Abstract
Riboswitches are natural RNA sensors that regulate gene expression in response to ligand binding. Riboswitches have been identified in prokaryotes and eukaryotes but are unknown in organelles (mitochondria and plastids). Here we have tested the possibility to engineer riboswitches for plastids (chloroplasts), a genetic system that largely relies on translational control of gene expression. To this end, we have used bacterial riboswitches and modified them in silico to meet the requirements of translational regulation in plastids. These engineered switches were then tested for functionality in vivo by stable transformation of the tobacco chloroplast genome. We report the identification of a synthetic riboswitch that functions as an efficient translational regulator of gene expression in plastids in response to its exogenously applied ligand theophylline. This riboswitch provides a novel tool for plastid genome engineering that facilitates the tightly regulated inducible expression of chloroplast genes and transgenes and thus has wide applications in functional genomics and biotechnology.
Keywords: chloroplast, plastid transformation, translational regulation
Riboswitches are natural RNA sensors that mediate control of gene expression via their capacity to bind small molecules (metabolites). They fold into RNA secondary structures whose conformation switches between an “on” state and an “off” state in response to ligand binding (reviewed, e.g., in refs. 1–4). Riboswitches can be divided into two distinct structural domains: an aptamer and an expression platform. The aptamer domain binds the metabolite and this triggers conformational changes in the expression platform, which either permit or prevent gene expression. Depending on the nature of the response to metabolite binding, “on” switches and “off” switches are distinguished. In bacteria, riboswitches reside mainly in the 5′ untranslated regions (UTRs) of mRNAs and an emerging theme is that anabolic genes are mainly controlled by “off” switches (5–7), whereas catabolic genes are controlled by “on” switches (8), thus providing an efficient mechanism of feedback control of metabolic pathways. In bacteria, most riboswitches act at the transcriptional level [e.g., by resolution of rho-independent transcription termination hairpins (8, 9)], but a few translational switches have also been identified (5, 7). Translational riboswitches usually function via sequestration of the ribosome-binding site (Shine-Dalgarno (SD) sequence), thereby blocking translation initiation in a metabolite-dependent manner. The increasing understanding of the functional principles of bacterial riboswitches and the possibility to produce novel aptamers by in vitro evolution of RNA molecules has facilitated attempts to design synthetic switches (10–12), which potentially can provide versatile tools for genetic engineering and synthetic biology applications.
While, over the past years, numerous riboswitches have been discovered in prokaryotes (13) and also a few in eukaryotes including plants (14, 15), no riboswitches are known in organellar (plastid and mitochondrial) genomes. Plastids (chloroplasts) and mitochondria are derived from formerly free-living bacteria and have retained a largely prokaryotic gene expression machinery. However, in contrast to prokaryotes and eukaryotes, chloroplasts have largely abandoned transcriptional control and switched to predominantly translational control of their gene expression (16–18). Although some transcriptional regulation is known to occur (19, 20), its importance for determining plastid protein levels appears to be very much limited (16, 17). Translational control is much more prevalent (21, 22) and, in many cases, appears to largely override any changes that are seen in transcript abundance (17, 20). The posttranscriptional mechanisms of gene regulation in plastids are mediated by intricate plastid-nuclear interactions (18) and are still far from being fully understood. Their complexity has also hampered the development of tightly controlled inducible gene expression systems for plastids. Although recent progress has made it possible to genetically engineer the chloroplast genomes of an increasing number of higher plants (23), transgene expression from the plastid genome is usually constitutive and attempts to construct inducible gene expression systems have been afflicted with the requirement for additional nuclear transgenes and/or a substantial leakiness of gene expression in the uninduced state (24–26). This is highly unfortunate, because tightly controllable expression systems represent versatile tools in both functional genomics and biotechnology. The possibility to express transgenes from the plastid genome has stirred considerable excitement among plant biotechnologists (23, 27, 28), mainly due to the attainable enormous expression levels (29) and the increased transgene containment provided by maternal inheritance of plastids in most crops (30, 31). However, constitutive plastid transgene expression can cause severe mutant phenotypes (e.g., 32, 33) and, also for this reason, the development of tightly controllable systems for inducible gene expression is of utmost importance. Here we have explored the possibility of engineering riboswitches to function as translational regulators of gene and transgene expression in plastids.
Results
Design of Synthetic Riboswitches for Plastids.
To engineer translational switches for inducible gene expression from the plastid genome, several riboswitches known to function in bacteria were selected and modified to meet the requirements of plastid translational regulation. From the relatively few translational riboswitches identified so far, we chose the Escherichia coli thiamine pyrophosphate (TPP) riboswitch [translational “off”’ switch (5, 34)] and the synthetic theophylline (theo) riboswitch [translational “on” switch (12)]. We also selected two transcriptional “on” switches and attempted to convert them into translational switches: the glycine (gly) switch (8) and the adenine (ade) switch (9) (Fig. 1 and Fig. S1). Both are from Bacillus subtilis and operate by a transcriptional antitermination mechanism (1, 13). The riboswitches were first engineered in silico to improve the translation initiation signals for expression in plastids (Fig. 1 and Fig. S1 A and B) and convert the transcriptional terminator structures of the gly and ade switches into Shine-Dalgarno/anti-Shine-Dalgarno structures theoretically suitable to confer translational regulation. This was necessary because there is no efficient transcription termination occurring in plastids (35) and, moreover, bacterial transcription terminators have been demonstrated to not function in plastids (36). We, therefore, replaced the terminator structures of the gly and ade switches by a secondary structure containing the Shine-Dalgarno sequence (SD). Similar to the terminator structure of the natural gly and ade switches (Fig. S1 A and B), metabolite binding should resolve the base-pairing between the SD and a complementary anti-Shine-Dalgarno sequence (Fig. 1 A and B) and, in this way, facilitate access of the 70S ribosomes to the mRNA. The sequences of the designed riboswitches were synthesized as double-stranded DNAs and cloned into an expression cassette containing the reporter gene gfp (encoding the green fluorescent protein, GFP) under the control of the plastid ribosomal operon promoter Prrn (Fig. S2 and Fig. 3B). In addition to the synthetic riboswitches designed to act as translational switches, we also included the natural gly and ade transcriptional switches. Thus, altogether six riboswitch constructs were produced: a synthetic gly switch (s.gly-RS; Fig. 1A), a synthetic ade switch (s.ade-RS; Fig. 1B), a synthetic theo switch (s.theo-RS; Fig. 1C), the natural gly switch (gly-RS; Fig. S1A), the natural ade switch (ade-RS; Fig. S1B), and the natural TPP switch (tpp-RS; Fig. S1C). All six riboswitch-containing gfp expression cassettes were cloned into a plastid transformation vector suitable to target the transgenes to an intergenic spacer region in the tobacco plastid genome by homologous recombination (37). The vector contains a chimeric spectinomycin resistance gene that facilitates the selection of plants with transgenic chloroplast genomes [transplastomic plants (38)]. As chloroplasts evolved from cyanobacteria and have retained a largely prokaryotic-type gene expression machinery, plastid expression elements (promoters and untranslated regions, UTRs) are usually also active in bacteria. This allowed us to test all riboswitch constructs also in E. coli and compare their regulatory properties between bacteria and plastids.
Fig. 1.
Secondary structural models for synthetic riboswitches tested in this study. Nucleotides changed with respect to the original sequence are shown in red. SD sequences are boxed. Restriction sites (BamHI, NsiI) are underlined. The start codons are part of NsiI restriction sites. All switches are designed as translational “on” switches and are shown in the proposed off mode (i.e., in the absence of the metabolite). In this mode the SD sequence is masked and, therefore, inaccessible to the ribosome. (A) A synthetic glycine riboswitch (s.gly-RS) containing an anti-Shine-Dalgarno (ASD) sequence designed to allow translational regulation by base-pairing with the SD sequence. The switch is derived from the glycine riboswitch (gly-RS) from Bacillus subtilis (8) (Fig. S1A). (B) A synthetic adenine riboswitch (s.ade-RS) designed as translational “on” switch. The switch is derived from the ydhL adenine riboswitch (ade-RS) from Bacillus subtilis (9) (Fig. S1B). (C) The synthetic theophylline responsive riboswitch (s.theo-RS) based on helix slipping (12). The one-nucleotide theophylline-induced slipping in the secondary structure is indicated by the red arrow. The 5′UTR contains two possible SD sequences (boxed).
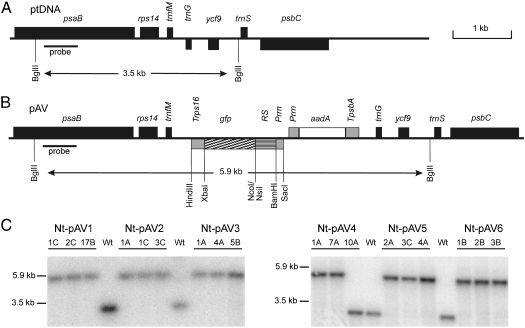
Fig. 3.
Generation of plastid transformants with vectors carrying gfp gene constructs under the control of riboswitch elements. (A) Physical map of the targeting region in the plastid genome. (B) Structure of plastid transformation vectors of the pAV series harboring riboswitch-containing GFP expression cassettes. Relevant restriction sites are marked. The transgenes are targeted to the intergenic region between the trnfM and trnG genes (37). The GFP expression cassette consists of the ribosomal RNA operon promoter (Prrn) fused to the riboswitch (RS) element (see Fig. 1 and Fig. S1) and the 3′UTR from the plastid rps16 gene (Trps16). The expected sizes of DNA fragments in restriction fragment length polymorphism analyses with the enzyme BglII are indicated. The location of the RFLP probe is shown as a black bar. The selectable marker gene aadA is driven by a chimeric ribosomal RNA operon promoter (Prrn) and fused to the 3′UTR from the plastid psbA gene [(TpsbA (38)]. (C) RFLP analysis of transplastomic tobacco lines. Total cellular DNA was digested with BglII and hybridized to a radiolabeled probe detecting the region of the plastid genome that flanks the transgene insertion site. Fragment sizes for the wild-type and the transplastomic lines are indicated. Absence of a hybridization signal for the wild-type genome indicates homoplasmy of the transplastomic lines. Line Nt-pAV4-10A shows the hybridization signal for the wild-type genome suggesting that this line represents a spontaneous antibiotic-resistant mutant. The transplastomic lines harbor the following riboswitches: Nt-pAV1: gly-RS; Nt-pAV2: ade-RS; Nt-pAV3: tpp-RS; Nt-pAV4: s.gly-RS; Nt-pAV5: s.ade-RS; Nt-pAV6: s.theo-RS.
Analysis of Riboswitch Function in E. coli.
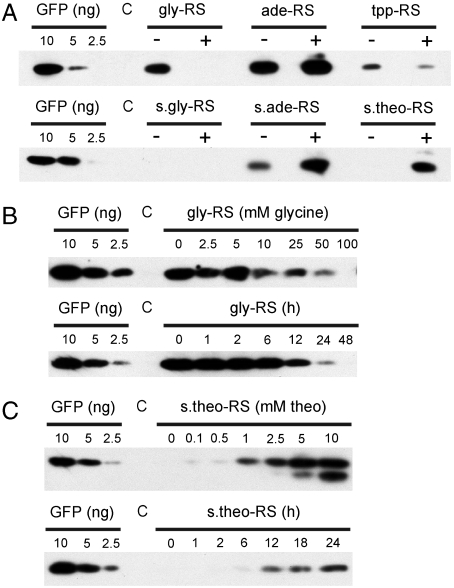
To analyze the functionality of the riboswitches in bacteria, we introduced the six constructs into E. coli cells. We first tested the switch properties on minimal medium with or without the regulatory metabolite (glycine, adenine, TPP, or theophylline). No GFP expression was detectable with the synthetic glycine switch, possibly indicating that the designed secondary structure masking the SD is too stable to be melted by metabolite binding (Fig. 2A). The natural adenine switch showed relatively poor inducibility by adenine, whereas the synthetic adenine switch responded much stronger, possibly suggesting that we have successfully converted the ade switch from a transcriptional into a translational riboswitch (Fig. 2A). To confirm the translational nature of the induction of GFP accumulation by adenine in the s.ade-RS construct, we compared RNA accumulation in the presence vs. the absence of adenine (Fig. S3A). No adenine dependence of gfp mRNA accumulation was detectable, confirming that the designed s.ade-RS functions at the level of translation initiation. As expected, the TPP riboswitch showed a repression of GFP accumulation in the presence of TPP, and the s.theo-RS showed efficient and fast induction of GFP expression by theophylline (Fig. 2 A and C). Unexpectedly, the natural gly riboswitch, acting as a transcriptional “on” switch in Bacillus subtilis, behaved like an “off” switch when expressed from our construct in E. coli (Fig. 2A). gly-RS switching required relatively high concentrations of glycine (10–100 mM) and became evident ≥12 h after metabolite addition (Fig. 2B). Analysis of a control construct lacking a riboswitch sequence confirmed that addition of none of the four metabolites appreciably affected GFP expression (Fig. S3B).
Fig. 2.
Test of metabolite dependence of GFP expression from riboswitch constructs in E. coli by western blotting. Cells were grown in minimal medium. An E. coli strain harboring a luciferase gene (51) instead of gfp was used as negative control. To allow for quantitative comparisons, a dilution series of purified GFP (10 ng, 5 ng, 2.5 ng) was included in all blots. (A) GFP accumulation in the absence (-) versus the presence (+) of the regulatory metabolite. In the upper panel, 3 μg of total soluble protein (TSP) were loaded for the gly-RS, ade-RS, tpp-RS, and the control strain (C). In the lower panel, 9 μg TSP were loaded for the s.gly-RS, s.ade-RS, s.theo-RS, and the C strain. Cells were grown to midexponential phase. For metabolite-induced switching, the medium was supplemented with 100 mM glycine, 5 mM adenine, 1 mM thiamine pyrophosphate, or 10 mM theophylline. (B) GFP expression controlled by the glycine riboswitch. In the upper panel, the GFP accumulation in dependence on the glycine concentration is shown. The lower panel shows the time course of GFP accumulation following addition of 100 mM glycine. 3 μg TSP were loaded in all lanes. (C) GFP expression controlled by the theophylline riboswitch. The upper panel shows the GFP accumulation in dependence on the theophylline concentration in the medium. The lower panel shows the time course of GFP accumulation following addition of 10 mM theophylline. 9 μg TSP were loaded in all lanes.
As a control, we also analyzed all riboswitch constructs in bacterial cultures grown in Luria-Bertani (LB) medium. LB is a rich medium, which contains most bacterial metabolites in significant concentrations. As expected, this resulted in loss of switchability of all riboswitches, with the notable exception of the s.theo-RS (Fig. S3C). This is because theophylline is a product of plant metabolism and, therefore, is not present in LB medium (which is produced from bacterial and yeast cell extracts).
Integration of Riboswitches into the Plastid Genome.
To analyze riboswitch function in plastids, we introduced all six constructs into tobacco plants by stable transformation of the chloroplast genome (38, 39). As the functioning of riboswitches depends on a multitude of factors, including endogenous metabolite concentrations, proper RNA folding, RNA stability, and ribosome-binding to the mRNA, we decided to test also those riboswitches in plastids that were not properly regulated by metabolite addition in E. coli.
Plastid transformation was carried out by particle bombardment followed by selection of spectinomycin-resistant cell lines. Spectinomycin resistance is conferred by the chimeric selectable marker gene aadA (38), which is driven by plastid expression signals (Fig. 3 A and B). For each construct, several putative transplastomic lines were obtained and subjected to additional rounds of regeneration under antibiotic selection to eliminate residual wild-type copies of the plastid genome and isolate homoplasmic lines (39). Successful plastid transformation and integration of the transgenes into the chloroplast genome by homologous recombination was verified by restriction fragment length polymorphism (RFLP) analyses (Fig. 3C). Inheritance assays revealed homogeneous populations of spectinomycin-resistant seedlings in all crosses using transplastomic lines as a maternal crossing partner, thus ultimately demonstrating that homoplasmic lines had been obtained and all wild-type copies of the plastid genome had been successfully eliminated.
Analysis of Riboswitch Function in Plastids.
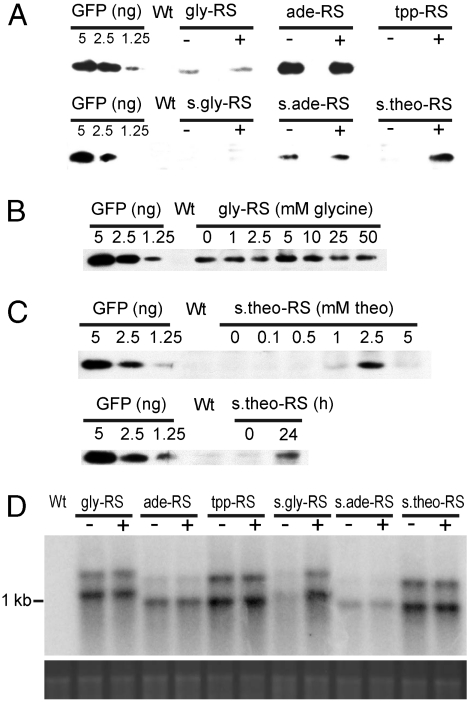
Having obtained stable homoplasmic transplastomic plants, we next wanted to test metabolite dependence of GFP expression from the chloroplast genome. To this end, we germinated seeds on medium with or without the regulatory metabolite and comparatively analyzed GFP accumulation by western blotting using a GFP-specific antibody. GFP was undetectable in the tpp-RS and s.gly-RS lines (Fig. 4A), although gfp transcripts accumulated to normal levels (Fig. 4D). This may suggest that, neither in the presence nor in the absence of the metabolite, the tpp-RS and s.gly-RS structures permit access of the plastid ribosome to the SD sequence in the 5′UTR. In contrast, GFP accumulated in a metabolite-independent manner in the gly-RS, ade-RS, and s.ade-RS transplastomic lines, suggesting that these riboswitches either do not function in plastids or, alternatively, the endogenous metabolite levels of glycine and adenine are too high to allow suppression of gene expression in the absence of exogenously supplied metabolite. Testing of a wide range of metabolite concentrations confirmed the insensitivity of GFP expression to the regulatory metabolite in these transplastomic lines (Fig. 4B). Interestingly, the s.theo-RS lines showed strong induction of GFP expression by theophylline and no background expression in the absence of the metabolite (Fig. 4A). Induction was strongest with a theophylline concentration of 2.5 mM (Fig. 4C). Theophylline concentrations of 5 mM and higher were toxic to tobacco plants (as evidenced by aberrant growth and a pale-green phenotypic) and resulted in a concomitant decline in GFP expression. When theophylline was sprayed onto plants raised in the absence of the metabolite, nearly full induction was seen after 24 h (Fig. 4C). RNA gel blot analyses revealed theophylline-independent accumulation of gfp transcripts (Fig. 4D) confirming that the s.theo-RS acts as a translational switch also in plastids.
Fig. 4.
Metabolite-dependent mRNA and foreign protein accumulation in transplastomic plants. (A) GFP accumulation in transplastomic tobacco plants. Total soluble protein was extracted from pools of 10–20 seedlings (28 d old) grown on medium without inducers (-) or medium supplemented with 10 mM glycine, 5 mM adenine, 0.1 mM thiamine pyrophosphate, or 2.5 mM theophylline (+). 10 μg TSP were loaded for the gly-RS, tpp-RS, and s.ade-RS lines; 1 μg TSP for the ade-RS; and 20 μg TSP for the s.gly-RS and s.theo-RS lines. For comparison, a dilution series of purified GFP was included. Wt: wild-type. (B) GFP expression in transplastomic lines harboring the glycine riboswitch (Nt-pAV1) in dependence on the glycine concentration in the medium. 10 μg TSP were loaded in all lanes. As glycine in high concentrations is toxic to plants (and results in pale phenotypes), concentrations higher than 50 mM were not tested. (C) GFP expression in transplastomic lines harboring the synthetic theophylline riboswitch (Nt-pAV6). The upper panel shows the dependence of GFP accumulation on the theophylline concentration in the medium. Induction is detectable at 1 mM theophylline and peaks at 2.5 mM. Application of 5 mM theophylline is toxic to plants and results in aberrant growth and reduced induction of GFP expression. 20 μg TSP were loaded in all lanes. The lower panel shows the induction of GFP expression 24 h after spraying with a solution of 50 mM theophylline. (D) Analysis of metabolite dependence of mRNA accumulation in transplastomic lines harboring riboswitch constructs. Plants were grown on medium without inducer (-) medium or supplemented with 10 mM glycine, 5 mM adenine, 0.1 mM thiamine pyrophosphate, or 2.5 mM theophylline (+). Samples of 2 μg total RNA were blotted and hybridized to a gfp-specific probe. The size of the gfp transcripts varies slightly due to length differences of the riboswitches (Fig. S2). The major band represents monocistronic gfp mRNA and the upper band originates from read-through transcription, as observed before with transgene inserted into the same genomic location (52).
Discussion
In the course of this work, we have tested the possibility to adapt bacterial riboswitches to the gene expression system in plastids. Although plastids have a prokaryotic past, the regulatory mechanisms of their gene expression are very different from those operating in bacteria (18). Plastid gene expression is extensively regulated at the posttranscriptional level, and translational regulation can override even big changes in transcription and/or RNA stability (17). Because of the small number of natural riboswitches acting at the translational level, we attempted to convert transcriptional riboswitches into translational riboswitches. Although the s.ade-RS appears to function as a translational switch in E. coli at least to some extent (Fig. 2A), the limited success of this approach indicates that conversion of transcriptional into translational riboswitches is far from straightforward and much is yet to be learned about the structural and sequence requirements determining riboswitch efficiency. However, as transcription initiation from the plastid Prrn promoter has not been characterized in E. coli, and we also cannot exclude the possibility that aberrant long-range secondary structural interactions prevented proper RNA folding in E. coli and/or in plastids; the exact reasons for the lack of metabolite responsiveness currently remain unknown.
When tested in chloroplasts of tobacco plants, the synthetic theophylline riboswitch displayed excellent switching properties. No basal expression was detectable in the absence of the metabolite and expression was rapidly inducible by application of theophylline. The tightness of the switch in both bacteria and chloroplasts may be attributable to the fact that, unlike the other regulatory metabolites tested here, theophylline is not endogenously produced by either E. coli or tobacco plants. The GFP accumulation level reached by induction with theophylline in tobacco chloroplasts was in the range of 0.01–0.02% of the total soluble protein (Fig. 4C). This is significantly lower than upon constitutive expression of GFP from the same promoter [which is at least 10-fold higher (40)] and may be due to the Shine-Dalgarno sequence within the theophylline switch being less efficient and/or less accessible.
The identification of a riboswitch that functions in plastids provides opportunities for both functional genomics and plastid biotechnology. The highly efficient homologous recombination system in plastids has been successfully employed to study the functions of plastid genome-encoded genes and open reading frames by reverse genetics (e.g., refs. 41–43). However, the plastid genome of higher plants contains many essential genes that are not amenable to functional analysis by targeted inactivation (i.e., knockout by insertional or deletional mutagenesis). These essential plastid genes include components of the gene expression machinery (44–46), essential metabolic enzymes (47), as well as several open reading frames of unknown function (48). The synthetic theophylline riboswitch can now be used to construct inducible knockout lines for essential plastid genes by simply placing the target gene under the control of the s.theo-RS and transforming this construct into the plastid genome to replace the resident gene copy. Selection of transplastomic lines in the presence of theophylline will keep the essential gene active and allow isolation of homoplasmic transplastomic lines. Subsequent removal of theophylline will then allow to gradually repress the gene activity and analyze the phenotype of the plant in the absence of the essential gene product.
Transgene expression from the plant’s plastid genome has unique attractions to biotechnologists (28), including the plastids’ potential to accumulate foreign proteins to extraordinarily high levels [to up to more than 70% of the plant’s total soluble protein (29)] and the increased biosafety provided by the maternal mode of plastid inheritance, which greatly reduces unwanted transgene transmission via pollen (30, 31). However, constitutive expression of pharmaceutical proteins or unique metabolic pathways from the plastid genome can result in mutant phenotypes and/or severe growth retardation of transplastomic plants due to metabolite toxicities, interference with photosynthesis or disturbance of the plastid endomembrane system (e.g., refs. 32, 33). Solutions to these problems have long been sought (24–26), but progress has been hampered so far by the lack of tightly regulated inducible gene expression systems for plastids that are independent of additional nuclear transgenes. The theophylline riboswitch offers a “plastid-only” solution to inducible gene expression from the chloroplast genome that does not require additional (nuclear or plastid) transgenes and thus should be widely applicable.
Materials and Methods
Bacterial Strains and Growth Conditions.
E. coli strains Top10F’ (Invitrogen) or BL21(DE3) (Stratagene) were used for transformation with plasmid constructs. Transgenic strains were grown aerobically to midexponential phase in either LB medium or M9 minimal medium with ampicillin (100 μg/ml) at 37 °C under continuous shaking (180 rpm). For metabolite induction of riboswitches, the medium was supplemented with 100 mM glycine, 5 mM adenine, 1 mM thiamine pyrophosphate, or 10 mM theophylline, as indicated. To this end, cultures were diluted 1∶30 in 3 ml of medium and grown to midexponential phase in the presence or absence of the regulatory metabolite. To determine the time course of metabolite-dependent repression or induction of gene expression, bacteria were precultured in M9 minimal medium in the absence of the metabolite and then diluted in fresh medium. The starting cell density was set to OD600 = 1. Glycine or theophylline was added to a final concentration of 100 mM or 10 mM, respectively, and samples were taken for protein isolation at the indicated time points. Cultures were continuously diluted in fresh medium supplemented with the regulatory metabolite to keep them in the midexponential phase.
Plant Material.
Sterile tobacco (Nicotiana tabacum cv. Petit Havana) plants were grown on agar-solidified MS medium (49) supplemented with sucrose (20 g/l). Regenerated shoots from homoplasmic transplastomic lines were rooted and propagated on the same medium, then transferred to soil and grown to maturity under standard greenhouse conditions. For inheritance assays, seeds were surface sterilized and germinated on MS medium with or without spectinomycin (500 mg/l). For analysis of riboswitch function, the medium was supplemented with 10 mM glycine, 5 mM adenine, 0.1 mM thiamine pyrophosphate, or 2.5 mM theophylline.
Cloning Procedures.
All vectors containing 5′UTR riboswitches from Bacillus subtilis or E. coli are based on a pBS SK(+) vector harboring a Prrn–5′UTR–gfp–Trps16 expression cassette (50). Details are provided in SI Materials and Methods. The riboswitch-containing Prrn–5′UTR–gfp–Trps16 expression cassettes were subsequently excised with SacI and HindIII and inserted into the similarly cut plastid transformation vector pRB95, resulting in plasmids pAV4 (s.gly-RS), pAV5 (s.ade-RS), and pAV6 (s.theo-RS).
Plastid Transformation.
Plastid transformation was carried out by the biolistic protocol (38) using 0.6 μm gold particles and a helium-driven biolistic gun (PDS1000He; BioRad). Transplastomic lines were selected on regeneration medium containing 500 mg/l spectinomycin (38). Primary transplastomic lines were subjected to additional regeneration rounds on spectinomycin-containing medium to obtain homoplasmic tissue.
Metabolite Induction Experiments, Protein Extraction, and Immunoblotting.
Transformed E. coli cells of strains Top10F’ or BL21(DE3) were grown to midexponential phase in either LB or M9 minimal medium supplemented with the regulatory metabolite as indicated. Cells were diluted 1∶30 into 3 ml of the appropriate medium in the presences or absence of the metabolite, further grown to midexponential phase and finally brought to an OD600 of 1.0 (in LB medium). Cells were sedimented by centrifugation, the pellets were frozen in liquid nitrogen, resuspended in 1,000 μl lysis buffer (50 mM HEPES, 300 mM NaCl, 0.5% SDS, pH 8.0) and incubated for 30 min on ice. Subsequently, the cells were disrupted by sonication (amplitude 10%, 15 s; Sonifier®, W-250 D, Heinemann Ultraschall & Labortechnik) and centrifuged for 12 min at 12.000 g. The protein concentration was measured by the bicinchoninic acid protein assay (Pierce). Plant total soluble protein was extracted from 10 to 20 pooled seedlings (28 d old) grown on agar-solidified MS medium containing 2% sucrose. To test metabolite dependence of GFP expression, plants were grown on MS medium containing 2% sucrose with or without the regulatory metabolite. Total soluble protein was extracted from samples homogenized in a buffer containing 50 mM HEPES (pH 7.5), 10 mM potassium acetate, 5 mM magnesium acetate, 1 mM EDTA, 1 mM dithiothreitol, and 1 mM Pefabloc. After centrifugation for 10 min at 12.000 g at 4 °C, the protein concentration in the supernatant was determined using the Bradford assay (Roth) and known concentrations of bovine serum albumin as standard. To determine the time course of theophylline-dependent induction of gene expression, transplastomic seedlings were grown on agar-solidified MS medium containing 2% sucrose for 28 d. Theophylline was sprayed (5 ml of 50 mM theophylline dissolved in water) on the plate to a final concentration of 2.5 mM and samples were taken for protein isolation after 24 h. For western blotting, protein samples were separated by electrophoresis in denaturating SDS-polyacrylamide gels and transferred to Hybond P PVDF membranes (GE Healthcare). Transfer was carried out using a semidry blotting apparatus (SEDEC-M, PeqLab) and a standard transfer buffer (25 mM Tris-HCl, 192 mM glycine, pH 8.3). Protein detection was performed with a monoclonal mouse antibody raised against GFP (JL-8; Clontech) using the ECL Plus detection system (GE Healthcare). To exclude biological or technical variation, all results were confirmed by three biological replicas.
Supplementary Material
Acknowledgments.
We thank Dr. Bodo Rak (University of Freiburg, Germany) for bacterial strains, Drs. Juliane Neupert and Ning Shao for vectors, Dr. Stephanie Ruf and Claudia Hasse (MPI-MP) for plant transformation, and Drs. Wiebke Apel and Oliver Drechsel for discussion. This research was supported by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914423107/DCSupplemental.
References
- 1.Mandal M, Breaker RR. Gene regulation by riboswitches. Nat Rev. 2004;5:451–463. doi: 10.1038/nrm1403. [DOI] [PubMed] [Google Scholar]
- 2.Serganov A, Patel DJ. Ribozymes, riboswitches and beyond: Regulation of gene expression without proteins. Nat Rev Genet. 2007;8:776–790. doi: 10.1038/nrg2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakeman CA, Winkler WC, Dann CE., III Structural features of metabolite-sensing riboswitches. Trends Biochem Sci. 2007;32:415–424. doi: 10.1016/j.tibs.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henkin TM. Riboswitch RNAs: Using RNA to sense cellular metabolism. Gene Dev. 2008;22:3383–3390. doi: 10.1101/gad.1747308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler W, Nahvi A, Breaker RR. Thiamine derivates bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 6.Barrick JE, et al. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc Natl Acad Sci USA. 2004;101:6421–6426. doi: 10.1073/pnas.0308014101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nahvi A, Barrick JE, Breaker RR. Coenzyme B12 riboswitches are widespread genetic control elements in prokaryotes. Nucleic Acids Res. 2004;32:143–150. doi: 10.1093/nar/gkh167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal M, et al. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science. 2004;306:275–279. doi: 10.1126/science.1100829. [DOI] [PubMed] [Google Scholar]
- 9.Mandal M, Breaker RR. Adenine riboswitches and gene activation by disruption of a transcription terminator. Nat Stuct Mol Biol. 2004;11:29–35. doi: 10.1038/nsmb710. [DOI] [PubMed] [Google Scholar]
- 10.Bayer TS, Smolke CD. Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat Biotechnol. 2005;23:337–343. doi: 10.1038/nbt1069. [DOI] [PubMed] [Google Scholar]
- 11.Müller M, Weigand JE, Weichenrieder O, Suess B. Thermodynamic characterization of an engineered tetracycline-binding riboswitch. Nucleic Acids Res. 2006;34:2607–2617. doi: 10.1093/nar/gkl347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suess B, Fink B, Berens C, Stentz R, Hillen W. A theophylline responsive riboswitch based on helix slipping controls gene expression in vivo. Nucleic Acids Res. 2004;32:1610–1614. doi: 10.1093/nar/gkh321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkler WC, Breaker RR. Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- 14.Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- 15.Thore S, Leibundgut M, Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- 16.Deng X-W, Gruissem W. Control of plastid gene expression during development: The limited role of transcriptional regulation. Cell. 1987;49:379–387. doi: 10.1016/0092-8674(87)90290-x. [DOI] [PubMed] [Google Scholar]
- 17.Eberhard S, Drapier D, Wollman F-A. Searching limiting steps in the expression of chloroplast-encoded proteins: Relations between gene copy number, transcription, transcript abundance, and translation rate in the chloroplast of Chlamydomonas reinhardtii. Plant J. 2002;31:149–160. doi: 10.1046/j.1365-313x.2002.01340.x. [DOI] [PubMed] [Google Scholar]
- 18.Barkan A, Goldschmidt-Clermont M. Participation of nuclear genes in chloroplast gene expression. Biochimie. 2000;82:559–572. doi: 10.1016/s0300-9084(00)00602-7. [DOI] [PubMed] [Google Scholar]
- 19.Klein RR, Mullet JE. Light-induced transcription of chloroplast genes. J Biol Chem. 1990;265:1895–1902. [PubMed] [Google Scholar]
- 20.Kahlau S, Bock R. Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: Chromoplast gene expression largely serves the production of a single protein. Plant Cell. 2008;20:856–874. doi: 10.1105/tpc.107.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staub JM, Maliga P. Translation of the psbA mRNA is regulated by light via the 5′-untranslated region in tobacco plastids. Plant J. 1994;6:547–553. doi: 10.1046/j.1365-313x.1994.6040547.x. [DOI] [PubMed] [Google Scholar]
- 22.Hauser CR, Gillham NW, Boynton JE. Translational regulation of chloroplast genes. J Biol Chem. 1996;271:1486–1497. doi: 10.1074/jbc.271.3.1486. [DOI] [PubMed] [Google Scholar]
- 23.Maliga P. Plastid transformation in higher plants. Annu Rev Plant Biol. 2004;55:289–313. doi: 10.1146/annurev.arplant.55.031903.141633. [DOI] [PubMed] [Google Scholar]
- 24.McBride KE, Schaaf DJ, Daley M, Stalker DM. Controlled expression of plastid transgenes in plants based on a nuclear DNA-encoded and plastid-targeted T7 RNA polymerase. Proc Natl Acad Sci USA. 1994;91:7301–7305. doi: 10.1073/pnas.91.15.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mühlbauer SK, Koop H-U. External control of transgene expression in tobacco plastids using the bacterial lac repressor. Plant J. 2005;43:941–946. doi: 10.1111/j.1365-313X.2005.02495.x. [DOI] [PubMed] [Google Scholar]
- 26.Buhot L, Horvàth E, Medgyesy P, Lerbs-Mache S. Hybrid transcription system for controlled plastid transgene expression. Plant J. 2006;46:700–707. doi: 10.1111/j.1365-313X.2006.02718.x. [DOI] [PubMed] [Google Scholar]
- 27.Ma JK-C, et al. Molecular farming for new drugs and vaccines. EMBO Rep. 2005;6:593–599. doi: 10.1038/sj.embor.7400470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bock R. Plastid biotechnology: Prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr Opin Biotechnol. 2007;18:100–106. doi: 10.1016/j.copbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Oey M, Lohse M, Kreikemeyer B, Bock R. Exhaustion of the chloroplast protein synthesis capacity by massive expression of a highly stable protein antibiotic. Plant J. 2009;57:436–445. doi: 10.1111/j.1365-313X.2008.03702.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruf S, Karcher D, Bock R. Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci USA. 2007;104:6998–7002. doi: 10.1073/pnas.0700008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svab Z, Maliga P. Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc Natl Acad Sci USA. 2007;104:7003–7008. doi: 10.1073/pnas.0700063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lössl A, Eibl C, Harloff H-J, Jung C, Koop H-U. Polyester synthesis in transplastomic tobacco (Nicotiana tabacum L): Significant contents of polyhydroxybutyrate are associated with growth reduction. Plant Cell Rep. 2003;21:891–899. doi: 10.1007/s00299-003-0610-0. [DOI] [PubMed] [Google Scholar]
- 33.Hennig A, Bonfig K, Roitsch T, Warzecha H. Expression of the recombinant bacterial outer surface protein A in tobacco chloroplasts leads to thylakoid localization and loss of photosynthesis. FEBS J. 2007;274:5749–5758. doi: 10.1111/j.1742-4658.2007.06095.x. [DOI] [PubMed] [Google Scholar]
- 34.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature. 2006;441:1167–1171. doi: 10.1038/nature04740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern DB, Gruissem W. Control of plastid gene expression: 3′ inverted repeats act as mRNA processing and stabilizing elements but do not terminate transcription. Cell. 1987;51:1145–1157. doi: 10.1016/0092-8674(87)90600-3. [DOI] [PubMed] [Google Scholar]
- 36.Oey M, Lohse M, Scharff LB, Kreikemeyer B, Bock R. Plastid production of protein antibiotics against pneumonia via a new strategy for high-level expression of antimicrobial proteins. Proc Natl Acad Sci USA. 2009;106:6579–6584. doi: 10.1073/pnas.0813146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruf S, Hermann M, Berger IJ, Carrer H, Bock R. Stable genetic transformation of tomato plastids and expression of a foreign protein in fruit. Nat Biotechnol. 2001;19:870–875. doi: 10.1038/nbt0901-870. [DOI] [PubMed] [Google Scholar]
- 38.Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA. 1993;90:913–917. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bock R. Transgenic chloroplasts in basic research and plant biotechnology. J Mol Biol. 2001;312:425–438. doi: 10.1006/jmbi.2001.4960. [DOI] [PubMed] [Google Scholar]
- 40.Zhou F, Karcher D, Bock R. Identification of a plastid Intercistronic Expression Element (IEE) facilitating the expression of stable translatable monocistronic mRNAs from operons. Plant J. 2007;52:961–972. doi: 10.1111/j.1365-313X.2007.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi Y, Goldschmidt-Clermont M, Soen S-Y, Franzen LG, Rochaix J-D. Direct chloroplast transformation in Chlamydomonas reinhardtii: Insertional inactivation of the psaC gene encoding the iron-sulfur protein destabilizes photosystem I. EMBO J. 1991;10:2033–2040. doi: 10.1002/j.1460-2075.1991.tb07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruf S, Kössel H, Bock R. Targeted inactivation of a tobacco intron-containing open reading frame reveals a novel chloroplast-encoded photosystem I-related gene. J Cell Biol. 1997;139:95–102. doi: 10.1083/jcb.139.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schöttler MA, Flügel C, Thiele W, Stegemann S, Bock R. The plastome-encoded PsaJ subunit is required for efficient photosystem I excitation, but not for plastocyanin oxidation in tobacco. Biochem J. 2007;403:251–260. doi: 10.1042/BJ20061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogalski M, Ruf S, Bock R. Tobacco plastid ribosomal protein S18 is essential for cell survival. Nucleic Acids Res. 2006;34:4537–4545. doi: 10.1093/nar/gkl634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogalski M, Karcher D, Bock R. Superwobbling facilitates translation with reduced tRNA sets. Nat Struct Mol Biol. 2008;15:192–198. doi: 10.1038/nsmb.1370. [DOI] [PubMed] [Google Scholar]
- 46.Rogalski M, Schöttler MA, Thiele W, Schulze WX, Bock R. Rpl33, a nonessential plastid-encoded ribosomal protein in tobacco, is required under cold stress conditions. Plant Cell. 2008;20:2221–2237. doi: 10.1105/tpc.108.060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kode V, Mudd EA, Iamtham S, Day A. The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 2005;44:237–244. doi: 10.1111/j.1365-313X.2005.02533.x. [DOI] [PubMed] [Google Scholar]
- 48.Drescher A, Ruf S, Calsa T, Jr., Carrer H, Bock R. The two largest chloroplast genome-encoded open reading frames of higher plants are essential genes. Plant J. 2000;22:97–104. doi: 10.1046/j.1365-313x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 49.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 50.Neupert J, Karcher D, Bock R. Design of simple synthetic RNA thermometers for temperature-controlled gene expression in Escherichia coli. Nucleic Acids Res. 2008;36:e124. doi: 10.1093/nar/gkn545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao N, Bock R. A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Curr Genet. 2008;53:381–388. doi: 10.1007/s00294-008-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou F, et al. High-level expression of HIV antigens from the tobacco and tomato plastid genomes. Plant Biotechnol J. 2008;6:897–913. doi: 10.1111/j.1467-7652.2008.00356.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.