Abstract
Brain activity in sleep plays a crucial role in memory consolidation, an offline process that determines the long-term strength of memory traces. Consolidation efficacy differs across individuals, but the brain activity dynamics underlying these differences remain unknown. Here, we studied how interindividual variability in fear memory consolidation relates to neural activity in brain structures that participate in Pavlovian fear learning. From the end of training to testing 24 h later, some rats showed increased and others decreased conditioned fear responses. We found that overnight bidirectional changes in fear memory were selectively correlated with modifications in theta coherence between the amygdala, medial prefrontal cortex, and hippocampus during paradoxical sleep. Thus, our results suggest that theta coordination in the limbic system may influence interindividual differences in memory consolidation of aversive experiences.
Keywords: amygdala, fear conditioning, prefrontal cortex, hippocampus, Granger causality
Recently formed memories undergo a period of consolidation (1, 2), and neuronal activity taking place during sleep is thought to play a critical role in this process (3–7). Consolidation of emotional memories determines their long-term retention, and interindividual variations in memory performance have been related to various factors affecting consolidation, including neuromodulatory transmission (8), levels of circulating stress hormones (9), or genetic factors (10). Several strongly interconnected brain structures are involved in the formation and maintenance of emotional memories, including the hippocampus (Hi), medial prefrontal cortex (mPFC), and basolateral amygdala (BLA) (11–13). The latter structure in particular was shown to mediate the facilitating effects of emotions on memory consolidation (14). Indeed, we form more vivid and enduring memories for emotionally arousing experiences (15). Via the release of peripheral stress hormones (16), emotional arousal causes long-lasting increases in the firing rate of BLA neurons (17), and pharmacologic manipulations that prevent this increased activity interfere with memory for events that took place shortly before, in many learning tasks (18). Importantly, in most learning paradigms, the same interventions performed shortly before testing long-term memory recall have no effect, indicating that the BLA can facilitate the consolidation of memories in other brain structures (14, 18).
Potentially related to the facilitation of emotional memories by BLA activity, various lines of evidence implicate posttraining paradoxical sleep (PS) in memory consolidation (6, 19). These include PS reactivation of brain areas implicated in prior learning (20) and spontaneous replay of waking activity patterns during PS (21). In fact, it was suggested that the distinct pattern of neuronal activity occurring during PS favors memory consolidation (4, 22) and that emotional memories are particularly susceptible to this effect (5, 23). Indeed, after fear conditioning, the responsiveness of amygdala and thalamic neurons to conditioned stimuli is enhanced during PS (24). Moreover, aversive events increase PS amounts (25, 26), whereas PS deprivation after training impairs consolidation of aversive memories (27). Therefore, the present study investigated whether interindividual variations in the consolidation of emotional memories are related to BLA, mPFC, and Hi interactions during PS.
Results
We focused on the consolidation of classically conditioned fear responses to auditory cues because this form of learning was shown to cause synaptic plasticity in a widely distributed network of structures, including different amygdala nuclei (BLA and central amygdala) (28–31), multiple stages of the auditory pathways (32, 33), Hi (34), and mPFC (35). Thus, auditory fear conditioning constitutes an ideal model to study the role of sleep activity in system-level memory consolidation.
In our experimental paradigm (Fig. 1A), rats (n = 12) were subjected to a classic fear conditioning protocol in which an auditory conditioned stimulus (CS) coterminated with a noxious unconditioned stimulus (US). Before and after fear conditioning, spontaneous unit activity and local field potentials (LFPs) were recorded during waking and sleep in the BLA, mPFC, and Hi. Twenty-four hours later, recall of the fear memory was tested by presenting the CS in a different context (Fig. S1).
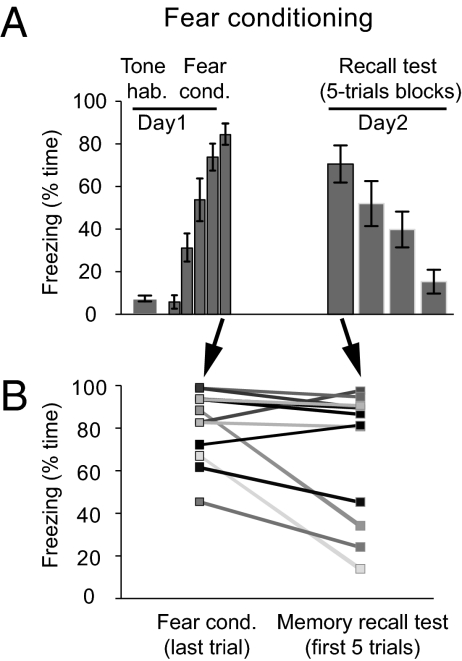
Fig. 1.
Interindividual variations in the efficacy of memory consolidation in Pavlovian fear conditioning. (A) Time course of percentage time spent freezing (mean ± SEM) to the CS in rats (n = 12) during habituation, fear conditioning (day 1), and memory recall test (day 2). (B) Overnight changes in individual performance are bidirectional and are not determined by the level of freezing at the end of the conditioning.
From the last CS of the training session to the recall test the next day, there were marked variations in the retention of the conditioned fear response (CR), with some rats showing inflation and others reduction in the time spent freezing during the CS (Fig. 1B). Importantly, there was no correlation between the change in CR seen from training to recall and freezing levels during tone habituation (r = 0.25, P = 0.43, n = 12) or the training session (last CS; r = 0.27, P = 0.40, n = 12). Thus, variations in retention were not related to baseline anxiety levels or the strength of the CS-US association at the end of training. We therefore tested whether interindividual variations in consolidation, measured as the difference in freezing between the last CS of the fear conditioning session vs. the first five CSs of the recall test, correlated with pre- to posttraining changes in neuronal activity during sleep. Because theta oscillations in the Hi-BLA (36) and mPFC (23, 37) network have been implicated in memory, and because neuronal activity (38) and theta oscillations are prominent during PS in the Hi (39), BLA (22), and mPFC (40), we examined whether fluctuations in PS theta activity correlated with variations in memory consolidation.
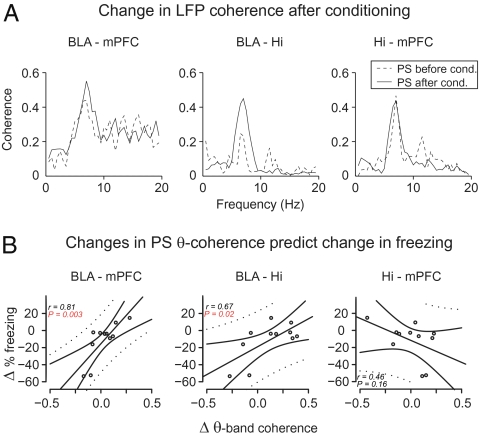
Theta oscillations were present in Hi, BLA, and mPFC LFPs during PS (Fig. S2), with rhythmic theta-related unit activity at these sites (40–42) (Fig. S3), indicating that phase-locked synaptic activity likely contributes to local theta LFP generation in these structures. The amplitude of the theta oscillations in BLA and mPFC was weakly correlated with that of hippocampal theta (mean ± SEM: BLA: r2 = 0.098 ± 0.028; mPFC: r2 = 0.013 ± 0.005; n = 12), indicating that volume conduction between these structures contributed marginally to BLA and mPFC theta oscillations. Theta oscillations at the three sites were coherent with one another, as shown by peaks at the theta frequency in the coherence spectra (Fig. 2A). However, coherent theta occurred only intermittently during PS, with interleaved bouts of correlated and uncorrelated activity coinciding with similarly high-amplitude hippocampal theta (Fig. S2). Remarkably, interindividual variations in fear memory consolidation strongly correlated with changes in theta coherence from pre- to posttraining PS episodes (Fig. 2B, n = 11) for BLA-mPFC (r = 0.81, P = 0.003) and BLA-Hi, (r = 0.67, P = 0.025) but not for Hi-mPFC (r = −0.46, P = 0.16). In contrast, such correlations were not found in other frequency bands or vigilance states (see Table S1, although a statistical trend was found for the correlation between consolidation and changes of delta slow-wave sleep coherence in the BLA-Hi) nor with changes in LFP power. Although there was no relationship between consolidation and the latency (36.1 ± 2.4 min) of posttraining PS episodes (r = 0.25, P = 0.42), there was a trend for a correlation with the duration of posttraining PS episodes (duration: r = 0.53, P = 0.09). This result is consistent with earlier reports of increased PS after learning (19).
Fig. 2.
Changes in LFP theta coherence from pre- to postconditioning PS epochs correlate with interindividual variations in the efficacy of memory consolidation. (A) Examples of pre- to posttraining shifts in LFP coherence spectra in amygdala-Hi-cortex network during PS. (B) Linear regression of overnight change in freezing vs. shifts in PS theta coherence. Positive values in the y and x axes indicate increases in freezing levels and theta coherence from training to testing, respectively. Each point corresponds to one animal (n = 11). The plain and dotted curves correspond to the 95% tolerance and confidence bands of the linear regression, respectively. The correlation in B (Left) remained significant even after omitting the two most extreme animals.
Moreover, for all pairs of recording sites, changes in theta LFP coherence from pre- to posttraining PS were not correlated with freezing during the last CS of the training session (BLA-Hi: r = 0.15, P = 0.29; BLA-mPFC: r = 0.21, P = 0.47; Hi-mPFC: r = 0.25, P = 0.46). Overall, these results indicate that the changes in theta coherence from pre- to posttraining PS episodes were not related to baseline anxiety levels, to the strength of the CS-US association at the end of training, or to the timing of PS epochs, and are thus specifically correlated with the efficacy of the consolidation process (Table S2).
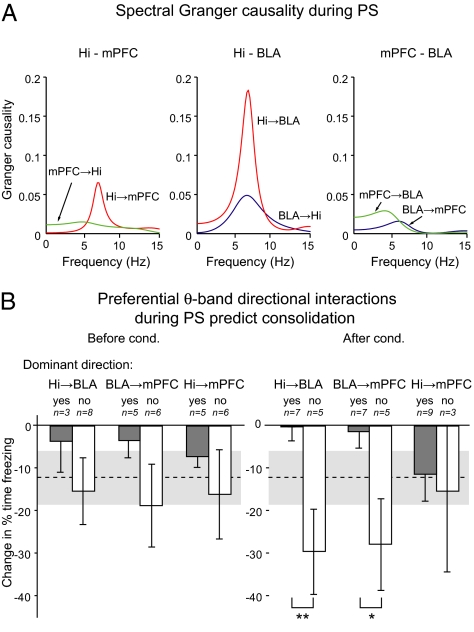
To analyze how theta oscillations circulate in the Hi-BLA-mPFC network, we used Granger causality analysis (43, 44) (Fig. 3), a method that measures whether one time series is causal to another by testing whether past values of the former help predict future values of the latter. This causality can be expressed in the frequency domain (43) and has been used to study the directionality of interactions between cortical areas for specific frequency bands (45, 46). When present, peaks in the theta band in the causality spectra (Fig. 3A) predominantly indicated an influence of Hi theta over mPFC and BLA theta before and after conditioning, indicating a rhythmic entrainment of these structures by the Hi (Fig. S4). To examine whether the directionality of theta interactions was associated with interindividual differences in the efficacy of memory consolidation, we compared the performance of the rats exhibiting predominant theta interactions in causality spectra with the performance of the other rats (Fig. 3B). This analysis revealed that the directionality of theta interactions among the three structures after (Fig. 3B, Right) but not before (Fig. 3B, Left) training was differentially related to the efficacy of the consolidation process (Table S3). Indeed, in the posttraining PS episodes, causality analyses revealed that rats with a stronger influence of Hi over BLA theta or of BLA over mPFC theta than the opposite direction displayed a significantly higher efficacy of overnight consolidation (Hi-BLA: t test, P = 0.009; BLA-mPFC: t test, P = 0.024; n = 12). In contrast, the directionality of Hi-mPFC theta interactions was not predictive of consolidation efficacy (t test, P = 0.79).
Fig. 3.
Granger causality analysis in the Hi-mPFC-BLA network during PS. (A) Examples of spectral Granger causality between pairs of structures. Each curve corresponds to a pair of channels (e.g., Hi → BLA), and peaks indicate the frequencies for which the largest fraction of the power of the second channel may be attributed to a causal influence of activity in the first channel. (B) Overnight change in freezing (mean ± SEM) is predicted by the preferential directional theta interactions in the Hi-mPFC-BLA network during PS after (Right) but not before (Left) conditioning. Words “yes” and “no” indicate whether the preferential direction of interaction in the group corresponds to the label indicated above. The average change in freezing from training to testing is indicated by the dashed line (mean) and gray band (mean ± SEM).
Discussion
It was previously reported that the amygdalohippocampal network exhibits synchronized theta activity during CS presentations after conditioning (36), and it was proposed that this activity supports system consolidation of fear memories (47). However, because this increased coherence was observed during CS presentations, its potential involvement in fear memory consolidation vs. expression could not be disentangled. Subsequent work revealed that this amygdalohippocampal theta synchrony is not a mere reflection of fear expression because it was selectively observed during the recall of long-term but not short-term fear memories (48). The present study lends further support to this view. Indeed, despite a relatively small sample size, we found that postlearning changes in theta coordination occur in PS, involve the mPFC, and are tightly correlated with interindividual variations in efficacy of the consolidation process. Our study notably suggests that the BLA-to-mPFC pathway (11, 49) is strongly involved in consolidation, an effect mirroring the role of the mPFC-to-BLA pathway in fear memory expression and extinction (49). Finally, our study indicates that the increased PS duration observed in earlier reports (19) might facilitate memory consolidation by allowing for prolonged theta-band interactions in limbic networks.
Although previous studies emphasized that conditioned fear memories are stored in the amygdala (29, 30), our results instead imply that consolidation of this form of memory involves coordinated interactions in a distributed network of structures. This is consistent with previous data indicating that fear conditioning leads to synaptic plasticity outside the amygdala, including various stages of the auditory pathways (32, 33), Hi (34), and mPFC (35).
Variations in consolidation of emotional memories had been previously described as a function of circulating hormones or neuromodulators, which are thought to affect the intracellular processes of plasticity (1). Our study provides a complementary view by indicating a direct link between consolidation efficacy and neuronal activity in a limbic network. Therefore, our results suggest that theta coordination of the fear network during PS participates in the consolidation of Pavlovian fear memories.
Materials and Methods
Procedures were conducted in accordance with National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Rutgers University Animal Care and Use Committee. Adult male Sprague-Dawley rats (Charles River) were housed individually with ad libitum access to food and water and maintained on a 12-h light/dark cycle. Rats were anesthetized with a mixture of isoflurane and O2 and administered atropine methyl nitrate to reduce secretions and aid breathing. In aseptic conditions, rats were mounted in a stereotaxic apparatus with nonpuncture ear bars. A local anesthetic (bupivacaine, s.c.) was injected in the region to be incised. The scalp was incised, small burr holes were made in the skull above the BLA, mPFC, and Hi, and bundles of eight microwires were inserted in each of these structures under stereotaxic guidance [from the bregma in mm: BLA: anteroposterior (AP) −3.3, mediolateral (ML) 5.0, dorsoventral (DV) 8.7; mPFC: AP +2.7, ML 0.5, DV 4.6; Hi: AP −5.6, ML 5.0, DV 8.0, and AP −3.3, ML 1.8, DV 2.7]. Electromyographic recordings were performed by means of wires inserted in the neck muscles. The rats were allowed 1 week to recover from the surgery.
Fear conditioning and recall testing occurred in different contexts (context A and B). For fear conditioning (context A), rats were placed in a conditioning chamber with a metal grid floor (Coulbourn Instruments) that was enclosed within a sound attenuating chamber. The chamber was dimly illuminated by a single house light. For testing recall, the chamber contained a black Plexiglas floor washed with peppermint soap (context B). The rats were first habituated to context A and B for 15 min each in a counterbalanced manner. The next day, the rats received a tone habituation session consisting of five CS presentations (20 s, 4 kHz, 80 dB). This was followed by a fear conditioning session in which the rats received presentations of five CS, each coterminating with a footshock US (0.5 mA, 1 s). Before and after fear conditioning, spontaneous unit and LFP activity was recorded with a sampling rate of 25 kHz during the different states of vigilance (in one rat, no PS was obtained before conditioning). Recall was tested 24 h later in context B with multiple CS presentations. Behavior was recorded by a video camera and scored offline. Time spent freezing (immobility, with the exception of breathing) was measured.
At the end of behavioral experiments, the animals were given an overdose of pentobarbital (100 mg kg−1, i.p.) and perfused intracardially with 0.9% saline, followed by paraformaldehyde (4%). The brains were then removed, stored in paraformaldehyde (4%), sectioned at a thickness of 100 μm, and the sections counterstained with cresyl violet to assess the position of the recording sites. This report only includes data from recording sites histologically confirmed to be in the structures of interest.
Detailed information on data analysis is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grant RO1 MH073610 (to D. Paré) and by the Institut National de la Santé et de la Recherche Médicale (C.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913016107/DCSupplemental.
References
- 1.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 3.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Wagner U, Born J. Memory consolidation during sleep: Interactive effects of sleep stages and HPA regulation. Stress. 2008;11:28–41. doi: 10.1080/10253890701408822. [DOI] [PubMed] [Google Scholar]
- 6.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 8.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 9.Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 10.de Quervain DJ, et al. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- 11.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 13.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 14.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 15.Christianson SA. Handbook of Emotion and Memory: Current Research and Theory. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 16.Gold PE, McGaugh JL. A single-trace, two process view of memory storage processes. In: Deutsch D, Deutsch JA, editors. Short-Term Memory. New York: Academic Press; 1975. [Google Scholar]
- 17.Pelletier JG, Likhtik E, Filali M, Paré D. Lasting increases in basolateral amygdala activity after emotional arousal: Implications for facilitated consolidation of emotional memories. Learn Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 19.Smith C. Sleep states, memory processes and synaptic plasticity. Behav Brain Res. 1996;78:49–56. doi: 10.1016/0166-4328(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 20.Maquet P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 21.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 22.Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- 23.Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennevin E, Maho C, Hars B. Neuronal plasticity induced by fear conditioning is expressed during paradoxical sleep: Evidence from simultaneous recordings in the lateral amygdala and the medial geniculate in rats. Behav Neurosci. 1998;112:839–862. doi: 10.1037//0735-7044.112.4.839. [DOI] [PubMed] [Google Scholar]
- 25.Marinesco S, Bonnet C, Cespuglio R. Influence of stress duration on the sleep rebound induced by immobilization in the rat: A possible role for corticosterone. Neuroscience. 1999;92:921–933. doi: 10.1016/s0306-4522(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 26.Popa D, Léna C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: Evidence from sleep, stress, and behavior. J Neurosci. 2008;28:3546–3554. doi: 10.1523/JNEUROSCI.4006-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestri AJ. REM sleep deprivation affects extinction of cued but not contextual fear conditioning. Physiol Behav. 2005;84:343–349. doi: 10.1016/j.physbeh.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 29.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosi. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberger NM. Learning-induced changes of auditory receptive fields. Curr Opin Neurobiol. 1993;3:570–577. doi: 10.1016/0959-4388(93)90058-7. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: Cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- 35.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 37.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maquet P, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 39.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 40.Sirota A, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paré D, Gaudreau H. Projection cells and interneurons of the lateral and basolateral amygdala: Distinct firing patterns and differential relation to theta and delta rhythms in conscious cats. J Neurosci. 1996;16:3334–3350. doi: 10.1523/JNEUROSCI.16-10-03334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 43.Geweke J. Measurements of linear-dependance and feedback between multiple time-series. J Am Stat Assoc. 1982;77:304–313. [Google Scholar]
- 44.Granger CWJ. Investigating causal relations by economic models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- 45.Bernasconi C, König P. On the directionality of cortical interactions studied by structural analysis of electrophysiological recordings. Biol Cybern. 1999;81:199–210. doi: 10.1007/s004220050556. [DOI] [PubMed] [Google Scholar]
- 46.Brovelli A, et al. Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by Granger causality. Proc Natl Acad Sci USA. 2004;101:9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- 48.Narayanan RT, et al. Dissociated theta phase synchronization in amygdalo- hippocampal circuits during various stages of fear memory. Eur J Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- 49.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.