Abstract
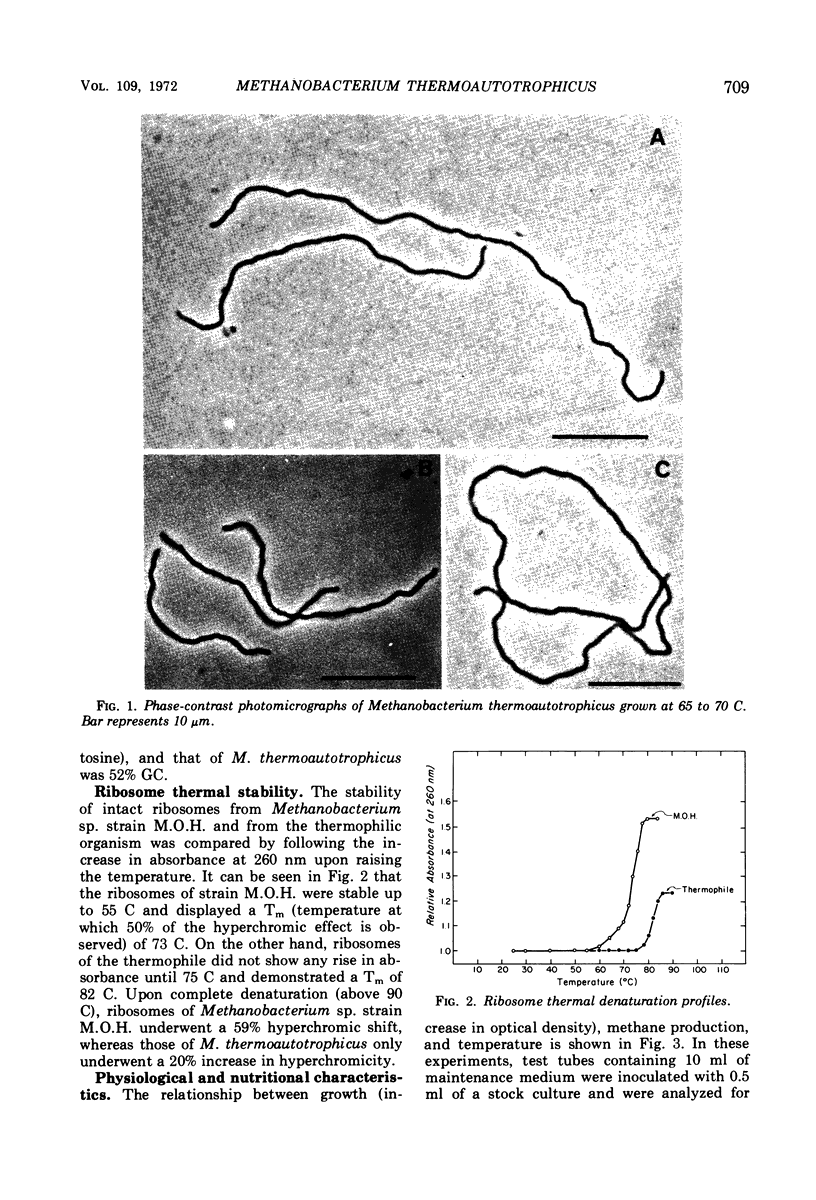
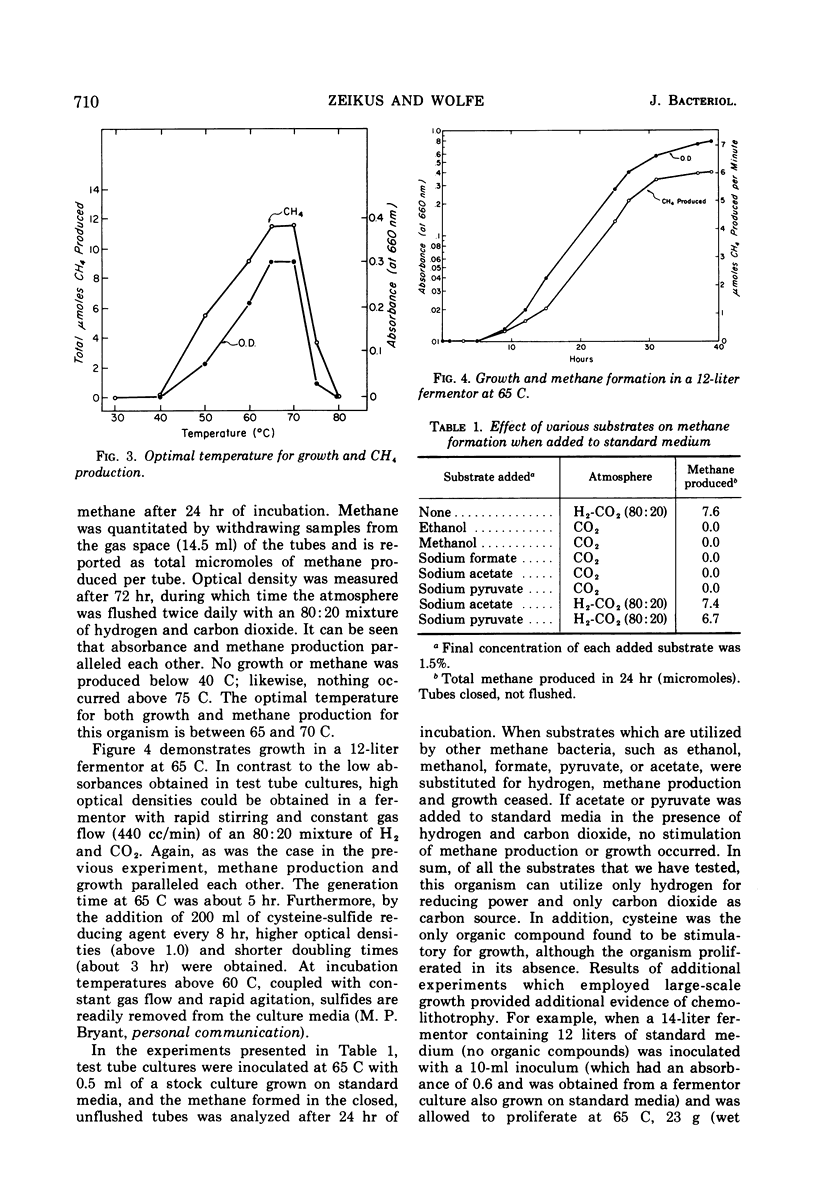
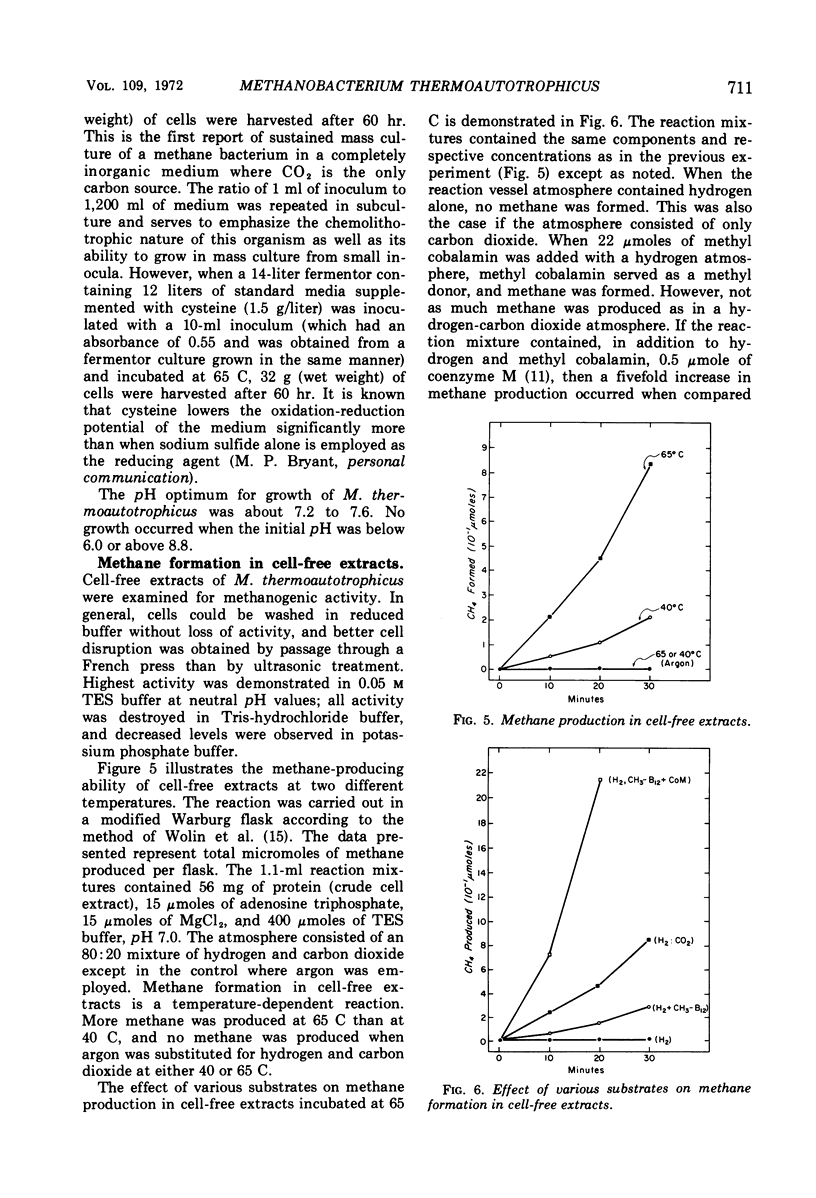
The isolation of a new methanogenic bacterium, Methanobacterium thermoautotrophicus sp. n., is described. Successful isolation required a medium containing inorganic salts, an atmosphere consisting of an 80:20 mixture of hydrogen-carbon dioxide, and incubation temperatures of 65 to 70 C. Isolates of M. thermoautotrophicus were gram-positive, nonmotile, irregularly curved rods which frequently formed long filaments. The organism was found to be an autotroph and a strict anaerobe, and to have a pH optimum of 7.2 to 7.6. The optimal temperature for growth was 65 to 70 C, the maximum being 75 C and the minimum about 40 C. The generation time at the optimum was about 5 hr. The deoxyribonucleic acid of M. thermoautotrophicus had a guanine plus cytosine (GC) content of 52 moles per cent, whereas Methanobacterium sp. strain M.O.H. had a GC content of 38%. When heated, intact ribosomes of Methanobacterium sp. strain M.O.H. were stable up to 55 C and had a Tm of 73 C. In contrast, ribosomes of M. thermoautotrophicus were stable up to 75 C and had a Tm of 82 C. Upon complete thermal denaturation, ribosomes of strain M.O.H. underwent a 59% hyperchromic shift, whereas those of the thermophile showed only a 20% increase in hyperchromicity. Methane formation in cell-free extracts of M. thermoautotrophicus was temperature-dependent and required hydrogen and carbon dioxide; methyl cobalamin served as a methyl donor, and addition of coenzyme M stimulated methanogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bott T. L., Brock T. D. Bacterial growth rates above 90 degrees C in Yellowstone hot springs. Science. 1969 Jun 20;164(3886):1411–1412. doi: 10.1126/science.164.3886.1411. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen W. Growth conditions and temperature-dependent substrate specificity of two extremely thermophilic bacteria. Arch Mikrobiol. 1971;76(1):2–17. doi: 10.1007/BF00409310. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McBEE R. H. The anaerobic thermophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):51–63. doi: 10.1128/br.14.1.51-63.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Pace B., Campbell L. L. Correlation of maximal growth temperature and ribosome heat stability. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1110–1116. doi: 10.1073/pnas.57.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Zeikus J. G., Brock T. D. Protein synthesis at high temperatures: aminoacylation of tRNA. Biochim Biophys Acta. 1971 Feb 11;228(3):736–745. doi: 10.1016/0005-2787(71)90739-8. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Taylor M. W., Brock T. D. Thermal stability of ribosomes and RNA from Thermus aquaticus. Biochim Biophys Acta. 1970 Apr 15;204(2):512–520. doi: 10.1016/0005-2787(70)90171-1. [DOI] [PubMed] [Google Scholar]