Abstract
T-regulatory cells (Treg) and mast cells (MC) are abundant in colorectal cancer (CRC) tumors. Interaction between the two is known to promote immune suppression or loss of Treg functions and autoimmunity. Here, we demonstrate that in both human CRC and murine polyposis the outcome of this interaction is the generation of potently immune suppressive but proinflammatory Treg (ΔTreg). These Treg shut down IL10, gain potential to express IL17, and switch from suppressing to promoting MC expansion and degranulation. This change is also brought about by direct coculture of MC and Treg, or culture of Treg in medium containing IL6 and IL2. IL6 deficiency in the bone marrow of mice susceptible to polyposis eliminated IL17 production by the polyp infiltrating Treg, but did not significantly affect the growth of polyps or the generation of proinflammatory Treg. IL6-deficient MC could generate proinflammatory Treg. Thus, MC induce Treg to switch function and escalate inflammation in CRC without losing T-cell–suppressive properties. IL6 and IL17 are not needed in this process.
Keywords: interleukin 17, interleukin 6
Enhancing the anti-tumor immune response through depletion or inactivation of Treg is an attractive therapeutic option for treatment of cancer, given that Treg expansion and infiltration into the tumor correlates with poor prognosis, as previously reviewed (1). Similarly, MC accumulation in human tumors is linked with poor outcome, which is in part attributed to release of mediators that promote angiogenesis and recruitment of proinflammatory cells (2, 3). An essential role for MC in tumor progression was documented in a number of murine models of cancer including polyposis (4), pancreatic islet tumors (5), and squamous carcinogenesis (6).
MC have an intricate interaction with Treg that regulates the functions of both cell types in a reciprocal manner. MC play an indispensable role in allograft acceptance, where they are required to sustain the peripheral tolerance mediated by Treg (7). Treg can inhibit MC differentiation (8) and hinder degranulation by contact-dependent mechanisms (9) and production of soluble factors, such as IL10 (10). Conversely, the activation and subsequent degranulation of MC breaks peripheral tolerance. MC degranulation (11) or direct cell contact and secretion of IL6 (12) promotes TH17 conversion of Treg with loss of both Foxp3 expression and T-cell–suppressive properties.
We have reported accumulation of MC in adenomatous polyps in both mice and humans and linked this to progressive polyp growth (4). The adoptive transfer of Treg derived from healthy mice to mice with polyposis suppressed MC numbers and led to the regression of preexisting polyps. However, Treg derived from polyp-ridden mice did not hinder polyp growth and promoted the expansion of MC, suggesting diversion of the Treg to a proinflammatory phenotype (8). Interestingly, the benefit or hazard of Treg expansion in CRC is currently under debate. Although the majority of studies correlate increased Treg density (13, 14) or Treg to effector CD4 ratio (15) in cancer with poor prognosis, a recent report has concluded that a high density of FOXP3+ Tregs in tumor tissue is associated with improved survival (16).
Here we provide evidence for systemic skewing of Treg from anti- to proinflammatory functions in both human CRC and murine polyposis. MC are target of suppression by Treg but at the same time play a critical role to reverse the anti-inflammatory function of the Treg. We predict that targeting the pathogenic cross-talk between Treg and MC to allow recovery of Treg antiinflammatory functions will help to control CRC.
Results
Cytokine Milieu of CRC Tumors Favors TH17 Skewing.
Cytokine bias in the tumor microenvironment can be predictive of disease outcome. Expression of cytokines that favor commitment of T cells to production of IL17, including TGFβ, IL6, TNFα, and IL1β, can generate potent T-Helper-17 (TH17) proinflammatory T cells. In chronic inflammatory diseases, TH17 cytokines may favor tumor growth (17); but in sporadic cancer, TH17 bias is commonly linked with protective effector T-cell responses (18, 19). We reported earlier that mice harboring adenomatous polyps have elevated levels of cytokines that are typically associated with a TH17 response (4, 8). To elucidate the trend in human CRC we carried out cytokine ELISA on extracts prepared from fresh human CRC tumor specimens. To this aim, and for further studies detailed below, we obtained fresh tissue samples and peripheral blood (PB) from patients undergoing surgical resection. A total of 31 patients (median age 66, range 31–87 years, mean 63.8 ± 15 years) were enrolled for this study (Table S1). A large proportion (67.7%) had T3 disease, 61% of the total population had lymph-node–positive primary tumors, and 32.3% of patients had metastatic disease (Table S1). In a study of 11 individuals, the tumor as compared with marginal tissue of the same patients showed significant increases in the levels of IL17, TGFβ, IL6, and IL1β (Fig. 1) with corresponding reductions in IL10 and INFγ. Marginal tissue is defined by us as a separate piece of tissue obtained from the same surgical resection but distant from the tumor that has been diagnosed as pathologically normal. This pattern is compatible with a TH17-biased microenvironment.
Fig. 1.
Proinflammatory cytokines are elevated in CRC tumors. Cytokine levels in fresh tumor lysates from CRC patients were determined by ELISA; n = 11 in triplicate, P < 0.0003.
CRC Tumors Are Enriched in Treg.
Treg are known to systemically expand in CRC and accumulate in tumors (20). Treg are also known to have particular plasticity and tendency to convert to TH17 cells in a permissive cytokine environment, as reviewed elsewhere (21). This normally should lead to the loss of immune suppressive Treg functions and to generation of potent proinflammatory helper CD4 T cells. We therefore proceeded to characterize the tumor infiltrating Foxp3+ cells.
Initially, in situ antibody staining from 13 independent T3 tumors was used to visualize tumor infiltrating T cells (Fig. S1 A–D). There was no increase in the density of CD4+ cells (cell count in histologic fields of view) but, rather, a trend to reduced frequency (cell count in relation to total cells) in the tumor relative to marginal tissue (Fig. 2A). In contrast, the density and frequency of infiltrating Foxp3+ cells were significantly higher as compared with the marginal tissue (Fig. 2B). The ratio of Foxp3+ to total CD4 cells was 2-fold higher in the tumor as compared with the margin (Fig. 2C). The frequency of these cells was significantly higher in the tumor as compared with the healthy surrounding tissue. FACS analysis of total MNC from the fresh surgical samples revealed that ∼70% of tumor infiltrating CD4+Foxp3+ T cells had elevated levels of cell surface bound CD25 (Fig. 2D), with a 10% average of the total tumor infiltrating lymphocytes being CD4+CD25+Foxp3+ and potentially Treg. (Fig. 2E). In contrast, the frequency of Foxp3+ cells with low cell surface CD25 was similar in the tumor and marginal tissue (Fig. 2F). The CD4+Foxp3+CD25− T cells may be effector CD4 T cells or Treg that have down-regulated CD25. The accumulation CD4+Foxp3+CD25+ T cells in tumors is consistent with the behavior of Treg.
Fig. 2.
Foxp3+ T cells accumulate in human CRC tumors. Total number and frequency of CD4+ (A) or Foxp3+ (B) cells per total number of cells in each 200× field of vision. Total: CD4, margin 121 ± 6 cells vs. tumor 119 ± 6 cells; Foxp3, margin 17 ± 2 cells vs. tumor 37 ± 2 cells, P = 0.0001; Frequency: CD4, margin 15 ± 0.8% vs. tumor 12.2 ± 0.6%, P = 0.009; Foxp3, margin 2.2 ± 0.2% vs. tumor 3.8 ± 0.3%, P = 0.0001, two-tailed unpaired t test with Welch’s correction; n = 13, three to five fields per slide. (C) Frequency of Foxp3+ cells to CD4+ cells determined from serial section stainings, margin 13.7 ± 1.1% vs. tumor 29.9 ± 1.6%, P = 0.0001. (D) Representative FACS plot of tumor-derived MNC gated on CD4 and analyzed for Foxp3 and CD25. (E) Compiled frequencies of CD4+CD25+Foxp3+ cells and (F) CD4+CD25+Foxp3− tumor-infiltrating T cells; n = 9 patients. P values: (E) P = 0.0063 for margin 5.3 ± 1.2% vs. tumor 10.3 ± 1.5%, P = 0.0452 for tumor vs. blood 4.8 ± 1.9%; (F) not significant.
CRC Tumors Are Enriched in MC.
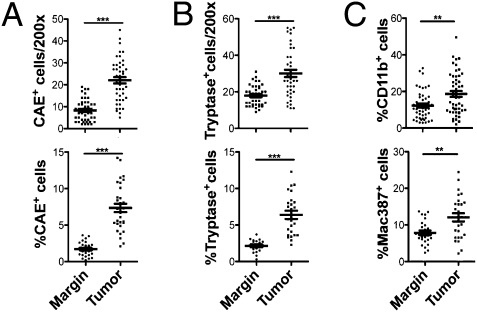
Numerous reports exist of accumulation of MC in tumors, but only recently has this accumulation been convincingly linked with poor prognosis (22, 23) and experimentally associated with carcinogenesis (4–6), as reviewed elsewhere (2, 3). Our earlier study suggested that there is a 3-fold enrichment of MC in human polyps compared with marginal tissue (4). To determine whether MC also accumulated in CRC, tumor and healthy marginal tissue from 13 T3 CRC patients were stained in paraffin sections with chloroacetate esterase (CAE) and tryptase antibody immunohistochemistry (Fig. S1 E–H). There was an approximate 4.5% increase in CAE+ cells and 3% increase in tryptase+ cells in the tumor compared with healthy tissue (Fig. 3 A and B). This increase was more significant than that of total myeloid cells as defined by CD11b+ cells and of Mac+ macrophages (Fig. 3C and Fig. S1 I–L). Thus, in both polyps and CRC tumors there was a preferential accumulation of MC in areas of dysplasia and cancer.
Fig. 3.
Increased MC infiltrate in CRC tumors. Total number and frequency of CAE+ (A) or tryptase+ (B) cells per total number of cells in each 200× field of vision. Total number: CAE, margin 8 ± 1 cells vs. tumor 22 ± 1 cells, P = 0.0001; tryptase, margin 17 ± 1 cells vs. tumor 30 ± 2 cells, P = 0.0001. Frequency: CAE, margin 1.7 ± 0.2% vs. tumor 7.4 ± 0.6%, P = 0.0001. (C) Frequency of CD11b+ (Upper, margin 12.3 ± 1.1% vs. tumor 18.6 ± 1.6, P = 0.0013) and Mac+ (Lower, margin 7.8 ± 0.6 vs. tumor 12.1 ± 1.1%, P = 0.0022) myeloid cells in CRC. For all, n = 13, three to five fields per slide, two-tailed unpaired t test with Welch's correction.
CRC Treg Are Unable to Suppress MC Degranulation.
The concomitant expansion of Treg and MC in the tumor is surprising in view of the reputed anti-inflammatory properties of Treg, but is in line with our earlier finding that in mice the polyp infiltrating Treg are unable to control inflammation (8). We therefore proceeded to analyze the suppressive properties of the CRC patient–derived Treg.
A hallmark of Treg is their ability to compete for IL2 in proliferation assays with effector CD4+ T cells. To test the tumor-infiltrating cells functionally, CD4+CD25+ T cells were purified from total MNC of fresh surgical tumor samples as well as from the PB of CRC patients. As control, we isolated CD4+CD25− and CD4+CD25+ cells from the PB of healthy donors. CD4+CD25+ T cells always hindered proliferation of the naive CD4 T cells (Fig. 4A). Therefore, we concluded that, despite the heavily TH17-biased tumor microenvironment, the CD4+CD25+ T cell in CRC patients largely consisted of Treg that had maintained their T-cell–suppressive properties and thus had not undergone classical TH17 differentiation.
Fig. 4.
Treg from CRC patients are proinflammatory. (A) Treg from healthy donors PB and CRC PB, tumor, and margin inhibit T-cell proliferation; n = 2 for PB and n = 1 for tissue, tested in triplicate. (B) Compiled values of % LAD2-MC degranulation sensitized (IgE) and cross-linked with anti-(IgE+Ag = 40.1 ± 3.2%) incubated with CD4 effector (Teff) or CD4+CD25+ (Treg) T cells. Healthy donors Treg vs. healthy donors Teff, 34.4 ± 2.8% vs. 45.3 ± 3.3%, P = 0.0003; healthy donors Treg vs. CRC PB, 34.4 ± 2.8% vs. 48.8 ± 4.6%, P = 0.0354; healthy donors Treg vs. CRC tumor, 34.4 ± 2.8% vs. 50.4 ± 2.2%, P = 0.0179; healthy donors Treg vs. CRC margin, 34.4 ± 2.8% vs. 47.1 ± 3%, P = 0.0185; n = 5, in duplicate.
Two recently reported assays allow evaluation of the anti-inflammatory properties of murine Treg by quantitative analysis of their ability to suppress MC progenitor (MCp) expansion/differentiation (8) and to suppress degranulation and release of prestored granules from primary murine bone marrow–derived mast cells (BMMC) (9). First, to establish the relevance of the MC degranulation assay to human CRC, we stained tumor sections with Toluidine Blue and identified MC and their granules under high magnification. MC degranulation was readily visible in CRC tumors (Fig. S1). This encouraged us to adapt the inhibition of MC degranulation assay to human cells. To this aim, we used the LAD2 established human MC line (24). LAD2 cocultured with healthy donors’ Treg were inhibited in degranulation, whereas Treg from tumor, marginal tissue, lymph node, or PB of CRC patients failed to suppress degranulation (Fig. 4B). CRC Treg exhibited similar properties to effector CD4 T cells from the patients, both showing tendencies to promote LAD2 degranulation (Fig. 4B). The findings are completely in agreement with our earlier studies using polyp-ridden mice (8) and suggest a systemic reversal in the ability of Treg in CRC to suppress inflammation.
Treg in CRC Patients Down-Regulate IL10 and Express IL17.
Expression of IL10 by Treg is critical for maintaining their suppressive and hence protective functions in colitis (25). Accordingly, intracellular staining after a short, 4-h stimulation ex vivo with PMA and ionomycin in the presence of GolgiPlug revealed that expression of IL10 is significantly lower among the tumor-infiltrating Foxp3+ T cells as compared with those infiltrating the healthy margins (Fig. 5 A and B). Also, Treg from the PB of CRC patients expressed significantly less IL10 than from healthy donors (Fig. 5 A and B). These differences were more obvious for the CD25+ T cells as compared with CD25− T cells (Fig. 5 B and C). Foxp3− T cells did not produce much IL10 (Fig. 5D). There was a corresponding increase in expression of IL17 among tumor-infiltrating Foxp3+ cells compared with those in the margins of the tumor (Fig. 5 E and G). The fraction of tumor infiltrating TH17 CD4+ T cells was slightly less to that of the tumor infiltrating IL17+ Treg (Fig. 5 E and F). Therefore, in human CRC as in murine polyposis, Treg lose anti-inflammatory properties, and those infiltrating the tumor share characteristics of TH17 proinflammatory T cells. We therefore propose that this may be a distinct sublineage of Treg with split inhibitory and effector properties, and designate it as ΔTreg. It remaine to be shown whether expression of IL17 indicated that the tumor infiltrating ΔTreg were different from the circulating ΔTreg, as both shared proinflammatory properties. This was addressed below.
Fig. 5.
Tumor-infiltrating Treg from CRC lack IL10 but produce IL17. (A) Representative FACS plots of cytokine production by live CD4+CD25+Foxp3+ cells isolated from CRC patients and healthy donor PB. (B–D) Compiled frequencies among total MNC of IL10-producing T cells derived from CRC patients or healthy donors; n = 5. CD4+CD25+Foxp3+, for margin 5.9 ± 1.9% vs. tumor 2 ± 0.7%, P = 0.0418; for CRC PB 2.2 ± 0.6% vs. healthy donors PB 5.7 ± 0.7%, P = 0.0022; CD4+CD25+Foxp3−, CRC PB 1.1 ± 0.3% vs. HD PB 4.3 ± 0.9, P = 0.0481; CD4+CD25−Foxp3+ not significant. (E–G) Compiled frequencies of T cells expressing IL17; n = 9; CD4+CD25+Foxp3+, margin 4 ± 1.3% vs. tumor 9.9 ± 2.5%, P = 0.0050; CD4+CD25+Foxp3−, not significant; CD4+CD25−Foxp3+, margin 3.8 ± 1.6% vs. tumor 10.5 ± 3.3%, P = 0.0179.
IL6 Induces Diversion of Healthy Human Treg to a Proinflammatory Phenotype with Ability to Inhibit CD4 T Cells.
Because IL6 is a major product of activated MC and the predominant cytokine involved in TH17 skewing of T cells, we tested the effect of this cytokine on the ex vivo function of PB Treg derived from healthy donors. In these assays we included IL2, as we found this to be elevated both in CRC tumors and murine polyps as compared to healthy margin (Fig. S2). Elevated levels of IL2 in human CRC has been also reported by others (26). Nonadherent cells derived from PB of healthy donors were incubated with IL2 together with IL6 or IL2 alone for 5 days (with no stimulation), and then the Treg were purified and tested for phenotypic alterations and their ability to suppress MC degranulation.
When cultured in medium containing IL6 and IL2, Treg switched from expressing IL10 to IL17 (Fig. 6A) and expressed elevated levels of pSTAT3 (Fig. 6B). The expression of IL17 is regulated through the activity of STAT3 (27), a transcription factor that is induced by IL6 (28) and has a demonstrated importance in tumor progression, as reviewed elsewhere (29). IL6Rα expression on Treg exceeds that of effector T cells, and both populations respond to IL6 by phosphorylating STAT3 (30). These observations are consistent with the Stat3-dependent expression of IL17 by the Treg. Untreated Treg from healthy donors suppressed the degranulation of LAD2 cells, but IL2 + IL6–treated Treg failed to suppress, and even showed a tendency to promote, LAD2 degranulation (Fig. 6C). Both untreated and IL6-treated Treg successfully inhibited the proliferation of naive CD4 T cells from healthy donors (Fig. 6D). These results suggest that elevated levels of IL6 in CRC can divert Treg to a proinflammatory phenotype without affecting their ability to suppress CD4 T cells.
Fig. 6.
Treg become proinflammatory in the presence of IL6. Typical FACS plot of cytokine production (A) or pSTAT3 (B) expression by Treg from healthy donors PB untreated or treated with IL6 in complete medium containing IL2 for 5 days, n = 3. (C) Compiled values of % LAD2-MC degranulation and impact of Treg from healthy donors untreated or pretreated with IL6; 34.4 ± 2.8% vs. 47.8 ± 4.9%, P = 0.0025, n = 4 in duplicate. (D) Treg cultured for 5 days with or without IL6 assayed for suppressing CD4 T cell proliferation; n = 2 in duplicate.
pSTAT3+ Proinflammatory Treg Can Be Generated by Coculture of Naive Treg with Primary MC.
To investigate further a direct role for the role MC in altering Treg functions, we resorted to working with primary murine MC that are more amenable to manipulation than primary human MC. First we tested whether ex vivo expanded BMMC alone can change the phenotype of Treg derived from healthy C57BL/6 (wt) mice. MC and Treg were cultured together (1:1) in the presence of IL2 and SCF, with or without IL6. Over the course of 5 days the Treg shifted from producing IL10 to IL17 (Fig. 7A). The coculture of Treg with BMMC in medium containing IL2 resulted in significant increases in the Treg levels of pSTAT3 (Fig. 7B). We observed a comparable increase in pSTAT3 when the Treg were incubated with IL6 + IL2 in the absence of BMMC (Fig. 7B). These Treg were no longer able to suppress MC degranulation (Fig. 7C), but continued to suppress the proliferation of naive CD4 T cells (Fig. 7D) and were thus functionally distinct from Treg-derived TH17 cells induced by MC under conditions in which IL2 is limiting (12). Interestingly, the ΔTreg generated by IL6 + IL2 were less potent than Treg freshly isolated from the spleen of polyp-bearing mice, as the latter but not the former actually promoted MC degranulation (Fig. 7E). We confirmed these results in a MC progenitor assay and showed that Treg derived from healthy mice suppressed, whereas ΔTreg from polyp-bearing mice promoted, MC differentiation and expansion (Fig. 7F). Of note, the APCΔ468 spleen-derived ΔTreg are poor in expression of IL17.
Fig. 7.
MC and IL6 alter murine Treg functions. (A) Typical FACS plots of intracellular cytokines in Treg derived from wt spleen cultured without or with BMMC for 3 or 5 days. Live cells were gated on CD4+CD25+Foxp3+, n = 5. (B) Compiled frequencies of % pSTAT3 expressing Treg, cultured alone (white bar), with IL6 (striped bar), or with BMMC (gray bar; 1:1), striped compared with white, 6.6 ± 0.6% vs. 2.7 ± 0.4%, P = 0.0106; gray compared with white, 9.1 ± 0.6% vs. 2.7 ± 0.4%, P = 0.003; n = 3). (C) MC degranulation with no Treg (black 31.2 ± 1.3%), with Treg (white 18.8 ± 1.8%, P = 0.0035), or with IL6 pretreated Treg (striped 25.9 ± 1.7%, P = 0.0046); n = 3. (D) Suppression of T cell proliferation by untreated or IL6 pretreated Treg, n = 2 in triplicate. (E) BMMC degranulation assessed in absence (IgE+Ag) or presence of Treg from healthy mice (wt) or polyp-ridden mice (APCΔ468, P = 0.0007 compared with wt Treg and compared with IgE+Ag, P = 0.0427), n = 3 in duplicate. (F) Frequency of MCp among total MNCs isolated from the intestine of Rag−/− mice in absence of Treg (black bar 1,339 ± 58 MCp) or on inclusion of Treg from spleen and MLN of healthy mice (white, wt 838 ± 28MCp) or polyp-ridden mice (gray, APCΔ468 2300 ± 119 MCp); n = 3 in duplicate, P = 0.0001.
IL6 Focal Mastocytosis and Polyposis Are Independent of Bone Marrow–Derived IL6.
It has been reported that IL6−/− APCMin/+ mice have moderately attenuated polyposis (31). To test the notion that MC derived IL6 was contributing to polyposis, we lethally irradiated 6-week-old APCΔ468 mice and reconstituted them with bone marrow (BM) from IL6−/− mice or from wt control mice. The mice were allowed to age to 4 months. Surprisingly, the lack of IL6 had little impact on the frequency of the polyps (Fig. S3A), with only a small difference in polyp size (Fig. S3B). The average frequency of MCp in the polyps of the IL6−/− BM-reconstituted mice was only 1.3-fold less than that of the control wt BM-reconstituted mice (Fig. S3C), and there was a mere 1.2-fold reduction in the average number of mature MC infiltrating the polyps (Fig. S3D). Thus, IL6 deficiency in MC appeared to make a negligible impact on MC density and polyposis in the intestine.
MC-Mediated Diversion of Treg to Proinflammatory Phenotype Does Not Require IL6 or IL17.
We used the IL6-deficient BM chimeric APCΔ468 mice to test whether MC-derived IL6 was responsible for rendering the Treg proinflammatory. First, we confirmed the absence of IL6 in MC-derived from mice with IL6−/− BM (Fig. S4). Next, we tested whether MC IL6 was needed for committing polyp-infiltrating ΔTreg to IL17 production. Polyp-infiltrating Treg from polyp-ridden APCΔ468 mice that had been reconstituted with wt or IL6−/− BM were accessed for production of IL17. Absence of IL6 significantly ablated expression of IL17 by the Treg (Fig. 8 A and B) and normalized levels of IL17 in the serum as determined by ELISA (Fig. 8C). However, using the MC degranulation assay, we failed to detect any significant recovery of Treg anti-inflammatory functions in IL6−/− BM-reconstituted APCΔ468 mice (Fig. 8D), suggesting that MC-derived IL6 was not critical for altering Treg functions. Furthermore, addition of purified IL17, IL10, or TGFβ did not alter MC degranulation in ex vivo assays (Fig. S5). Previous studies have shown that IL10 can inhibit MC degranulation when cultured with MC for 4 days before Ag cross-linking (10). However, in the short, 30-min incubation used in this study, none of the cytokines had an impact. Thus, although IL17 may serve as a marker for altered Treg functions, its contribution to the proinflammatory phenotype of ΔTreg is not certain and needs more investigation.
Fig. 8.
Functional alteration of Treg by MC is independent of IL6 and IL17. (A) Typical FACS plot of IL17 production by polyp-infiltrating Treg from mice reconstituted with wt BM or IL6−/− BM. Total MNC were isolated from microdissected polyps, stimulated with anti-CD3/CD28 for 3 days and then stained for analysis. Cells were gated for CD4+CD25+. (B) Compiled % of IL17 producing Foxp3+ cells; wt BM 6 ± 1.5%, IL6−/− BM 1.8 ± 0.2%, n = 3, P = 0.0236, one-tailed unpaired t test. (C) IL17 ELISA of serum; white, IL6−/− BM (127 ± 12.9 pg/mL) compared with black, wt BM (213.3 ± 17.1pg/mL), P = 0.0007; gray, control healthy wt mouse (64.3 ± 7.8 pg/mL) compared with polyp-ridden with wt BM P = 0.0001, and compared with polyp-ridden IL6−/− BM mice P = 0.0.003. (D) wt Treg inhibited BMMC degranulation (control vs. IgE+Ag; 19.5 ± 1.2% vs. 30.9 ± 1.3%, P = 0.0001), whereas Treg from poly-ridden mice with wt (30 ± 1.9%) or IL6−/− BM (29.8 ± 1.7%) did not; P = 0.0036 wt BM compared with control wt Treg; P = 0.0150 IL6−/− BM compared with control wt Treg. (E) After 5 days in culture, wt B6 Treg inhibit BMMC degranulation (no MC compared with IgG+Ag; white = 16.8 ± 1.8% vs. black = 31.2 ± 1.3%, P = 0.0040), whereas preincubation for 5 days with BMMC (gray 26.2 ± 2%, P = 0.0212) or IL6−/− BMMC (striped 22.9 ± 1.7%, P = 0.0282) did not; n = 3 mice, in duplicate.
To test the hypothesis that MC can divert Treg functions independently of IL6, we cocultured Treg from healthy wt mice with wt BMMC, IL6−/− BMMC or no MC, in media containing IL2 and SCF for 5 days. Treg were then washed to remove cytokines, depleted of MC using magnetic isolation, and assayed for their ability to inhibit degranulation of MC using freshly IgE-sensitized wt BMMC. After 5 days in culture, untreated Treg cultured alone were able to inhibit degranulation (Fig. 8E). Treg cultured in the presence of MC lost their ability to inhibit β-hexosaminidase release, and IL6 deficiency of the MC made no impact on this. Based on these results, we conclude that, in the tumor microenvironment, MC were capable of overcoming Treg regulatory function and that although IL6 is sufficient, it is not ultimately necessary. Other, yet undefined, MC mediators are equally capable of converting Tregs from suppressing to promoting inflammation.
Altogether, our observations extend the phenomenon of T-cell dysfunction in cancer to the Treg and highlight the critical contribution of MC to the change in Treg function. MC modulate Treg function independently of IL6, a key cytokine for TH17 conversion. Furthermore, although lack of IL6 hinders expression of IL17, the altered Treg (ΔTreg) are nevertheless proinflammatory, as judged by their ability to promote MC differentiation and degranulation in mice, as well as degranulation in assays with human cells. Expression of IL17 appears to be limited to tumor-infiltrating ΔTreg. Our data do not support a role for IL17 in promoting the activation of MC. Indeed, restricted expression of IL17 by tumor-infiltrating ΔTreg and CD4 effector cells is in line with a defensive response to the breakdown of epithelial barrier in the tumor. IL10 is critical for maintaining Treg functions (25), and we propose that it is rather the loss of IL10 expression by the Treg that is responsible for loss of control of inflammation. The final effect is systemic.
We propose that focal chronic mastocytosis in CRC (4) achieves a pathologic shift in the natural role of Treg from controlling acute inflammation to promoting inflammation. This is consistent with reported loss of Treg functions in chronic inflammatory diseases such as arthritis (32), in which MC are also pathologically implicated. Our results agree with earlier reports that, in chronic inflammatory diseases, MC can reverse the anti-inflammatory function of Treg (11, 12). However, the Treg diversion in CRC is distinct from the classical TH17 conversion, as the ΔTreg maintain expression of Foxp3 and their ability to suppress effector T cells. The TH17-biased cytokine milieu, together with elevated levels of IL2 in CRC, favor this phenotype. ΔTreg do not produce IL2 but are dependent on IL2 for their survival and proliferation. IL2 also helps to maintain the suppressive Treg properties in a proinflammatory milieu by constraining TH17 cell generation (26, 33), as previously reviewed (34).
Identical changes in human and murine Treg indicate that Treg dysfunction in cancer is evolutionary conserved. Since Treg normally suppress inflammation, one cannot rule out the possibility that depletion of Treg will escalate inflammation and accelerate progression of CRC. MC are known to recruit Treg (35), to mediate Treg suppression of host-vs-graft rejection responses (7), and we have now demonstrated that they can also render the Treg proinflammatory. We predict that a safer therapeutic strategy in CRC may be to target the Treg and MC interaction to hinder the generation of ΔTreg.
Material and Methods
Patient Samples.
Informed consent was obtained from all participants. The protocols were approved by the Scientific Review Committee of Northwestern University and the Ethical Committed of the University of Heidelberg.
Animals.
All animal work was approved and conducted under the guidelines of Northwestern University's Animal Care and Use Committee.
Immunohistochemistry.
Paraffin sections (5-μm) were stained as follows. CAE staining: 20 min with naphthol-AS-D chloroacetate (Sigma), counterstained with hematoxylin Gill's II. Toludine Blue: in 10% solution (Sigma) 3 min. CD4 (1:200; Thermo Scientific), Foxp3 (1:100; Abcam), CD11b (1:200; Abcam), Mac387 (1:200; AbD Serotec) and tryptase (1:5,000; Thermo Scientific): 0.5% H2O2 block for 10 mins, IHC with Dako’s EnVision system. Images were collected with a Leica DCC camera, analyzed with ImageJ plug-in “threshold color” and the plug-in “nuclei counting.”
Isolation of MNC from Surgical Samples.
Tissues were washed (DMEM with 0.5% penicillin/streptomycin, 10 μg/mL gentamycin sulfate), minced with surgical blades, digested (750 U/mL type IV collagenase, Worthington Biochemical; 500 U/mg hyaluronidase, Sigma; 0.1 μg/mL DNase, Sigma) for 1 h at 37, and subjected to Percoll gradient centrifugation (40–80%). Interphase was collected.
Tumor Lysates and ELISAs.
Tumor tissue (30–50 mg) was homogenized in 1 mL PBS and centrifuged (13,000 rpm for 20min at 4 °C). Supernatant was filtered (0.22 μm), and protein was determined by the Bradford assay and subjected to ELISA (eBioscience).
MC Degranulation and Inhibition by Treg.
MC were presensitized with DNP-specific IgE and challenged in Tyrode's buffer with DNP (100 ng/mL, 30 min), then centrifuged. Supernatants and solubilized cell pellets (Triton X-100, 0.5%) were incubated with p-nitrophenyl N-acetyl-β-D-glucosaminide in 0.1 M sodium citrate (pH 4.5) for 60 min at 37 °, and stopped with 0.2 M glycine (pH 10.7). 4-p-Nitrophenol was detected by absorbance at 405 nm. Degranulation is a measure of the percent release of β-hexosaminidase, calculated as [absorbance of the supernatant /(absorbance of supernate + absorbance of pellet)] × 100. For Treg inbition, MC were preincubated at 1:1 with Treg for 10 min before addition of DNP, and then for a further 30 min after addition of DNP. Where indicated, Treg were pretreated with IL6 by incubating in complete medium containing IL6 (10 ng/mL) and IL2 (100 U/mL) for 5 days without stimulation. Cells were extensively washed to remove cytokines before assay.
Impact of Treg on Differentiation of MCp.
Murine intestine was washed with PBS, minced with surgical blades, digested (25 mL RPMI 1640, 10 U collagenase type IV for 20 min at 37 °C with agitation), subjected to Percoll gradient centrifugation (40–60%), and interface collected. A quantity of 20,000 cells/100 μL (complete medium, 10 ng/mL SCF, 20 ng/mL IL3, 50 U/mL of IL2, and 106/mL irradiated spleen cells) were serially diluted (1:2, in 96-well plates). Treg were added (1:1) for 10–15 days at 37 °C, 5% CO2 after which colonies of MC were scored.
T-Cell Purification and Proliferation/Inhibition.
Teff and Treg were enriched using T-cell–negative isolation kit (Invitrogen) and CD4+CD25+ Treg isolation kit (Miltenyi Biotec). Treg (40,000 cells/200 μL complete medium) and equal or increasing numbers of niïve CD4 were coincubated (72 h, 37 °C 5% CO2) in precoated (anti-CD3, 5 μg/mL) 96-well plates. A 1-μCi quanity of 3H-thymidine was added for 18 h and incorporation measured (Perklin-Elmer MicroBeta plate counter).
Antibody Staining and FACS.
Cells were preincubated 10 min with Fc Block (BD Bioscience). For intracellular stainings, GolgiPlug (BD Bioscience) was added 4 h before Fc block, fixation, and permeabilization (BD Bioscience). Dead cells were excluded using LIVE/DEAD Violet Dead cell Stain kit (Invitrogen). Human antibodies: CD4 PE-Cy5 (MT310) Dako, CD25 PE-Cy7 (M-A251) and IL10 APC (JES3-19F1) BD, Foxp3 FITC (PCH101) and IL17 PE (eBio64CAP17) (eBioscience). Murine antibodies, cKit APC (2B8), Sca1 PE (D7), FcεRI FITC (MAR1), CD4 PE-Cy5 (H129.19), CD25 PE-Cy7 (PC61), IL10 FITC (JES5-16E3), IL17 PE (TC11-18H10), pSTAT3 PE (pY705, 4/P-STAT3), IL6 PE (MP5-20F3) BD. Foxp3 expression was detected using either GFP-reporter mice or with Biolegend’s Foxp3 Alexa Fluor647 (MF14). Acquisition was performed with BD FACSCanto instrument. Data analyses used FlowJo software (Tree Star).
Statistical Analysis.
Prism 5 software was used for statistical analysis. Except were indicated, P values determined with two-tailed paired t test.
Supplementary Material
Acknowledgments
We thank Dr. Fotini Gounari, Dr. Silvia Piconese, Dr. Steven Rosen, and Dr. Terrence Barrett for critical comments and support. This work was supported by the American Cancer Society Research Scholar Award 113422 RSG, Circle of Service Foundation of the Robert H. Lurie Cancer Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913683107/DCSupplemental.
References
- 1.Khazaie K, Bonertz A, Beckhove P. Current developments with peptide-based human tumor vaccines. Curr Opin Oncol. 2009;21:524–530. doi: 10.1097/CCO.0b013e328331a78e. [DOI] [PubMed] [Google Scholar]
- 2.Crivellato E, Nico B, Ribatti D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008;269:1–6. doi: 10.1016/j.canlet.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 3.Maltby S, Khazaie K, McNagny KM. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim Biophys Acta. 2009;1796:19–26. doi: 10.1016/j.bbcan.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gounaris E, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soucek L, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 6.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 8.Gounaris E, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gri G, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29:771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy Norton S, et al. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 11.de Vries VC, et al. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piconese S, et al. Mast cells counteract regulatory T cell suppression through interleukin-6 and OX40/OX40L axis toward Th17 cell differentiation. Blood. 2009;114:2639–2648. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 13.Clarke SL, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS One. 2006;1:e129–135. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling KL, et al. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7–14. [PMC free article] [PubMed] [Google Scholar]
- 15.Sinicrope FA, et al. Intraepithelial effector (CD3(+))/regulatory (FoxP3(+)) T-cell ratio predicts adverse outcome of human colon carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama P, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonertz A, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009 doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang FC, et al. Nf1+/- mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nonomura N, et al. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br J Cancer. 2007;97:952–956. doi: 10.1038/sj.bjc.6603962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirshenbaum AS, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 25.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contasta I, Berghella AM, Pellegrini P, Adorno D. Passage from normal mucosa to adenoma and colon cancer: Alteration of normal sCD30 mechanisms regulating TH1/TH2 cell functions. Cancer Biother Radiopharm. 2003;18:549–557. doi: 10.1089/108497803322287628. [DOI] [PubMed] [Google Scholar]
- 27.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 28.Niemand C, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–3272. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 29.Devarajan E, Huang S. STAT3 as a central regulator of tumor metastases. Curr Mol Med. 2009;9:626–633. doi: 10.2174/156652409788488720. [DOI] [PubMed] [Google Scholar]
- 30.Goodman WA, et al. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baltgalvis KA, et al. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–R401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 32.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 35.Kashyap M, et al. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–2043. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.