Abstract
A proportion of classical Hodgkin lymphoma (HL) is believed to be causally related to infection with the ubiquitous lymphotropic EBV. The determining factors for development of EBV-related HL remain poorly understood, but likely involve immunological control of the viral infection. Accordingly, markers of the HLA class I region have been associated with risk of EBV-related HL. To study the host genetic component of EBV-related HL further, we investigated the lymphoma's association with HLA-A*01 and HLA-A*02 simultaneously in the setting of infectious mononucleosis (IM), a risk factor for EBV-related HL, in a case-series analysis including 278 EBV-related and 656 EBV-unrelated cases of HL. By logistic regression, HLA-A*01 alleles [odds ratio (OR) per allele, 2.15; 95% CI, 1.60–2.88] were associated with increased and HLA-A*02 alleles (OR per allele, 0.70; 95% CI, 0.51–0.97) with decreased risk of EBV-related HL. These allele-specific associations corresponded to nearly 10-fold variation in risk of EBV-related HL between HLA-A*01 and HLA-A*02 homozygotes. History of IM was also associated with risk of EBV-related HL (OR, 3.40; 95% CI, 1.74–6.66). The association between history of IM and EBV-related HL was not seen in the presence of HLA-A*02 because this allele appeared to neutralize the effect of IM on EBV-related HL risk. Our findings suggest that HLA class I-restricted EBV-specific cytotoxic T-cell responses and events in the early immune response to EBV infection in IM play critical roles in the pathogenesis of EBV-related HL.
Keywords: case series, epidemiology
There is compelling evidence that 30% to 40% of all cases of classical Hodgkin lymphoma (HL) are causally associated with EBV (1). EBV-related HL cases are distinguished from the group of EBV-unrelated HL cases by the presence of the virus in the malignant Hodgkin/Reed-Sternberg cells. EBV is an extremely efficient transforming agent and growth-transforming infection in vitro is associated with expression of eight EBV latent antigens, including six EBV nuclear antigens (EBNAs) and two latent membrane proteins (LMPs) (2). A restricted group of EBV latent antigens, comprising EBNA1, LMP1, and LMP2, is expressed by Hodgkin/Reed-Sternberg cells, and these have a plausible function in disease pathogenesis (3–6).
More than 90% of the world's adult population is infected with EBV (7). Following primary infection, which is usually clinically silent, the virus establishes a reservoir in memory B cells (8). Infected memory B cells escape immune detection by down-regulation of viral antigens (8). Activation of replicative (i.e., lytic) infection, and outgrowth of latently infected cells, is kept under tight control by HLA-restricted, cytotoxic T lymphocytes (CTLs) (8, 9). A host:virus equilibrium is established and the number of EBV-infected B cells within an individual appears stable over time (10). In 20% to 40% of persons who experience EBV infection after childhood, primary infection manifests as infectious mononucleosis (IM) (11). IM is associated with a striking expansion of EBV-specific CTLs, most of which are reactive with epitopes from lytic cycle antigens (8, 9, 12). Following the acute disease, there is a rapid culling of these T cells and the convalescent and memory T-cell pool contain a relatively higher proportion of CTLs reactive with latent antigens (8, 12). There are therefore qualitative as well as quantitative differences between the CTL response in acute and persistent infection (8, 12). Responses to latent antigens demonstrate a hierarchy of immunodominance with responses to the EBNA3 family of proteins dominating responses to other proteins (8, 9). The latent antigens expressed by Hodgkin/Reed-Sternberg cells elicit only subdominant or weak CTL responses (8, 9).
Although HL risk is not normally associated with overt immune deficiency, immune suppression as seen in AIDS or organ transplantation is associated with an increased risk of EBV-related HL, whereas risk of EBV-unrelated HL does not appear to be increased (13). Evidence suggesting that genetically determined variation in the cell-mediated immune response to EBV infection also influences the risk of EBV-related HL is accumulating. Markers in the HLA class I locus, including the SNPs rs2530388 and rs6457110, were recently found to be associated with risk of EBV-related HL (14, 15). These SNPs are in strong linkage disequilibrium with HLA-A alleles, and it was subsequently shown that HLA-A*01 was associated with an increased and HLA-A*02 with a decreased risk of EBV-related HL (16). The independence of these two associations was not assessed and, theoretically, both could reflect the same association, i.e., the increased risk associated with HLA-A*01 could simply result from lack of HLA-A*02. In addition, HLA typing at the four digit level, which is required to define specific alleles or subtypes (http://hla.alleles.org/nomenclature/naming.html), was not reported (16). This is important because HLA-A*02 subtypes bind and present different epitopes (9, 17); therefore, associations with EBV-related HL could vary between these subtypes.
Both self-reported and laboratory-confirmed prior IM have been associated with an increased risk of EBV-related HL (18–21). The risk increase follows an incubation period–like distribution and is of a transient nature (20, 21), suggesting that events related to control of primary EBV infection have an important impact on disease risk. However, propensity to develop IM has been associated with the HLA class I polymorphisms that were originally linked with EBV-related HL, thus raising the possibility that the association between EBV-related HL and prior IM simply reflects shared genetic susceptibility (22).
Understanding the association and interaction between risk factors for EBV-related HL could have significant implications for both disease prevention and treatment. We therefore explored the effect of HLA-A alleles on risk of EBV-related HL in the context of other risk factors including history of IM. As there is no evidence for an association between EBV-unrelated HL and both prior IM (18–21, 23, 24) and HLA genotype (14, 16), our investigation focused on the comparison of 934 patients with either EBV-related or EBV-unrelated HL.
Results
Case Distribution by HL EBV Status, Sex, and Age.
The analysis included 934 patients with HL, of whom 278 (30%) had EBV-related HL (Table 1). The proportion of EBV-related HL cases was higher among men (n = 185; 37%) than women (n = 93; 21%), and this difference was statistically significant [odds ratio (OR) for male sex, 2.18; 95% CI, 1.63–2.92; Table 1]. The proportion of EBV-related HL increased with age from 23% in the age group of 15 to 34 y to 28% in the age group of 35 to 49 y and 43% in the age group of 50 to 80 y (Table 1). Differences between the oldest and the youngest age groups were statistically significant (OR for age ≥50 y, 2.51; 95% CI, 1.81–3.47; Table 1). These trends were all observed within the individual data sets, as shown in the breakdown of patient characteristics by study in Table S1.
Table 1.
Cases analyzed by EBV status, sex, age, and history of IM
| HL cases | ||||
| Group | All | EBV-related (%) | EBV-unrelated (%) | OR (95% CI) |
| All cases | 934 | 278 (30) | 656 (70) | – |
| Sex | ||||
| Female | 436 | 93 (21) | 343 (79) | 1.00 (reference) |
| Male | 498 | 185 (37) | 313 (63) | 2.18 (1.63–2.92)* |
| Age, y | ||||
| 15–34 | 484 | 113 (23) | 371 (77) | 1.00 (reference) |
| 35–49 | 196 | 55 (28) | 141 (72) | 1.28 (0.88–1.87) |
| ≥50 | 254 | 110 (43) | 144 (57) | 2.51 (1.81–3.47)* |
| IM | ||||
| No | 634 | 173 (27) | 461 (73) | 1.00 (reference) |
| Yes | 87 | 35 (40) | 52 (60) | 1.79 (1.13–2.85)* |
| Not available | 213 | 70 (33) | 143 (67) | – |
Odds ratio for EBV-related versus EBV-unrelated HL with 95% CI by sex, age group, and self-reported history of IM.
*Significant results.
HLA-A*01 and HLA-A*02 and Risk of EBV-Related HL.
The main focus of this analysis was a comparison of EBV-related and EBV-unrelated HL cases. To confirm that the EBV-unrelated HL cases were an appropriate control group, we first compared the prevalence of HLA-A*01 and HLA-A*02 in this group of cases with the corresponding prevalence in the background populations of the countries studied in a series of tests (25–27). These showed similar distributions of the HLA alleles in the patients and the background populations, as tests for homogeneity of all genotypes combined (P = 0.22), allele frequencies (HLA-A*01, P = 0.32; HLA-A*02, P = 0.84) and HLA-A*02 genotypes (P = 0.88) were all unremarkable. The test for homogeneity of HLA-A*01 genotypes was formally statistically significant (P = 0.02), primarily reflecting a small deficit of HLA-A*01 heterozygotes among the EBV-unrelated HL cases (Table S2).
Because our main interest was in HLA-A*01 and A*02 alleles, we combined all other alleles into one group, HLA-A*xx, and analyzed HLA-A genotype in six groups (01/01, 01/xx, 01/02, xx/xx, 02/xx, 02/02). In crude analyses, HLA-A genotypes 01/01, 01/02, and 01/xx, and the rs2530388 SNP (genotypes A/A and A/T), were associated with increased risk of EBV-related HL whereas HLA-A genotype 02/02 and the rs6457110 SNP (genotypes A/A and A/T) were associated with decreased risk (Table 2). All differences were statistically significant (Table 2). When HLA-A and SNP genotypes were analyzed together, i.e., in a mutually adjusted analysis, HLA-A genotypes 01/01 and 01/xx retained their association with increased risk and HLA-A genotypes 02/02 and 02/xx were associated with decreased risk of EBV-related HL (Table 2). In contrast, the two SNPs were no longer associated with risk of EBV-related HL (P = 0.34; Table 2). This suggests that the significant associations between the SNPs and EBV-related HL risk observed in the crude analyses were a result of linkage disequilibrium with HLA-A. Further statistical analyses showed that the associations between EBV-related HL and HLA-A*01 and HLA-A*02 were independent of each other and also that they followed dose–response associations, i.e., could be modeled as linear trends in the number of alleles.
Table 2.
Crude and mutually adjusted case-series ORs by HLA-A genotype
| Number of HLs | OR (95% CI) | |||
| Genotype | EBV-related | EBV-unrelated | Crude | Adjusted |
| HLA-A | ||||
| 01/01 | 40 | 27 | 4.05 (2.29–7.17)* | 3.47 (1.65–7.29)* |
| 01/02 | 40 | 63 | 1.74 (1.06–2.85)* | 1.55 (0.84–2.86) |
| 01/xx | 74 | 107 | 1.89 (1.24–2.87)* | 2.02 (1.25–3.28)* |
| 02/02 | 7 | 67 | 0.29 (0.12–0.66)* | 0.21 (0.07–0.59)* |
| 02/xx | 56 | 226 | 0.68 (0.45–1.03) | 0.55 (0.33–0.90)* |
| xx/xx | 60 | 164 | 1.00 (reference) | 1.00 (reference) |
| rs2530388 | ||||
| A/A | 72 | 73 | 3.60 (2.40–5.42)* | 1.29 (0.67–2.48) |
| A/T | 123 | 278 | 1.62 (1.17–2.24)* | 0.84 (0.54–1.32) |
| T/T | 81 | 296 | 1.00 (reference) | 1.00 (reference) |
| rs6457110 | ||||
| A/A | 19 | 97 | 0.34 (0.20–0.58)* | 1.57 (0.70–3.53) |
| A/T | 122 | 321 | 0.65 (0.49–0.88)* | 1.43 (0.90–2.28) |
| T/T | 136 | 234 | 1.00 (reference) | 1.00 (reference) |
*Significant results.
As epitopes presented by different HLA-A*02 subtypes may evoke different immunological responses, we performed exploratory analyses using four-digit HLA-A typing results. All the HLA-A*01 alleles included in this study were HLA-A*0101. The majority of HLA-A*02–positive patients possessed the HLA-A*0201 allele; however, 15 individuals were heterozygous for A*0205 and three were heterozygous for A*0206. Exclusion of these individuals from the analysis had little effect, indicating that HLA-A*0201 is driving the protective effect (Table S3).
Self-Reported IM and Risk of EBV-Related HL.
Self-reported information about IM was available for 721 patients. EBV-related HL was more common in those who reported a history of IM (n = 35; 40%) than in those who did not (n = 173; 27%), and differences by prior IM were statistically significant (OR, 1.79; 95% CI, 1.13–2.85; Table 1). The breakdown of cases by HLA-A genotype, EBV status, and history of IM is shown in Table 3. The positive association between EBV-related HL and history of IM was present in persons who lacked both HLA-A*01 and HLA-A*02 (OR, 2.82; 95% CI, 1.15–6.90; Table 3). Furthermore, among cases with EBV-unrelated HL, we found no evidence for an association between history of IM and HLA-A*01 or HLA-A*02, whether analyzed as phenotype or as number of alleles (OR per HLA-A*01 allele, 0.88; 95% CI, 0.48–1.59; OR per HLA-A*02 allele, 1.04; 95% CI, 0.66–1.63; Table 3). These results indicate that the association between history of IM and EBV-related HL is not simply explained by an association between both diseases and HLA-A*01 and HLA-A*02 alleles.
Table 3.
HLA-A genotype by HL EBV status and self-reported history of IM
| EBV-related | EBV-unrelated | |||
| HLA-A genotype | No prior IM | Prior IM | No prior IM | Prior IM |
| 01/01 | 22 | 4 | 19 | – |
| 01/02 | 26 | 6 | 42 | 6 |
| 01/xx | 41 | 11 | 65 | 8 |
| 02/02 | 6 | – | 54 | 5 |
| 02/xx | 38 | 3 | 161 | 20 |
| xx/xx | 39 | 11 | 120 | 12 |
Interaction Between Risk Factors for EBV-Related HL.
As a next step in the development of a unifying statistical model of risk factors for EBV-related HL, we examined whether the association between EBV-related HL and HLA-A*01 and/or HLA-A*02 varied by sex, age, and history of IM. These analyses showed that the effect on EBV-related HL risk per HLA-A*01 allele was similar in men and women (OR for men, 2.25; 95% CI, 1.54–3.30; OR for women, 1.93; 95% CI, 1.26–2.96; P = 0.59), across age groups (OR for age 15–34 y, 2.19; 95% CI, 1.46–3.28; OR for age 35–49 y, 2.24; 95% CI, 1.19–4.22; OR for age 50–80 y, 1.90; 95% CI, 1.16–3.11; P = 0.88), and in persons with and without a history of IM (OR with IM, 2.96; 95% CI, 1.25–6.99; OR without IM, 2.01; 95% CI, 1.48–2.74; P = 0.40). Similarly, the effect on EBV-related HL risk per HLA-A*02 allele was similar in men and women (OR for men, 0.65; 95% CI, 0.45–0.94; OR for women, 0.59; 95% CI, 0.35–0.97; P = 0.75) and across age groups (OR for age 15–34 y, 0.57; 95% CI, 0.37–0.89; OR for age 35–49 y, 0.80; 95% CI, 0.41–1.56; OR for age 50–80 y, 0.61; 95% CI, 0.37–1.01; P = 0.71). In contrast, the effect on EBV-related HL risk per HLA-A*02 allele differed between persons with and without a history of IM (OR with IM, 0.27; 95% CI, 0.10–0.69; OR without IM, 0.70; 95% CI, 0.51–0.97; P = 0.05). In other words, HLA-A*02 modified the association between history of IM and EBV-related HL.
Statistical Modeling of Risk of EBV-Related HL Versus Risk of EBV-Unrelated HL.
The final unifying statistical model of risk factors for EBV-related HL included the main effects: sex, age, country, history of IM, number of HLA-A*01 alleles, number of HLA-A*02 alleles, and the interaction between IM history and HLA-A*02. In this model, sex (OR for male vs. female, 2.12; 95% CI, 1.48–3.04) and age (OR for age ≥50 vs. 15–34 y, 2.60; 95% CI, 1.72–3.93) retained their independent associations with EBV-related HL, as did history of IM (OR, 3.40; 95% CI, 1.74–6.66; Fig. 1). The OR for EBV-related HL conveyed by each additional HLA-A*01 allele was 2.15 (95% CI, 1.60–2.88) whereas the OR for each HLA-A*02 allele was 0.70 (95% CI, 0.51–0.97), i.e., essentially identical to the unadjusted estimates (Table 2 and Fig. 1). When distributed across genotypes, the associations translated into an almost 10-fold variation in odds of EBV-related HL between HLA-A*01 and HLA-A*02 homozygotes with no history of IM (OR, 9.45; 95% CI, 4.60–19.4). The OR for the interaction between history of IM and HLA-A*02 allele count was 0.38 (95% CI, 0.14–1.04; P = 0.05) in the fully adjusted model.
Fig. 1.
Case-series ORs with 95% CIs for EBV-related HL by non-HLA and HLA risk factors. IM, self-reported history of IM.
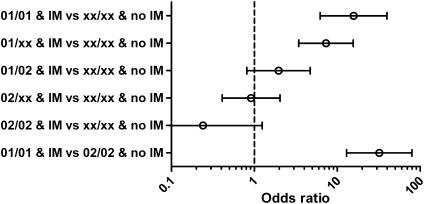
The combined effects of HLA-A alleles and history of IM on odds of EBV-related HL are illustrated in Fig. 2. The interaction between history of IM and HLA-A*02 implies that the positive association between history of IM and EBV-related HL is essentially abrogated in HLA-A*02 carriers [OR, 0.91 (95% CI, 0.41–2.04) for HLA-A*02/xx persons with a history of IM vs. HLA-A*xx/xx persons without a history of IM; Fig. 2]. The range of risks suggested by our findings is illustrated by HLA-A*01 homozygotes with a history of IM being at a 32-fold (95% CI, 13–80) higher odds of EBV-related HL than HLA-A*02 homozygotes without a history of IM (Fig. 2); although the 95% CI is wide, even the lower estimate suggests a very large variation in risk by HLA-A genotype combined with history of IM.
Fig. 2.
Case-series ORs with 95% CIs for EBV-related HL by selected combinations of IM and HLA-A genotype. IM, self-reported history of IM.
Discussion
In this study we show that HLA-A*01 and HLA-A*02 contribute independently to the risk of EBV-related HL in a dose-dependent fashion. This extends previous analyses reporting an association between HLA-A*01 and HLA-A*02 and EBV-related HL (16). Following adjustment for the effects of HLA-A alleles, associations with two SNPs in the HLA class I region (rs2530388 and rs6457110), which were previously shown to be associated with EBV-related HL (15), are no longer present; thus providing further evidence that HLA-A is the biologically important locus. Each HLA-A*01 allele was associated with a twofold increased odds of EBV-related HL, whereas each HLA-A*02 allele was associated with a 30% reduced odds of EBV-related HL. As a result of these independent associations, HLA-A*01 homozygous patients were nearly 10 times more likely to have EBV-related HL than patients who were HLA-A*02 homozygous. To our knowledge, this is the strongest association ever reported between HLA class I and a virus-associated malignancy.
The observed associations with HLA-A alleles suggest that EBV-specific CTL responses, restricted through HLA class I, play a key role in development of EBV-related HL. At least two different mechanisms are conceivable. EBV-specific CTL responses could be important for the control or early elimination of Hodgkin/Reed-Sternberg cells or their precursors. In this scenario, we would envisage a protective response directed against EBV antigens expressed by Hodgkin/Reed-Sternberg cells, i.e., EBNA1, LMP1, and LMP2. Hodgkin/Reed-Sternberg cells express HLA class I and TAP1/2 and therefore should be able to process antigen for presentation to CTLs (28, 29); however, EBV-specific CTLs do not appear to accumulate or expand within tumors (30). Secretion of immunomodulatory cytokines, such as interleukin 10 and transforming growth factor β, and skewing of the infiltrating T cells toward T-helper 2/T-regulatory subsets by CCL17 and galectin 1 could all contribute to a local immunosuppressive effect within tumors (31–33). CTL responses to subdominant EBV antigens may therefore have an insignificant effect. An alternative possibility is that the protective CTL response is directed at B cells undergoing occasional lytic or growth-transforming latent infection, and that this response limits the viral load within the infected individual.
HLA-A*02, and HLA-A*0201 in particular, is known to present peptides from a wide range of EBV lytic and latent antigens, including LMP2 and LMP1 (9, 17). The majority of the HLA-A*02 alleles analyzed in this study were HLA-A*0201 and exclusion of non–HLA-A*0201 alleles had little effect on the overall results (Table S3); therefore, the main effects of HLA-A*02 were driven by HLA-A*0201. A protective, HLA-A*0201–restricted CTL response is biologically plausible but available data on HLA-A*0201–restricted epitopes do not allow us to predict whether this is directed against Hodgkin/Reed-Sternberg cells or B cells. In contrast, there are no confirmed HLA-A*01–restricted EBV epitopes (17, 34), and a recent study failed to demonstrate proliferative CTL responses to EBV antigens presented by HLA-A*01–positive stimulator cells (35). This could help to explain the increased risk associated with HLA-A*01; however, HLA-A*01 is in linkage disequilibrium with HLA-B*08 and immunodominant HLA-B*08–restricted EBV-specific CTL responses against lytic antigens are well documented (36). An HLA-B*08–restricted EBNA1 epitope has also been described (37). The biological basis for the increased risk associated with HLA-A*01 is therefore unclear and requires further investigation.
To better understand the role of HLA in the natural history of EBV-related HL, we investigated HLA-A alleles in the context of other risk factors. Older age and male sex were independently associated with an increased risk of EBV-related HL and significant interactions between these risk factors and HLA-A were not detected, i.e., the effect of HLA-A alleles was similar in older and younger adults, and in male and female subjects. The relationship between prior IM and HLA-A was investigated for two reasons: first, to determine whether this association simply results from shared genetic susceptibility; and second, to determine, albeit indirectly, whether the HLA class I–restricted CTL response during IM has an impact on subsequent risk of EBV-related HL. We found little support for the idea of shared genetic susceptibility. Accordingly, history of IM was associated with a greater than twofold risk of EBV-related HL in patients who were both HLA-A*01– and HLA-A*02–negative, and the association remained in adjusted analyses. Furthermore, we did not detect an association between either HLA-A*01 or HLA-A*02 and self-reported history of IM in patients with EBV-negative HL. In contrast, our results provide some support for the idea that the CTL response during IM has an influence on risk of EBV-related HL, as described here later.
Although prior IM was associated with risk of EBV-related HL independently of HLA-A*01 and HLA-A*02, we also observed a statistically significant interaction between HLA-A*02 and history of IM. The effect of this was to effectively abrogate the increased risk of EBV-related HL in individuals who were HLA-A*02–positive, i.e., persons with the HLA-A*02 phenotype were not at increased risk of EBV-related HL following IM. These results, taken together with our previous findings (20, 21), are consistent with the idea that a short-lived event occurring as a consequence of IM results in an increased risk of developing EBV-related HL, and that this “event” can be modified by HLA-A*02–restricted responses. Acute IM is associated with high numbers of EBV-infected B cells, which gradually decrease over time (38). Patients with EBV-related HL have been shown to have a higher frequency of circulating EBV-infected B cells than patients with EBV-unrelated HL at diagnosis (39). We, therefore, propose a disease model in which the number of EBV-infected B cells is a critical determinant of risk of EBV-related HL. We speculate that the rate of decrease of EBV-infected B cells following IM is modulated by HLA class I–restricted EBV-specific CTL responses, e.g., HLA-A*0201–restricted responses. By regulating the dynamics of EBV infection following IM, the CTL response modifies disease risk. This model suggests that events occurring early after primary infection have an important impact on subsequent risk of EBV-related HL, and predicts that HLA-restricted lytic responses will be more important than latent responses. We cannot, however, exclude the possibility that IM results in the generation of a population of Hodgkin/Reed-Sternberg cell precursors that, like Hodgkin/Reed-Sternberg cells, express the viral proteins EBNA1, LMP1, and LMP2, and are the target of the protective CTL response.
Our study has a number of strengths and weaknesses. We included more than 900 cases of HLA- and EBV-typed HL, rendering it the largest and statistically most robust epidemiological study of HL to date as far as we are aware. Because information on selected risk factors was available for more than 700 cases, it was possible to conduct gene–environment interaction analyses with reasonable statistical power. Our primary design was that of case–case comparisons, which rested on presumed etiological heterogeneity between EBV-related and EBV-unrelated HL. This approach was supported by analyses showing that the distributions of age, sex, HLA-A*01, HLA-A*02, and history of IM clearly differed between the two groups of patients with HL. Indeed, from the perspective of etiological heterogeneity, the differences observed between EBV-related and EBV-unrelated HL may in fact constitute stronger evidence than respective comparisons with the general population (40). Specifically, case-only comparisons such as the present are likely to be less affected by potential selection or participation bias arising from the recruitment of controls without HL, which is of concern in case-control studies; for instance, a case-only comparison of the effect of self-reported prior IM is less likely to suffer from recruitment or recall bias than a case:control analysis. Importantly, in the absence of associations between a history of IM and HLA-A alleles and EBV-unrelated HL, the observed case-series odds ratios may be taken as valid estimates of the ORs of EBV-related HL relative to the general population (40).
In conclusion, we report compelling evidence that HLA-A*01 and HLA-A*02 are associated with strikingly increased and decreased risks of EBV-related HL, respectively. We found no evidence of a strong association between HLA-A*01 or HLA-A*02 and history of IM, and prior IM was independently associated with an increased risk of EBV-related HL. The observed interaction between prior IM and HLA-A*02 suggests that events occurring during or following acute IM have an important impact on subsequent risk of EBV-related HL. The data provide further evidence that EBV-related and EBV-unrelated HL have different natural histories and suggest that the CTL-mediated control of EBV is critical in the development of EBV-related HL.
Materials and Methods
Study Populations.
Patients with HL were identified from three case-control investigations and a local case series. The case-control studies were the Scandinavian Lymphoma Etiology Study (SCALE) (21, 41), the Scotland and Newcastle Epidemiological Study of Hodgkin Disease (SNEHD) (19, 42), and the Young adult Hodgkin Case-Control Study (YHCCS) (18). In brief, the SCALE study was carried out in Denmark and Sweden between 1999 and 2002 and included 586 patients with classical HL (participation rate, 91%) (21, 41). SNEHD, carried out in Scotland and the Northern Region of England between 1993 and 1997, included 408 patients with incident classical HL (participation rate, 78%) (19, 42). Finally, the YHCCS investigation recruited a total of 118 newly diagnosed HL patients aged 16 to 24 y (participation rate, 90%) in parts of Yorkshire, Cumbria, and Lancashire in the United Kingdom between 1991 and 1995 (18). In each of the studies, participation involved structured telephone (i.e., SCALE) or face-to-face (i.e., SNEHD and YHCCS) interviews and blood sampling. Tumor specimens were retrieved for diagnostic validation and EBV typing, which was accomplished for 958 cases [499 cases in SCALE (85% of participants), 356 cases in SNEHD (87% of participants), and 103 cases in YHCCS (87% of participants)]. Germline DNA was available from 720 of the EBV-typed cases [430 (86%) of SCALE cases, 283 (80%) of SNEHD cases, and 41 (40%) of YHCCS cases; Table S1]. The “local case series” was a prospectively collected series of 210 patients from Scotland and the north of England who had HL of known EBV status and from whom germline DNA was available. History of IM was not available for these cases (Table S1). HLA-A*02 phenotype and rs2530388 and rs6457110 genotypes were previously reported for some of the United Kingdom data (15, 16, 43). All contributing studies were approved by regional scientific ethics committees and data protection agencies, and all participants provided informed consent.
Genetic Analyses—HLA Typing and SNP Analyses.
HLA-A typing (medium-level resolution) was performed by PCR sequence specific oligonucleotide assay using Luminex xMAP technology and commercial kits (LABType SSO; One Lambda). HLA-A results were available at the four-digit level, but statistical analysis largely involved comparisons at the two-digit level.
Two SNPs located in the HLA class I region (rs2530388 and rs6457110) were analyzed in all patients with HL as previously described (15, 22). These particular SNPs were chosen from a group of HLA class I polymorphisms previously shown to be associated with EBV-related HL because they are amenable to analysis using TaqMan allelic discrimination assays (Applied Biosystems) (15).
Statistical Analyses.
All associations were estimated as ORs using logistic regression analyses with 95% CIs based on Wald tests. P values were based on likelihood-ratio tests. Three combinations of outcomes and data sets were examined: (i) the risk of EBV-related HL among HL cases (i.e., case-series analysis) (40); (ii) the risk of EBV-unrelated HL by HLA-A genotype relative to the general population; and (iii) the occurrence of history of IM by genotype among cases with EBV-unrelated HL. Because our a priori focus was on HLA-A*01 and HLA-A*02, all other HLA-A alleles were combined into one group (HLA-A*xx) (16). The representation of HLA-related genetic information was determined through model selection in the case series starting from a model including only main effects of HLA-A genotype (01/01, 01/xx, 01/02, xx/xx, 02/xx, 02/02), rs2530388 (T/T, T/A, A/A), rs6457110 (T/T, T/A, A/A), and sex, age group (15–34, 35–49, and ≥50 y), and country. The model selection criterion was the corrected Akaike information criterion with the number of EBV-related cases as n (44). The analyses included testing of whether the genetic effects should be treated categorically or as linear effects (i.e., trends) of the number of alleles on the logit scale.
The final model for the case series analysis was arrived at after including self-reported history of IM (yes, no) and examining whether non-HLA risk factors modified the main effects of the studied genetic markers. Specifically, we considered interactions with country, age groups, sex, and history of IM. To this end we tested whether the effects of each of the non-HLA factors should be further modified by age group, sex, and country. Potential modification of non-HLA effects by HLA markers was sequentially evaluated in models including both main effects of risk factors (HLA and non-HLA) and their interaction terms for each combination of HLA and non-HLA factors.
The risk of EBV-unrelated HL relative to the general population was assessed for the HLA-A allele frequencies and genotypes by comparison with the expected frequencies in the general population assuming independent allele mixing. Estimates of population genotype frequencies were obtained from previous reports (25–27). We used χ2 tests to assess homogeneity in the distribution of HLA-A genotypes.
Supplementary Material
Acknowledgments
We thank Katrina Farrell, Karen McAulay and Maggie Conacher for critical reading of the manuscript. This work was supported by Leukaemia Research Fund Grants 08031 and 05045, Danish Cancer Research Foundation Grant 41-08, Lundbeck Foundation Grant R19-A2364, and Danish Cancer Society Grant DP 08-155; and the Kay Kendall Leukaemia Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915054107/DCSupplemental.
References
- 1.Mueller N, Grufferman S. Hodgkin lymphoma. In: Schottenfeld D, Fraumeni J Jr, editors. Cancer Epidemiology and Prevention. Oxford: Oxford Univ Press; 2006. pp. 872–897. [Google Scholar]
- 2.Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2511–2573. [Google Scholar]
- 3.Wu TC, et al. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin's disease. Int J Cancer. 1990;46:801–804. doi: 10.1002/ijc.2910460509. [DOI] [PubMed] [Google Scholar]
- 4.Pallesen G, Hamilton-Dutoit SJ, Rowe M, Young LS. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991;337:320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- 5.Grässer FA, et al. Monoclonal antibodies directed against the Epstein-Barr virus-encoded nuclear antigen 1 (EBNA1): Immunohistologic detection of EBNA1 in the malignant cells of Hodgkin's disease. Blood. 1994;84:3792–3798. [PubMed] [Google Scholar]
- 6.Küppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 7.Ambinder RF, Cesarman E. Clinical and pathological aspects of EBV and KSHV infection. In: Arvin A, et al., editors. Human Herpesviruses—Biology, Therapy and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. pp. 885–902. [PubMed] [Google Scholar]
- 8.Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2655–2700. [Google Scholar]
- 9.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Khan G, Miyashita EM, Yang B, Babcock GJ, Thorley-Lawson DA. Is EBV persistence in vivo a model for B cell homeostasis? Immunity. 1996;5:173–179. doi: 10.1016/s1074-7613(00)80493-8. [DOI] [PubMed] [Google Scholar]
- 11.Crawford DH, et al. A cohort study among university students: Identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis. 2006;43:276–282. doi: 10.1086/505400. [DOI] [PubMed] [Google Scholar]
- 12.Callan MF. The immune response to Epstein-Barr virus. Microbes Infect. 2004;6:937–945. doi: 10.1016/j.micinf.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin's lymphoma. Br J Haematol. 2004;125:267–281. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- 14.Diepstra A, et al. Association with HLA class I in Epstein-Barr-virus-positive and with HLA class III in Epstein-Barr-virus-negative Hodgkin's lymphoma. Lancet. 2005;365:2216–2224. doi: 10.1016/S0140-6736(05)66780-3. [DOI] [PubMed] [Google Scholar]
- 15.Niens M, et al. The human leukocyte antigen class I region is associated with EBV-positive Hodgkin's lymphoma: HLA-A and HLA complex group 9 are putative candidate genes. Cancer Epidemiol Biomarkers Prev. 2006;15:2280–2284. doi: 10.1158/1055-9965.EPI-06-0476. [DOI] [PubMed] [Google Scholar]
- 16.Niens M, et al. HLA-A*02 is associated with a reduced risk and HLA-A*01 with an increased risk of developing EBV+ Hodgkin lymphoma. Blood. 2007;110:3310–3315. doi: 10.1182/blood-2007-05-086934. [DOI] [PubMed] [Google Scholar]
- 17.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: Lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 18.Alexander FE, et al. Risk factors for Hodgkin's disease by Epstein-Barr virus (EBV) status: Prior infection by EBV and other agents. Br J Cancer. 2000;82:1117–1121. doi: 10.1054/bjoc.1999.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander FE, et al. An epidemiologic study of index and family infectious mononucleosis and adult Hodgkin's disease (HD): Evidence for a specific association with EBV+ve HD in young adults. Int J Cancer. 2003;107:298–302. doi: 10.1002/ijc.11156. [DOI] [PubMed] [Google Scholar]
- 20.Hjalgrim H, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 21.Hjalgrim H, et al. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–2388. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 22.McAulay KA, et al. HLA class I polymorphisms are associated with development of infectious mononucleosis upon primary EBV infection. J Clin Invest. 2007;117:3042–3048. doi: 10.1172/JCI32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang ET, et al. Childhood social environment and Hodgkin's lymphoma: New findings from a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1361–1370. [PubMed] [Google Scholar]
- 24.Glaser SL, et al. Exposure to childhood infections and risk of Epstein-Barr virus-defined Hodgkin's lymphoma in women. Int J Cancer. 2005;115:599–605. doi: 10.1002/ijc.20787. [DOI] [PubMed] [Google Scholar]
- 25.Brynedal B, et al. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS One. 2007;2:e664. doi: 10.1371/journal.pone.0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darke C, et al. HLA class I (A, B) and II (DR, DQ) gene and haplotype frequencies in blood donors from Wales. Exp Clin Immunogenet. 1998;15:69–83. doi: 10.1159/000019057. [DOI] [PubMed] [Google Scholar]
- 27.Svejgaard A. Vaevstyper (Tissue types) In: Thaysen JH, Lorenzen I, Christensen LK, editors. Medicinsk Kompendium. Copenhagen: Nyt Nordisk Forlag Arnold Busck; 1986. pp. 44–67. (in Danish) [Google Scholar]
- 28.Murray PG, Constandinou CM, Crocker J, Young LS, Ambinder RF. Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein-Barr virus-positive Hodgkin's disease. Blood. 1998;92:2477–2483. [PubMed] [Google Scholar]
- 29.Diepstra A, et al. HLA-G protein expression as a potential immune escape mechanism in classical Hodgkin's lymphoma. Tissue Antigens. 2008;71:219–226. doi: 10.1111/j.1399-0039.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- 30.Chapman AL, et al. Epstein-Barr virus-specific cytotoxic T lymphocyte responses in the blood and tumor site of Hodgkin's disease patients: Implications for a T-cell-based therapy. Cancer Res. 2001;61:6219–6226. [PubMed] [Google Scholar]
- 31.Herbst H, et al. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin's disease. Blood. 1996;87:2918–2929. [PubMed] [Google Scholar]
- 32.Poppema S. Immunobiology and pathophysiology of Hodgkin lymphomas. Hematology (Am Soc Hematol Educ Program) 2005;1:231–238. doi: 10.1182/asheducation-2005.1.231. [DOI] [PubMed] [Google Scholar]
- 33.Rodig SJ, et al. AP1-dependent galectin-1 expression delineates classical Hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin Cancer Res. 2008;14:3338–3344. doi: 10.1158/1078-0432.CCR-07-4709. [DOI] [PubMed] [Google Scholar]
- 34.Straathof KC, et al. Characterization of latent membrane protein 2 specificity in CTL lines from patients with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol. 2005;175:4137–4147. doi: 10.4049/jimmunol.175.6.4137. [DOI] [PubMed] [Google Scholar]
- 35.Brennan RM, Burrows SR. A mechanism for the HLA-A*01-associated risk for EBV+ Hodgkin lymphoma and infectious mononucleosis. Blood. 2008;112:2589–2590. doi: 10.1182/blood-2008-06-162883. [DOI] [PubMed] [Google Scholar]
- 36.Tan LC, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 37.Bell MJ, et al. Widespread sequence variation in Epstein-Barr virus nuclear antigen 1 influences the antiviral T cell response. J Infect Dis. 2008;197:1594–1597. doi: 10.1086/587848. [DOI] [PubMed] [Google Scholar]
- 38.Hadinoto V, et al. On the dynamics of acute EBV infection and the pathogenesis of infectious mononucleosis. Blood. 2008;111:1420–1427. doi: 10.1182/blood-2007-06-093278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan G, et al. Phenotype and frequency of Epstein-Barr virus-infected cells in pretreatment blood samples from patients with Hodgkin lymphoma. Br J Haematol. 2005;129:511–519. doi: 10.1111/j.1365-2141.2005.05483.x. [DOI] [PubMed] [Google Scholar]
- 40.Begg CB, Zhang ZF. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev. 1994;3:173–175. [PubMed] [Google Scholar]
- 41.Smedby KE, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97:199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 42.Jarrett RF, et al. The Scotland and Newcastle epidemiological study of Hodgkin's disease: Impact of histopathological review and EBV status on incidence estimates. J Clin Pathol. 2003;56:811–816. doi: 10.1136/jcp.56.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryden H, et al. Determination of HLA-A*02 antigen status in Hodgkin's disease and analysis of an HLA-A*02-restricted epitope of the Epstein-Barr virus LMP-2 protein. Int J Cancer. 1997;72:614–618. doi: 10.1002/(sici)1097-0215(19970807)72:4<614::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 44.Hurvich CM, Tsai CL. Model selection for extended quasi-likelihood models in small samples. Biometrics. 1995;51:1077–1084. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.