Abstract
Finding our way in spatial environments is an essential part of daily life. How do we come to possess this sense of direction? Extensive research points to the hippocampus and entorhinal cortex (EC) as key neural structures underlying spatial navigation. To better understand this system, we examined recordings of single-neuron activity from neurosurgical patients playing a virtual-navigation video game. In addition to place cells, which encode the current virtual location, we describe a unique cell type, EC path cells, the activity of which indicates whether the patient is taking a clockwise or counterclockwise path around the virtual square road. We find that many EC path cells exhibit this directional activity throughout the environment, in contrast to hippocampal neurons, which primarily encode information about specific locations. More broadly, these findings support the hypothesis that EC encodes general properties of the current context (e.g., location or direction) that are used by hippocampus to build unique representations reflecting combinations of these properties.
Keywords: electrophysiology, navigation, entorhinal cortex, direction, place cell
Convergent evidence from electrophysiological, lesion, and imaging studies shows that the hippocampus plays an important role in spatial navigation and episodic memory, both in humans and in animals (1–3). An important example of this comes from recordings of rodent hippocampal place cells during spatial navigation (4). During navigation, the network of place cells encodes the animal's spatial location because each one activates when the animal is at a particular location in the environment, that cell's “place field” (5). However, in addition to location, subsequent studies showed that hippocampal neurons also respond to other aspects of the current context, including the navigational goal (6, 7), the phase of the behavioral task (8), and the direction of movement (9).
In particular, the current direction of movement often has a dramatic effect on place-cell activity. In environments where animals follow distinct routes, place cells often behave in a unidirectional manner in which one cell's place fields remap to different locations according to the direction of movement (10). In contrast, when animals navigate unconstrained through open environments, the location of each place field is typically fixed, regardless of the animal's direction. It is unknown whether direction-related place-cell remapping is caused by an afferent directional signal outside the hippocampus or whether it originates from intrahippocampal computations (11–13). Examining this issue is important to better understand hippocampal processing.
A powerful way to probe hippocampal computation is to compare the neuronal activity observed in the hippocampus with activity recorded from its primary input, entorhinal cortex (EC). Previous research in animals using this technique revealed a fundamental dissociation between the types of information encoded by neurons in each of these regions (14–16). When a hippocampal neuron activates, it typically represents a specific behavioral context, such as a place cell that activates when the animal is at a particular location. In contrast, the spiking of EC neurons typically represents attributes of the current setting that are not exclusively linked to one location. For example, EC “grid cells” indicate whether the animal is positioned at one of various equally spaced locations (17, 18), EC “border cells” indicate the orientation and distance to the environment's walls (19), and EC “path equivalent cells” indicate a location's relative position along a common type of route (14). The different types of information that are encoded by hippocampal and EC neurons play a critical role in computational models of human spatial navigation and memory (20, 21). However, this system is difficult to study in detail experimentally, because direct reports of EC neuronal activity are relatively rare, especially in humans. In particular, patterns of EC activity that could lead to direction-related place-cell remapping have not been observed.
Here we examined neuronal coding in the human medial temporal lobe—including EC and hippocampus—using recordings of single-neuron activity from neurosurgical patients playing a virtual-navigation game. Our primary goal was to compare the types of spatial information encoded by neuronal spiking in various brain regions. To do this, we designed a paradigm in which patients navigated a relatively simple virtual environment. In each trial of this game, patients drove along a narrow, square-shaped virtual road in either a clockwise or a counterclockwise direction to reach a destination landmark. In these recordings we observed place cells, which indicated the patient's presence at certain virtual locations, and we also observed a unique phenomenon, EC path cells, which varied their firing rate according to whether the patient was driving in a clockwise or counterclockwise direction, regardless of location. During navigation, path cells encoded directional attributes of the current context and may be an important input to unidirectional hippocampal place cells.
Results
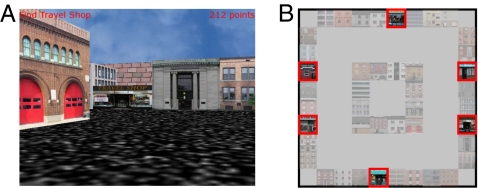
We recorded 1,419 neurons from widespread brain regions of neurosurgical patients implanted with intracranial depth electrodes. Electrodes were implanted to identify the seizure focus for potential surgical treatment for drug-resistant epilepsy. During each recording session patients played Yellow Cab, a virtual-reality video game. In Yellow Cab, patients used a handheld joystick to drive a taxicab through a virtual town to a randomly selected destination. The virtual town had six destination stores arranged along the outside of a narrow square road; the center of the environment was obstructed to force patients to drive in a clockwise or counterclockwise direction around the road to their destination (Fig. 1). In each trial, the destination store was randomly selected, ensuring that patients repeatedly traversed every part of the environment across multiple deliveries.
Fig. 1.
The Yellow Cab virtual-navigation video game. (A) A patient's on-screen view of the environment during the game. (B) Overhead map of the environment. Possible destination stores are brightly colored and outlined in red. Pale-colored buildings form the remainder of the outer and inner walls of the environment.
To characterize the patterns of neuronal activity during this task, we examined the relation between each neuron's firing rate and the patient's simultaneous behavior. We identified two functional classes of neurons: path cells and place cells. Path cells encode information about the patient's clockwise or counterclockwise direction of movement, continuously representing directional information while the patient is at widespread locations. Place cells encode the patient's presence at a specific location, spiking only when the patient is at a particular position and facing a certain direction. The critical difference between path and place cells is that path cells continuously encode direction across the environment, whereas place cells activate only at specific locations and otherwise are silent (4, 6, 22).
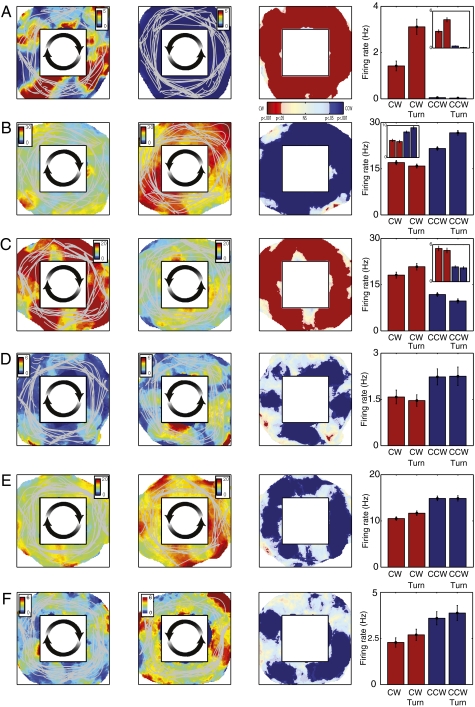
To identify each path cell, we calculated the proportion of the locations in the environment where neuronal activity significantly varied between clockwise and counterclockwise movements. To do this, we performed a two-sided rank-sum test at each location and calculated the proportion of the environment (Adir) where this test indicated that spiking rate varied with direction. If Adir was significantly greater than chance, then we designated the neuron as a path cell (SI Materials and Methods). Our analysis identified 79 path cells (6% of all cells). Figure 2A depicts the activity of a path cell from patient 2’s right EC (Movie S1). Here we plotted the firing rate of this neuron as a function of the patient's virtual location and direction (clockwise or counterclockwise). This neuron's activity appeared to closely relate to the patient's direction because it was highly active during clockwise movements throughout the environment (Left) but showed little activity during counterclockwise movements (Middle Left). To illustrate this directional activity more specifically, at each location we plotted the result of a comparison between the neuron's firing rates between clockwise and counterclockwise traversals (Middle Right). This analysis indicated that this cell had a significantly higher firing rate during clockwise movements at 94% of the traversed locations in the environment (i.e., Adir = 0.94). Thus, this neuron's activity encoded the patient's clockwise or counterclockwise direction of movement regardless of the current spatial location. Next, by analyzing this neuron's activity across all locations, we found that this cell's clockwise-specific firing was not only present when the patient was turning (angular velocity >15°/s), but also when the patient was driving straight (rank-sum tests, P values <10−12; Fig. 2A, Right). Thus, this neuron's activity appeared to be a cognitive correlate of the “clockwiseness” of the current path, rather than a perceptual or motor-related response to right turns. In addition to this cell, we also observed other path cells that consistently encoded the patient's clockwise or counterclockwise direction throughout the environment (Fig. 2 B–F).
Fig. 2.
Clockwise and counterclockwise path cell activity. (A) Firing rate of a clockwise path cell from patient 2’s right entorhinal cortex (microelectrode 13) during testing session 2. (Left) Mean firing rate during clockwise movement at each location in the virtual environment. Color indicates the mean firing rate (in Hz), and gray lines indicate the path of the patient. (Middle left) Neuronal activity during counterclockwise movements. (Middle right) Indication of whether firing rate at each location was statistically greater (rank-sum test) during clockwise movements (red) or during counterclockwise movements (blue). (Spatial statistics: Adir = 0.94 and Dpref = 1; SI Materials and Methods). (Right) Firing rate of this neuron combined across all regions of the environment for clockwise (“CW”) and counterclockwise (“CCW”) movements during straight movements and turns (“Turn”). (Inset) Activity of a neuron recorded from this same microelectrode in a different testing session; the two cells have very different firing rates and thus are likely distinct, nearby neurons. (B) Activity of a counterclockwise path cell recorded from patient 2’s right entorhinal cortex (microelectrode 15). (Adir = 0.78 and Dpref = 1.) (C) Activity of a clockwise path cell recorded from patient 2’s right entorhinal cortex (microelectrode 9). (Adir = 0.81 and Dpref = 1.) (D) Activity of a counterclockwise path cell recorded from the left entorhinal cortex of patient 5. (Adir = 0.29 and Dpref = 0.99.) (E) Activity of a counterclockwise path cell from patient 4’s right parahippocampal gyrus. (Adir = 0.32 and Dpref = 1.) (F) Activity of a counterclockwise path cell from patient 13’s right orbitofrontal cortex. (Adir = 0.45 and Dpref = 1.)
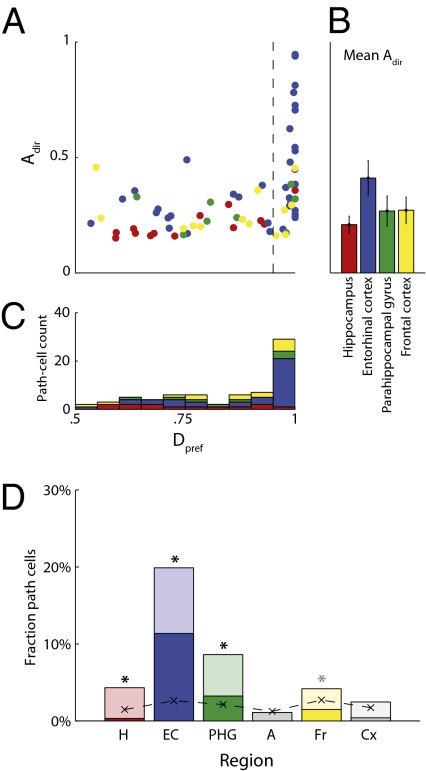
Next, we sought to measure the properties of path cells in more detail. We identified each path cell's preferred direction as the direction (clockwise or counterclockwise) where the cell had a greater firing rate across all locations, and then we computed a quantity called Dpref. Dpref indicates the proportion of the environment where the cell had elevated firing during movement in the preferred direction, out of all locations exhibiting significant direction-related spiking in either direction. Thirty path cells (38%) had Dpref ≥ 0.95 (Fig. 3A). This indicates that they consistently had elevated firing during movement in the cell's preferred direction throughout at least 95% of the environment—we refer to these as clockwise path cells or counterclockwise path cells. In one notable patient, in the right EC (Fig. S1) we observed a number of both clockwise path cells (Fig. 2 A and C) and counterclockwise path cells (Fig. 2B). Notably, across testing sessions conducted in this patient on different days, we sometimes repeatedly observed path cells at the same microelectrodes. In 10 of these 11 instances (binomial test, P < 0.001), we found that path cells observed in the second or third testing session had the same preferred direction as the path cell observed at that electrode in the first session (Fig. 2 A–C, Right, Inset). Unfortunately, because of the limitations of the clinical testing environment, we cannot determine whether the same neurons were indeed recorded across multiple sessions.
Fig. 3.
Characteristics of path cells. (A) Each path cell is indicated by a dot positioned to indicate its value of Adir, the fraction of the environment at which it exhibited directional firing, and Dpref, the proportion of these directional locations that were in the cell's preferred direction (Materials and Methods). Color indicates the brain region where the cell was observed. Black dashed line separates clockwise or counterclockwise path cells (Dpref ≥ 0.95) and complex path cells (Dpref < 0.95). Only path cells from brain regions where significant numbers of path cells were observed are included. (B) Mean value of Adir for all path cells in each region; error bars denote 95% confidence intervals. (C) Histogram for values of Dpref from path cells in each region. Horizontal axis (Dpref) has the same scale for A and C. (D) Regional distribution of path cells: Bars depict percentage of neurons observed in each brain area that were path cells. Dark shading indicates clockwise or counterclockwise path cells; light shading indicates complex path cells. Region key: A, amygdala; Cx, parietal and temporal cortices; EC, entorhinal cortex; Fr, Frontal cortices; H, hippocampus; PHG, parahippocampal gyrus. Black dashed line and letter x indicate the percent of path cells expected by chance (Type 1 error rate). Asterisks indicate regions in which the number of observed path cells significantly exceeded the Type 1 error rate (one-sided binomial test; black asterisks, P < 0.05; gray asterisk, P = 0.06).
In addition to clockwise or counterclockwise path cells, our statistical framework also identified 49 complex path cells (62%), which exhibited directional activity at widespread positions in the virtual environment but had different preferred directions across these locations. Thus, these complex path cells had large values of Adir, but had Dpref < 0.95 (Fig. S2). Complex path cells may be related to other navigation-related neuronal phenomena, such as head-direction cells (23), goal-related place cells (6, 7), and spatial-view cells (24). For example, a “north” head-direction cell might appear as a complex path cell that is active at clockwise headings in the west part of the environment and active at counterclockwise headings in the east part of the environment (Fig. S2D). In all, 29 of the complex path cells (59%) exhibited activity that significantly varied with the patient's absolute “compass” heading (P values <0.01, ANOVA), which is consistent with them behaving as head-direction cells (23).
Next, we aimed to determine, across our entire dataset, whether path cells were clustered in particular brain regions or whether they were homogeneously distributed throughout the brain. We computed the proportions of path cells observed in each brain region (Fig. 3D), and we found that path cells were not distributed uniformly [χ2(5) = 87, P < 10−13]. Rather, they were especially prevalent in the EC, where 35 of the recorded neurons (20%) were path cells—this was significantly greater than the Type 1 error rate of our statistical framework (Bonferroni-corrected one-sided binomial test, P < 10−10). We also observed significant numbers of path cells in hippocampus (13 cells, 1.5%, P < 0.02) and in parahippocampal cortex (eight cells, 2%, P < 0.02), and also many path cells in frontal cortex (14 cells, 3%, P = 0.10, uncorrected).
One possibility is that path cells from different brain regions have divergent properties. To examine this, we compared the properties of the path cells observed in hippocampus, EC, parahippocampal cortex, and frontal cortex. We found that path cells in each of these regions exhibited significantly different areal extents of directional activity, as measured via Adir [Kruskal–Wallis nonparametric ANOVA, H(3) = 18, P < 0.0005; Fig. 3 A–C]. Specifically, EC path cells had significantly larger values of Adir than path cells in hippocampus, parahippocampal cortex, and frontal cortex (posthoc rank-sum test, P < 0.0002). This indicates that EC path cells exhibited directional firing at a greater proportion of the locations in each environment than path cells in other regions. We also tested for regional differences in the tendency for path cells to have a single clockwise or counterclockwise preferred direction, as assessed through Dpref (Fig. 3C). Here, we found that path cells in EC had significantly larger values of Dpref than path cells in other regions [H(3) = 9.4, P < 0.03; posthoc test, P < 0.01]. Thus, path cells in EC were more likely to encode a single clockwise or counterclockwise preferred direction across the environment, whereas complex path cells were more prevalent in other regions. By analyzing each neuron's spike waveform, mean firing rate, and interspike intervals (25), we designated 9% of the cells in our dataset as putative interneurons. We compared the prevalence and properties of path cells between interneurons and noninterneurons, but we did not find any significant differences in the properties of the path cells between these cell populations (SI Results).
We performed a series of follow-up analyses to elucidate the properties of path cells and to test alternate explanations for their behavior (SI Results). Path-cell activity does not appear to be an artifact of neuronal responses to turns in a particular direction, because we found that path cells reliably encoded their preferred direction during various types of clockwise and counterclockwise driving, including straight movements and both left and right turns. We also compared the activities of path cells with the expected behaviors of retrospective- and prospective-coding neurons, a phenomenon in which neuronal activity encodes the direction of upcoming or previous turns at certain locations (14). We found that path-cell activity was most closely linked to the current direction rather than future or past headings (Fig. S3), which indicates that path cells are not performing retrospective or prospective coding. Finally, we found that path cells’ directional activity was present immediately when each task session began. Because significant path-cell directional activity appeared before the environment's layout was learned, it indicates that path cells encode a directional signal that is not directly related to the patient's memory for the environment's landmarks.
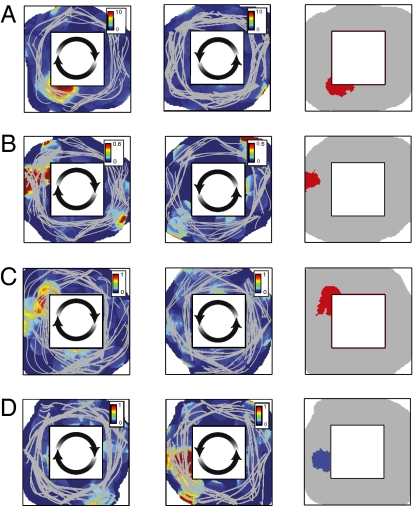
In addition to path cells, we identified 118 place cells (8%). Each place cell had a significantly elevated firing rate when the patient was at a particular location and facing in a certain direction, compared with all other locations. Thus, the activity of these place cells was much more spatially focused than path cells. All of the place cells that we observed were unidirectional, meaning that they only increased their firing rate when a particular location was traversed in a certain direction. For example, Fig. 4A depicts the activity of one hippocampal place cell, which had a significantly elevated firing rate when traveling clockwise through a location in the southwest corner of the environment. These direction-sensitive place cells have not been reported before in humans (6). However, they are often observed in rodents performing directionally organized tasks (9, 26, 10), like the square virtual road in our task. We identified statistically significant quantities of place cells in hippocampus, EC, parahippocampal gyrus, amygdala, and frontal cortex (one-sided binomial tests, all P values <0.03; Fig. S4). To examine whether place cells from different regions had different properties, we compared the spatial-firing properties of the place cells observed in each of these regions. There was a trend for place cells from hippocampus to encode more location-related information than place cells from other regions (nonparametric ANOVA, P = 0.1); we did not observe a significant region-related difference in place-field size (P > 0.5). Consistent with findings in animals (27), we observed that 18% of place cells exhibited a significant positive correlation (P < 0.05) between firing rate and the speed of virtual movement. We compared the directionality indices of place and path cells and found that both complex and noncomplex path cells encoded directional information more robustly than place cells (rank-sum tests, P values <10−10).
Fig. 4.
Example place cells. (A) Activity of a place cell from the hippocampus of patient 1. (Left) Depiction of this cell's activity during clockwise movement. (Middle) Depiction of this cell's activity during counterclockwise movement. Color scale indicates each cell's firing rate (in Hz). (Right) Computed place field of this cell (Materials and Methods). Red place field indicates that this cell activated when the place field was traversed while traveling clockwise. (B) Example place cell from the hippocampus of patient 5. (C) Place cell from the hippocampus of patient 13. (D) Place cell from the entorhinal cortex of patient 5. (Right) Blue place field indicates that this cell was activated when this position was traversed while moving counterclockwise.
Discussion
The primary finding in the current study is that the human EC contains path cells that encode the clockwise or counterclockwise direction of the current route during navigation in circular environments. Below we comment on the relation between path cells and the current body of knowledge on the electrophysiology of spatial navigation, and we discuss these findings more broadly in relation to modern theories on the role of the EC in cognition.
Largely based on brain recordings from navigating rodents, researchers theorized that the EC plays a critical role in navigation and memory by encoding attributes of the current behavioral context (20, 21). Unlike downstream hippocampal neurons, which typically activate only at specific locations, EC neurons encode information in a less sparse manner so that they may activate at multiple locations. An example of this phenomenon is the EC “path equivalent” cell, which encodes an animal's relative position along common types of routes (14). For example, one path-equivalent cell might activate immediately before making a right turn, exhibiting this pattern before a right curve in any environment. Because these cells activate at multiple spatial locations, they are fundamentally different from hippocampal place cells. Human clockwise and counterclockwise path cells may be a more general version of path-equivalent cells. In contrast to path-equivalent cells, which only encode directional information at particular spots (e.g., positions preceding right turns), clockwise and counterclockwise path cells exhibit this directional activity throughout the environment. Furthermore, we note that the sustained activations of path cells during movement in one direction (Movie S1) are reminiscent of intracellular recordings from EC neurons that revealed sustained patterns of spiking for many seconds (28, 29).
Our finding, in conjunction with reports of EC activity in navigating rodents (14, 17–19, 30), supports the view that the hippocampal–entorhinal system operates in this manner: Each EC neuron encodes a perceptual, spatial, or cognitive attribute of the current behavioral context. This occurs in a nonspecific manner, so that similar but nonidentical contexts have comparable EC representations. During navigation, path cells are one part of this scheme, such that each path cell encodes the “clockwiseness” of the current context. Then, in the hippocampus, this contextual information is used to form conjunctive neural patterns, such as directional place cells (Fig. 4), which are sensitive to the precise combination of currently active EC neurons (20, 21, 31).
In addition to path-equivalent cells, several other findings from research in animals share characteristics with path cells. Frank et al. (14) described EC retrospective- and prospective-coding neurons, which encode information about the direction of previous or upcoming turns. This phenomenon is generally related to clockwise and counterclockwise path cells, as both cell types encode information that is correlated with the direction of an upcoming or previous turn. Critically, these cells encode information only at certain locations—such as before or after turns (14) or at specific positions where navigation decisions are made (32)—which is different from the behavior of clockwise and counterclockwise path cells.
A second related cell type from the rodent navigation literature is the head-direction cell (33), the activities of which are determined by the orientation of an animal's head. Conceptually, head-direction cells seem related to path cells, as their activity is determined solely by a measure of direction, rather than by location. However, the fundamental nature of the directional signals encoded by these cell classes differ: Head-direction cells encode absolute compass-like orientations (e.g., northwest), whereas path cells encode direction in a manner that depends on the environment's shape. Human clockwise and counterclockwise path cells may be related to head-direction cells, but they appear to represent a more abstract version of this phenomenon.
A third related cell class from the animal literature are grid cells (17) and grid × direction cells (34), both of which have been observed in the rodent EC. Grid cells activate when an animal is positioned at one of various equally spaced locations, and grid × direction cells spike when an animal is at one of these locations and also facing in a specific direction. At first glance, the spatial firing pattern of the path cell depicted in Fig. 2C is reminiscent of a grid cell (17) because it was especially active at specific locations near the corners of the environment during clockwise movement. In fact, a recent study by Derdikman et al. reported that EC grid cells in rodents could exhibit a similar pattern in a “hairpin” maze, as they observed that the grid vertices were aligned to the maze's turns (35). However, there are two reasons why we do not believe that our results directly correspond to grid cells or grid × direction cells (even if the grid vertices were linked to the environment's layout). First, whereas grid × direction cells are active when certain locations are traversed at a fixed compass-like bearing (e.g., when driving north), the direction at which this cell activates varies depending on the instantaneous location (e.g., driving south in the east part of the environment and driving north in the west part of the environment). Second, whereas grid cells are typically silent when the animal is between the grid's vertices, this cell also exhibited a robust directional signal between the locations exhibiting peak activity (Fig. 2, Middle Right).
Some clockwise or counterclockwise path cells exhibited spatial variations in their firing rates, in addition to their overall direction coding. For example, the cell the activity of which is depicted in Fig. 2C appeared to have an especially elevated firing rate during clockwise movements when approaching turns. However, the magnitude of these location-related variations was smaller than the magnitude of this cell's overall directional activity. This phenomenon may be explained because at some locations increased visual information causes patients to increase their attention to the task. This may cause path cells to experience an attention-related gain modulation of their underlying directional activity (36). Although some path cells fulfilled our criterion for exhibiting a place field (Fig. S5), this location-related activity explained only a small component of these cells’ spiking, compared with the direction-related spiking that appeared throughout the environment.
One significant difference between the current findings and recent literature concerns place cells. Whereas most studies report that place cells are especially prevalent in hippocampus, here we found place cells in various brain regions (Fig. S4). This relatively low number of hippocampal place cells, relative to previous studies (6, 37), may be explained by potential differences in the navigational demands of this task. Previous human studies show that the use of a spatial or nonspatial strategy to perform a particular task can dramatically affect the resultant hippocampal activity (38). Because our task involved a relatively simple, mostly one-dimensional environment, some participants may have relied more heavily on nonspatial tactics, such as learning the relative ordering of adjacent stores, to perform the task. This could account for the relatively small number of hippocampal place cells we observed. A further difference in our results was that all of the place cells we observed were unidirectional, meaning that they were only active when their place field was traversed in a certain direction. In contrast, a previous study reported human place-cell responses in a grid-like virtual environment that were not sensitive to the direction of movement (6). However, our observation of directional place cells is consistent with studies where rodents performed directionally organized tasks, similar to the circular road in our task. In these studies, movement at each location is typically observed in one of two main directions and different place fields appear for each direction (9). These results are often explained by the hypothesis that different “cognitive maps” are used for each direction of movement (10). Here we suggest that the same phenomenon may be occurring, because patients navigate the circular road in one of two main directions (clockwise or counterclockwise) and because the high walls around the road encourage patients to use local cues for navigation. Therefore, this environment may induce patients to use different cognitive maps for clockwise movements than for counterclockwise movements. The activity of EC neurons, such as path cells, may be one of the factors that determine which cognitive map is used.
Our discovery of EC path cells provides a striking demonstration of the broad range of information that may be encoded in EC. In addition to directional information encoded by path cells during navigation, recent studies showed that EC contains neurons encoding a broad range of cognitive information in both spatial and nonspatial tasks, including characteristics of the current behavioral task (8), future or past movements (14, 32), the contents of working memory (39), the objects currently being viewed (6, 40), the distance to nearby walls (19), and the current spatial location (17, 30, 34). Most of these studies analyzed recordings from rodents, and thus it is possible that similar patterns do not exist in humans. Nonetheless, taken together, these findings support the view that the EC plays a pivotal role in memory formation because EC neurons encode attributes of the current context that are subsequently stored by the hippocampus as memories (20, 21, 41). Going forward, we suggest that more fully characterizing the diverse information-coding properties of EC neurons is an important step toward better understanding the neural basis of human cognition.
Materials and Methods
Behavioral Task.
The data for this study came from 13 patients undergoing surgical treatment for drug-resistant epilepsy (surgeries performed by I.F.). This study was in compliance with the guidelines of the Medical Institutional Review Board at the University of California–Los Angeles. We examined data from 34 testing sessions (30–50 min), which were conducted in patients’ spare time between standard clinical procedures. (Individual patients completed between one and four testing sessions.)
In each testing session, patients played Yellow Cab, a taxi-driver video game (6, 42), on a laptop computer in their hospital room. In Yellow Cab, patients used a handheld joystick to drive through a virtual three-dimensional environment delivering passengers to their requested destinations. The virtual environment contained six possible destination stores arranged on the perimeter of a narrow square road. The center region of this environment was obstructed by buildings, so patients had to choose to drive in either a clockwise or counterclockwise direction around the road. Two stores were located on each of the east and west walls, and one store was on each of the north and south walls (Fig. 1). Each destination store is marked by a brightly colored storefront with a sign displaying its name. The patient delivers a passenger by driving the cab into the front of the store. After each delivery, text appears on-screen displaying the name of the next, randomly selected destination.
Electrophysiology.
In each patient, we recorded spiking activity at 28–32 kHz using 40-μm platinum–iridium microwire electrodes (43) connected to a Cheetah recording system (Neuralynx). Nine microwires extended from the tip of each clinical depth electrode. The first eight wires were insulated except for their tip and were used to record action potentials. The ninth microwire had its insulation stripped for ≈1 cm and served as the voltage reference for the other eight microwires. Action potentials were manually isolated using spike shape, clustering of wavelet coefficients, and interspike intervals (44).
Data Analysis.
To determine how neuronal activity related to patients’ virtual navigation, we computed the firing rate of each neuron in 100-ms epochs throughout the task. We then labeled each epoch according to the patient's instantaneous location, whether they were currently pointed in a clockwise or counterclockwise direction, and whether they were turning left, turning right, or driving straight. The patient's direction (clockwise, counterclockwise, or neither) was calculated from the patient's bearing and location. For example, if a patient was pointed south in the east part of the environment, then the direction was clockwise. Our initial visual observations revealed path cells that significantly varied their activity depending on the patient's direction throughout large regions of the virtual environment. To examine this phenomenon in detail, we designed a statistical framework to measure the direction- and location-related characteristics of each neuron's activity (SI Materials and Methods). For cells that did not meet our path-cell criteria, we also conducted a series of analyses to identify place cells.
Supplementary Material
Acknowledgments
We thank Igor Korolev for help with cluster cutting; Christoph Weidemann, Sean Polyn, Marieke van Vugt, and Per Sederberg for helpful discussions; and Brian Jacobs for technical assistance. This work was sponsored by National Institutes of Health Research Grants MH61975, MH062196, NS033221, NS054575, and NS50067 and National Science Foundation Grant SBE0354378.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911213107/DCSupplemental.
References
- 1.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford University Press; 1978. [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 4.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 6.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–187. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 7.Ainge JA, Tamosiunaite M, Woergoetter F, Dudchenko PA. Hippocampal CA1 place cells encode intended destination on a maze with multiple choice points. J Neurosci. 2007;27:9769–9779. doi: 10.1523/JNEUROSCI.2011-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin A, Eichenbaum H, Hasselmo M. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;27:2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNaughton BL, Barnes CA, O'Keefe J. The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res. 1983;52:41–49. doi: 10.1007/BF00237147. [DOI] [PubMed] [Google Scholar]
- 10.Markus EJ, et al. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacucci F, Lever C, Wills T, Burgess N, O'Keefe J. Theta-modulated place-by-direction cells in the hippocampal formation of the rat. J Neurosci. 2004;24:8265–8277. doi: 10.1523/JNEUROSCI.2635-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 13.Huxter J, Senior T, Allen K, Csicsvari J. Theta phase–specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat Neurosci. 2008;11:587–594. doi: 10.1038/nn.2106. [DOI] [PubMed] [Google Scholar]
- 14.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 15.Hargreaves E, Rao G, Lee I, Knierim J. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- 16.Fyhn M, et al. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- 17.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 18.Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solstad T, Boccara C, Kropff E, Moser M, Moser E. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 20.Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: Toward a common explanation of medial temporal lobe function across domains. Psychol Rev. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori E, et al. Place-related neural responses in the monkey hippocampal formation in a virtual space. Hippocampus. 2005;15:991–996. doi: 10.1002/hipo.20108. [DOI] [PubMed] [Google Scholar]
- 23.Taube J, Muller R, Ranck J. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolls E. Spatial view cells and the representation of place in the primate hippocampus. Hippocampus. 1999;9:467–480. doi: 10.1002/(SICI)1098-1063(1999)9:4<467::AID-HIPO13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Viskontas I, Ekstrom A, Wilson C, Fried I. Characterizing interneuron and pyramidal cells in the human medial temporal lobe in vivo using extracellular record-ings. Hippocampus. 2007;17:49–57. doi: 10.1002/hipo.20241. [DOI] [PubMed] [Google Scholar]
- 26.Muller R, Bostock E, Taube J, Kubie J. On the directional firing properties of hippocampal place cells. J Neurosci. 1994;14:7235–7251. doi: 10.1523/JNEUROSCI.14-12-07235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huxter J, Burgess N, O'Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- 29.Hasselmo M, Stern C. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10:487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr The positional firing properties of medial entorhinal neurons: Description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasselmo M, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton PA, White JA, Eichenbaum H. Disambiguation of overlapping experiences by neurons in the medial entorhinal cortex. J Neurosci. 2007;27:5787–5795. doi: 10.1523/JNEUROSCI.1063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blair HT, Sharp PE. Anticipatory head direction signals in anterior thalamus: Evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J Neurosci. 1995;15:6260–6270. doi: 10.1523/JNEUROSCI.15-09-06260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sargolini F, et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 35.Derdikman D, et al. Fragmentation of grid cell maps in a multicompartment environment. Nat Neurosci. 2009;12:1325–1332. doi: 10.1038/nn.2396. [DOI] [PubMed] [Google Scholar]
- 36.Salinas E, Thier P. Gain modulation: A major computational principle of the central nervous system. Neuron. 2000;27:15–21. doi: 10.1016/s0896-6273(00)00004-0. [DOI] [PubMed] [Google Scholar]
- 37.Muller R. A quarter of a century of place cells. Neuron. 1996;17:813–822. doi: 10.1016/s0896-6273(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 38.Maguire E, Valentine E, Wilding J, Kapur N. Routes to remembering: The brains behind superior memory. Nat Neurosci. 2003;6:90–95. doi: 10.1038/nn988. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- 40.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 41.Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary learning systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 42.Newman EL, et al. Learning your way around town: How virtual taxicab drivers learn to use both layout and landmark information. Cognition. 2007;104:231–253. doi: 10.1016/j.cognition.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Fried I, et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. J Neurosurg. 1999;91:697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- 44.Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.