Abstract
Although the exact cause of multiple sclerosis (MS) is unknown, a number of genetic and environmental factors are thought to influence MS susceptibility. One potential environmental factor is sunlight and the subsequent production of vitamin D. A number of studies have correlated decreased exposure to UV radiation (UVR) and low serum 25-hydroxyvitamin D3 [25(OH)D3] levels with an increased risk for developing MS. Furthermore, both UVR and the active form of vitamin D, 1α,25-dihydroxyvitamin D3, suppress disease in the experimental autoimmune encephalomyelitis (EAE) animal model of MS. These observations led to the hypothesis that UVR likely suppresses disease through the increased production of vitamin D. However, UVR can suppress the immune system independent of vitamin D. Therefore, it is unclear whether UVR, vitamin D, or both are necessary for the putative decrease in MS susceptibility. We have probed the ability of UVR to suppress disease in the EAE model of MS and assessed the effect of UVR on serum 25(OH)D3 and calcium levels. Our results indicate that continuous treatment with UVR dramatically suppresses clinical signs of EAE. Interestingly, disease suppression occurs with only a modest, transient increase in serum 25(OH)D3 levels. Further analysis demonstrated that the levels of 25(OH)D3 obtained upon UVR treatment were insufficient to suppress EAE independent of UVR treatment. These results suggest that UVR is likely suppressing disease independent of vitamin D production, and that vitamin D supplementation alone may not replace the ability of sunlight to reduce MS susceptibility.
Keywords: calcium, immune, multiple sclerosis, sunlight
Multiple sclerosis (MS) is a chronic and often debilitating disease affecting ≈2.5 million people worldwide (1). The hallmark pathological characteristic of MS is the formation of inflammatory plaques in the central nervous system. The plaques contain a number of immune cells, which are believed to orchestrate the autoimmune-mediated destruction of the myelin sheath surrounding neuronal axons (2). Demyelination leads to altered neuronal signal conduction and a myriad of adverse neurological symptoms. Although the exact cause of MS is unknown, a number of genetic and environmental factors are thought to influence MS development (3). Epidemiological studies have demonstrated that MS incidence typically follows a latitudinal gradient in both hemispheres. In Europe and North America, MS is more common in the northern regions, whereas MS is more prevalent in the southern part of Australia (4).
In general, sunlight exposure decreases with increasing latitude, leading to speculation that decreased sunlight exposure may be an underlying cause of the MS latitude gradient (5). Findings that the average annual hours of sunlight exposure in an individual’s place of birth is inversely correlated with MS development support this hypothesis (5, 6). Furthermore, individuals with the highest residential and occupational solar exposure have the lowest rate of MS incidence (7). These results suggest that decreased sunlight exposure may be a significant environmental factor contributing to the development of MS.
The sun emits a wide range of electromagnetic radiation, including UV (UVR) (100–400 nm), visible (400–800 nm), and infrared (≥800 nm) radiation. Exposure to UVR has profound effects on human health. UVR can cause direct damage to DNA and is a leading cause of skin carcinomas. In addition to directly damaging DNA, UVR can induce carcinogenesis by suppressing the immune system (8, 9). The absorption of UVR by photoreceptors leads to the release of a number of secondary mediators capable of suppressing cell-mediated immunity through multiple mechanisms (10). These mechanisms lead to both local and systemic immunosuppression, thereby eliminating natural defense mechanisms against aberrant cell growth. Although UV-induced immunosuppression clearly has detrimental effects in the context of skin cancer, it may have beneficial effects on organ-specific autoimmune diseases, such as MS (11). Indeed, a recent study demonstrated that MS relapse rates are lower in the summer than in the winter, suggesting that decreased UV exposure may be a contributing factor in relapses (12). Furthermore, experiments conducted in the experimental autoimmune encephalomyelitis (EAE) animal model of MS have demonstrated that 7-day pretreatment with UVR prevents disease induction in SJL mice (13). Thus, although avoiding UVR exposure may reduce the risk of various skin cancers, it could inadvertently increase the risk of developing autoimmune diseases such as MS.
UVR also modulates the immune response by stimulating the endogenous production of vitamin D in the skin. UVB wavelengths between 270 and 300 nm stimulate the production of previtamin D3 from the cholesterol derivative 7-dehydrocholesterol (14). Previtamin D3 undergoes a spontaneous isomerization to produce vitamin D3. Vitamin D3 undergoes two successive hydroxylation steps to form the active hormone 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3]. The first activation step occurs in the liver, where vitamin D3 is hydroxylated at carbon-25 to generate 25-hydroxyvitamin D3 [25(OH)D3] (15). The 25(OH)D3 metabolite is the primary circulating form of vitamin D3 and is commonly used as a clinical indicator of vitamin D status (16). The second activation step occurs in the kidney and involves the stereospecific hydroxylation of 25(OH)D3 at carbon-1 to yield 1,25(OH)2D3 (17, 18). The classic biological function of 1,25(OH)2D3 is to maintain sufficient serum calcium and phosphorus levels for proper mineralization of bone and neuromuscular function.
In addition to its role in regulating serum calcium levels, vitamin D may also be an environmental factor in MS and other autoimmune diseases (19). The potential link between vitamin D insufficiency and MS was first proposed by Goldberg, based on the geographic distribution of MS and the relationship between UVR and vitamin D production (20). Goldberg postulated that decreased exposure to UVR and subsequent vitamin D insufficiency at higher latitudes predisposes individuals residing in these regions to developing MS. Much of the evidence supporting this hypothesis is derived from epidemiological data demonstrating an association between UVR and MS prevalence and the assumption that the immunosuppressive effects of UVR are mediated through vitamin D production. However, UVR suppresses the immune system through mechanisms independent of vitamin D (21). Therefore, this assumption may not be valid. Additional evidence suggesting that vitamin D may play a role in MS stems from population-based studies that have correlated high serum 25(OH)D3 levels with a decreased risk for developing MS (22). However, because 25(OH)D3 levels largely reflect an individual’s exposure to UVR, it is impossible to determine if the decreased risk is attributable specifically to vitamin D or UVR.
Perhaps the most compelling evidence supporting a role for vitamin D in MS is derived from studies conducted in the EAE model. A number of in vivo studies have demonstrated that 1,25(OH)2D3 can suppress disease induction and progression in the EAE model of MS (23–25). However, complete disease suppression is only achieved using supraphysiological doses of 1,25(OH)2D3, which cause vitamin D toxicity and hypercalcemia (26). Vitamin D toxicity and hypercalcemia do not typically occur upon exposure to sunlight because of a number of factors that limit the endogenous production of vitamin D. These factors include the photochemical conversion of previtamin D3 into biologically inert compounds, skin pigmentation, and latitude (27). Thus, the levels of 1,25(OH)2D3 required to suppress EAE are well above those that can be produced naturally upon exposure to sunlight. Furthermore, results from our laboratory suggest that hypercalcemia is more than simply an unfortunate consequence of 1,25(OH)2D3 treatment, and may play an essential role in the immunosuppressive effects of 1,25(OH)2D3 (28).
In summary, although exposure to UVR and the subsequent production of vitamin D appear to be important environmental factors in MS susceptibility, the relative contribution of each is unknown. This article analyzes the effect of UVR on both the progression of EAE and vitamin D production. Because the levels of 25(OH)D3 obtained upon UVR treatment are insufficient to suppress EAE, UVR likely suppresses EAE independent of vitamin D production.
Results
UV Pretreatment Slightly Increases 25(OH)D3 Levels but Does Not Suppress EAE.
A previous study demonstrated that pretreatment with 2.5 kJ/m2 UVB prevented induction of EAE in SJL mice (13). However, the effects on vitamin D production and serum calcium levels were not determined. We sought to confirm these findings in the myelin oligodendrocyte glycoprotein (MOG) model of EAE and to determine what effect UV treatment might have on vitamin D production and serum calcium levels. Female C57BL/6 mice were treated once daily for 7 days with either 2.5 or 5.0 kJ/m2 UVB. Mice were immunized with MOG35–55 following the last UV treatment and monitored daily for clinical signs of EAE. In contrast to the previous report, treatment with 2.5 kJ/m2 UVB had no significant effect on any of the clinical parameters that were tested (Fig. 1A and Table 1). Although the lamps used were not identical, we placed the mice so they received the reported 2.5 kJ/m2 used by Hauser et al. (13), to be sure we doubled the exposure to 5.0 kJ/m2. The higher dose of 5.0 kJ/m2 UVB also had no significant effect on clinical signs of EAE, although the onset appeared to be slightly delayed.
Fig. 1.
UVB pretreatment fails to suppress EAE and causes a slight increase in serum 25(OH)D3 levels. Mice were treated for 7 days before immunization with the indicated doses of UVB. (A) Average clinical EAE scores were determined daily for control and UVB treated mice (n = 7–12). (B) Mice were weighed weekly (±SD) throughout the study to monitor disease-associated weight loss and toxicity. (C) Serum calcium levels (±SD) were determined at the end of the experiment using a clinical chemistry analyzer. (D) Serum 25(OH)D3 levels (±SD) were determined at the end of UV treatment and at the termination of the experiment.
Table 1.
UV pretreatment does not suppress clinical signs of EAE
| Treatment | Incidence | Day of onset | Peak severity | CDI |
| Control | 100% (7/7) | 11 ± 1 | 3.3 ± 0.4 | 43 ± 7 |
| 2.5 kJ/m2 | 100% (11/11) | 12 ± 3 | 3.3 ± 0.5 | 42 ± 9 |
| 5.0 kJ/m2 | 92% (11/12) | 14 ± 3 | 3.4 ± 0.6 | 36 ± 15 |
Female C57BL/6 mice on a regular chow diet were treated once daily for 7 days with either 2.5 or 5.0 kJ/m2 UVB before immunization with MOG35–55. The cumulative disease score (CDI) was calculated by summing all of the clinical scores for the entire experiment and dividing by the number of mice for each group. The clinical data demonstrate the mean ± SD from one representative of three individual experiments.
Vitamin D toxicity causes weight loss and a dramatic rise in serum calcium levels. To assess the effect of UV treatment on these parameters, mice were weighed at selected time-points throughout the study and serum calcium levels were determined at the termination of the experiment. UV pretreatment did not significantly affect the weight of the mice (Fig. 1B). Furthermore, there were no differences in serum calcium levels at the termination of the experiment (Fig. 1C). Serum 25(OH)D3 levels were determined at the end of the UV treatment period and at the termination of the experiment. Treatment with 2.5 and 5.0 kJ/m2 UVB led to a slight increase in serum 25(OH)D3 levels (75 ng/mL) compared with the control group (67 ng/mL) at the end of the UV treatment period (Fig. 1D). Only the 5.0 kJ/m2 group remained elevated at the termination of the experiment. Thus, UVB pretreatment did not cause vitamin D toxicity or hypercalcemia and did not confer protection against the development or progression of EAE.
Continuous Treatment with UV Suppresses Clinical Signs of EAE.
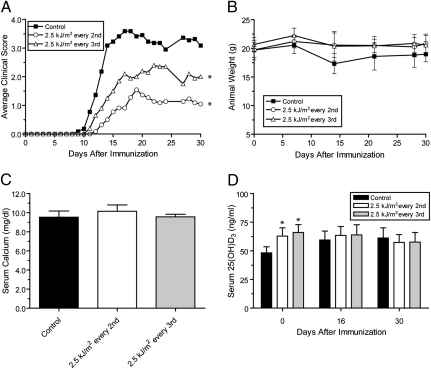
Individuals living in equatorial regions are exposed to UVR on a daily basis for much of their lives. Although it is not possible to mimic the effects of a lifetime of UVR exposure in the laboratory, we reasoned that continuous treatment with UVR throughout the experiment would provide a more realistic representation of UVR exposure in these regions. To determine the effect of continuous UVR treatment on EAE, mice were treated once daily with 2.5 kJ/m2 UVB for 7 days before immunization with MOG35–55. Following immunization, mice were either treated every other day or every third day with 2.5 kJ/m2 UVB for the duration of the experiment. The incidence of EAE was not significantly decreased in either treatment group (Table 2). However, treatment with 2.5 kJ/m2 every third day did cause a slight reduction in disease severity and a decrease in the cumulative disease index (CDI). A significant reduction in the average clinical EAE scores was also noted in this group (Fig. 2A). Increasing the frequency of UVB exposure to every other day enhanced the suppressive effect of the UVB treatment. Treatment with 2.5 kJ/m2 UVB every other day significantly delayed the onset of the disease, reduced the peak severity, and decreased the CDI compared to the control group (Table 2). Increasing the frequency of UVB exposure also caused a further decrease in the average clinical EAE scores (Fig. 2A). Thus, UVB was far more effective at suppressing EAE when treatment was delivered throughout the experiment, as opposed to discontinuing after immunization.
Table 2.
Continuous UV treatment inhibits EAE
| Treatment | Incidence | Day of onset | Peak severity | CDI |
| Control | 100% (11/11) | 12 ± 1 | 3.8 ± 0.7 | 54 ± 12 |
| 2.5 kJ/m2 every second day | 82% (9/11) | 17 ± 3* | 2.3 ± 0.9* | 17 ± 16* |
| 2.5 kJ/m2 every third day | 90% (9/10) | 14 ± 3 | 3.1 ± 0.9 | 32 ± 19* |
Female C57BL/6 mice on a regular chow diet were treated once daily for seven days with 2.5 kJ/m2 UVB before immunization with MOG35–55. After immunization, mice were treated either every other or every third day with 2.5 kJ/m2 UVB. The clinical data demonstrate the mean ± SD from one representative of two individual experiments.
*, P < 0.05 compared with the control group.
Fig. 2.
Continuous UVB treatment suppresses EAE and causes a transient increase in serum 25(OH)D3 levels. After immunization, mice were treated either every other or every third day with 2.5 kJ/m2 UVB. (A) Average clinical EAE scores were determined daily for control and UVB treated mice (n = 10–11). (B) Mice were weighed weekly (±SD) throughout the study to monitor disease-associated weight loss and toxicity. (C) Serum calcium levels (±SD) were determined at the end of the experiment using a clinical chemistry analyzer. (D) Serum 25(OH)D3 levels (±SD) were determined at selected time-point throughout the experiment. *, P < 0.05 compared to control group.
In addition to weight loss caused by vitamin D toxicity, mice can also lose weight because of muscle wasting and decreased food ingestion secondary to paralysis during the clinical course of EAE. The loss in weight correlated with severity of the disease in mice displaying more severe signs of disease. Mice treated every other day or every third day with 2.5 kJ/m2 UVB did not lose as much weight as the control group (Fig. 2B). Furthermore, the serum calcium levels in both UVB-treated groups were normal (Fig. 2C). Serum 25(OH)D3 levels were significantly elevated on the day of immunization in both UVB-treated groups (Fig. 2D). However, 25(OH)D3 levels did not remain elevated, despite the continuation of UVB treatment. Thus, continuous UVB treatment caused significant suppression of clinical signs of EAE without elevating serum calcium levels and caused only a transient elevation of serum 25(OH)D3 levels.
25(OH)D3 Fails to Prevent EAE at Doses That Cause Severe Hyper-calcemia.
After establishing that continuous treatment with UVB suppresses EAE without dramatically increasing serum 25(OH)D3 levels, we sought to determine if 25(OH)D3 levels obtained upon UVB treatment were sufficient to suppress EAE without UVB treatment. Female C57BL/6 mice were treated with either 10, 500, or 1,000 μg/kg 25(OH)D3 per day and compared to mice treated with vehicle or 2.5 μg/kg 1,25(OH)2D3 per day. Previous studies indicated that treatment with 2.5 μg/kg of 1,25(OH)2D3 per day caused a dramatic suppression of clinical signs of EAE and was associated with severe hypercalcemia (24, 26). Consequently, this dose of 1,25(OH)2D3 served as a useful treatment group with which to compare the clinical and calcemic effects of 25(OH)D3.
Treatment with 10 μg/kg of 25(OH)D3 per day had no significant effect on the incidence, onset, severity, or progression of EAE (Fig. 3A and Table 3). Increasing the dose to 500 μg/kg per day caused a significant delay in the onset of disease and a slight suppression of clinical EAE scores. Further increasing the dose to 1,000 μg/kg 25(OH)D3 per day only slightly enhanced the suppressive effects seen in the 500 μg/kg 25(OH)D3 treatment group, causing a significant decrease in the CDI as well as a delay in the onset of clinical signs of disease when compared with the vehicle group. Thus, even at a dose as high as 1,000 μg/kg per day, 25(OH)D3 caused only a modest suppression of EAE. In contrast, treatment with 2.5 μg/kg of 1,25(OH)2D3 led to a significant decrease in the disease incidence, delayed the onset, and dramatically decreased the CDI when compared with the vehicle and 25(OH)D3-treated groups.
Fig. 3.
25(OH)D3 only modestly suppresses EAE at doses that cause severe hypercalcemia. Beginning 10 days before immunization, mice were fed a purified 0.87% calcium diet delivering the indicated doses of either 25(OH)D3 or 1,25(OH)2D3. Treatment continued for the duration of the experiment. (A) Average clinical EAE scores were determined daily for vehicle, 25(OH)D3-, and 1,25(OH)2D3-treated mice (n = 15–17). (B) Mice were weighed weekly (±SD) throughout the study to monitor weight loss and toxicity. (C) Serum calcium levels (± SD) were determined at the end of the experiment using a clinical chemistry analyzer. (D) Serum 25(OH)D3 levels (±SD) were determined at the termination of the experiment. *, P < 0.05 compared with control group.
Table 3.
25(OH)D3 only modestly suppresses EAE
| Treatment | Incidence | Day of onset | Peak severity | CDI |
| Vehicle | 87% (13/15) | 13 ± 2 | 2.7 ± 0.8 | 25 ± 10 |
| 10 μg/kg 25 D3 | 88% (15/17) | 14 ± 3 | 2.9 ± 0.9 | 23 ± 16 |
| 500 μg/kg 25 D3 | 82% (14/17) | 16 ± 4* | 2.7 ± 0.6 | 19 ± 12 |
| 1,000 μg/kg 25 D3 | 82% (14/17) | 16 ± 3* | 2.6 ± 0.6 | 17 ± 11* |
| 2.5 μg/kg 1,25 D3 | 35% (6/17)† | 20 ± 3† | 2.3 ± 0.6 | 4 ± 7† |
Female C57BL/6 mice were treated with either 25(OH)D3 or 1,25(OH)2D3 in the indicated doses delivered in purified diet. All mice were immunized with MOG35–55 10 days after initiating therapy with the vitamin D metabolites. Mice were monitored daily for 25 days and assessed clinically for signs of EAE. The clinical data demonstrate the mean ± SD from one representative of three individual experiments.
*P < 0.05 compared with the vehicle group.
†P < 0.05 compared with all other groups.
Treatment with 1,000 μg/kg of 25(OH)D3 and 2.5 μg/kg of 1,25(OH)2D3 caused a significant decrease in the weight of the mice at the termination of the study (Fig. 3B). However, the drop in weight developed more slowly and was reduced in magnitude in the 1,000 μg/kg 25(OH)D3 group. Serum calcium levels were unchanged in mice treated with 10 μg/kg of 25(OH)D3 (9.9 mg/dL) compared to the vehicle group (10.1 mg/dL) (Fig. 3C). In contrast, treatment with 500 μg/kg 25(OH)D3 (12.9 mg/dL), 1,000 μg/kg 25(OH)D3 (14.2 mg/dL), and 2.5 μg/kg 1,25(OH)2D3 (14.9 mg/dL) all caused hypercalcemia. Although the elevation in serum calcium levels was similar in the 1,000 μg/kg 25(OH)D3- and 2.5 μg/kg 1,25(OH)2D3-treated groups, only 1,25(OH)2D3 prevented the induction of EAE (Fig. 3A). Thus, even at doses that dramatically elevated serum calcium levels and caused weight loss, 25(OH)D3 provided only modest suppression of EAE. It is known that at high plasma levels of 25(OH)D3, it acts as an analog of 1,25(OH)2D3 and increases serum calcium levels (29). Although 25(OH)D3 may act as an analog elevating serum calcium levels, it may not express all of the functions of 1,25(OH)2D3, such as immunomodulation.
Analysis of serum 25(OH)D3 levels revealed that dietary administration of 25(OH)D3 led to a dose-dependent increase of the 25(OH)D3 metabolite in the serum of treated mice (Fig. 3D). Treatment with 10 μg/kg of 25(OH)D3 resulted in serum 25(OH)D3 levels similar to those seen upon continuous UVB treatment (Figs. 2D and 3D). Notably, unlike with continuous UVB treatment, dietary administration of 10 μg/kg 25(OH)D3 had no effect on EAE progression. This finding suggests that the serum 25(OH)D3 levels obtained upon treatment with UVB are insufficient to suppress EAE and that UVB likely suppresses EAE independent of vitamin D production.
Discussion
In contrast to a previous report (13), 7-day pretreatment with 2.5 kJ/m2 UVB did not suppress clinical signs of EAE. This discrepancy is potentially because of differences in mouse strains and antigens used in these studies or because of differences in UV administration. Although UVB pretreatment failed to show an effect on EAE progression, continuous UVB treatment throughout the duration of the experiment caused significant inhibition of EAE. This finding suggests that increasing the frequency of UVB exposure enhances its suppressive effects, and that the mechanisms underlying disease suppression may be transient and reversible.
Surprisingly, continuous UVB treatment only slightly elevated serum 25(OH)D3 levels. Daily treatment with 2.5 kJ/m2 UVB for 7 days caused a modest increase of 16 ng/mL of 25(OH)D3 in the serum. However, there was no difference in serum 25(OH)D3 levels at later time-points, despite continued exposure to UVB. Further increases in 25(OH)D3 levels may have been inhibited by mechanisms meant to prevent vitamin D toxicity. Interestingly, clinical signs of EAE were suppressed throughout the duration of the study, even when 25(OH)D3 levels were no longer elevated compared to control mice. This suggests that sustained elevations of 25(OH)D3 levels were not critical for the suppressive effects of UVB on EAE. This observation led us to explore the ability of 25(OH)D3 delivered in the diet to suppress EAE independent of UVB exposure. Our results indicate that treatment with 10 μg/kg of 25(OH)D3 had no effect on EAE, despite causing an elevation in serum 25(OH)D3 levels similar to that seen in the UVB-treated mice. Furthermore, treatment with up to 1,000 μg/kg of 25(OH)D3 caused only a modest suppression of EAE and was associated with severe hypercalcemia. In contrast, continuous treatment with 2.5 kJ/m2 UVB led to greater disease suppression and had no effect on serum calcium levels. In humans, the normal range of serum 25(OH)D3 levels is between 20 and 100 ng/mL (30). Vitamin D toxicity occurs at serum 25(OH)D3 levels above 200 ng/mL (30). The 25(OH)D3 doses required to suppress EAE were well above this level. Thus, our data suggest that the 25(OH)D3 levels obtained upon treatment with UVB are insufficient to suppress EAE, and that UVB is likely suppressing disease through mechanisms that are independent of vitamin D production.
The current model used to explain the relationship between increased UV exposure and decreased MS incidence is that UVR is critical for producing vitamin D, which is then converted into 25(OH)D3. Provided sufficient 25(OH)D3 levels are present, 25(OH)D3 can be converted to 1,25(OH)2D3 and perform immunoregulatory functions that suppress autoimmune mechanisms. Support for this hypothesis is derived from studies indicating that decreased exposure to UVR and decreased 25(OH)D3 levels are associated with a higher risk for developing MS (22, 31). However, our results suggest that the levels of 25(OH)D3 required to suppress EAE cannot feasibly be produced upon exposure to UVR.
UVR can suppress the immune system through a number of mechanisms independent of vitamin D, including inhibiting antigen presentation, altering inflammatory cytokine levels, and inducing suppressor T-cell populations (32). Therefore, we suggest that UVR is likely playing a role in immunosuppression independent of vitamin D production. Potential caveats to this hypothesis include important differences between the immune systems of mice and humans (33), as well as between MS and EAE (34). Additionally, the electromagnetic radiation spectrum emitted by UV bulbs is not representative of sunlight and delivers a much higher proportion of UVB (35). Despite these potential caveats, our data suggest that the putative benefits associated with exposure to UVR cannot be completely recapitulated by simple supplementation with vitamin D. In fact, the benefits of 25(OH)D3 levels below the threshold that causes vitamin D toxicity and hypercalcemia would likely be negligible. Thus, at least some exposure to UVR may be necessary to prevent MS development. More work is required to determine the optimal levels of UVR exposure that provide the beneficial aspects of UVR, while avoiding the detrimental effects associated with chronic UVR exposure.
Additional evidence linking vitamin D and MS is the observation that treatment with the active form of vitamin D, 1,25(OH)2D3 suppresses EAE (23, 24). However, the efficacy of 1,25(OH)2D3 treatment is closely linked with the hormone’s ability to increase serum calcium levels; complete disease suppression only occurs using doses of 1,25(OH)2D3 that cause severe hypercalcemia (26). Prolonged hypercalcemia can lead to the calcification of soft tissues, such as kidney, heart, and liver, ultimately leading to organ failure. The hypercalcemic effects of 1,25(OH)2D3 have precluded its usage as a therapeutic agent in the treatment MS. A number of investigators have tried to overcome this limitation by developing less calcemic vitamin D analogs in hopes of reducing the calcemic effects, while retaining the suppressive effects of the natural hormone (36–38). Despite modest success, no treatment involving 1,25(OH)2D3 or vitamin D analogs has conclusively shown prevention of EAE without elevation of serum calcium levels. Moreover, results from our laboratory suggest that calcium may be playing an essential mechanistic role in 1,25(OH)2D3-mediated suppression of EAE (26, 28). These results diminish but do not eliminate the chance that an analog of 1,25(OH)2D3 can be found that may suppress MS. In contrast, continuous treatment with UVB suppresses EAE without altering serum calcium levels. Furthermore, there are no reported cases of hypercalcemia caused by excessive sunlight exposure (30). This observation suggests that disease suppression with UVR is independent of calcium, and that UVR is likely suppressing disease through different mechanisms than 1,25(OH)2D3.
In summary, our results indicate that continuous treatment with UVB suppresses clinical signs of EAE. Although UVB treatment causes a slight increase in serum 25(OH)D3 levels, this elevation is insufficient to contribute to disease suppression. Furthermore, treatment with UVB did not elevate serum calcium levels, which appears to be a critical step in 1,25(OH)2D3-mediated suppression of EAE. Therefore, we conclude that UVB is likely suppressing EAE independent of vitamin D production.
Materials and Methods
Compounds.
The compounds 25(OH)D3 and 1,25(OH)2D3 were synthesized by Sigma-Aldrich Fine Chemicals. Compounds were dissolved in absolute ethanol, and the concentration was determined with an UV spectrophotometer using λmax of 264 nm and an extinction coefficient of 18,200 M−1 cm−1 for both compounds. Compounds were added to vegetable oil in the indicated concentrations and delivered in the purified diet, as described below.
Animals and Diet.
Female C57BL/6 mice between 7 and 9 weeks of age were purchased from The Jackson Laboratory. All mice were housed at the University of Wisconsin–Madison Biotron animal facility under specific pathogen-free conditions and exposed to 12-h light-dark cycles. Before administration of experimental diets, mice were fed ad libitum standard rodent Labdiet 5008 chow (Purina Mills International). In the indicated experiments, 8-week-old mice were switched to a purified diet containing all of the essential nutrients for normal growth (39). Next, 25(OH)D3 and 1,25(OH)2D3 were added to the purified diet at doses ranging from 0 to 1,000 μg per kilogram body weight per day. The diet was delivered in solidified agar form three times per week beginning 10 days before immunization and continued until the termination of the experiment. Animal protocols were approved by the University of Wisconsin–Madison Institutional Animal Care and Use Committee.
UV Irradiation.
Mice from the control and UV-treated groups were shaved with electric clippers 1 day before initiating UV therapy. UV-treated mice were irradiated with a bank of four unfiltered FS20T12 fluorescent sunlamps (Solarc Systems) emitting a broad band of UVR from 280 to 360 nm. Approximately 65% of the output was in the UVB range (290–320 nm). The radiation output was measured before each treatment using a UVX radiometer equipped with a 302-nm sensor (UVP). Mice were individually irradiated in a specially designed 16-chamber Plexiglas cage to prevent mice from sheltering each other from the UVR. Because the UVB output was unequal in the different chambers, mice were rotated through the different chambers on successive days. Mice were irradiated daily for either 13 min (2.5 kJ/m2) or 26 min (5.0 kJ/m2) at a distance of 40 cm from the UV source. In the pretreatment study, mice were treated once daily with either 2.5 or 5.0 kJ/m2 for a total of 7 days. In the continuous UV study, mice were treated once daily with 2.5 kJ/m2 for 7 days, then either every other day or every third day with 2.5 kJ/m2 UVB for the duration of the experiment.
Induction of EAE.
Myelin oligodendrocyte glycoprotein peptide (MOG35–55) (MEVGWYRSPFSRVVHLYRNGK) was synthesized at the University of Wisconsin–Madison Biotechnology Center and purified to ≥95% by reverse-phase HPLC. The MOG35–55 peptide was resuspended in sterile PBS to a concentration of 4 mg/mL, then emulsified with an equivalent volume of complete Freund's adjuvant (CFA) supplemented with 5 mg/mL inactivated Mycobacterium tuberculosis H37Ra (DIFCO Laboratories). EAE was induced in 9-week-old C57BL/6 mice by s.c. injection of 100 μL of MOG35–55/CFA homogenate delivering 200 μg of MOG35–55 peptide. On the day of immunization and 48 h later, mice were injected intraperitoneally with 200 ng of pertussis toxin (List Biological Laboratories) diluted in sterile PBS. Mice were scored daily for clinical signs of EAE using the following scale: 0, no clinical disease; 1, loss of tail tone; 2, unsteady gait; 3, hind limb paralysis; 4, forelimb paralysis; 5, death. Scoring was performed by the same individual throughout the experiment to ensure consistency. On selected days, mice were independently scored by a different individual for comparison purposes, but the scores were not counted in the final analysis.
Serum Calcium Analysis.
Blood was collected at the termination of the experiments. Blood samples were spun at 2,938 × g for 15 min, followed by a second spin at 16,883 × g for 1 min. Serum calcium levels were determined using the calcium L3K reagent (Genzyme Diagnostics) and the ABX Pentra 400 clinical chemistry analyzer (Horiba-ABX Diagnostics).
Serum 25(OH)D3 Analysis.
Blood was collected at selected time points throughout the experiment. Red blood cells were removed through two successive centrifugation steps, as described above. Serum 25(OH)D3 levels were determined using a 125I-radioimmunological assay following the manufacturer's instructions (DiaSorin). Samples above the range of the standard curve were diluted before analysis. Radioactivity was quantified using a Cobra 5002 gamma scintillation counter (PerkinElmer).
Data Analysis.
Individual mice were scored daily for signs of EAE, and the mean clinical score was calculated for each group. Average onset and severity were calculated in affected mice displaying a clinical score of ≥ 1.0 for a minimum of 2 consecutive days. Onset was calculated by averaging the first day when clinical signs appeared. Severity was determined by averaging the maximum disease score reached during the entire experiment. The CDI was calculated by summing the clinical scores for each group for all time-points collected and dividing by the number of mice per group. Statistical analysis was performed using the two-tailed Fisher’s exact probability test for incidence rates, the Mann-Whitney nonparametric test for clinical scores, and the unpaired Student’s t test for all other measurements. A value of P < 0.05 was considered statistically significant.
Acknowledgments
The authors thank Dr. James Kim and Chad Johnson for their help in constructing the UVR apparatus, Bradley James and Wendy Hellwig for technical assistance, and Pat Mings for assistance in the preparation of this manuscript. This work was funded in part by the Wisconsin Alumni Research Foundation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7:268–277. doi: 10.1016/S1474-4422(08)70042-5. [DOI] [PubMed] [Google Scholar]
- 4.Ebers GC, Sadovnick AD. The geographic distribution of multiple sclerosis: a review. Neuroepidemiology. 1993;12:1–5. doi: 10.1159/000110293. [DOI] [PubMed] [Google Scholar]
- 5.Acheson ED, Bachrach CA, Wright FM. Some comments on the relationship of the distribution of multiple sclerosis to latitude, solar radiation, and other variables. Acta Psychiatr Scand Suppl. 1960;35:132–147. doi: 10.1111/j.1600-0447.1960.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland JM, Tyrer JH, Eadie MJ. The prevalence of multiple sclerosis in Australia. Brain. 1962;85:149–164. doi: 10.1093/brain/85.1.149. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Dosemeci M, Alavanja MC. Mortality from multiple sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med. 2000;57:418–421. doi: 10.1136/oem.57.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 10.Leitenberger J, Jacobe HT, Cruz PD., Jr Photoimmunology—illuminating the immune system through photobiology. Semin Immunopathol. 2007;29:65–70. doi: 10.1007/s00281-007-0063-6. [DOI] [PubMed] [Google Scholar]
- 11.McMichael AJ, Hall AJ. Does immunosuppressive ultraviolet radiation explain the latitude gradient for multiple sclerosis? Epidemiology. 1997;8:642–645. doi: 10.1097/00001648-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Tremlett H, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31:271–279. doi: 10.1159/000166602. [DOI] [PubMed] [Google Scholar]
- 13.Hauser SL, et al. Prevention of experimental allergic encephalomyelitis (EAE) in the SJL/J mouse by whole body ultraviolet irradiation. J Immunol. 1984;132:1276–1281. [PubMed] [Google Scholar]
- 14.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 15.Blunt JW, DeLuca HF, Schnoes HK. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968;7:3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6) Suppl:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF, Schnoes HK, DeLuca HF. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci USA. 1971;68:803–804. doi: 10.1073/pnas.68.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 19.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003;49:277–300. [PubMed] [Google Scholar]
- 20.Goldberg P. Multiple Sclerosis: Vitamin D and calcium as environmental determinants of prevalence (a viewpoint). Part 1: Sunlight, dietary factors, and epidemiology. Int J Environ Stud. 1974a;6:19–27. [Google Scholar]
- 21.Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006;92:140–149. doi: 10.1016/j.pbiomolbio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 23.Lemire JM, Archer DC. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nashold FE, Hoag KA, Goverman J, Hayes CE. Rag-1-dependent cells are necessary for 1,25-dihydroxyvitamin D(3) prevention of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001;119:16–29. doi: 10.1016/s0165-5728(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 26.Cantorna MT, Humpal-Winter J, DeLuca HF. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr. 1999;129:1966–1971. doi: 10.1093/jn/129.11.1966. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 28.Meehan TF, Vanhooke J, Prahl J, Deluca HF. Hypercalcemia produced by parathyroid hormone suppresses experimental autoimmune encephalomyelitis in female but not male mice. Arch Biochem Biophys. 2005;442:214–221. doi: 10.1016/j.abb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Shephard RM, Deluca HF. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys. 1980;202:43–53. doi: 10.1016/0003-9861(80)90404-x. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Mei IA, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–590. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 32.Norval M, McLoone P, Lesiak A, Narbutt J. The effect of chronic ultraviolet radiation on the human immune system. Photochem Photobiol. 2008;84:19–28. doi: 10.1111/j.1751-1097.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 33.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 34.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Brown DB, et al. Common fluorescent sunlamps are an inappropriate substitute for sunlight. Photochem Photobiol. 2000;72:340–344. [PubMed] [Google Scholar]
- 36.Lemire JM, Archer DC, Reddy GS. 1,25-Dihydroxy-24-OXO-16ene-vitamin D3, a renal metabolite of the vitamin D analog 1,25-dihydroxy-16ene-vitamin D3, exerts immunosuppressive activity equal to its parent without causing hypercalcemia in vivo. Endocrinology. 1994;135:2818–2821. doi: 10.1210/endo.135.6.7988477. [DOI] [PubMed] [Google Scholar]
- 37.Mattner F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.van Etten E, et al. Novel insights in the immune function of the vitamin D system: synergism with interferon-beta. J Steroid Biochem Mol Biol. 2007;103:546–551. doi: 10.1016/j.jsbmb.2006.12.094. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]