier in vitro analyses demonstrated that APα significantly increased proliferation of both rodent and human neural progenitor cells (1). APα-induced proliferation was mediated via GABAA receptor-activated voltage-gated L-type Ca2+ channels (1) leading to a rapid rise in intracellular calcium in neural progenitors (38). In neural progenitor cells, the high intracellular chloride content leads to an efflux of chloride through the GABAA channels upon opening, which leads to depolarization of the membrane and influx of Ca2+ through voltage dependent L-type Ca2+ channels and activation of the CREB transcription factor (1, 38, 39). Through this pathway, APα stimulation of GABA-mediated excitation and CREB signaling can activate a key pathway in adult hippocampal neurogenesis, to promote the proliferation, survival, and differentiation of neural progenitor cells. Though this APα-activated pathway is relevant to induction of NPC proliferation, the mechanisms by which APα promotes survival of rNPCs that could involve either delayed or prolonged actions of APα on gene expression and neuron survival mechanisms remain to be determined. More generally, it must be acknowledged that possible actions of APα on other brain regions and actions other than neurogenesis may be involved, i.e., other delayed and prolonged actions of APα involving as yet unknown actions on gene expression, etc., issues to be explored in the future.
An important mechanistic consideration is the impact of behavioral training on neural progenitor cell proliferation (17). We elected to use the trace eye-blink conditioning paradigm based on the studies of Shors et al. (17), who demonstrated that newly generated neurons within the dentate gyrus contribute to the association of stimuli that are separated in time, which is a hippocampal-dependent associative learning and memory function (22). Unlike the high number of training trials used in the Shors analyses (800 trials over 3 days) (40), which increased the survival of newly generated neural progenitors, we intentionally used a lower number of training trials (2 × 30 trials/day for 5 days), which our data show does not change the number of surviving BrdU+ cells. Verification that the behavioral paradigm used in this study did not induce proliferation was evident in the lack of difference between proliferation in the trained and untrained vehicle-treated 3xTgAD mice. As most of the vehicle 3xTgAD mice failed to learn (average CRs of 28%), the subthreshold training regime would not be expected to increase the number of surviving cells, which is consistent with Waddell and Shors (41). Analyses to determine the impact of APα on a broader array of behavioral measures are underway.
In summary, results of the current analysis show that APα reversed deficits in SGZ neurogenesis, learning, and memory in the 3xTgAD mouse model of Alzheimer's disease to restore both regenerative and cognitive function to that of the normal nontransgenic mouse. From a translational perspective, blood brain penetrance of APα, efficacy of a single exposure, and mechanism of APα action make it an ideal CNS-targeted molecule and a promising regenerative therapeutic candidate for promoting neural regeneration and reversing cognitive deficits associated with the prodromal stage of Alzheimer's disease.
Materials and Methods
Breeding pairs of the triple-transgenic Alzheimer's disease mouse (3xTgAD, homozygous mutant of human APPswe and tauP301L and PS1M146V) and its background strain (129/Sv × C57BL/6) were obtained from Frank LaFerla (Univ of California–Irvine) and the colonies were established at University of Southern California. Characterization of amyloid and tau pathologies, as well as synaptic dysfunction, has been described previously (15) and confirmed in our laboratory. 3xTgAD mice were regularly genotyped to confirm the purity of the colony. Experiments were performed using 3-month-old male 3xTgAD and non-Tg. Mice were maintained under a 12-h light/12-h dark cycle with continuous access to food and water.
Allopregnanolone (APα; 3α-hydroxy-5α-pregnan-20-one; aka AP, Allo, or THP) stock solution was prepared in pure ethanol and diluted in PBS before injection (with a final ethanol concentration of 0.002% of the body weight). Mice received an s.c. injection of APα at different concentrations or vehicle as indicated. One hour after APα injection, mice were i.p. injected with BrdU at a concentration of 100 mg/kg. In all acute experiments, mice were killed the next day after injection and sampled as described in SI Text. All experiments used a minimal number of animals and conformed to the Animal Welfare Act, Guide to Use and Care of Laboratory Animals, and the U.S. Government Principles of the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training guidelines on the ethical use of animals.
See SI Text for detailed description of animal treatments, tissue collection, immunohistochemistry, real-time reverse-transcription PCR, Western blot, unbiased stereology, GC/MS, and trace eye-blink conditioning.
Supplementary Material
Acknowledgments
We thank Dr. Frank LaFerla (UCI) for the gift of 3xTgAD and nonTg mice and Sean Iwamoto for excellent technical assistance. This research was supported by grants from the Alzheimer Drug Development Foundation, National Institute on Aging Grants PO1 AG02657 (Project 3) and U01AG031115, and the Kenneth T. and Eileen L. Norris Foundation (R.D.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001422107/DCSupplemental.
References
- 1.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton RD. The neurosteroid 3 alpha-hydroxy-5 alpha-pregnan-20-one induces cytoarchitectural regression in cultured fetal hippocampal neurons. J Neurosci. 1994;14:2763–2774. doi: 10.1523/JNEUROSCI.14-05-02763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 4.Mellon SH, Griffin LD. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 5.Gago N, et al. 3alpha,5alpha-Tetrahydroprogesterone (allopregnanolone) and gamma-aminobutyric acid: Autocrine/paracrine interactions in the control of neonatal PSA-NCAM+ progenitor proliferation. J Neurosci Res. 2004;78:770–783. doi: 10.1002/jnr.20348. [DOI] [PubMed] [Google Scholar]
- 6.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessberger S, Gage FH. Fate plasticity of adult hippocampal progenitors: Biological relevance and therapeutic use. Trends Pharmacol Sci. 2009;30:61–65. doi: 10.1016/j.tips.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genazzani AR, et al. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 10.Weill-Engerer S, et al. Neurosteroid quantification in human brain regions: Comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- 11.Marx CE, et al. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006;60:1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29:129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: Role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 14.Lauber ME, Lichtensteiger W. Ontogeny of 5 alpha-reductase (type 1) messenger ribonucleic acid expression in rat brain: Early presence in germinal zones. Endocrinology. 1996;137:2718–2730. doi: 10.1210/endo.137.7.8770891. [DOI] [PubMed] [Google Scholar]
- 15.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 16.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 17.Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996;93:13438–13444. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez JJ, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shors TJ. Memory traces of trace memories: Neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan MH, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 24.Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 25.Feng R, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 26.Wen PH, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki TR, et al. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci. 2007;27:11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Wang JM, Irwin RW, Chen S, Brinton RD. Washington, DC: Society for Neuroscience Program No. 554.8; 2008. Neuroscience. November 15-19, 2008. [Google Scholar]
- 29.Lustbader JW, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 30.Yang SY, He XY, Schulz H. Multiple functions of type 10 17beta-hydroxysteroid dehydrogenase. Trends Endocrinol Metab. 2005;16:167–175. doi: 10.1016/j.tem.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Takuma K, et al. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19:597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- 32.Brinton RD, et al. Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JM, Irwin RW, Liu L, Chen S, Brinton RD. Regeneration in a degenerating brain: Potential of allopregnanolone as a neuroregenerative agent. Curr Alzheimer Res. 2007;4:510–517. doi: 10.2174/156720507783018262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: Oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varvel NH, et al. Abeta oligomers induce neuronal cell cycle events in Alzheimer's disease. J Neurosci. 2008;28:10786–10793. doi: 10.1523/JNEUROSCI.2441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopes JP, Blurton-Jones M, Yamasaki TR, Agostinho P, LaFerla FM. Activation of cell cycle proteins in transgenic mice in response to neuronal loss but not amyloid-beta and tau pathology. J Alzheimers Dis. 2009;16:541–549. doi: 10.3233/JAD-2009-0993. [DOI] [PubMed] [Google Scholar]
- 38.Wang JM, Brinton RD. Allopregnanolone-induced rise in intracellular calcium in embryonic hippocampal neurons parallels their proliferative potential. BMC Neurosci. 2008;9(Suppl 2):S1–S10. doi: 10.1186/1471-2202-9-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jagasia R, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci. 2009;29:7966–7977. doi: 10.1523/JNEUROSCI.1054-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leuner B, et al. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.