Abstract
Extracellular ATP has been proposed as a paracrine signal in rodent islets, but it is unclear what role ATP plays in human islets. We now show the presence of an ATP signaling pathway that enhances the human β cell's sensitivity and responsiveness to glucose fluctuations. By using in situ hybridization, RT-PCR, immunohistochemistry, and Western blotting as well as recordings of cytoplasmic-free Ca2+ concentration, [Ca2+]i, and hormone release in vitro, we show that human β cells express ionotropic ATP receptors of the P2X3 type and that activation of these receptors by ATP coreleased with insulin amplifies glucose-induced insulin secretion. Released ATP activates P2X3 receptors in the β-cell plasma membrane, resulting in increased [Ca2+]i and enhanced insulin secretion. Therefore, in human islets, released ATP forms a positive autocrine feedback loop that sensitizes the β cell's secretory machinery. This may explain how the human pancreatic β cell can respond so effectively to relatively modest changes in glucose concentration under physiological conditions in vivo.
Keywords: extracellular ATP, human pancreatic β cell, insulin secretion, P2X receptor, positive autocrine feedback
Glucose homeostasis is tightly controlled by hormone secretion from the endocrine pancreas, the islets of Langerhans. Even small physiological deviations (e.g., 10%) in plasma glucose are effectively counteracted by sharp (e.g., 3-fold) increases in the secretion of the islet hormones insulin and glucagon (1). Intraislet autocrine and paracrine signaling are pivotal mechanisms for proper function of the islet, making islet cells extremely sensitive and responsive to plasma glucose fluctuations. The roles of different compounds such as GABA, glutamate, Zn2+, insulin, and ATP as autocrine and paracrine regulators of islet hormone release have been examined extensively (2–8). Extracellular ATP seems important because it is present in insulin-containing secretory granules and is released during glucose stimulation in sufficient amounts to stimulate ATP receptors (9–12).
Extracellular ATP is an important neurotransmitter signal in the brain as well as in vascular, immune, and endocrine cells (13–15). The purinergic system comprises receptors for extracellular ATP and adenosine, the P2 and P1 receptors, respectively. P2 purinergic receptors can be divided into metabotropic P2Y receptors (G protein coupled) and ionotropic P2X receptors (ligand-gated ion channels) (16). The ionotropic P2X family comprises seven subtypes named P2X1–P2X7 that regulate cell function by opening cation channels permeable to Na+, K+, and Ca2+ (15, 17). Activation of these channels regulates the release of neurotransmitters and hormones, either through direct Ca2+ influx or by promoting membrane depolarization and thereby inducing action potentials (18–21).
The role of ATP signaling in the physiology of pancreatic islets has been studied in rodent models, but the results in the literature are conflicting (22–28). In rat islets, purinergic agonists have been reported to increase insulin secretion (22, 28). This contrasts with a report on rat islets showing that extracellular ATP provides excitatory as well as inhibitory feedback loops for insulin secretion (23). In mouse islets, extracellular ATP has been consistently reported to decrease glucose-induced insulin secretion (24–26). In the two reports on human islets, purinergic agonists were shown to evoke inward currents in β cells and to stimulate insulin release (29, 30), but the receptors involved were not identified. More importantly, the physiological contexts under which these receptors are activated have not been investigated. Because islets from different species are strikingly different in terms of structure and function (31, 32), we decided to study, in detail, the role of purinergic signaling in human β cells. We were interested in defining the role of endogenously released ATP during stimulation of β cells with increases in glucose concentration. We examined the effects of ATP signaling by performing dynamic hormone-release assays, RT-PCR, immunohistochemistry, and in situ hybridization as well as imaging [Ca2+]i. We now show that human β cells express P2X receptors of the P2X3 type. Our results show that, on activation, P2X receptors promote Ca2+ influx and insulin secretion in human β cells, establishing an autocrine positive feedback loop during glucose-induced insulin release. This is important because it enables effective activation of the insulin secretory machinery despite relatively modest changes in blood glucose concentration.
Results
In rodent islets, insulin granules contain ATP, and ATP is coreleased with insulin during high glucose stimulation, reaching extracellular concentrations >25 μM (9–12, 33). Recent papers have provided evidence that smaller molecules such as ATP can be released by a kiss-and-run exocytotic mechanism, whereas insulin is retained in the granule (12, 34). Furthermore, insulin secretion shows a lower activation threshold in human islets than in mouse islets, and slight increases in insulin secretion already occur at 3 mM glucose (Fig. S1; see also ref. 35). Thus, ATP is likely to be coreleased with insulin at relatively low glucose concentrations. ATP is therefore an excellent signaling candidate for modulating the β-cell responsiveness to increases in glucose around the threshold.
To infer the role of ATP as an autocrine/paracrine signal, we manipulated ATP degradation and thus the concentration of endogenously released ATP in isolated human islets. We recorded changes in hormone secretion by using a perifusion assay of dynamic secretory responses (36). Released ATP is rapidly cleared by membrane ecto-ATPases, such as apyrase, that convert ATP into adenosine (37, 38). Ecto-ATPases are crucial in the duration and magnitude of purinergic signaling (39). A functional apyrase (CD39) has been shown to be expressed in human β cells (40). Application of the apyrase inhibitor ARL67156 (50 μM) (41, 42) increased basal insulin secretion from islets incubated at low glucose concentration (3 mM; Fig. 1 A and B), revealing that human islet cells released ATP. Under these conditions, the endogenous ecto-ATPases are fully effective, explaining why exogenously added apyrase (5 U/mL) did not reduce basal insulin secretion (Fig. 1 A and B).
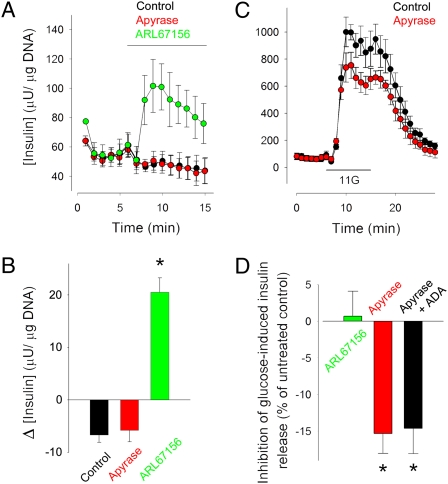
Fig. 1.
ATP is secreted by human islets at low glucose concentrations, and it amplifies insulin secretion during glucose stimulation. (A) The ectonucleotidase inhibitor ARL67156 (50 μM) increased insulin secretion at a low glucose concentration (3 mM; green symbols). Apyrase (5 U/mL) did not change basal insulin secretion (red symbols). Average traces of insulin secretion are shown (n = 4 perifusions). Control, black symbols. Bar indicates drug application. Data in all figures are presented as average ± SEM. (B) Quantification of the results shown in A. Δ[Insulin] (μU/μg DNA), change in insulin secretion from prestimulus levels. (C) Insulin secretion induced by raising glucose from 3 mM to 11 mM (black symbols) was reduced in the presence of apyrase (5 U/mL; red symbols). Average traces of insulin secretion are shown (n = 4 perifusions). 11G indicates 10 min of elevated glucose (11 mM). (D) Quantification of the results shown in C. Reducing extracellular ATP levels with apyrase (5 U/mL) decreased glucose-induced insulin release by ∼15%. Adding adenosine deaminase (ADA; 1 U/mL) to degrade adenosine did not change the effect of apyrase on glucose-stimulated insulin secretion. Control is insulin secretion induced by elevating glucose from 3 mM to 11 mM. Asterisks denote statistical significance (ANOVA followed by multiple comparisons versus control group in Bonferroni t test; P < 0.05).
Because ATP is already released at low glucose concentrations and has the potential to evoke insulin secretion, we hypothesized that ATP potentiates glucose-induced insulin secretion at early stages of the response. Accordingly, adding apyrase (5 U/mL) during a step increase in glucose concentration from 3 mM to 11 mM reduced insulin release by ∼15% (Fig. 1 C and D), indicating that endogenously released ATP contributed to the β-cell response. Adding the competitive apyrase inhibitor ARL67156 during glucose stimulation, however, did not amplify the β-cell response (Fig. 1D), suggesting that the concentration of endogenously released ATP was high enough to saturate its potentiating effect. Hence, stimulating with exogenous ATP while the glucose concentration was increased did not add to the insulin response.
Apyrase may decrease glucose-induced insulin release either by reducing extracellular ATP or by increasing adenosine, which may act on P1 receptors to inhibit insulin release (43). Degrading adenosine with adenosine deaminase did not change the effect of apyrase on glucose-stimulated insulin secretion (Fig. 1D), indicating that the presence of adenosine did not contribute to the inhibition of the insulin response. Accordingly, neither the P1 receptor antagonist CGS15943 (10 μM) nor adenosine (100 μM) altered glucose-induced insulin secretion (Discussion). Because nerves are severed and neuronal remnants that could be additional sources or targets for ATP do not survive under our experimental conditions (32, 44), the most likely interpretation is that ATP secreted by β cells provides a positive autocrine feedback loop to amplify insulin secretion.
To examine the receptors involved in this autocrine feedback loop, we blocked purinergic receptors with specific receptor antagonists during stimulation with an increase in glucose concentration from 3 mM to 11 mM (Fig. 2A). Insulin secretory responses to glucose stimulation were reduced in the presence of suramin (50 μM; a broad antagonist of P2 receptors), iso-pyridoxal-phosphate-6-azophenyl-2′, 4′-disulfonate (PPADS) (50 μM; an antagonist for P2X1, P2X2, P2X3, and P2X5 receptors), and oxidized ATP (oATP; 500 μM; an antagonist for P2X2, P2X3, and P2X7 receptors) by 40%, 30%, and 65%, respectively (Fig. 2B). Insulin secretory responses to glucose stimulation in the presence of the specific P2X1 antagonist MRS2159 (10 μM) and the two P2X7 receptor antagonists brilliant Blue G (1 μM) and KN-62 (1 μM) were not significantly reduced (Fig. 2B). Antagonists for P2Y receptors [reactive blue 2 (50 μM) and MRS2179 (10 μM); specific for the P2Y1 receptor; Fig. 2B)] or the P1 receptor [CGS15943 (10 μM)] did not inhibit glucose-induced insulin release.
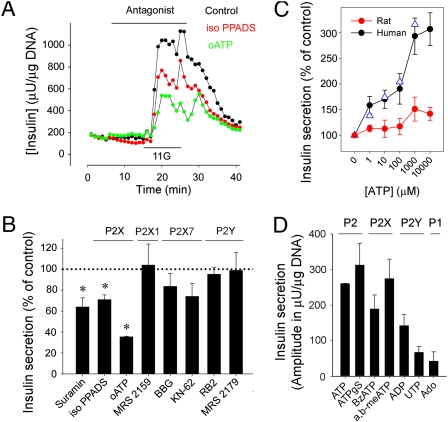
Fig. 2.
Endogenously released ATP amplifies glucose-induced insulin secretion in human islets through P2X receptors. (A) Insulin secretion induced by raising glucose from 3 mM to 11 mM was reduced in the presence of the P2X receptor antagonists iso-PPADS (50 μM; red symbols) and oATP (500 μM; green symbols; representative traces of at least three perifusions). Bar denotes antagonist application. 11G indicates 10 min of elevated glucose (11 mM). (B) Quantification of the results shows the effects of suramin (100 μM), iso-PPADS (50 μM), oATP (500 μM), MRS2159 (10 μM), Brilliant Blue G (BBG; 1 μM), KN-62 (1 μM), reactive blue 2 (RB2; 50 μM), and MRS2179 (10 μM) on the magnitude of glucose-induced insulin response (peak amplitudes; n ≥ 3). Suramin, iso-PPADS, and oATP reduced insulin release by 40%, 30%, and 65%, respectively. The specificity of the antagonists is indicated at the top of the panel. Asterisks denote statistical significance (ANOVA followed by multiple comparisons versus control group in Bonferroni t test; P < 0.05). (C) ATP concentration–response relationships for insulin secretion in human (n = 3 islet preparations; black and blue symbols are 3 mM and 11 mM glucose, respectively) and rat islets (n = 3; red symbols) are shown. Control is nonstimulated basal insulin secretion. (D) The purinergic agonists ATP (100 μM), ATPγS (50 μM), BzATP (50 μM), and ADP (100 μM) elicited insulin secretory responses in human islets at low glucose concentrations (3 mM). The P2Y agonist UTP (100 μM) and the P1 receptor agonist adenosine (Ado; 100 μM) did not evoke strong insulin responses (n ≥ 3 islet preparations).
To determine the direct effects of purinergic receptor activation on insulin secretion, we applied exogenous ATP and other agonists. In human islets, application of ATP, the universal agonist of P2 purinergic receptors, stimulated increases in insulin release concentration dependently with similar thresholds at low (3 mM) and high glucose concentrations (11 mM) (Fig. 2C). The concentration–response relationship showed a high affinity component (∼0.5 μM) that compared well with the reported EC50 for the human P2X3 receptor (∼0.39 μM) and a second increase between 100 and 1000 μM that might correspond to activation of P2X7 receptors (EC50 ∼ 100 μM) (45). Increasing extracellular ATP > 1 mM did not further raise insulin release (Fig. 2C). The insulin responses to ATP were similar to responses stimulated by glucose. Compared with the increase in insulin secretion elicited by ATP (1 mM), the response to high glucose (11 mM) was 101% ± 30% or almost identical. Similar results were obtained using monkey islets. By contrast, neither ATP nor any of the other tested purinergic agonists stimulated insulin release in pig, mouse, or rat islets (Fig. 2C and Fig. S2). In rat islets, only high concentrations of ATP (1 mM and 10 mM) induced small increases in insulin release (Fig. 2C).
ATPγS (50 μM; a nonhydrolysable ATP analog), the specific P2X receptor agonist BzATP (50 μM), and the P2X1 and P2X3 agonist α,β-methylene ATP (50 μM) elicited strong insulin responses (Fig. 2D). P2Y receptors were not involved in the response to endogenously released ATP during glucose stimulation (Results) but could be directly activated by the selective agonists UTP (100 μM; an agonist of P2Y2, P2Y4, and P2Y6) and ADP (100 μM; an agonist of P2Y1, P2Y12, and P2Y13) to increase insulin release (Fig. 2D), suggesting the presence of multiple ATP receptor subtypes in the human β cell. Adenosine had a minor effect on insulin release, indicating that P1 receptors were only modestly involved (Fig. 2D). The magnitudes of the insulin responses to ATP (100 μM), ATPγS (50 μM), BzATP (50 μM), UTP (100 μM), and ADP (100 μM) in islets kept at high glucose (11 mM) were similar to the magnitudes of insulin responses to these agonists that were recorded in islets kept at low glucose concentrations, indicating that the effects of purinergic receptor activation are not altered at higher glucose levels.
Our results suggest that human islets express P2X receptors whose activation strongly stimulates insulin secretion. By using RT-PCR, we found that all P2X receptor genes were expressed in human islets, confirming results from the Beta Cell Biology Consortium database (http://www.betacell.org/resources/data/epcondb/). To localize P2X receptor expression in the islet, we performed in situ hybridization on human pancreatic sections. Strong hybridization signals in human islets were detected for P2X3, P2X5, and P2X7 (Fig. 3A). By combining in situ hybridization with immunofluorescence for islet hormones, we found that these receptors were expressed in β cells (Fig. 3A). No signals could be detected with P2X1, P2X2, P2X4, P2X6, or control sense riboprobes. Immunofluorescence and Western blots further showed that the P2X3 protein was present in β cells (Fig. 3 B and C). Although P2X5 and P2X7 immunoreactivities were seen in islets, they could not be blocked by control peptide preadsorption. Therefore, it was not possible to determine if the staining could be considered a reliable indication of P2X5 and P2X7 receptor protein expression.
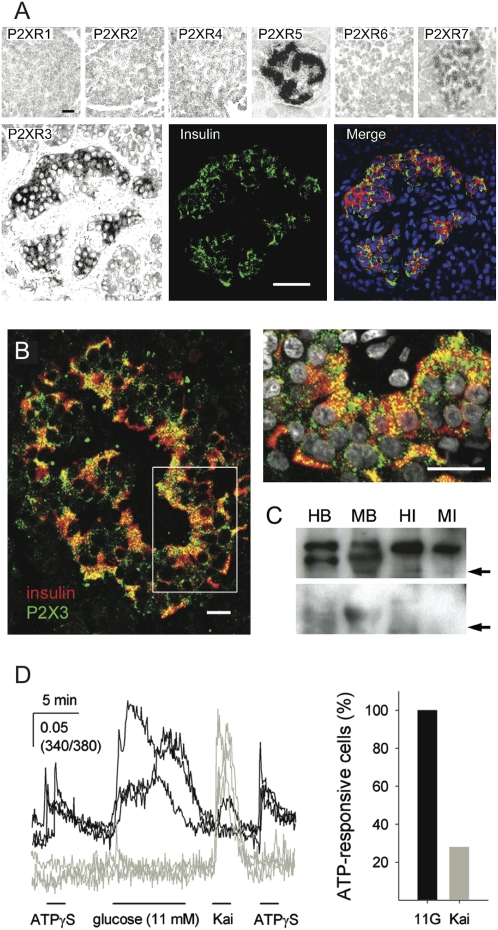
Fig. 3.
P2X expression profiles in human islets. (A) In situ hybridization on human pancreatic sections with riboprobes for all P2X receptors showed expression of P2X3, P2X5, and P2X7 mRNA in islets (Upper). No hybridization signal could be detected for P2X1, P2X2, P2X4, or P2X6. The hybridization signal for P2X3 colocalized with insulin immunoreactivity (Lower). (Scale bar, 50 μm.) Images are representative of three human pancreata. (B) Confocal images of human pancreatic sections showing immunoreactivity for P2X3 in islets. P2X3 immunoreactivity (green) localized to insulin-expressing β cells [red; Right, higher magnification image of region indicated by Left) is shown. Cell nuclei are shown in gray. Images are representative of five human pancreata. (Scale bar, 20 μm.) (C) Western blotting analysis of lysates from human (HI) and monkey islets (MI) with human (HB) and monkey brain (MB) used as positive controls. A band for P2X3 receptors is visible at ∼65 kDa (Upper). Specific bands disappeared when primary antibodies were preadsorbed with their cognate protein (Lower). Arrows indicate 50 kDa molecular weight (n = 3 islet preparations). A molecular marker was run in parallel. (D) ATPγS (50 μM) induced [Ca2+]i responses in individual human islet cells loaded with Fura-2. These cells responded to stimulation with high glucose (11 mM; black traces, representative of 8 cells). Most of the alpha cells, identified by their response to kainate (100 μM), did not respond to ATPγS (gray traces; representative of 25 cells). Bars indicate the duration of the stimulus. The graph (Right) shows the percentages of cells that responded to ATPγS in the glucose-responsive (11G; n = 8) and kainate-responsive cell populations (Kai; n = 25). Recorded at low glucose concentration (3 mM).
Isolated human islet cells were examined for the presence of functional P2X receptors using measurements of [Ca2+]i. Beta cells, identified by their response to high glucose (11 or 16 mM) (8), responded to ATPγS (50 μM) and BzATP (50 μM) with rapid [Ca2+]i increases (Fig. 3D). A fraction of the cells (30%) that responded to the alpha cell-specific stimulus kainate (100 μM) (8) responded to ATPγS (50 μM) or BzATP (50 μM) with rapid [Ca2+]i increases (Fig. 3D). In line with these results, ATP stimulated small increases in glucagon secretion in human, monkey, and mouse islets, and a subset of human alpha cells expressed P2X4 receptors (Fig. S3).
What are the mechanisms by which ATP induces insulin secretion in human β cells? Insulin responses to ATP (10 μM) were inhibited by the general P2 receptor antagonist suramin (100 μM) and the specific P2X antagonist iso-PPADS (50 μM; ∼95% inhibition; Fig. 4A). In the nominal absence of extracellular Ca2+, insulin responses to ATP (Fig. 4B) and α, β meATP (100 μM) were strongly diminished. By contrast, blocking the contribution of Ca2+ release from intracellular stores with thapsigargin (1 μM) had no effect on insulin responses to ATP (Fig. 4B). The Ca2+ needed for ATP-induced insulin secretion could enter through the P2X receptor pore or voltage-dependent Ca2+ channels, which are activated as a consequence of P2X receptor-mediated membrane depolarization. The broad-spectrum voltage-gated Ca2+ channel blocker Cd2+ (100 μM; a concentration not affecting Ca2+ influx through P2X receptors) (46, 47) and the L-type Ca2+ channel blocker nifedipine (10 μM) abolished insulin responses to ATP (Fig. 4B) or α, β meATP. That ATP failed to increase insulin secretion in the presence of Cd2+ or nifedipine indicates that P2X receptor activation caused sufficient depolarization to activate voltage-dependent Ca2+ channels (15, 17, 47), particularly L-type Ca2+ channels critical to action potential firing in human β cells (48).
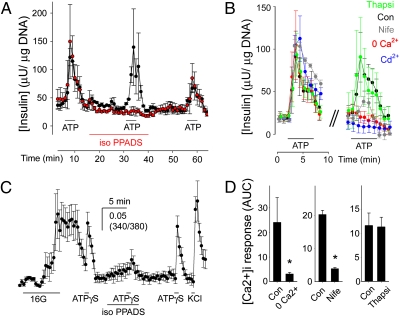
Fig. 4.
ATP-induced insulin release by human β cells requires P2X receptor activation and Ca2+ influx through voltage-gated Ca2+ channels. (A) Insulin secretion induced by ATP (10 μM) was inhibited in the presence of iso-PPADS (50 μM). Average traces from three islet preparations ± SEM with (red symbols) and without (black symbols) incubation in iso-PPADS. Bars indicate drug or antagonist application. (B) Insulin secretion induced by ATP (10 μM) was reduced in nominal 0 Ca2+ (+1 mM EGTA; red symbols) or in the presence of the Ca2+ channel blockers Cd2+ (100 μM; blue symbols) or nifedipine (Nife; 10 μM; gray symbols). Thapsigargin treatment (Thapsi; 1μM; green symbols) did not affect insulin responses. Average insulin response of three islet preparations (± SEM) before (Left) and during treatment (Right). Con, control insulin response to ATP (black symbols). (C) Iso-PPADS reduced [Ca2+]i responses induced by ATPγS (50 μM) in human β cells. Only islet cells that responded to high glucose (16 mM) were examined. Bars indicate the duration of the stimulus or antagonist application. Average trace is shown (7 cells ± SEM). (D) [Ca2+]i responses induced by ATPγS (50 μM) were reduced in nominal 0 Ca2+ (+1 mM EGTA) or in the presence of nifedipine (10 μM). [Ca2+]i responses to ATPγS were not decreased in the presence of thapsigargin (1 μM). Shown is the average peak response amplitude ± SEM of ≥3 cells from three human islet preparations. Asterisks denote statistical significance (Student t test; P < 0.05). Con, control [Ca2+]i response to ATPγS before treatment; AUC, area under the curve.
ATP and the P2X receptor agonists BzATP and α, β meATP elicited repeatable [Ca2+]i responses in β cells that were comparable with responses to glucose or KCl stimulation (Fig. 4C). [Ca2+]i responses to ATP were blocked by isoPPADS by ∼80% in human β cells (Fig. 4C). Thapsigargin (1 μM) did not affect [Ca2+]i responses to ATP, indicating little contribution of Ca2+ released from intracellular stores (Fig. 4D). The nominal absence of extracellular Ca2+ or the addition of nifedipine (10 μM) reduced [Ca2+]i responses to ATP (Fig. 4D), indicating a major Ca2+ influx through the β-cell plasma membrane.
Discussion
Our results show that human β cells express receptors for extracellular ATP that mediate an essential positive autocrine feedback loop for insulin secretion. We have presented evidence that this autocrine feedback loop is present in human and nonhuman primate islets but not in the other species that we examined. Although we cannot completely rule out the role of P2Y receptors, our results suggest that, in primates, P2X receptors predominate in the ATP (purinergic) signaling pathway that amplifies the secretion of insulin in response to rapid increases in glucose concentration (Fig. 5).
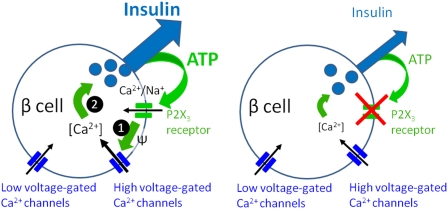
Fig. 5.
Proposed model for the positive autocrine feedback loop mediated by ATP in human β cells. ATP, coreleased with insulin, activates ionotropic P2X3 receptors in the β-cell plasma membrane. This opens the cation selective P2X3 channel pore to let Na+ and Ca2+ flow into the cell (1). The resultant membrane depolarization and increase in action potential frequency increases Ca2+ flux through high voltage-gated Ca2+ channels. Increased [Ca2+]i (2) stimulates insulin secretion. In the absence of P2X3 activation, insulin secretion is diminished (Right).
The role of purinergic signaling in the regulation of insulin secretion has been investigated in rodent models, but only two reports have been published on human islets. Our findings have revealed a signaling pathway for ATP in human β cells. We have found that ATP is already released at low glucose concentrations, which is in agreement with recent studies in rodents showing that ATP can be released from secretory granules while insulin is retained (12, 34). Therefore, ATP signaling may precede secretion of insulin, sensitizing the β cell to respond appropriately to glucose stimulation. This notion is in line with studies showing that ATP facilitates neurotransmitter release in presynaptic nerve terminals (49, 50). Our results further suggest that ATP release seems to be strongest during sharp increases in glucose concentration. Although exogenous ATP promoted strong responses in islets kept at constant glucose concentrations (3 mM or 11 mM), it was not effective during abrupt increases in glucose concentration, indicating that the receptors were fully activated by endogenously released ATP under these conditions. Thus, we showed that ATP is a signal serving in an autocrine positive feedback loop for insulin release subsequent to glucose stimulation.
Our results showing substantial differences between human β cells and rodent β cells in terms of ATP signaling reiterate that the structure and function of the human islets are distinctive (31, 32). Our studies revealed that ATP is a potent stimulator of insulin release in islets of primate species but not in those of the other examined species. Because we used the same technical approach for all species tested, the most likely explanation is that ATP signaling differs between species.
The differences in purinergic signaling suggest that β cells of various species express different subsets of purinergic receptors. Our results show that both P2X and P2Y receptors can be activated in human β cells, but the responses mediated by P2X receptors predominate. In mice, ATP elicits [Ca2+]i responses in β cells predominantly through P2Y receptors, not P2X receptors (26, 51). There are only a few studies examining the expression of P2X receptors in the endocrine pancreas of any species. Recently, P2X1 and P2X3 receptors were identified in isolated single mouse β cells (30), and P2X1, P2X2, P2X3, P2X4, and P2X6 have been detected in the mouse and rat pancreas (28, 52, 53). The expression of these receptors in rodent β cells needs to be confirmed with in situ hybridization or single-cell RT-PCR studies. It is important to stress, however, that ATP did not evoke insulin responses at low glucose concentrations in mouse islets (Fig. S1), suggesting that, even if expressed, P2X receptors in mouse β cells may not be activated to amplify the early insulin response to glucose stimulation. This is consistent with reports showing that extracellular ATP does not increase insulin secretion in mouse islets (24–26).
P2X receptors most likely contribute to shape the electric activity of human β cells. Direct application of ATP at 3 mM glucose elicited large insulin and [Ca2+]i responses that were comparable with those elicited by high glucose or KCl depolarization. Blocking ATP receptors with P2X receptor antagonists reduced the insulin response to high glucose by up to 65% (Fig. 2), revealing a strong contribution of ATP receptor activation to the response. Our results further indicated that most of the human β-cell response to ATP was mediated by ionotropic P2X receptors (Fig. 4). This activation promotes considerably large inward currents in the nA range and thereby depolarizes the β-cell membrane, which results in increased electric activity (30). However, the exact magnitude of the currents will depend on the amount of ATP released, the receptor density, and/or their localization.
By using a combination of technical approaches, we have consistently identified P2X3 receptors in human β cells. P2X1, P2X2, P2X4, and P2X6 receptors, reported to be expressed in rodent β cells (28, 30, 52, 53), could not be detected in human β cells. In contrast, our studies revealed the presence of P2X5 and P2X7. Therefore, P2X receptors in human β cells may exist as monomers or heteromers of combinations of P2X3, P2X5, and P2X7. The presence of a polymorphism at a critical position in the human P2X5 gene indicates that only a small subset of humans (∼14%) will process and translate a functional protein (54, 55), ruling out a contribution of P2X5 to ATP signaling in β cells in most human beings. P2X7 receptors are unlikely to form heteromeric receptors with P2X3 (17) but may work as homomeric receptors. Homomeric P2X7 receptors, however, likely do not participate in normal β-cell physiology, because their activation requires ATP concentrations >100 μM (17). This is in agreement with our results showing that P2X7 receptor antagonists did not affect the positive autocrine feedback loop mediated by ATP. If P2X7 receptors are activated to promote human β-cell death during pathophysiological processes, as described in other systems (13), remains to be determined. Under physiological conditions, the most likely scenario is that P2X3 homomeric receptors are mediating the positive autocrine feedback loop for insulin release that we are describing.
Autocrine loops with positive feedback allow cells to modulate the amplitude and the duration of the signaling response to external stimuli (56). We propose that ATP functions in an automodulatory system that, when activated by an increase in blood glucose, adds speed and sensitivity to the β-cell secretory response. The β cell secretes ATP along with insulin when the glucose concentration increases. Released ATP then activates P2X3 receptors in the β-cell plasma membrane. Activation of P2X3 receptors leads to membrane depolarization mediated by Ca2+ and Na+ influx (17) and subsequent opening of voltage-gated Ca2+ channels. This results in increased [Ca2+]i and enhanced insulin secretion. This positive feedback allows the β cell to translate small changes in plasma glucose into large alterations in insulin release. Thus, positive ATP autocrine signaling may explain how adequate and fast insulin release can be achieved in response to modest physiological changes in blood glucose concentration.
Experimental Procedures
A detailed discussion can be found at SI Experimental Procedures.
Pancreatic Islets.
Human pancreatic islets were obtained from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami Miller School of Medicine or from the Islet Cell Resource basic science islet distribution program, Islet Cell Resource Centers (ICRs) Consortium, Division of Clinical Research, National Center for Research Resources, National Institutes of Health.
[Ca2+]i Imaging.
[Ca2+]i imaging was performed as previously described (8, 36).
Insulin and Glucagon Secretion.
Insulin and glucagon secretion were measured as previously described (8, 36).
Immunohistochemistry.
In Situ Hybridization.
In situ hybridization using digoxigenin (DIG)-labeled RNA probes for mRNA detection of human P2XRs (1–7) was performed as described (60).
Western Blotting.
Immunoblot analysis was carried out by standard methods with the antibodies used for P2X immunohistochemistry (1:1,000). In control experiments, primary antibodies were incubated with corresponding control peptide (Alomone Labs) at a ratio of 50 μg antigenic peptide to 1 μg antibody at room temperature for 5 h.
Statistical Analyses.
For statistical comparisons, we used a Student t test or a one-way ANOVA followed by multiple comparison procedures with the Bonferroni t test. Throughout the manuscript, data are presented as average ± SEM.
Supplementary Material
Acknowledgments
We thank the members of the Human Cell Processing Facility, Translational Research Laboratory of the Cell Transplant Center, Clinical Islet Transplant Center, Organ Procurement Organizations, the Islet Cell Resource Center Basic Science Islet Distribution Program, and Administrative Offices at the Diabetes Research Institute. This work was supported by National Institutes of Health Grants for General Clinical Research Center M01RR16587 and 1R01-DK55347-IU42RR016603, Islet Cell Resources Grants 5U42RR016603 and 1R03DK075487-01 (to A.C.), Juvenile Diabetes Research Foundation International Grant 4-2004-361, Juvenile Diabetes Research Foundation International Grant 3-2006-853 (to M.C.J.-S.), the Diabetes Research Institute Foundation, the Swedish Research Council, Novo Nordisk Foundation, the Swedish Diabetes Association, the Berth von Kantzow's Foundation, and the Family Erling-Persson Foundation. M.C.J.-S. was the recipient of a postdoctoral fellowship from Comissao de Aperfeicoamento de Pessoal de Nivel Superior/Brazil.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908935107/DCSupplemental.
References
- 1.Conn PM, Goodman HM, Kostyo JL. The Endocrine System. New York: Oxford University Press; 1998. pp. 1–5. [Google Scholar]
- 2.Doyle ME, Egan JM. Pharmacological agents that directly modulate insulin secretion. Pharmacol Rev. 2003;55:105–131. doi: 10.1124/pr.55.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Franklin IK, Wollheim CB. GABA in the endocrine pancreas: Its putative role as an islet cell paracrine-signaling molecule. J Gen Physiol. 2004;123:185–190. doi: 10.1085/jgp.200409016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- 5.Kisanuki K, et al. Expression of insulin receptor on clonal pancreatic alpha cells and its possible role for insulin-stimulated negative regulation of glucagon secretion. Diabetologia. 1995;38:422–429. doi: 10.1007/BF00410279. [DOI] [PubMed] [Google Scholar]
- 6.Leibiger IB, Leibiger B, Berggren PO. Insulin feedback action on pancreatic β-cell function. FEBS Lett. 2002;532:1–6. doi: 10.1016/s0014-5793(02)03627-x. [DOI] [PubMed] [Google Scholar]
- 7.Rorsman P, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341:233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera O, et al. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. 2008;7:545–554. doi: 10.1016/j.cmet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detimary P, Jonas JC, Henquin JC. Stable and diffusible pools of nucleotides in pancreatic islet cells. Endocrinology. 1996;137:4671–4676. doi: 10.1210/endo.137.11.8895332. [DOI] [PubMed] [Google Scholar]
- 10.Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic β cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- 11.Leitner JW, Sussman KE, Vatter AE, Schneider FH. Adenine nucleotides in the secretory granule fraction of rat islets. Endocrinology. 1975;96:662–677. doi: 10.1210/endo-96-3-662. [DOI] [PubMed] [Google Scholar]
- 12.MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic β cells. Cell Metab. 2006;4:283–290. doi: 10.1016/j.cmet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Fields RD, Burnstock G. Purinergic signaling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 16.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 17.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 18.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 19.Knott TK, Velázquez-Marrero C, Lemos JR. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflugers Arch. 2005;450:381–389. doi: 10.1007/s00424-005-1471-x. [DOI] [PubMed] [Google Scholar]
- 20.Tomić M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 21.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 22.Petit P, Manteghetti M, Puech R, Loubatieres-Mariani MM. ATP and phosphate-modified adenine nucleotide analogues. Effects on insulin secretion and calcium uptake. Biochem Pharmacol. 1987;36:377–380. doi: 10.1016/0006-2952(87)90297-8. [DOI] [PubMed] [Google Scholar]
- 23.Salehi A, Qader SS, Quader SS, Grapengiesser E, Hellman B. Inhibition of purinoceptors amplifies glucose-stimulated insulin release with removal of its pulsatility. Diabetes. 2005;54:2126–2131. doi: 10.2337/diabetes.54.7.2126. [DOI] [PubMed] [Google Scholar]
- 24.Léon C, et al. The P2Y(1) receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal. 2005;1:145–151. doi: 10.1007/s11302-005-6209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petit P, et al. Evidence for two different types of P2 receptors stimulating insulin secretion from pancreatic B cell. Br J Pharmacol. 1998;125:1368–1374. doi: 10.1038/sj.bjp.0702214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poulsen CR, et al. Multiple sites of purinergic control of insulin secretion in mouse pancreatic β-cells. Diabetes. 1999;48:2171–2181. doi: 10.2337/diabetes.48.11.2171. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand G, Chapal J, Loubatières-Mariani MM, Roye M. Evidence for two different P2-purinoceptors on β cell and pancreatic vascular bed. Br J Pharmacol. 1987;91:783–787. doi: 10.1111/j.1476-5381.1987.tb11276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards-Williams C, Contreras JL, Berecek KH, Schwiebert EM. Extracellular ATP and zinc are co-secreted with insulin and activate multiple P2X purinergic receptor channels expressed by islet β-cells to potentiate insulin secretion. Purinergic Signal. 2008;4:393–405. doi: 10.1007/s11302-008-9126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Alvarez J, Hillaire-Buys D, Loubatières-Mariani MM, Gomis R, Petit P. P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas. 2001;22:69–71. doi: 10.1097/00006676-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Silva AM, et al. Electrophysiological and immunocytochemical evidence for P2X purinergic receptors in pancreatic β cells. Pancreas. 2008;36:279–283. doi: 10.1097/MPA.0b013e31815a8473. [DOI] [PubMed] [Google Scholar]
- 31.Brissova M, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 32.Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun M, et al. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic β cells. J Gen Physiol. 2007;129:221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermüller S, et al. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- 35.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 36.Cabrera O, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 2008;16:1039–1048. [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 38.Cunha RA. Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem Res. 2001;26:979–991. doi: 10.1023/a:1012392719601. [DOI] [PubMed] [Google Scholar]
- 39.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Kittel A, Garrido M, Varga G. Localization of NTPDase1/CD39 in normal and transformed human pancreas. J Histochem Cytochem. 2002;50:549–556. doi: 10.1177/002215540205000412. [DOI] [PubMed] [Google Scholar]
- 41.Crack BE, et al. Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br J Pharmacol. 1995;114:475–481. doi: 10.1111/j.1476-5381.1995.tb13251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westfall TD, Kennedy C, Sneddon P. The ecto-ATPase inhibitor ARL 67156 enhances parasympathetic neurotransmission in the guinea-pig urinary bladder. Eur J Pharmacol. 1997;329:169–173. [PubMed] [Google Scholar]
- 43.Hillaire-Buys D, Gross R, Parés-Herbuté N, Ribes G, Loubatières-Mariani MM. In vivo and in vitro effects of adenosine-5′-O-(2-thiodiphosphate) on pancreatic hormones in dogs. Pancreas. 1994;9:646–651. [PubMed] [Google Scholar]
- 44.Karlsson S, Myrsén U, Nieuwenhuizen A, Sundler F, Ahrén B. Presynaptic sympathetic mechanism in the insulinostatic effect of epinephrine in mouse pancreatic islets. Am J Physiol. 1997;272:R1371–R1378. doi: 10.1152/ajpregu.1997.272.5.R1371. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi BR, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, Koizumi S, Nakazawa K. Glutamate-evoked release of adenosine 5′-triphosphate causing an increase in intracellular calcium in hippocampal neurons. Neuroreport. 1995;6:437–440. doi: 10.1097/00001756-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Khakh BS, Henderson G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol Pharmacol. 1998;54:372–378. doi: 10.1124/mol.54.2.372. [DOI] [PubMed] [Google Scholar]
- 48.Braun M, et al. Voltage-gated ion channels in human pancreatic β-cells: Electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 49.Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- 50.Dorostkar MM, Boehm S. Presynaptic lonotropic receptors. Handb Exp Pharmacol. 2008;184:479–527. doi: 10.1007/978-3-540-74805-2_15. [DOI] [PubMed] [Google Scholar]
- 51.Hellman B, Dansk H, Grapengiesser E. Pancreatic β-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab. 2004;286:E759–E765. doi: 10.1152/ajpendo.00452.2003. [DOI] [PubMed] [Google Scholar]
- 52.Coutinho-Silva R, Parsons M, Robson T, Burnstock G. Changes in expression of P2 receptors in rat and mouse pancreas during development and ageing. Cell Tissue Res. 2001;306:373–383. doi: 10.1007/s004410100458. [DOI] [PubMed] [Google Scholar]
- 53.Coutinho-Silva R, Parsons M, Robson T, Lincoln J, Burnstock G. P2X and P2Y purinoceptor expression in pancreas from streptozotocin-diabetic rats. Mol Cell Endocrinol. 2003;204:141–154. doi: 10.1016/s0303-7207(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 54.Lê KT, Paquet M, Nouel D, Babinski K, Séguéla P. Primary structure and expression of a naturally truncated human P2X ATP receptor subunit from brain and immune system. FEBS Lett. 1997;418:195–199. doi: 10.1016/s0014-5793(97)01380-x. [DOI] [PubMed] [Google Scholar]
- 55.Bo X, et al. Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol. 2003;63:1407–1416. doi: 10.1124/mol.63.6.1407. [DOI] [PubMed] [Google Scholar]
- 56.Shvartsman SY, et al. Autocrine loops with positive feedback enable context-dependent cell signaling. Am J Physiol Cell Physiol. 2002;282:C545–C559. doi: 10.1152/ajpcell.00260.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.