Abstract
Glutamate is the major neurotransmitter in the brain, mediating point-to-point transmission across the synaptic cleft in excitatory synapses. Using a glutamate imaging method with fluorescent indicators, we show that synaptic activity generates extrasynaptic glutamate dynamics in the vicinity of active synapses. These glutamate dynamics had magnitudes and durations sufficient to activate extrasynaptic glutamate receptors in brain slices. We also observed crosstalk between synapses—i.e., summation of glutamate released from neighboring synapses. Furthermore, we successfully observed that sensory input from the extremities induced extrasynaptic glutamate dynamics within the appropriate sensory area of the cerebral cortex in vivo. Thus, the present study clarifies the spatiotemporal features of extrasynaptic glutamate dynamics, and opens up an avenue to directly visualizing synaptic activity in live animals.
Keywords: synapse, spillover, fluorescence imaging, two-photon microscopy, in vivo
Glutamate is the major excitatory neurotransmitter in the mammalian brain. The conventional view is that glutamate mediates synaptically confined point-to-point transmission at excitatory synapses. However, glutamate has also been suggested to escape from the synaptic cleft, generating extrasynaptic glutamate dynamics (often referred to as glutamate spillover) (1–4). Extrasynaptic glutamate dynamics has been implicated in the activation of extrasynaptic glutamate receptors via volume transmission to regulate a variety of important neural and glial functions including synaptic transmission (5, 6), synaptic plasticity (7), synaptic crosstalk (8–11), nonsynaptic neurotransmission (12, 13), neuronal survival (14), gliotransmitter release (15–17), and hemodynamic responses (18–20).
Despite the immense potential physiological importance of glutamate spillover, the spatiotemporal dynamics of extrasynaptic glutamate concentration have been only inferred indirectly, and their characteristics remain elusive because of a lack of appropriate technology. Indeed, the magnitude and spatiotemporal distribution of extrasynaptic glutamate concentrations are the key determinants of physiological functions of glutamate spillover, and they are the essential factors for understanding extrasynaptic glutamate signaling. However, we have had to indirectly estimate the spatiotemporal dynamics of the glutamate spillover from its end effects mediated by glutamate receptors using electrophysiological and other means. To overcome this problem, we set out to image extrasynaptic glutamate dynamics in the brain.
We developed glutamate indicators derived from the E (glutamate) optical sensor (EOS) (21). EOS is a hybrid-type fluorescent indicator consisting of the glutamate-binding domain of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunit GluR2 and a fluorescent small molecule conjugated near the glutamate-binding pocket. EOS changes its fluorescence intensity upon binding of glutamate, for which it has both high affinity and high selectivity (21). We successfully used EOS in cultured hippocampal neurons to observe spontaneous and evoked synaptic activities (21). Because EOS has a high affinity to glutamate, we considered whether the indicator could be used to detect extrasynaptic glutamate activities in acute slice preparations. To this end, we improved the signal-to-noise ratio of EOS-based glutamate imaging, developing sister indicators of EOS with increased dynamic range and different affinities. Extrasynaptically labeled sister EOS indicators in brain slices enabled imaging of extrasynaptic glutamate dynamics during synaptic activity. This EOS imaging method revealed that physiologically significant levels of extrasynaptic dynamics were generated upon repetitive inputs in the vicinity of active synapses. To further establish whether glutamatergic volume transmission takes place under physiological conditions, we carried out in vivo glutamate imaging in the brain in response to sensory input from the extremities, and successfully imaged glutamate dynamics that were mapped within the appropriate sensory area of the cerebral cortex. Thus, the present results shed light on the extrasynaptic glutamate dynamics during physiological synaptic activities.
Results
Distribution of Labeled EOS.
We developed EOS sister indicators by introducing site-directed amino acid substitutions (Fig. 1A and Fig. S1A). K716A-EOS, in which lysine residue at position 716 was replaced by alanine, has a high affinity [dissociation constant (Kd) = 174 nM; Fig. 1B] similar to that of the original EOS but has a greater amplitude of the maximal change in fluorescence intensity (29.1 ± 1.7%, n = 9; measured after labeling on the cell surface, mean ± SEM; SI Text) (21). This improvement allowed for glutamate imaging with a higher signal-to-noise ratio. L401C-EOS has a lower affinity to glutamate (Kd = 1.57 μM; Fig. 1B), but its maximal signal amplitude (48.2 ± 1.8%, n = 9; measured after labeling on the cell surface; SI Text) is greater than that of K716A-EOS. These properties are suitable for imaging glutamate dynamics at micromolar concentrations. We also generated Y711A-EOS, which is insensitive to glutamate and was used for control experiments (Fig. 1B).
Fig. 1.
Glutamate sensitivity of EOS and extracellular distribution of labeled EOS. (A) Schematic primary structures of K716A-EOS, Y711A-EOS, and L401C-EOS. Replaced amino acids and labeled fluorescent dyes are indicated. The amino acid positions denote those in the original GluR2. (B) Steady-state dose–response relationship between the glutamate concentration and fractional change in the fluorescence intensity (ΔF/F0) of EOS sister indicators measured in vitro using a spectrofluorometer. Estimated Kd of K716A-EOS and L401C-EOS were 174 nM and 1.57 μM, respectively. (C) K716A-EOS labeling in the molecular layer of cerebellar slices. Magnified images show a roughly even distribution of K716A-EOS in the extracellular space. (D and E) Analysis of ultrastructural distribution of K716A-EOS by pre-embedding (D) and post-embedding (E) immunoelectron microscopy. Gold particles were predominantly observed in extrasynaptic and perisynaptic regions. Cyan indicates presynaptic terminal, and magenta indicates postsynaptic spine. Gold particles were observed within the clefts in only four synapses among 189 synapses analyzed in pre-embedding immunoelectron microscopy. Only 28 gold particles among 1,178 particles analyzed were observed within the clefts in post-embedding immunoelectron microscopy.
These indicators labeled the extracellular space of acute brain slices through the tight biotin–streptavidin interaction described in ref. 21 (Fig. S1C). We first used K716A-EOS in sagittal cerebellar slices to study glutamatergic parallel fiber (PF) synapses that are tightly ensheathed by astrocytic processes (22). Two-photon microscopic observation of the K716A-EOS-labeled cerebellar cortex showed roughly even staining with scattered negative staining silhouettes of the cellular components (Fig. 1C). A fluorescence recovery after photobleaching (FRAP) experiment showed that K716A-EOS was essentially immobilized in the slice (Fig. S1D). Electron microscopic observation indicated that K716A-EOS labeling had no obvious effect on the structure of the synaptic components (Fig. S1E). Pre-embedding immunoelectron microscopic analysis confirmed the extracellular distribution of K716A-EOS, which was observed primarily at extrasynaptic sites (Fig. 1D). The same distribution was observed by post-embedding immunoelectron microscopy (Fig. 1E). We quantified the number of immunogold particles and found only 2.4% of gold particles (28 out of 1,178 particles analyzed in 24 specimens) in the synaptic clefts. This value was compared with the fraction of synaptic membranes in brain tissue. Given the average postsynaptic density area of PF synapse (0.15 μm2) (23), the density of PF synapse (≈1 μm−3) (24), and the total membrane surface area within a unit volume of the cerebellar molecular layer (14 μm2 μm−3) (3, 25), the fraction of membranes facing the synaptic cleft is ≈2.1% (0.15 μm2 × 2 × 1 μm−3/14 μm2 μm−3 × 100%) of the total surface area. These considerations indicate that labeled K716A-EOS was distributed throughout the extracellular surface of cells without any accumulation in the synaptic cleft, and that >97% of the indicator molecules were present extrasynaptically.
Imaging of Extrasynaptic Glutamate Dynamics During Synaptic Activity.
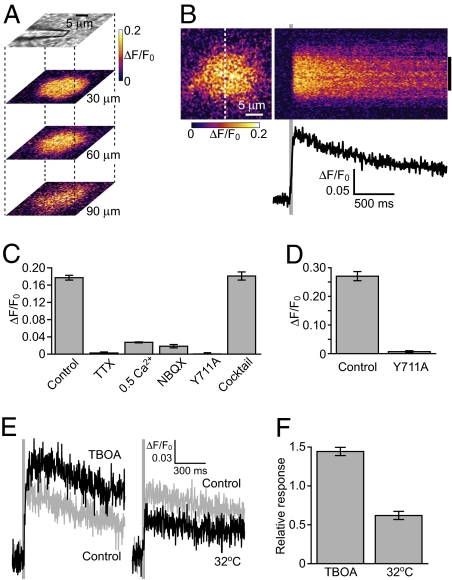
We then used a two-photon microscope to image glutamate dynamics in response to PF stimulation in K716A-EOS-labeled cerebellar slice preparations. When the PFs were electrically stimulated using a physiologically relevant pulse protocol (five pulses at 100 Hz) (26), the K716A-EOS fluorescence intensity increased within a circular area on a sagittal plane (Fig. 2A). Similar K716A-EOS signals were observed at different depths of the slice (Fig. 2A), consistent with the orientation of the PFs, which run perpendicular to the sagittal plane. The line-scan image across the center of the K716A-EOS signal showed a rapid increase and a slow decay (Fig. 2B), which was consistent with the dissociation kinetics of K716A-EOS (rate constant = 0.35 s−1).
Fig. 2.
Imaging of extrasynaptic glutamate dynamics during synaptic activity. (A) Imaging of K716A-EOS fluorescence at the indicated depths in response to PF stimulation (five pulses at 100 Hz, average of five successive trials). ΔF/F0 was color-coded as indicated. (B) Line-scan imaging across the center of a K716A-EOS signal. Plot of the mean ΔF/F0 within the region indicated by the right-hand side bar is shown. (C) K716A-EOS signals induced by PF stimulation (five pulses at 100 Hz) were blocked with 1 μM TTX, 0.5 mM Ca2+, and 10 μM NBQX, whereas a mixture of glutamate receptor antagonists [50 μM GYKI-52466, 50 μM D-AP5, and 500 μM (RS)-MCPG] had no effect. Y711A-EOS showed no response to PF inputs. Mean ± SEM, n = 6. (D) Perfusion of 1 mM glutamate induced an increase in the fluorescence intensity of K716A-EOS but not that of Y711A-EOS (mean ± SEM, n = 6). (E and F) Contribution of glutamate transporters to extrasynaptic glutamate dynamics. (E) K716A-EOS signal in response to PF stimulation (three pulses at 100 Hz) was enhanced in the presence of 200 μM TBOA and reduced at a higher temperature (32 °C). Average of five successive trials each. (F) Amplitude of K716A-EOS signals in both conditions normalized by that in control condition (mean ± SEM, n = 6).
We confirmed that the observed K716A-EOS signals directly reported glutamate released from the PFs through the following experiments. Blockade of synaptic glutamate release by tetrodotoxin (TTX) or low extracellular Ca2+ concentration inhibited the PF-induced K716A-EOS signal (Fig. 2C). Blockade of glutamate binding of K716A-EOS by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), a competitive AMPAR antagonist, also inhibited the signal (Fig. 2C). The glutamate-insensitive indicator Y711A-EOS (Fig. 1 A and B) did not produce any signal during PF stimulation (Fig. 2C). These results indicate that glutamate release from PFs is necessary for observed K716A-EOS signals. In contrast, perfusion of a saturating concentration of glutamate over the preparation increased the fluorescence intensity of K716A-EOS by an amplitude comparable with maximal response (Fig. 2D, and see above). This glutamate-induced signal was not observed with glutamate-insensitive Y711A-EOS (Fig. 2D). These results indicate that glutamate is sufficient to induce K716A-EOS signals. Thus, glutamate released from PFs is necessary and sufficient to induce K716A-EOS signals. A mixture of an AMPAR/kainate receptor noncompetitive antagonist, an N-methyl-D-aspartate receptor (NMDAR) antagonist and a metabotropic glutamate receptor (mGluR) antagonist had no effect on the K716A-EOS signal, showing that it was not generated by an event secondary to glutamate receptor activation (Fig. 2C).
The observed K716A-EOS signal should be predominantly reporting extrasynaptic glutamate concentration changes because most of the labeled K716A-EOS was localized in the extrasynaptic space as shown by the immunoelectron microscopy (Fig. 1 D and E). To evaluate the contribution of K716A-EOS molecules localized within the synaptic clefts on the observed signals, we calculated possible maximal response derived from intrasynaptic K716A-EOS. If all of the intrasynaptic K716A-EOS molecules (2.4% of total K716A-EOS) were exposed to a saturating glutamate concentration to give off the maximal response (≈0.29 of ΔF/F0), while extrasynaptic K716A-EOS showed no response, the expected magnitude of fluorescence response normalized to the total K716A-EOS fluorescence should be ≈0.007 ΔF/F0. This is far smaller than observed K716A-EOS signals during PF inputs. Thus, the contribution of intrasynaptic K716A-EOS should be negligible in the following experiments.
Uptake of glutamate via glutamate transporters is critically involved in the regulation of extrasynaptic glutamate dynamics (27). We thus examined whether glutamate transporter activities affect PF-induced extrasynaptic glutamate dynamics reported by the K716A-EOS signal. When glutamate transporters were blocked by DL-threo-β-benzyloxyaspartate (TBOA), a glutamate transporter inhibitor, the fluorescence intensity of K716A-EOS was slightly increased (ΔF/F0 = 0.054 ± 0.0076, n = 6), being consistent with an increase in the ambient glutamate concentration. Furthermore, the amplitude of PF-induced K716A-EOS signals increased in the presence of TBOA (Fig. 2 E and F). However, when the activity of glutamate transporters was increased by raising the temperature to 32 °C (28), the PF-induced K716A-EOS response decreased (Fig. 2 E and F). These results indicated the regulatory effects of glutamate transporters on extrasynaptic glutamate dynamics. Glutamate trans-porters are expected to restrict the lateral diffusion of glutamate. Indeed, after five pulses of PF stimulation at 100 Hz (Figs. 2B and 3B), the width of K716A-EOS signals was constant during the imaging period, showing no significant time-dependent lateral spread. However, when 10–20 pulses of PF stimulation were applied, delayed signals exhibiting latency were observed at the periphery of K716A-EOS signals (Fig. S2). This suggested that when the glutamate transporters are saturated by the intense accumulation of glutamate, the lateral spread of glutamate becomes detectable by K716A-EOS.
Fig. 3.
Microdomain of extrasynaptic glutamate dynamics. (A) Simultaneous measurement of PF-induced EOS signal and EPSC. (A Left) Purkinje cell within K716A-EOS-labeled region (green) filled with Alexa Fluor 594 (red) via the whole-cell patch pipette. (Middle) Magnified image of the region indicated by the white box. (A Right) Line-scan image of K716A-EOS signal across the dotted white line and corresponding EPSC induced by PF stimulation (five pulses at 100 Hz). (B) The simultaneous measurement of K716A-EOS signals and EPSCs upon the stimulation of a small number of PFs (five pulses at 100 Hz, average of ten successive trials each). (C) Relationship between the amplitude of the K716A-EOS signal and the time-integral of the corresponding EPSC. Data were rank-ordered with the value of the EPSC time-integral, and four to six results were averaged and plotted (mean ± SEM). (D) Spatial distribution of the amplitude of the K716A-EOS signal (gray) was fitted with a Gaussian function (black line). K716A-EOS signals with >0.03 of ΔF/F0 amplitude were analyzed. FWHM values of the Gaussian function were plotted (open circles, n = 6 and filled circle, mean ± SEM).
Labeled EOS might buffer glutamate to lower the extrasynaptic glutamate concentration. However, decreasing the K716A-EOS concentration by a factor of five resulted in only ≈30% increase in K716A-EOS signal amplitude (Fig. S3). This limited effect may be due to the presence of high concentration of intrinsic glutamate buffers including glutamate transporters (25, 29). Thus, labeled K716A-EOS should not significantly affect extrasynaptic glutamate dynamics, confirming that EOS imaging is a faithful reporter of glutamate concentration. K716A-EOS signals indicated and analyzed above are the average of five successive trials to improve the signal-to-noise ratio. This averaging procedure was justified by the reproducibility of K716A-EOS signals across trials (Fig. S4).
Extrasynaptic Glutamate Dynamics Are Locally Induced by a Small Number of Neighboring Synapses.
Crosstalk between synapses—i.e., summation of glutamate released from neighboring synapses—is one of the critical determinants of extrasynaptic glutamate dynamics (2, 8, 9, 11, 30). Thus, we evaluated the intensity of PF inputs required to induce detectable levels of glutamate signals and examined the spatial distribution of extrasynaptic glutamate dynamics in response to a minimal level of PF inputs. We simultaneously measured K716A-EOS signals and PF-induced EPSCs in whole-cell patch-clamped Purkinje cells to assess the intensity of PF inputs (Fig. 3A). A small number of neighboring PFs were then electrically stimulated with near-threshold stimulus intensity (31). The K716A-EOS signal confined to a narrow region and a corresponding EPSC with small amplitude was observed in response to very weak PF stimulation (Fig. 3B). K716A-EOS signal amplitude increased with the time-integral of the EPSC, showing the dependence of extrasynaptic glutamate dynamics on the density of stimulated PFs (Fig. 3C) (8, 11, 30). It is thus strongly suggested that glutamate released from neighboring synapses can be integrated in the extrasynaptic space. K716A-EOS signals with >0.03 of ΔF/F0 amplitude were selected to analyze the signal's spatial distribution. The full width at half maximum (FWHM) of these K716A-EOS signals was ≈7 μm (Fig. 3D). These results suggest that extrasynaptic glutamate dynamics are locally generated by the summation of glutamate released from neighboring synapses activated within a narrow time window.
Magnitude and Time Course of Extrasynaptic Glutamate Dynamics.
We then studied whether the extrasynaptic glutamate generated by PF stimulation reaches a concentration sufficient for the activation of glutamate receptors. Considering the EC50 values of NMDAR (≈2 μM) (32) and mGluR (≈10 μM) (33), glutamate concentrations at micromolar levels are necessary for the activation of these high-affinity glutamate receptors. We therefore used the lower-affinity glutamate indicator L401C-EOS (Kd = 1.57 μM) to measure PF-induced extrasynaptic glutamate dynamics. Single stimulation pulses generated a faint but statistically significant L401C-EOS signal (P < 0.05 against no stimulation condition; Fig. 4 A and B). Two to five pulses at 100 Hz induced a sizable L401C-EOS response (Fig. 4 A and B). Thus, the extrasynaptic glutamate dynamics undergo temporal summation (8, 9, 11, 30). In response to five pulses of stimulation, the L401C-EOS signal increased by ≈0.28 F0 at its peak (Fig. 4 A and B). This value amounts to ≈58% of the maximal L401C-EOS response induced by the perfusion of saturating concentration of glutamate (Fig. 4B). Based on the steady state calibration of L401C-EOS (Fig. 1B), the observed peak signal corresponds to glutamate concentration of ≈2 μM. Notably, this value was an underestimate of the true glutamate concentration because the L401C-EOS signal did not reach the steady state during the observed period because of the finite glutamate association and dissociation rates.
Fig. 4.
Magnitude of extrasynaptic glutamate dynamics. (A) Time course of ΔF/F0 of L401C-EOS induced by one to five pulses of PF stimulation (100 Hz, average of five successive trials). (B) Peak amplitude of the L401C-EOS signal in response to one to five pulses of PF stimulation and to perfusion of 1 mM glutamate (mean ± SEM, n = 6). (C) Estimation of extrasynaptic glutamate concentration. Upper traces show time courses of L401C-EOS signal (average of five successive trials each, n = 6 slices); lower traces show the corresponding time courses of glutamate concentration estimated by the deconvolution method (see text). Magenta traces indicate averaged data. (D) Average of estimated glutamate concentration within 50-ms time window starting from the first PF stimulation (mean ± SEM, n = 6).
To estimate the time course of glutamate transients, we numerically deconvolved the L401C-EOS signals assuming first-order glutamate-indicator association kinetics (SI Text). The time course of the estimated glutamate transients reached micromolar concentrations for tens of milliseconds in response to two to five pulses of stimulation (Fig. 4C, lower trace). Indeed, the average glutamate concentration within the 50-ms time window after stimulation was 1–8 μM depending on the number of stimulation pulses (Fig. 4D). Thus, two or more pulses generated extrasynaptic glutamate dynamics with concentrations and durations sufficient to activate high-affinity glutamate receptors.
Extrasynaptic Glutamate Dynamics in Neocortex and Hippocampus.
Observations indicative of the presence of extrasynaptic glutamate dynamics have been reported in various regions of the brain. We therefore examined extrasynaptic glutamate dynamics in the cerebral cortex and hippocampus. Layer 2/3 of neocortical slices and the stratum radiatum of the CA1 region in hippocampal slices were labeled with L401C-EOS (Fig. 5 A and C). Focal stimulation of synaptic inputs induced significant L401C-EOS signals in layer 2/3 of neocortical slices (Fig. 5B). Stimulation of Schaffer collaterals also induced significant L401C-EOS signals in hippocampal slices (Fig. 5D). The deconvolution method applied to L401C-EOS signals in response to two pulses of stimulation showed extrasynaptic glutamate dynamics exceeding micromolar concentrations in both neocortical and hippocampal slices (Fig. 5 E and F).
Fig. 5.
Extrasynaptic glutamate dynamics in neocortical and hippocampal slices. (A and C Left) L401C-EOS labeling in layer 2/3 of a neocortical slice (A) or in the stratum radiatum in CA1 in a hippocampal slice (C). (A and C Right) Magnified image of the region indicated by the white box. (B and D Left) Peak L401C-EOS signal induced by focal stimulation (five pulses at 100 Hz) applied via a stimulating electrode (indicated by white lines). (B and D Right) Line-scan imaging of L401C-EOS signal across the white dotted line in the left image. (E) Extrasynaptic glutamate dynamics upon focal stimulation (two pulses at 100 Hz) in the neocortical and hippocampal slices estimated as in Fig. 4C (average of five successive trials each, n = 6 slices). Magenta traces indicate averaged data. (F) Average of estimated glutamate concentration within 50-ms time window starting from the first stimulation (mean ± SEM, n = 6).
Extrasynaptic Glutamate Dynamics Induced by the Sensory Input in Vivo.
We next analyzed glutamate dynamics in vivo in the rat somatosensory cortex while receiving sensory input from the hind limb (Fig. 6A). We first determined the sensory area of the hind paw using flavoprotein autofluorescence imaging (34) through a cranial window over the sensory cortex (Fig. S5). The area was then labeled with L401C-EOS using the same method as in the slice experiments (Fig. 6C). Using epifluorescence microscopic observation, we found that the L401C-EOS signal exhibited a significant response to a 200-ms tactile stimulation of the hind paw (Fig. 6 D, E, and G). However, glutamate-insensitive Y711A-EOS exhibited no response (Fig. 6 F and G). After hind paw stimulation, the spatial distribution of the L401C-EOS signal was comparable to that of neuronal activity detected by calcium-sensitive dye imaging (Fig. S6). Interestingly, there was a significant fluctuation of the L401C-EOS signal before or without tactile stimulation (Fig. 6 E and H). Such fluctuations were not observed with the Y711A-EOS signal (Fig. 6 F and H) and might reflect spontaneous synaptic activity. Thus, in vivo EOS measurements clearly showed that physiological synaptic activity generates extrasynaptic glutamate signaling, and the imaging method also suggests the involvement of spontaneous synaptic activities.
Fig. 6.
Extrasynaptic glutamate dynamics upon sensory input from the hind paw. (A) Schema of glutamate imaging in the somatosensory cortex. (B) Flavoprotein fluorescence in the sensory cortex corresponding to the hind paw. (C) L401C-EOS labeling in the same area of the cortex as in (B). (D) Two-dimensional peak L401C-EOS signal induced by 200-ms tactile stimulation to the hind paw. (E and F) Time courses of ΔF/F0 of L401C-EOS or Y711A-EOS in the presence or absence of hind-paw stimulation (gray bar). Traces shown are the results of 12 successive trials in each condition, and magenta traces indicate averaged data. (G) Peak amplitude of the L401C-EOS signal upon hind-paw stimulation (mean ± SEM, n = 4 animals each). **, P < 0.01. (H) Basal fluctuation (coefficient of variation) of fluorescence intensity of L401C-EOS and Y711A-EOS (mean ± SEM, n = 4 animals each). **, P < 0.01.
Discussion
In the present study, using the high signal-to-noise ratio of glutamate indicators, we successfully mapped and estimated local extrasynaptic glutamate dynamics during physiologically relevant nerve inputs. Although glutamate spillover has been expected to take place to explain electrophysiological, pharmacological, and other results, the present study provides direct evidence that synaptic activity generates increases in the local glutamate concentration that exceed micromolar levels in the extrasynaptic space in both cerebrum and cerebellum. Such extrasynaptic glutamate dynamics are sufficient to activate NMDAR and mGluR that are located on the extrasynaptic membrane to mediate volume transmission.
This study showed that extrasynaptic glutamate dynamics summate both temporally and spatially, providing a basis for understanding synaptic crosstalk or collaborative activation of extrasynaptic glutamate receptors by adjacent synapses (2, 9, 11, 30). Due to this summation effect, two or more repetitive pulses generated extrasynaptic glutamate dynamics with concentrations and durations sufficient to activate high-affinity glutamate receptors. This is consistent with the previous findings that at least two pulses of repetitive PF input is required to activate perisynaptic mGluRs in Purkinje cells (11, 35–37). Furthermore, the present results directly demonstrated that brief tactile stimulation to the extremities generates an EOS signal in the corresponding sensory cortex in vivo. Together with the recent finding that sensory input from whiskers induces mGluR-mediated astrocytic Ca2+ responses at corresponding cortical sensory areas in vivo (38), these results show that physiological sensory stimulation is sufficient to induce volume transmission via high-affinity glutamate receptors in vivo. On the other hand, the EC50 value of AMPAR (≈500 μM) (39) suggests that extrasynaptic AMPARs must be activated by spatiotemporally confined glutamate dynamics with submillimolar to millimolar concentrations at the close vicinity of synaptic clefts or ectopic glutamate release sites (10, 40–43). Although such signals were difficult to resolve by the present EOS imaging method, the development of sister indicators of EOS with much lower affinity to glutamate and a method to control EOS localization may provide more information about the spatial profile of glutamate dynamics.
Whereas PF stimulation induced a beam-like extrasynaptic glutamate dynamics extending over 100 μm along the fiber path in cerebellar slices (Fig. 2A), those upon stimulation of Schaffer collaterals decayed within tens of micrometers from the stimulating electrode in hippocampal slices (Fig. 5D). Multiple factors including the length of fibers, density of presynaptic boutons, release probability, and capacity of glutamate transporters may underlie the difference. The functional anatomy of extrasynaptic glutamate dynamics provides an important basis for the study of glutamate spillover in these brain regions (1, 8, 9, 11, 28, 30).
Not only does the EOS-based glutamate imaging provide information about the magnitude of glutamate spillover, it provides a unique and direct method for mapping excitatory synaptic activity in the brain, which should be useful for studying the functional structures of neuronal circuits in the neocortex or other brain regions. It can be a useful addition to currently available methods including calcium and voltage-sensitive dye imaging, intrinsic optical imaging, and the flavoprotein autofluorescence imaging. Thus, the present study opens various avenues to understanding brain function.
Materials and Methods
Animal use procedures were in accordance with the guidelines established by the Animal Welfare Committee of the University of Tokyo. EOS sister indicators were produced and evaluated as described in ref. 21. Cerebellar, neocortical and hippocampal slices were prepared from postnatal day 20–24 C57/B6 mice (44). Slices were biotinylated in artificial cerebrospinal fluid (ACSF) containing 50–100 μM sulfo-NHS-SS-biotin (45), followed by injection of the EOS-biotin-streptavidin complex. EOS-labeled slices were imaged with a two-photon microscope, and stimulation-induced EOS signals were imaged with a one- or two-dimensional time-lapse scanning configuration. Glutamate concentration was calculated by deconvolving the time course of ΔF/F0 upon synaptic inputs considering the kinetic parameters of the EOS–glutamate interaction. For in vivo imaging, a craniotomy was performed on postnatal day 16–28 Sprague–Dawley rats. Sulfo-NHS-SS-biotin was injected into the somatosensory cortex via a glass pipette, followed by the injection of EOS-biotin-streptavidin. EOS fluorescence was imaged with an epifluorescence microscope through a cranial window. ΔF/F0 upon hind-paw stimulation was analyzed. The ultrastructural analysis of the EOS distribution was carried out by pre- and post-embedding immunogold electron microscopy. EOS-labeled slices were fixed (46) and stained with antistreptavidin antibodies and secondary antibodies linked to gold particles. Ultrathin sections (70 nm) were imaged with an electron microscope. For more detail, see SI Text.
Supplementary Material
Acknowledgments
We thank Ayami Nakanishi and Tomoyuki Kazuta for assisting in the development of L401C-EOS. This work was supported by Grant-in-Aid for Scientific Research, Ministry of Education, Culture, Sports, Science, and Technology (Japan); the Global COE Programs “Integrative Life Science Based on the Study of Biosignaling Mechanisms” and “Global Center of Education and Research for Chemical Biology of the Diseases,” Ministry of Education, Culture, Sports, Science, and Technology (Japan); and grants from the Uehara Memorial Foundation and Takeda Science Foundation. A part of this study is the result of “Development of biomarker candidates for social behavior” carried out under the Strategic Research Program for Brain Sciences, Ministry of Education, Culture, Sports, Science, and Technology (Japan).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913154107/DCSupplemental.
References
- 1.Kullmann DM, Erdemli G, Asztély F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 2.Barbour B, Häusser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- 3.Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–3170. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour B. An evaluation of synapse independence. J Neurosci. 2001;21:7969–7984. doi: 10.1523/JNEUROSCI.21-20-07969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- 7.Humeau Y, Shaban H, Bissière S, Lüthi A. Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature. 2003;426:841–845. doi: 10.1038/nature02194. [DOI] [PubMed] [Google Scholar]
- 8.Carter AG, Regehr WG. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci. 2000;20:4423–4434. doi: 10.1523/JNEUROSCI.20-12-04423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 10.DiGregorio DA, Nusser Z, Silver RA. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron. 2002;35:521–533. doi: 10.1016/s0896-6273(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 11.Marcaggi P, Attwell D. Endocannabinoid signaling depends on the spatial pattern of synapse activation. Nat Neurosci. 2005;8:776–781. doi: 10.1038/nn1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 13.Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- 14.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 15.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 16.Bezzi P, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JM, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 18.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 19.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 20.Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, Mac Vicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namiki S, Sakamoto H, Iinuma S, Iino M, Hirose K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur J Neurosci. 2007;25:2249–2259. doi: 10.1111/j.1460-9568.2007.05511.x. [DOI] [PubMed] [Google Scholar]
- 22.Xu-Friedman MA, Harris KM, Regehr WG. Three-dimensional comparison of ultrastructural characteristics at depressing and facilitating synapses onto cerebellar Purkinje cells. J Neurosci. 2001;21:6666–6672. doi: 10.1523/JNEUROSCI.21-17-06666.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris KM, Stevens JK. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1988;8:4455–4469. doi: 10.1523/JNEUROSCI.08-12-04455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274:168–177. doi: 10.1002/cne.902740204. [DOI] [PubMed] [Google Scholar]
- 25.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- 27.Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- 28.Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 29.Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcaggi P, Billups D, Attwell D. The role of glial glutamate transporters in maintaining the independent operation of juvenile mouse cerebellar parallel fibre synapses. J Physiol. 2003;552:89–107. doi: 10.1113/jphysiol.2003.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SS, Denk W, Häusser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- 32.Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, et al. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- 34.Shibuki K, et al. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 36.Takechi H, Eilers J, Konnerth A. A new class of synaptic response involving calcium release in dendritic spines. Nature. 1998;396:757–760. doi: 10.1038/25547. [DOI] [PubMed] [Google Scholar]
- 37.Okubo Y, Kakizawa S, Hirose K, Iino M. Cross talk between metabotropic and ionotropic glutamate receptor-mediated signaling in parallel fiber-induced inositol 1,4,5-trisphosphate production in cerebellar Purkinje cells. J Neurosci. 2004;24:9513–9520. doi: 10.1523/JNEUROSCI.1829-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 39.Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol. 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzubay JA, Jahr CE. The concentration of synaptically released glutamate outside of the climbing fiber-Purkinje cell synaptic cleft. J Neurosci. 1999;19:5265–5274. doi: 10.1523/JNEUROSCI.19-13-05265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iino M, et al. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- 42.Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 43.Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 45.Thomas-Crusells J, Vieira A, Saarma M, Rivera C. A novel method for monitoring surface membrane trafficking on hippocampal acute slice preparation. J Neurosci Methods. 2003;125:159–166. doi: 10.1016/s0165-0270(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 46.Jensen FE, Harris KM. Preservation of neuronal ultrastructure in hippocampal slices using rapid microwave-enhanced fixation. J Neurosci Methods. 1989;29:217–230. doi: 10.1016/0165-0270(89)90146-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.