Abstract
KcsA is a prokaryotic pH-dependent potassium (K) channel. Its activation, by a decrease in the intracellular pH, is coupled with its subsequent inactivation, but the underlying mechanisms remain elusive. Here, we have investigated the conformational changes and equilibrium of KcsA by using solution NMR spectroscopy. Controlling the temperature and pH of KcsA samples produced three distinct methyl-TROSY and NOESY spectra, corresponding to the resting, activated, and inactivated states. The pH-dependence of the signals from the extracellular side was affected by the mutation of H25 on the intracellular side, indicating the coupled conformational changes of the extracellular and intracellular gates. K+ titration and NOE experiments revealed that the inactivated state was obtained by the replacement of K+ with H2O, which may interfere with the K+-permeation. This structural basis of the activation-coupled inactivation is closely related to the C-type inactivation of other K channels.
Keywords: potassium channel, solution NMR, gating, inactivation
KcsA is a pH-dependent potassium (K) channel from Streptomyces lividans. Its crystal structure provided the structural basis for the permeation and the selectivity for K+ ions (1). It forms a homotetramer, in which the transmembrane region of each subunit is composed of three helices, referred to as the outer, pore, and inner helices, respectively (1). The C termini of the four inner helices in the KcsA tetramer tightly interact with each other, forming a helix-bundle crossing. A selectivity filter, consisting of the highly conserved TVGYG sequence that follows the pore helix, resides on the extracellular side of the K+-permeation pathway.
The macroscopic current behavior of KcsA can be described by dividing it into four stages (2–4): (i) no K+ current is observed when the pH on the intracellular side is neutral (the resting state); (ii) the maximum peak current is observed within a few tens of milliseconds after the intracellular pH drops below 5.0 (activation); (iii) the current exponentially decays with a time constant of a few seconds (inactivation), until it reaches the stationary K+ current, about 10–20% of the peak current; and (iv) the current stops within a few tens of milliseconds after the intracellular pH returns to neutral. The activation and subsequent inactivation are referred to as activation-coupled inactivation, which is also observed for the eukaryotic voltage-dependent K (Kv) channels (2–4). In vertebrates, the inactivation of Kv channels is important for determining the action potential duration and the shape and characteristics of any action potential plateau (5). Therefore, KcsA would serve as a good prototype of Kv channels, and a structural analysis of KcsA could clarify the mechanism of the Kv channel function, including the activation-coupled C-type inactivation.
At the molecular level, these electrophysiological properties of KcsA are explained by the transitions between the resting, activated, and inactivated states. These states are distinguished by the conformations of the two gates within KcsA: One resides on the intracellular side of the transmembrane region, including the helix-bundle crossing (intracellular gate), and the other is on the extracellular side, including the selectivity filter (extracellular gate). The crystal structure under neutral conditions revealed that, in the resting state, the intracellular gate is closed and the extracellular gate is open (1). Under acidic conditions, the intracellular gate is considered to be open, based on various analyses including EPR, fluorescence, diffracted X-ray tracking method, and NMR (6–10), while the extracellular gate is proposed to exist in an equilibrium between the open and closed conformations, based upon the results from mutational and electrophysiological analyses (3, 4). Together, the activated and inactivated states are considered to be as the states under acidic conditions, where the extracellular gate is open and closed, respectively, while the intracellular gate is open. Although quantitative characterization of the dynamics as well as structural information about the two gates is requisite to understand the gating of KcsA, little is known about the conformational changes and dynamics of the extracellular gate.
In this study, we analyzed the conformational changes and equilibrium within KcsA by using solution NMR spectroscopy. Using a KcsA sample prepared in dodecyl maltoside (DDM), we successfully observed three different spectra, corresponding to the resting, activated, and inactivated states, at different pH values and temperatures. The NMR spectroscopic results reported here provide the structural basis for the dual gate properties, including the activation-coupled inactivation, which is applicable to the C-type inactivation of eukaryotic Kv channels.
Results
The pH-Dependent Spectral Changes in the Methyl-TROSY Spectra of KcsA.
In order to obtain structural probes that are widely distributed on the protein structure, we utilized the methyl-TROSY technique (11), in which the methyl groups of Ile δ1, Leu δ1/2, and Val γ1/2 are selectively labeled with 1H and 13C in an otherwise highly deuterated background (12, 13). KcsA possesses 3 isoleucine, 24 leucine, and 16 valine residues in its primary sequence of 160 residues, thus providing 83 probes in the methyl-TROSY spectrum.
At 45 °C in the presence of 120 mM K+, KcsA in the DDM micelles exhibited different methyl-TROSY spectra at pH 6.7 and 3.2 (Fig. 1 A and B), where some weak signals that do not correspond to those observed at pH 6.7 were observed at pH 3.2 (Red Arrows in Fig. 1B inset and Table S1). As the pH decreased, a set of signals decreased the intensities to zero, and another set of signals showed up and increased their intensities, preventing the transfer of the assignments from one state to the other by the pH titration experiments. Therefore, the assignments of the signals were independently accomplished at pH 6.7 and 3.2, by site-directed mutagenesis (14), with the aid of intraresidual NOE information (15). 61 and 66 methyl groups among the 83 methyl groups from all of the Ile (δ1), Leu, and Val residues were assigned at pH 6.7 and 3.2, respectively (Table S1). The two methyl groups of the leucine and valine residues, which were not stereospecifically assigned, were distinguished by the labels (A) and (B), based on their 13C chemical shift values (Table S1). Under acidic conditions, the minor signals of L59 δ(A) and δ(B), V76 γ(A) and γ(B), L81 δ(A), V84 γ(B), and L86 δ(A) were assigned in a similar way. The exchange between these minor peaks and the corresponding major signals was also confirmed by the 13C-edited EXSY-HMQC spectrum (Fig. S1), in which the higher intensities of the cross peaks than those of the auto peak and the temperature dependence of the cross peak intensities indicated that the cross peak is due to chemical exchange, and not to an NOE.
Fig. 1.
Methyl-TROSY spectra of KcsA. (A and B) Methyl-TROSY spectra of  KcsA acquired at pH 6.7 (A) and 3.2 (B), at 45 °C and with 120 mM K+. Representative assignments are shown. Signals with asterisks are not from KcsA, but from an unknown contaminant molecule in the DDM reagent. See Table S1 for the complete assignment table. In panel (B), the selected region represented in the green box is magnified as an inset, with a deeper contour level. The red arrows indicate the weak signals observed at pH 3.2. Suffixes (A) and (B) are used to discriminate the two diastereotopic methyl groups, where (A) represents the methyl group in the lower field in the 13C dimension at pH 6.7. (C) Relative signal intensity ratios of the methyl-TROSY signals plotted against pH. The solid lines represent the best fit curve of a Hill equation to the data. Signals of the residues in the transmembrane region (the extra- and intracellular gates) exhibited averaged pH1/2 values of 5.0 ± 0.3, while those from the cytoplasmic region were 6.4 ± 0.4. (D) Distribution of the residues subjected to the Hill plot analyses (C) in the structure of KcsA. A structural model of full-length KcsA (created from PDB ID: 1F6G) under neutral conditions is shown, with one subunit in the tetramer colored green. The heavy atoms of the residues subjected to the analyses (C) are shown as red spheres.
KcsA acquired at pH 6.7 (A) and 3.2 (B), at 45 °C and with 120 mM K+. Representative assignments are shown. Signals with asterisks are not from KcsA, but from an unknown contaminant molecule in the DDM reagent. See Table S1 for the complete assignment table. In panel (B), the selected region represented in the green box is magnified as an inset, with a deeper contour level. The red arrows indicate the weak signals observed at pH 3.2. Suffixes (A) and (B) are used to discriminate the two diastereotopic methyl groups, where (A) represents the methyl group in the lower field in the 13C dimension at pH 6.7. (C) Relative signal intensity ratios of the methyl-TROSY signals plotted against pH. The solid lines represent the best fit curve of a Hill equation to the data. Signals of the residues in the transmembrane region (the extra- and intracellular gates) exhibited averaged pH1/2 values of 5.0 ± 0.3, while those from the cytoplasmic region were 6.4 ± 0.4. (D) Distribution of the residues subjected to the Hill plot analyses (C) in the structure of KcsA. A structural model of full-length KcsA (created from PDB ID: 1F6G) under neutral conditions is shown, with one subunit in the tetramer colored green. The heavy atoms of the residues subjected to the analyses (C) are shown as red spheres.
The intensities of the signals observed at 45 °C in the presence of 120 mM K+ under neutral conditions were decreased at a lower pH, reflecting the decrease in the population of the corresponding conformation. To investigate the pH-dependence of this transition, the intensities were plotted against the pH, for representative residues in the transmembrane region, containing the extra- and intracellular gates, and the cytoplasmic region (Fig. 1 C and D). Fitting of the data to the Hill equation generated the pH1/2 value of 5.0 ± 0.3, at which the intensity of a signal becomes half of that observed at pH 6.7, and the Hill coefficient value of 4.2 ± 1.1 for the signals from the transmembrane region (average of L59 δ(B), V76 γ(B), V91 γ(A), and L105 δ(B)). Meanwhile, the signals of the residues in the cytoplasmic region exhibited a larger pH1/2 value of 6.4 ± 0.4 and the equivalent Hill coefficient of 1.9 ± 0.1 (average of V126 γ(B) and L155 δ(A)). These results indicated that the whole transmembrane region changes the conformation cooperatively, which is independent of the cytoplasmic region.
Conformational Equilibrium Under Acidic Conditions in the Presence of K+ Ions.
The residues L59, V76, L81, and L86, which exhibit two slowly exchanging signals under acidic conditions (pH 3.2) at 45 °C in the presence of 120 mM K+, are located on the extracellular side of the transmembrane region (Fig. S2). It should be noted that the signal splitting was not observed for the signals from the intracellular region. The methyl groups with the largest chemical shift difference between the two signals are γ(A) and γ(B) from V76, which resides in the selectivity filter. As the temperature was decreased from 45 to 20 °C by 5 °C steps, the intensities of these minor signals at 45 °C increased, while the intensities of the corresponding major signals decreased (Fig. 2A). Almost equal intensities between these signals were observed at 30 °C.
Fig. 2.
Two slowly exchanging signals at the extracellular gate under acidic conditions. (A) The temperature dependence of the methyl-TROSY spectra of  KcsA at pH 3.2, in the presence of 120 mM K+. The populations of the two slowly exchanging signals shift drastically upon a change in the temperature, where 45 °C and 25 °C are sufficient to shift the equilibrium almost completely to one state. (B) Methyl-TROSY spectra of the wild type (Left) and the E71A (Middle) and Y82A (Right) mutants at pH 3.2 and 25 °C, in the presence of 120 mM K+, are shown. The numbers in parentheses are the relative intensities of the two signals of V76 γ(B). The E71A mutant does not exhibit the signals indicating the existence of two slowly exchanging conformations under acidic conditions, whereas the Y82A mutant exhibited a more biased equilibrium, as compared to the wild type.
KcsA at pH 3.2, in the presence of 120 mM K+. The populations of the two slowly exchanging signals shift drastically upon a change in the temperature, where 45 °C and 25 °C are sufficient to shift the equilibrium almost completely to one state. (B) Methyl-TROSY spectra of the wild type (Left) and the E71A (Middle) and Y82A (Right) mutants at pH 3.2 and 25 °C, in the presence of 120 mM K+, are shown. The numbers in parentheses are the relative intensities of the two signals of V76 γ(B). The E71A mutant does not exhibit the signals indicating the existence of two slowly exchanging conformations under acidic conditions, whereas the Y82A mutant exhibited a more biased equilibrium, as compared to the wild type.
To investigate the nature of these two slowly exchanging conformations on an NMR chemical shift time scale, the methyl-TROSY spectra were compared between the E71A and Y82A mutants. The E71A mutant is known as a noninactivating mutant, whereas Y82A is more deeply inactivated even under acidic conditions, where the intracellular gate is open (3). Fig. 2B shows that the signals from the E71A mutant at 25 °C correspond to the major wild-type signals present at temperatures higher than 30 °C (Fig. 2A, four left panels), while the spectrum of the Y82A mutant at 25 °C showed two sets of peaks, as observed for the wild type at 25 °C. The populations of one of the signals of V76 γ(B) (-0.13 and 17.7 ppm for 1H and 13C, respectively) at 25 °C were 1.0 (E71A), 0.31 (wild type), and 0.17 (Y82A), which correlate with their open probabilities, 1.0, 0.15, and 0.03 (approximate value read from Fig. 3C of ref. 3), respectively (3). Therefore, the signal observed at -0.13 and 17.7 ppm for 1H and 13C, respectively, corresponds to the open conformation of the extracellular gate under acidic conditions, and the other corresponds to the closed conformation. It should be noted that the population of the NMR signal of Y82A (0.17) deviates from the correlation with the open probability of 0.03 (3). One of the reasons for this discrepancy might be the difference in the buffer (MOPS in ref.3 and phosphate in this study), because the buffer type influences the peak current amplitude, and thus the estimation of the open probabilities from the macroscopic current profile (4).
Fig. 3.
Chemical shift perturbation by pH transition. (A) Chemical shift differences of the methyl groups between the resting (pH 6.7, 45 °C) and the activated (pH 3.2, 45 °C) states, in the presence of 120 mM K+. Chemical shift differences, Δδ, were calculated by the equation,  . The normalization factor (5.8) was determined from the ratio of the variance of the methyl 1H and 13C chemical shifts, deposited in the Biological Magnetic Resonance Data Bank. (B) Mapping of the chemical shift difference on the structural model of full-length KcsA. The heavy atoms of the residues containing the methyl groups assigned at both pH values are shown with spheres (Red: Δδ > 0.2, Orange: 0.2≥Δδ > 0.1, Gray: 0.1≥Δδ).
. The normalization factor (5.8) was determined from the ratio of the variance of the methyl 1H and 13C chemical shifts, deposited in the Biological Magnetic Resonance Data Bank. (B) Mapping of the chemical shift difference on the structural model of full-length KcsA. The heavy atoms of the residues containing the methyl groups assigned at both pH values are shown with spheres (Red: Δδ > 0.2, Orange: 0.2≥Δδ > 0.1, Gray: 0.1≥Δδ).
NMR Evidence of the Conformational Changes of the Selective Filter.
Three different methyl-TROSY spectra were observed, corresponding to the three states of KcsA under the conditions indicated in parentheses in the presence of 120 mM K+: the resting (pH 6.7, 45 °C), activated (pH 3.2, 45 °C), and inactivated (pH 3.2, 25 °C) states. We then carried out chemical shift perturbation (CSP) analyses to investigate the conformational changes of KcsA upon activation and inactivation.
To compare the conformations between the resting and activated states, the CSPs between these states were calculated and mapped on the structure (Fig. 3). The residues with methyl groups that were perturbed by more than 0.2 ppm were L24, L40, V95, I100, L105, and L155, and those with methyl groups perturbed between 0.1–0.2 ppm were V39, L59, V70, V76, V91, V126, and L151. These residues are widely distributed in the transmembrane region, and they include some within the extracellular gate (L59, V70, and V76) and the intracellular gate (L24 and L105). The most largely perturbed groups, V95 γ(A) and I100 δ1, are about 10 Å or farther away from the nearest Asp, Glu, or His residue, which is protonatable between pH 6.7 and 3.2, where the distances are 9.8 Å between V95 Cγ1 and E71 Cδ in the same subunit and 12.1 Å between I100 Cδ1 and E71 Cδ in the neighboring subunit. Therefore, the observed CSPs reflect the conformational change of the transmembrane region, rather than the protonation of the neighboring residues.
To compare the conformations between the activated and inactivated states, the CSPs between these states were calculated. Among all the assigned signals, V76 γ(A) and γ(B) exhibited the largest CSP values of 0.38 and 0.36 ppm, respectively, whereas the others were less than 0.1 ppm. Notably, V76, which is located in the selectivity filter, exhibited a significant CSP upon both activation and inactivation (Table S1). In order to investigate the conformational changes of the selectivity filter, the NOE patterns of V76 γ(B) were compared among the three states. We prepared a Tyr-selectively 1H-labeled deuterated sample together with 1H and 13C labels of the Leu and Val methyl groups,  KcsA, as we expected NOE cross peaks between V76 and Y78, which is located in close proximity to V76 in the crystal structure at neutral pH. The 1H-1H 2D planes of 13C-edited NOESY-HMQC spectra were acquired in the resting, activated, and inactivated states (Fig. S3). The cross peaks from Y78 were confirmed by the corresponding spectrum of the Y78F mutant. Furthermore, Hδ and Hε of Y78 were distinguished by using 2Hδ Tyr and 2Hε Tyr, instead of u-1H Tyr (Fig. S3).
KcsA, as we expected NOE cross peaks between V76 and Y78, which is located in close proximity to V76 in the crystal structure at neutral pH. The 1H-1H 2D planes of 13C-edited NOESY-HMQC spectra were acquired in the resting, activated, and inactivated states (Fig. S3). The cross peaks from Y78 were confirmed by the corresponding spectrum of the Y78F mutant. Furthermore, Hδ and Hε of Y78 were distinguished by using 2Hδ Tyr and 2Hε Tyr, instead of u-1H Tyr (Fig. S3).
In the resting and activated states, NOE cross peaks from Y78 Hδ and Hε were observed for V76 γ(B), each of which was also observed, when 2Hε or 2Hδ Tyr labeled KcsA were used, respectively (Fig. S3 A and B), indicating that Hδ and Hε are both proximal to V76, although the spin diffusion should occur in 1H Tyr labeled KcsA. Using the coordinates of hydrogen atoms generated from the crystal structure under neutral conditions (PDB ID: 1K4C), the distances from the average positions of Y78 Hδ1/2 and Hε1/2 to that of V76 Hγ1 are 3.9 and 3.4 Å, respectively, whereas those to V76 Hγ2 are 6.7 and 5.6 Å, respectively. This indicates that the NOE cross peaks reflect the conformation of the selectivity filter in the crystal structure.
In the inactivated state, the cross peak between V76 γ(B) and Y78 Hδ was also observed for 1H Tyr labeled KcsA (Fig. S3C, lower left). However, while the NOE cross peak from Y78 Hδ to V76 γ(B) was observed for 2Hε Tyr labeled KcsA, deuteration of Y78 Hδ diminished the cross peak of Y78 Hε to V76 γ(B) (Fig. S3C), indicating that the distance between the V76 γ(B) and Y78 Hε becomes longer upon transition from the activated to the inactivated state.
These CSP and NOE results indicated that the selectivity filter forms distinct conformations in the resting, activated, and inactivated states.
Activation is Triggered by the Protonation of H25.
We explored the residue(s) critical for the pH-dependent conformational change of the extracellular gate. Because Asp, Glu, and His residues could exhibit pKa values corresponding to the pH1/2 values of the pH-dependent conformational changes of the transmembrane region (5.0), we analyzed the effects of mutating these residues on the pH-dependent conformational changes of the extracellular gate, as monitored by the signals from V76 γ(B) and L59 δ(B) (Fig. S4).
The chymotrypsin-treated KcsA (residues 1–125, referred to as KcsA Δ126), including the entire transmembrane region (1) and retaining the same pH-dependent channel activity as the full-length KcsA (6, 16), exhibited the pH-dependent spectral change between the resting and activated states, which was essentially identical to that of the full-length KcsA (Fig. S4A). Therefore, we prepared alanine mutants of each of the Asp, Glu, and His residues within residues 1–125: H20, H25, E51, E71, D80, E118, E120, and H124 (Fig. S4B). Unfortunately, the mutations of residues E51 and D80 severely affected the expression levels of the proteins, and thus these residues were excluded from further analyses. The NMR signals from the extracellular gate, V76 γ(B) and L59 δ(B), showed similar pH-dependence among all of the prepared mutants, except for the H25A mutant, indicating that the protonation of these mutated residues has essentially no effect on the pH-dependent conformational changes (Fig. S4A). On the other hand, the H25A mutant exhibited both signals, corresponding to the resting and activated states of the wild type, indicating a decrease in the pH1/2 value, as compared to the wild type (Fig. S4A, Green Arrows). Therefore, the protonation of H25, which resides on the cytoplasmic side of the transmembrane region, has an important effect on the pH-dependent conformational change of the extracellular gate.
Interaction of K+ and H2O with the Selectivity Filter.
Thus far, we had investigated the pH and temperature dependence of KcsA at the constant K+ concentration of 120 mM. Next, to determine the effect of K+ on the selectivity filter, K+ titration experiments were conducted at pH 6.7 and 3.2, at 45 °C, in the presence of 10 mM Na+ as the counter cation of the phosphate (Fig. 4). At both pH conditions, the removal of K+ was accompanied by large CSPs. Because the sample was unstable at Na+ concentrations below 130 mM and in the absence of K+, the ionic strength of the buffer was not kept constant in the series of K+ titrations. Instead, we confirmed that a similar spectral change occurred in the presence of 120, 90, 60 mM K+ and 10, 40, 70 mM Na+, respectively, indicating that the observed spectral changes in Fig. 4 were not due to the difference in the ionic strength, but to the change in the K+ concentration (Fig. S5). Interestingly, the signals observed in the K+-bound and unbound states at pH 3.2 correspond to those from the open and closed conformations, respectively, of the extracellular gate (Fig. 4C), indicating that the conformational changes of the extracellular gate (Fig. 4B) occur together with the association and dissociation of K+ ion(s) in the selectivity filter.
Fig. 4.
K+ titration experiments. (A and B) Results of the K+ titration experiments at pH 6.7 (A) and 3.2 (B), at 45 °C. Methyl-TROSY spectra of  KcsA with the indicated K+ concentrations are shown. The signals of V76 γ(B) in the K+-bound state are highlighted with green and orange squares, respectively. (C) Methyl-TROSY spectra of
KcsA with the indicated K+ concentrations are shown. The signals of V76 γ(B) in the K+-bound state are highlighted with green and orange squares, respectively. (C) Methyl-TROSY spectra of 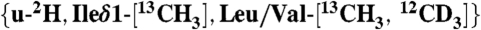 KcsA at pH 3.2 and 45 °C with 50 mM K+ (Black), and at pH 3.2 and 30 °C with 120 mM K+ (Red). The two spectra overlap well with each other, indicating that the open and closed conformations correspond to the K+-bound and unbound states, respectively. (D) The population of the V76 γ(B) in the K+-bound state plotted against the K+ concentration. The solid lines represent the best fit curves of Hill equations to the data. The apparent Kd values were 6.1 and 50.1 mM and the Hill coefficients were 1.0 and 2.8, respectively, at pH 6.7 and 3.2.
KcsA at pH 3.2 and 45 °C with 50 mM K+ (Black), and at pH 3.2 and 30 °C with 120 mM K+ (Red). The two spectra overlap well with each other, indicating that the open and closed conformations correspond to the K+-bound and unbound states, respectively. (D) The population of the V76 γ(B) in the K+-bound state plotted against the K+ concentration. The solid lines represent the best fit curves of Hill equations to the data. The apparent Kd values were 6.1 and 50.1 mM and the Hill coefficients were 1.0 and 2.8, respectively, at pH 6.7 and 3.2.
Next, we calculated the apparent dissociation constants (Kd) by using the relative signal intensity of the signal from V76 γ(B) in the K+-bound state, observed upon K+ titration. Fitting the data with a Hill equation yielded the Kd value of 6.1 mM and the Hill coefficient of 1.0 at pH 6.7, whereas the Kd value and the Hill coefficient were 50.1 mM and 2.8, respectively, at pH 3.2, indicating that the affinity of K+ to the selectivity filter decreased about 8-fold upon the transition from the resting state to the activated state (Fig. 4D).
NOESY analyses were conducted in order to investigate the interaction of H2O with the selectivity filter. Fig. 5 shows the NOESY spectra of  KcsA, recorded in the presence of 120 mM K+ and 90% H2O. In the resting and activated states, no NOE peak was observed between V76 γ(B) and H2O, whereas an NOE cross peak with H2O was observed for V76 γ(B) in the inactivated state. Then, we performed the NOE experiment at 30 °C, where the activated and inactivated states are observed simultaneously (Fig. S6). In the spectrum, an NOE to water was observed only for the signal from the inactivated state, and not from the activated state, indicating that the NOE is not due to the slower water exchange at lower temperature, but to the binding of water to the selectivity filter only in the inactivated state. In the resting state, the NOE pattern of V76 γ(B) with Y78 was unchanged in the presence or absence of H2O. However, the relative intensities of the cross peaks from Y78 Hδ and Hε became reversed in the activated state, and the cross peak from Y78 disappeared in the inactivated state, in the presence of H2O. It should be noted that the magnetization transfer pathway might be altered by the presence of H2O in the activated and inactivated states.
KcsA, recorded in the presence of 120 mM K+ and 90% H2O. In the resting and activated states, no NOE peak was observed between V76 γ(B) and H2O, whereas an NOE cross peak with H2O was observed for V76 γ(B) in the inactivated state. Then, we performed the NOE experiment at 30 °C, where the activated and inactivated states are observed simultaneously (Fig. S6). In the spectrum, an NOE to water was observed only for the signal from the inactivated state, and not from the activated state, indicating that the NOE is not due to the slower water exchange at lower temperature, but to the binding of water to the selectivity filter only in the inactivated state. In the resting state, the NOE pattern of V76 γ(B) with Y78 was unchanged in the presence or absence of H2O. However, the relative intensities of the cross peaks from Y78 Hδ and Hε became reversed in the activated state, and the cross peak from Y78 disappeared in the inactivated state, in the presence of H2O. It should be noted that the magnetization transfer pathway might be altered by the presence of H2O in the activated and inactivated states.
Fig. 5.
NOESY spectra in the presence of 90% H2O. (A) Selected region of the methyl-TROSY spectra of  KcsA in the resting (pH 6.7, 45 °C) (Black), activated (pH 3.2, 45 °C) (Red), and inactivated (pH 3.2, 25 °C) (Green) states, in the presence of 120 mM K+. (B) 1H-1H strips representing V76 γ(B), derived from 13C-edited NOESY-HMQC of
KcsA in the resting (pH 6.7, 45 °C) (Black), activated (pH 3.2, 45 °C) (Red), and inactivated (pH 3.2, 25 °C) (Green) states, in the presence of 120 mM K+. (B) 1H-1H strips representing V76 γ(B), derived from 13C-edited NOESY-HMQC of  KcsA in 90% H2O, in the resting (Black), activated (Red), and inactivated (Green) states, using a mixing time of 50 ms.
KcsA in 90% H2O, in the resting (Black), activated (Red), and inactivated (Green) states, using a mixing time of 50 ms.
Discussion
Structural and Dynamic Integrity of KcsA in DDM Micelles.
Our NMR analyses demonstrated that KcsA in DDM micelles exhibits spectroscopic properties that reflect its electrophysiological behaviors. Large spectral changes of methyl-TROSY were observed between the resting (pH 6.7, 45 °C) and activated (pH 3.2, 45 °C) states in the presence of 120 mM K+ (Figs. 1 and 3), with the typical pH1/2 value of 5.0 for the transmembrane region (Fig. 1C) that corresponds well to the electrophysiological pH1/2 (3, 4). Furthermore, the Hill coefficient of 4.2 for the transmembrane region suggests a cooperative conformational change between subunits, corresponding to the previous electrophysiological reports (4, 17). The differences in the pH1/2 and Hill coefficient values between the cytoplasmic and transmembrane regions suggest that the cytoplasmic region changes its conformation independently from the transmembrane region, consistent with the notion that the deletion of this region does not greatly affect the channel activity (6, 16). These results strongly suggest that the conformational changes, reflecting the activation of the channel, are induced upon the pH change.
Under acidic conditions, KcsA exists in an equilibrium between the activated and inactivated states, which provided the distinct NMR signals of the extracellular gate simultaneously in the presence of 120 mM K+ at 25–45 °C at pH 3.2 (Fig. 2), indicating that under the conditions the exchange between these conformations occurs more slowly than the difference in the resonance frequencies of the corresponding signals. Therefore, we conclude that KcsA in DDM micelles possesses the structural and dynamic integrity of the channel.
Inactivation Gating Mediated by H2O in the Selectivity Filter.
We found that the open and closed conformations of the extracellular gate, observed under acidic conditions, are the two discrete conformations of the selectivity filter, corresponding to the K+-saturated and K+-depleted conformations, respectively (Fig. 4C). Indeed, the populations of the open and closed conformations are influenced by the K+ concentration, as shown in the K+ titration experiment conducted at pH 3.2 and 45 °C (Fig. 4B). The crystal structures obtained with high-K+(200 mM) and low-K+ (2 mM) concentrations were reported under neutral conditions, where the structural differences were discussed (18). However, it should be noted that the chemical shifts and NOE patterns of the extracellular gate, as well as the effect of K+ on these NMR signals, are different between the neutral and acidic conditions (Fig. 4 and Fig. S3), suggesting that the conformational change between the activated and inactivated states that occurs under acidic conditions might be different from that observed between the high-K+ and low-K+ crystal structures under neutral conditions. The pH-dependent difference of the K+ effect on the conformation of the extracellular gate might be due to the coupling of the intracellular and extracellular gates (SI Discussion), since the intracellular gate adopts different structures at neutral and acidic pH values.
Another significant difference between the two conformations is the binding mode of H2O. An NOE signal between V76 γ(B) and H2O was observed in the inactivated state but not in the activated state (Fig. 5). It should be noted that the detection of the NOE cross peaks between water molecules and proteins is fraught with a number of problems, one of which is the residence time of water molecules (19). In case that a water molecule is bound to a large protein, such as KcsA, with a correlation time in the spin-diffusion limit, the NOE between protons of the water molecules and the protein will be positive and negative for the residence time of the water molecule shorter and longer than ∼300 ps, respectively (20). Therefore, the observation of a negative NOE signal, whose sign is the same as that of the diagonal peaks, with water from V76 γ(B) only in the inactivated state revealed that, in the inactivated state, at least one water molecule is trapped in the vicinity of V76 γ(B), with a residence time longer than 300 ps. In the activated state, where no NOE with water was detected for V76 γ(B), the water molecules near V76 γ(B) may be rapidly exchanging with the bulk water, with the residence time shorter than 300 ps.
Taken together, these results indicate that the inactivated state is achieved by the conformational change of the selectivity filter, together with the elimination of K+ ions and the association of H2O molecule(s). Considering the distance from the V76 γ methyl group, the carbonyl group of V76 seems to form hydrogen bonds with the H2O molecule in the selectivity filter. Based on the molecular dynamics simulation, K+ ions stay at each of the K+ binding sites in the selectivity filter for several tens to a few hundreds picoseconds before they finally permeate through the selectivity filter (21). Therefore, the residence time of the water molecules shorter than 300 ps might be beneficial for the efficient K+ permeation. On the other hand, the bound water molecule in the inactivated state, which resides within the selectivity filter for longer than 300 ps, might interfere with the efficient K+ permeation.
Molecular Basis of the Activation-Coupled Inactivation.
The spectroscopic evidence obtained here provides the molecular basis of the activation-coupled inactivation of KcsA. Under neutral conditions, where KcsA is in the resting state, the selectivity filter possesses higher affinity for K+ ions (the Kd value of 6 mM at 45 °C), which is consistent with the previous NMR results, using the KcsA sample in sodium dodecyl sulfate (SDS) micelles (22). This affinity enables the cell to retain K+ ions when the extracellular K+ concentration is low.
With a change to acidic conditions, the protonation of H25 triggers the opening of the intracellular gate, which is coupled with the initiation of the equilibrium between the open and closed conformations of the extracellular gate (SI Discussion). This transition reduces the affinity of KcsA for K+ ions, enabling the K+-eliminated, H2O-bound inactivated state. The equilibrium is initiated from the open conformation by keeping K+ ions in the extracellular gate, thus accounting for the strong peak current upon activation and its subsequent decay, and then the steady state of the equilibrium between the K+-bound activated state and the H2O-bound inactivated state is reached.
When the intracellular pH returns to neutral, the K+ current halts by the closing of the intracellular gate, which is coupled with the conformational change of the extracellular gate. Then, the extracellular gate releases the bound H2O and catches the K+ ion, finally returning to the resting state. The peak current is reportedly impaired when the interval between the deactivation and reactivation is less than a few seconds (2, 4), which might be the necessary dead time to restore the initial state, where all of the KcsA tetramers release H2O and catch K+ ions in the selectivity filters.
The electrophysiological properties of KcsA are similar to those of eukaryotic voltage-gated K+ (Kv) channels in the following points: (i) The peak current and the subsequent inactivation are observed upon activation (activation-coupled inactivation). (ii) It takes a few seconds to return from the inactivated state to the resting state that enables the maximum peak current upon activation (23). (iii) The inactivation rate decreases as the concentration of K+ ion increases (24). Together with the structural conservation of the selective filter, the molecular bases of these features, revealed here in terms of the affinity for K+ and H2O, also seem generally applicable to the eukaryotic Kv channels.
Materials and Methods
Construction and Sample Preparation.
The plasmid bearing the N-terminally decahistidine tagged wild-type KcsA was constructed as described previously (9). All KcsA mutants were constructed by PCR mutagenesis, using this plasmid as the template. For the 1H and 13C labeling of the Ile δ1, Leu δ1/2, and Val γ1/2 methyl groups with a deuterated background, the protocol established by Kay’s group (25) was utilized. Wild-type and mutant KcsA proteins were expressed and purified as reported previously (9). The chymotryptic cleavage of KcsA was conducted using chymotrypsin-immobilized agarose resin (Sigma). In brief, the resin was added to the sample and incubated overnight at room temperature with gentle tumbling. After confirming the selective and complete cleavage by SDS–PAGE, the resin was pelleted by centrifugation and the cleaved C-terminal polypeptide was removed by ultrafiltration.
NMR Spectroscopy.
All NMR spectra, except for the K+ titration, were recorded in buffer containing 10 mM K2HPO4 and 100 mM KCl, in 100% D2O or 10% D2O/90% H2O, at the indicated temperatures and pHs. For the K+ titration experiments, the sample was prepared using the buffer containing 10 mM NaH2PO4 in 100% D2O at the indicated pH, and the same buffer containing 1–3 M KCl was used for titration. The concentration of KcsA was 400 μM (100 μM as a tetramer), whereas that of DDM was 5 mM, as estimated from the signal intensity of the DDM signals in 1H 1D NMR spectra. For the 100% D2O samples, the pH values were calibrated by adding 0.4 pH unit to the reading on the pH meter (26). When needed, the tail-deuterated DDM (Anatrace) was used. Experiments were performed on Bruker Avance 500, 600, and 800 MHz spectrometers equipped with a cryogenic probe.
Assignments of the signals in the methyl-TROSY spectra were achieved mainly by site-directed mutagenesis. All Leu, Val and Ile residues in KcsA were mutated to Ile (for Leu/Val) or Val (for Ile) one by one, and the methyl-TROSY spectra of each mutant were compared to the wild-type spectra obtained at pHs 6.7 and 3.2. Methyl-methyl NOE cross peaks within residues (15), observed in the 13C-edited NOESY HMQC of  labeled KcsA, were also utilized to identify the pair of diastereotopic methyl groups within a Leu or Val residue.
labeled KcsA, were also utilized to identify the pair of diastereotopic methyl groups within a Leu or Val residue.
13C-edited NOESY/EXSY HMQC were acquired on a Bruker Avance 800 MHz spectrometer equipped with a cryogenic probe. To obtain the intraresidual methyl-methyl NOE cross peaks, measurements were conducted at 45 °C and pHs 6.7 and 3.2, using a mixing time of 200 ms. To obtain the NOE cross peaks between protons in the methyl groups and those in Tyr, measurements were conducted using a mixing time of 50 ms.
Supplementary Material
Acknowledgments.
This work was supported in part by grants from the Japan New Energy and Industrial Technology Development Organization (NEDO) and the Ministry of Economy, Trade, and Industry (METI) (to I.S.), a Grant-in-Aid for Scientific Research on Priority Areas from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to M.O. and I.S.), and a grant from Takeda Science Foundation (to M.O.). S.I. is a research fellow of the Japan Society for Promotion of Science (JSPS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911270107/DCSupplemental.
References
- 1.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 2.Gao L, et al. Activation-coupled inactivation in the bacterial potassium channel KcsA. Proc Natl Acad Sci USA. 2005;102:17630–17635. doi: 10.1073/pnas.0505158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordero Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 4.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: Macroscopic currents. J Gen Physiol. 2007;130:465–478. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedida D, Hesketh JC. Gating of voltage-dependent potassium channels. Prog Biophys Mol Biol. 2001;75:165–199. doi: 10.1016/s0079-6107(01)00006-2. [DOI] [PubMed] [Google Scholar]
- 6.Perozo E, Cortes DM, Cuello LG. Structural rearrangements underlying K+-channel activation gating. Science. 1999;285:73–78. doi: 10.1126/science.285.5424.73. [DOI] [PubMed] [Google Scholar]
- 7.Blunck R, et al. Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction. J Gen Physiol. 2006;128:569–581. doi: 10.1085/jgp.200609638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu H, et al. Global twisting motion of single molecular KcsA potassium channel upon gating. Cell. 2008;132:67–78. doi: 10.1016/j.cell.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi K, Takahashi H, Kawano S, Shimada I. Identification and characterization of the slowly exchanging pH-dependent conformational rearrangement in KcsA. J Biol Chem. 2007;282:15179–15186. doi: 10.1074/jbc.M608264200. [DOI] [PubMed] [Google Scholar]
- 10.Baker KA, et al. Conformational dynamics of the KcsA potassium channel governs gating properties. Nat Struct Mol Biol. 2007;14:1089–1095. doi: 10.1038/nsmb1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- 12.Goto NK, et al. A robust and cost-effective method for the production of Val, Leu, Ile (δ1) methyl-protonated 15N-, 13C-, 2H-labeled proteins. J Biomol NMR. 1999;13:369–374. doi: 10.1023/a:1008393201236. [DOI] [PubMed] [Google Scholar]
- 13.Tugarinov V, Kay LE. An isotope labeling strategy for methyl TROSY spectroscopy. J Biomol NMR. 2004;28:165–172. doi: 10.1023/B:JNMR.0000013824.93994.1f. [DOI] [PubMed] [Google Scholar]
- 14.Sprangers R, et al. Quantitative NMR spectroscopy of supramolecular complexes: Dynamic side pores in ClpP are important for product release. Proc Natl Acad Sci USA. 2005;102:16678–16683. doi: 10.1073/pnas.0507370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprangers R, Kay LE. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature. 2007;445:618–622. doi: 10.1038/nature05512. [DOI] [PubMed] [Google Scholar]
- 16.Cortes DM, Cuello LG, Perozo E. Molecular architecture of full-length KcsA: Role of cytoplasmic domains in ion permeation and activation gating. J Gen Physiol. 2001;117:165–180. doi: 10.1085/jgp.117.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson AN, Posson DJ, Parsa PV, Nimigean CM. Molecular mechanism of pH sensing in KcsA potassium channels. Proc Natl Acad Sci USA. 2008;105:6900–6905. doi: 10.1073/pnas.0800873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Morais Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 19.Otting G, Liepinsh E, Wuthrich K. Protein hydration in aqueous-solution. Science. 1991;254:974–980. doi: 10.1126/science.1948083. [DOI] [PubMed] [Google Scholar]
- 20.Clore GM, Bax A, Omichinski JG, Gronenborn AM. Localization of bound water in the solution structure of a complex of the erythroid transcription factor GATA-1 with DNA. Structure. 1994;2:89–94. doi: 10.1016/s0969-2126(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 21.Burykin A, Kato M, Warshel A. Exploring the origin of the ion selectivity of the KcsA potassium channel. Proteins. 2003;52:412–426. doi: 10.1002/prot.10455. [DOI] [PubMed] [Google Scholar]
- 22.Chill JH, Louis JM, Miller C, Bax A. NMR study of the tetrameric KcsA potassium channel in detergent micelles. Protein Sci. 2006;15:684–698. doi: 10.1110/ps.051954706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DI, Deutsch C. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys J. 1996;70:798–805. doi: 10.1016/S0006-3495(96)79619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: A tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- 25.Tugarinov V, Kanelis V, Kay LE. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc. 2006;1:749–754. doi: 10.1038/nprot.2006.101. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard JS. Buffers for enzymes. Methods Enzymol. 1984;104:404–414. doi: 10.1016/s0076-6879(84)04107-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







