Abstract
How does an animal age in natural conditions? Given the multifaceted nature of senescence, identifying the effects of age on physiology and behavior remains challenging. We investigated the effects of age on a broad array of phenotypic traits in a wild, long-lived animal, the wandering albatross. We studied foraging behavior using satellite tracking and activity loggers in males and females (age 6–48+ years), and monitored reproductive performance and nine markers of baseline physiology known to reflect senescence in vertebrates (humoral immunity, oxidative stress, antioxidant defenses, and hormone levels). Age strongly affected foraging behavior and reproductive performance, but not baseline physiology. Consistent with results of mammal and human studies, age affected males and females differently. Overall, our findings demonstrate that age, sex, and foraging ability interact in shaping aging patterns in natural conditions. Specifically, we found an unexpected pattern of spatial segregation by age; old males foraged in remote Antarctica waters, whereas young and middle-aged males never foraged south of the Polar Front. Old males traveled a greater distance but were less active at the sea surface, and returned from sea with elevated levels of stress hormone (corticosterone), mirroring a low foraging efficiency. In contrast to findings in captive animals and short-lived birds, and consistent with disposable soma theory, we found no detectable age-related deterioration of baseline physiology in albatrosses. We propose that foraging efficiency (i.e., the ability of individuals to extract energy from their environment) might play a central role in shaping aging patterns in natural conditions.
Keywords: senescence, foraging, immunity, oxidative stress, sex
Senescence, a decline in fitness with advancing age, has been documented across a wide range of wild animals (1 –3). There is an ongoing debate in the literature regarding the proximate mechanisms underpinning senescence. Age-associated immune dysfunction (referred to as immunosenescence) and increased susceptibility to oxidative stress are strong candidates as the major driving forces behind senescence in humans and laboratory animal models (4 –6), but their relevance in natural populations remains unclear. Because of their generally longer lifespan compared with mammals, birds have emerged as predominant models for studying aging (7, 8). The first studies on senescence were restricted almost entirely to investigations of age-dependent mortality or breeding performance (1). More recent pioneering studies that focused on proximal physiological patterns of aging in free-living birds yielded contrasting results; senescence was linked with decreased humoral immune response (9), increased oxidative stress (10), altered plasma levels of some hormones (refs. 2, 11; but see ref. 12), and decreased metabolic rate (ref. 13, but see ref. 14).
Foraging behavior, the set of processes by which organisms acquire energy and nutrients (15), merits specific attention, because it may play a key role in shaping patterns of age-specific reproduction in the wild (16). Basically, the ability of an individual to extract resources from the environment determines the amount of energy available to that individual to expend on fitness-related activities, such as self-maintenance and reproduction. Although foraging efficiency is known to be a major determinant of individual fitness (15), the examination of foraging behavior is fraught with limitations related to methodology and interpretation in captive animals and laboratory animal models.
Although a large body of literature reports that foraging skills improve at a young age through learning (17), there is a surprising lack of information on the potential links between foraging and aging. Most previous studies on the physiology of aging have focused on immunity, hormones, metabolic rates, or oxidative stress (9 –14). Only one study has reported a deterioration of foraging performance in old age (18), but this result was not controlled for immune, hormonal, and other physiological parameters that could covary.
Given the multifaceted nature of aging, physiological and behavioral traits might be inextricably linked. Examining only a single marker can possibly lead to misidentifying or overlooking some primary factors of aging. To the best of our knowledge, no previous attempts have been made to simultaneously monitor age-related patterns in a broad array of phenotypic markers in a single species under natural conditions. The present study was designed to investigate which physiological or behavioral trait would first decline with age in the free-living wandering albatross, Diomedea exulans, a long-lived seabird (50+ years). In this species, reproductive performance declines after about age 30 years; that is, old birds face “reproductive senescence,” which is arguably one of the best-documented phenomena in ornithology. The physiological mechanisms involved in mediating age-related changes in reproductive output remain unknown. What proximate factor determines the age-associated decline in reproductive performance in wandering albatrosses? Our main goal was to test whether we could detect age-related patterns in foraging behavior and baseline physiology in relation to reproductive senescence, while controlling for potential differences between males and females, given that senescence rates might be sex-specific (18, 19).
We selected nine markers describing a variety of immune indices, oxidative stress markers, and hormone levels susceptible to decline with age. We used satellite telemetry and immersion-activity loggers to simultaneously monitor 10 indices of foraging behavior. We performed a cross-sectional study on albatrosses age 8–47 years during the incubation stage, which is a suitable stage for exploring relationships among age, foraging performance, and reproductive performance (SI Text).
Results
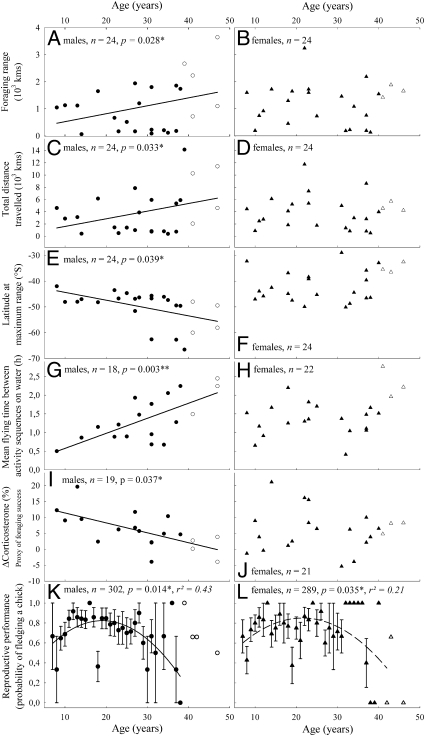
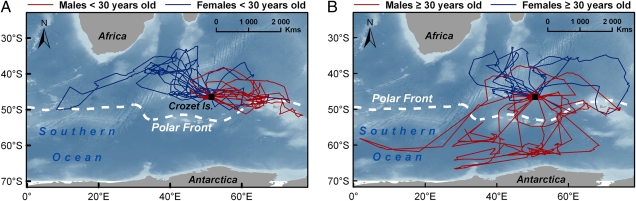
We detected various age-related patterns in the foraging behavior and reproductive performance of wandering albatrosses (Table 1 and Fig. 1). The most important finding was that young/middle-aged and old males partially segregated into different foraging areas (Fig. 2) and showed high levels of stress hormone on their return from the sea (Fig. 1I). Reproductive success also declined in old age, more sharply in males (Fig. 1K) than in females (Fig. 1L). There were no detectable effects of age on baseline physiology in either males or females.
Table 1.
Effects of age on foraging behavior, physiology, and reproductive performance in wandering albatross, age 6–48+ years
| Females | Males | Effect of age in selected model (males) | ||||||
| Independent variable | Date | Age | Date | Age | df | Estimate ± SEM | GLM | P |
| Foraging trip (24 males, 24 females) | ||||||||
| Total distance traveled, log | - | - | - | X | 22 | +0.047 ± 0.021 | z = 2.282 | 0.033* |
| Foraging range, log | - | - | - | X | 22 | +0.044 ± 0.019 | z = 2.350 | 0.028* |
| Latitude at maximum range | - | - | - | X | 22 | +0.304 ± 0.110 | F = 7.69 | 0.011* |
| Duration of foraging trip | X | - | - | - | ||||
| Activity at the sea surface (18 males, 22 females) | ||||||||
| Proportion of diurnal time spent on water | - | - | - | - | ||||
| Number of Diurnal Activity Sequences (DASs) per day | - | - | X | - | ||||
| Proportion of time spent on water during DASs | - | - | - | X | 16 | +0.113 ± 0.062 | F = 3.25 | 0.090 |
| Mean DAS duration | - | - | - | - | ||||
| Flying time between 2 DASs | - | - | - | X | 16 | +0.027 ± 0.012 | F = 5.16 | 0.037* |
| Variation of stress hormone levels over a foraging trip | ||||||||
| ∆Corticosterone (19 males, 21 females) | - | - | - | X | 17 | +0.312 ± 0.089 | F = 12.21 | 0.003** |
| Humoral immunity | ||||||||
| Haptoglobin levels (51 males, 37 females) | - | - | - | - | ||||
| Bactericidal activity (23 males, 24 females) | X | - | - | - | ||||
| Agglutination score (22 males, 22 females) | X | - | - | - | ||||
| Lysis score (21 males, 22 females) | - | - | - | - | ||||
| Oxidative stress and antioxidant defenses | ||||||||
| Antioxidant capacity of plasma (52 males, 47 females) | X | - | - | - | ||||
| Lipid peroxidation (53 males, 48 females) | X | - | - | - | ||||
| Superoxid dismutase activity (53 males, 48 females) | - | - | - | - | ||||
| Baseline hormone levels | ||||||||
| Stress hormone (corticosterone; 55 males, 49 females) | - | - | - | - | ||||
| Parental hormone (prolactin; 55 males, 47 females) | X | - | X | X | 52 | +0.660 ± 0.350 | t = 1.89 | 0.064 |
| Reproduction | ||||||||
| Laying date (116 nests) | - | - | - | - | ||||
| Reproductive performance (302 males, 289 females) | - | (age2) | - | age2 | 299 | −0.006 ± 0.001 | z = 6.36 | 0.018* |
Crosses denote which variable(s) appears in the most parsimonious model. Model selection (Tables S1, S2, and S3) shows a male-specific pattern of aging in foraging behavior, activity, and reproductive performance, independent of sampling date. Age2 denotes a quadratic effect of age, with greater statistical support in males than in females. ΔCorticosterone is a hormonal witness of foraging success (Discussion). df, degrees of freedom.
P < 0.05;
P < 0.01 (GLM).
Fig. 1.
Male-specific patterns of aging in foraging behavior and reproductive performance in wandering albatrosses. Age-related changes in foraging behavior were seen in males (circles, Left), but not in females (triangles, Right). Filled symbols represent exact known-age individuals and open symbols are individuals for which only a minimum age is known. Solid lines and P values denote significant linear (A– I) or quadratic (K and L) effects of age. There was a quadratic effect of age on reproductive performance, and a model with age of the male (K, solid line) had greater statistical support and better fit than a model with age of the female (L, dashed lines). Error bars represent SEs. *P < 0.05; **P < 0.01.
Fig. 2.
Age effects on foraging areas in wandering albatrosses. Young/middle-aged (A, red lines) and old (B, red lines) males were spatially segregated, whereas foraging areas of females largely overlapped at every age (blue lines). All of the birds that foraged south of the Polar Front were old males. (A) Young/middle-aged birds (males, n = 12, age 8–28 years; females, n = 12, age 8–25 years). (B) Old birds (males, n = 12, age 31–47+ years; females, n = 12, age 31–46+ years). All birds were satellite-tracked during the incubation stage.
Age and Foraging Behavior.
Satellite-tracked albatrosses foraged over a large oceanic area with high latitudinal range, from subtropical to high latitudes (35–65 °S). Old males (age ≥30 years) foraged significantly farther south compared with young males (n = 24; P = 0.011) (Table 1 and Fig. 1E), with some making extremely long foraging trips in remote Antarctic waters (55–65 °S, Fig. 2). In contrast, young/middle-aged males foraged between 35 and 50°S and never foraged south of the Polar Front. Foraging zones of females largely overlapped at every age (Fig. 2). Distance traveled and foraging range increased significantly with age in males (n = 24; P = 0.033 and 0.028, respectively) (Table 1 and Fig. 1 A and C), but not in females (n = 24; P > 0.5 for both parameters) (Table 1 and Fig. 1 B and D). Old males also exhibited less intense activity: mean flying time between two consecutive diurnal activity sequences (DASs) on water significantly increased with age in males (n = 22; P = 0.037) (Table 1 and Fig. 1G), but not in females (n = 22; P = 0.8) (Table 1 and Fig. 1H), independent of sampling date (age, wi = 57; age + date, wi = 14) (Table S1). ΔCorticosterone (the relative variation of stress hormone level over a foraging trip) was negatively correlated with age in males (n = 19; P = 0.003), but not in females (n = 21; P = 0.7; Table 1 and Fig. 1 C and J) meaning that old males, but not old females, failed to restore low baseline levels of stress hormone during a foraging trip, as albatrosses usually do. There was no confounding effect of departure date on any these parameters, and duration of trips was not linked to age in either males or females (Table 1 and Table S1).
Age and Physiological Parameters.
No age-related variations in humoral immunity, oxidative stress, or antioxidant defenses were detected (Table 1 and Table S2). Baseline levels of corticosterone (stress hormone) did not strongly decline with age (Table 1 and Table S2). Baseline levels of prolactin (parental hormone) increased slightly with age in males, but not in females (Table 1 and Table S2).
Age and Reproductive Performance.
A quadratic effect of age on re-productive performance of males in the study colony during the 2008 breeding season was seen [generalized linear model (GLM), n = 302 males, age2, P = 0.014] (Table 1). The probability of fledging a chick improved up to age 10–12 years, stabilized between 12 and 20–25 years, and declined after about age 25–30 years (Fig. 1F). This bell-shaped pattern was previously described in the wandering albatross based on a longitudinal analysis of breeding success in relation to the mean age of partners in the pair (20). Our sex-specific analysis demonstrated that a model with age of the male had better statistical support and better fit than a model with age of the female (males: r 2 = 0.43, wi = 82, 11.2-fold more supported than the intercept-only model, wi = 7.3; females, r 2 = 0.21, wi = 62.8, only 2.4-fold more supported than the intercept-only model, wi = 26) (Table S3). We found no confounding effect of laying date, detecting no effect of age of the female on laying date (GLM, n = 123 females, F 1,121 = 0.095, P = 0.758) (Table S3). Because males might influence laying date via copulation date or arrival date, we also examined the influence of age of the male on the laying date of the partner, but found no effect (GLM, n = 131 males, F 1,129 = 0.269, P = 0.607). Table S4 presents mean numerical values of all variables studied in male and female albatrosses.
Discussion
Our study of the behavior and physiology of albatrosses has re-vealed an age-related and male-specific pattern in foraging be-havior. There was a surprising pattern of spatial segregation by age; only old males foraged over remote Antarctic waters, whereas young males foraged over northerly waters and never foraged south of the polar front. Old males traveled a greater distance during foraging trips but were less active at the sea surface, and did not exhibit decreased baseline levels of stress hormone after foraging trips (as albatrosses and seabirds usually do). Reproductive senescence clearly occurred from age 30 years onward and was sharper in males. Interestingly, there was no detectable age-related decline in baseline physiology in either males or females, suggesting that the deterioration in foraging ability may be a proximal cause of reproductive senescence.
Age and Foraging Behavior: Elderly Go South!
Resource partitioning is known to occur among albatross species (21), as well as between sexes within a single species (22, 23). Here, we report a surprising pattern of a spatial partitioning by age in foraging areas of a wild animal. Because we specifically selected incubating albatrosses from Crozet Island, this pattern was controlled for breeding status, sex, and origin of the individuals. To ascertain that this result was not due to unusual environmental conditions during the study season, we further examined long-term satellite-tracking data. Sixty-four male albatrosses were previously satellite-tracked during the incubation stage at Crozet Island between 1989 and 2003 (Table S5). Eight out of nine males that foraged in Antarctic waters were age ≥25 years. Thus, long-term data suggest that birds progressively segregate from young birds with advancing age. Overall, our findings provide perspectives on, and raise questions about, the evolutionary bases of resource partitioning between young and old individuals.
Why do old males, but not younger males, forage in Antarctic waters? We have no clear explanation to offer for this unexpected and puzzling result. To the best of our knowledge, no previous ecological study has documented a pattern of segregation by age in foraging areas. It may be hypothesized that old albatrosses might exploit different wind regimes. Wind strength is known to increase with increasing latitudes in the Southern Ocean. Wandering albatrosses exploit the wind using gliding flight to minimize travel costs and are able to forage more economically in windy areas, where the cost of energetically expensive take-offs and landings might be reduced (24, 25). Assuming that old males would be energetically constrained by age, as a result of aging, they might feed in Antarctic waters in an attempt to encounter sufficient wind strength to reduce flight and take-off costs, resulting in longer trips and higher likelihood of nest desertion by the partner (22). There also could be a competitive exclusion between birds of different age classes. At-sea observations suggest that white (old) birds are dominant over darker (younger) birds (26). Conversely, old males might face competitive exclusion by younger males and need to forage further south. Subsequent studies should focus on this very surprising and as-yet unexplained result.
Alternatively, the observed pattern could result from an effect of differential survival between “southern foragers” and “northern foragers.” Longitudinal studies are needed to definitively differentiate the aging effects from the selection effects. But because only 1 out of 30 males age <25 years foraged in Antarctic waters (roughly 3%), whereas the population structure indicates that ∼16% of males survive to age >30 years (20), it seems reasonable to assume that segregation between older and younger males is an effect of age per se and not an effect of selection. Our results shed light on earlier observations based on ship surveys in the southern Indian Ocean demonstrating that the further south an albatross is seen, the whiter its plumage (27). Because the plumage of wandering albatrosses whitens progressively with age, only old birds were observed in the southern part of the observational range during the 1980s (27). Thus, the pattern of spatial segregation by age described in the present study likely already existed during the 1980s, although the at-sea observations could not be fully controlled for confounding effects of breeding status, origin, and sex.
Age-Related Decline in Activity and Foraging Success.
Here we report a decline of foraging activity with age in a wild animal. Old males spent longer time flying between two consecutive sequences of activity on water, and once engaged in an activity sequence (a series of take-offs and landings), they spent more time sitting on the water. Because taking off is the most energetically demanding activity for wandering albatrosses (24), our findings suggest that old males pursue an “energy-saving” strategy; however, further studies are needed to fully elucidate the nature of this age-related decline in activity. Do old individuals forage less effectively? Although immersion-activity loggers do not allow for the monitoring of prey capture at the sea surface, further information can be derived from corticosterone levels. Corticosterone, the primary avian glucocorticoid, mediates the stress response and plays a major role in energy mobilization (28). In seabirds, baseline corticosterone levels are known to decrease over a foraging trip (2, 29, 30) and to be negatively correlated with food abundance (31). In wandering albatrosses, we recently found a negative relationship between body mass change (namely, foraging success) and post-trip corticosterone; that is, birds that do not forage efficiently have high stress hormone levels when returning from the sea (2). In the present study, we found that old males returned from sea with high corticosterone levels relative to pre-trip levels, suggesting that old males had low foraging success, possibly ascribed to a physiological deterioration of soma (namely, aging).
No Age-Related Variation in Humoral Immunity or Oxidative Stress.
Previous research on free-living populations has yielded no strong conclusions regarding which physiological function first undergoes senescence. Our findings suggest that reproductive senescence might not be linked primarily to a decline in immune functions or to an increased susceptibility to oxidative stress in wild seabirds, contrary to the current paradigm in human studies and in laboratory animal models (4 –6). Indeed, an analysis of several indices that have been used in birds (32) showed no effect of age on immune, oxidative stress, or antioxidant defense markers in albatrosses. Many of these indices were found to vary throughout the incubation period in females, but not in males (Table 1). Thus, we are confident that the selected indices are sufficiently accurate to detect significant and meaningful variations among albatrosses. We could not detect any effect of age, however. We suggest that wandering albatrosses are likely to maintain a high level of somatic maintenance into old age. Interestingly, long-lived birds are known to maintain a high basal metabolic rate in old age (14, 33). In contrast, recent studies on short-lived birds reported an age-related decline in energy metabolism (34), humoral immune response (9), and plasma total antioxidant activity (35). Taken together, these results provide support for the disposable soma theory, in which species with different lifespans are expected to differ in their optimal investment in somatic maintenance and reproduction (36).
Do Old Males Sacrifice Reproduction for Somatic Maintenance?
Our finding of declining reproductive and foraging performance with age suggests that older adults invest less in parental care to maintain somatic function compared with younger adults. This is consistent with the idea that foraging performance mediates a crucial conflict between egg care and self-feeding in wandering albatrosses (22). A single incubation shift typically lasts 8–15 days (but can extend up to 40 days) and causes a 5%–15% (or possibly up to 30%) decrease in body mass (22). Between two fasts at the nest, albatrosses forage over thousands of kilometers at sea to catch patchily distributed prey (37), and they need to restore body reserves for the next incubation shift. Allocation of time and energy between fasting and self-feeding could be challenging for old, potentially energetically constrained individuals that maintain immune and antioxidant defenses, which are energetically demanding processes (38, 39). We can speculate that wandering albatrosses sacrifice parental investment to maintain a high level of physiological fitness until old age, consistent with the recently published concept that it might be optimal for organisms to decrease their investment in reproduction as they age (40).
Male Aging: Sex-Specific Patterns of Senescence.
Evolutionary research has recently highlighted the need for a detailed exploration of the links between sex and aging (41). Evidence for male reproductive senescence is scarce (but see ref. 42). In the present study, we detected a clear-cut male-specific senescence pattern in both foraging behavior and reproductive performance. Our results are consistent with predictions based on sex-specific selection of reproductive strategies (41, 43). Males are predicted to sacrifice longevity to maximize fitness by enhanced mating success, larger body size, or greater investment in reproduction. Of note, the wandering albatross is one of the few seabirds that exhibits significant sexual dimorphism in size, with males generally ∼20% larger than females (44, 45). Males also tend to invest more in the provisioning of larger chicks (24), although overall breeding duties are shared almost equally (22, 26). Why should aging occur earlier in males than in females? The long-standing idea that males are selected to pursue a “live fast, die young” reproductive strategy is supported by higher mortality rates in males over a broad range of taxa (41, 43, 46). In wild ungulates, compared with females, males have lower survival (46), have shorter life spans, reproduce earlier (47), and have earlier declines in body mass (47), possibly due to differences in the decline of foraging ability (tooth wear hypothesis). Human males live an average of 5 years less than females, with the sex difference in longevity decreasing with increasing resource availability (43). Previous studies might have failed to find a sex difference in aging patterns because food resources were not limited in the monitored populations (44). Thus, sex differences in survival might be driven by foraging ability and might be enhanced when resources are limited, as in albatrosses that rely on patchy, unpredictable, ephemeral prey (48).
Aging in the Wild: Foraging Ability as a Cornerstone?
Overall, our present findings demonstrate that age, sex, and foraging behavior interact in shaping aging patterns in wild animals. Consistent with some recent pioneering studies in free-living birds (18) and mammals (49), we propose that the ability of individuals to extract energy from their environment might be one of the first phenotypic traits to reflect aging in natural conditions, as opposed to immune or oxidative stress physiology. The trade-off between current reproduction versus future reproduction and survival is a central tenet of life history theory and might shape the evolution of life history traits. We emphasize that this hypothesis is based on the assumption that individuals must allocate a limited amount of energy to competing life-history traits, and that foraging ability directly determines the amount of energy available to an individual to expend on fitness-related activities.
To avoid confounding effects of interannual variability in available resources, our study was of a purely cross-sectional design. Although cross-sectional data are often used in aging research (9–11, 13, 18, 20), the reliability of conclusions drawn from such data has been called into question (50). Because aging is a within-individual process, only longitudinal studies can allow researchers to properly separate within-individual aging patterns from selection processes and between-individual heterogeneity (50). One of the key challenges facing researchers working to understand senescence is to compare physiology and behavior in the same individual at different ages, a difficult but promising benchmark for future work in extremely long-lived animals such as wandering albatrosses.
Materials and Methods
Study Site and Birds.
The field study was carried out on Possession Island, one of the Crozet Islands in the southwestern Indian Ocean (46.8 °S, 51.8 °E). To avoid potentially confounding effects of interannual environmental variability, all work was conducted during a single breeding season (2007–2008 austral summer). All birds had been ringed as part of the long-term mark recapture program (51), with chicks being banded before fledging since 1965. Because some very old birds had been ringed as adults in the early part of the long-term study, we calculated their minimum age from the year of banding and the minimum age at first breeding, which is 7 years in wandering albatrosses (51). All birds were handled during the incubation stage (SI Text). Laying dates were obtained from checks of the colony every 6 or 8 days. Reproductive performance was computed as the probability of each pair successfully raising a chick to fledging stage during the 2008 breeding season (i.e., breeding success).
Foraging Behavior.
The foraging trips of albatrosses (n = 48; age 7–46+ years) were studied using solar panel-powered satellite transmitters (Argos PTTs100, 22–30 g; Microwave Telemetry) attached to the back feathers by Tesa tape (52). Birds were captured by hand during the incubation period when they were about to leave their nest to forage at sea. Nests were monitored daily until the birds returned from sea to relieve their partners and start a new incubation shift. Data were filtered as described previously (53). We quantified trip duration, total distance traveled, and foraging range (the maximum distance from the colony). To monitor foraging activity, we used leg-mounted miniature light-level/immersion devices (GLS-MK4 loggers; British Antarctic Survey) mounted on plastic leg bands. This device records the proportion of time spent on the water every 10 minutes (SI Text and Table S6). The two devices’ total weight was only 0.8%–1.5% of the bird's body weight. The device was recovered after one foraging trip (1.7–38.8 days). The hatching success (i.e., the probability for each pair successfully incubating the egg until hatching) of the 48 birds fitted with satellite transmitter and activity loggers was 81.4% (n = 48 individuals), not significantly different from that in the control group (83.7%, n = 145 pairs; χ2 = 0.014, P = 0.91). To analyze activity patterns, we quantified the proportion of diurnal time spent on water and further analyzed DASs, which reflect foraging events on the water (SI Text). We quantified the number of DASs per day, percentage of time spent on the water during DASs, mean DAS duration, and mean flying time between two DASs (SI Text).
Blood Sampling, Hormone Assays, Immune Indices, and Oxidative Stress Assays.
Blood was sampled from the tarsus vein within 2 min of capture (SI Text), before departure to sea (pre-trip), and after return (post-trip). For ethical reasons, all tests were performed in vitro on plasma or RBCs, following procedures detailed in SI Text. Corticosterone and prolactin levels in pre-trip and post-trip blood samples were measured using RIA techniques developed at the Centre d'Etudes Biologiques de Chizé (SI Text). We quantified ∆Corticosterone, the relative decrease from baseline concentrations of corticosterone over a foraging trip, as a proxy of foraging success (Discussion and ref. 12). Seven indices were selected to probe various immune and oxidative stress status markers (32, 54) and were assessed in pre-trip blood samples (SI Text). Plasma antibacterial activity assesses the innate capacity of plasmatic proteins to kill invasive bacteria in a growth-inhibition test. Lysis score reflects the combined ability of lytic enzymes (complement) and natural antibodies to lyse exogenous rabbit RBCs in a standard avian hemagglutination and hemolysis assay (HHA). The agglutination score reflects the ability of natural antibodies to induce killing of exogenous cells. Haptoglobin, an acute-phase protein found in a wide range of taxa, including birds, is a well-known marker of inflammation. The plasma level of lipid peroxidation is a major indicator of oxidative damage. Total antioxidant capacity of plasma is a functional measure relying on the ability of total antioxidants in the plasma (e.g., vitamins E and C, carotenoids) to quench a free radical cation in vitro. Superoxide dismutase activity, an intracellular marker of antioxidant defense enzymatic activity, was assessed in RBCs. Because of ethical reasons, we could not challenge albatrosses; thus, all immune tests probed baseline immune status (i.e., innate immunity).
Statistical Analysis.
We investigated the age-related effects on foraging and physiological parameters separately for both sexes, because we know that male and female wandering albatrosses have different foraging strategies and areas during the incubation period (22). Because the date of departure is known to influence the duration of foraging trips (36) and physiological traits (2, 22), we included the Julian date as a covariate in all of our analyses. Age and experience were highly correlated in both males and females (SI Text). We used a second-order Akaike information criterion (AICc) to select the most parsimonious model. Model-ranking and mean values are reported in Tables S1–S4. GLMs with logarithm link were used to test for age and date effects on total distance traveled and foraging range.
Supplementary Material
Acknowledgments
We thank André Lacroix for the prolactin RIA, Hélène Maheo and Maud Berlincourt for their assistance in the field, Karine Delord and Maite Louzao for their assistance with data analysis, and Matthieu Authier, Annie Jenkins, Nicolas Hanuise, Vivien Lecomte, Patrick Pluton, and two anonymous referees for their helpful comments. We thank Liz Flavall for thoroughly editing the manuscript. All research was performed at Alfred Faure Station (Crozet Islands) and was supported by the French Polar Institute (IPEV; Program 109) and by the Fondation Albert II de Monaco. The field study was approved by the IPEV's Ethics Committee. B.F. and G.S. received financial support from the Agence Nationale de la Recherche (ANR-NT05-2-45491: STRESS OX & AGE).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0911181107/DCSupplemental.
References
- 1.Clutton-Brock TH. Reproductive Success. Chicago: Univ Chicago Press,; 1988. [Google Scholar]
- 2.Angelier F, Shaffer SA, Weimerskirch H, Chastel O. Effect of age, breeding experience and senescence on corticosterone and prolactin levels in a long-lived seabird: The wandering albatross. Gen Comp Endocrinol. 2006;149:1–9. doi: 10.1016/j.ygcen.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.McCleery RH, Perrins CM, Sheldon BC, Charmantier A. Age-specific reproduction in a long-lived species: The combined effects of senescence and individual quality. Proc R Soc Lond B Biol Sci. 2008;275:963–970. doi: 10.1098/rspb.2007.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annu Rev Entomol. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- 5.Larbi A, et al. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- 6.Partridge L, Gems D. Benchmarks for aging studies. Nature. 2007;450:165–167. doi: 10.1038/450165a. [DOI] [PubMed] [Google Scholar]
- 7.Nisbet ICT. Detecting and measuring senescence in wild birds: Experience with long-lived seabirds. Exp Gerontol. 2001;36:833–843. doi: 10.1016/s0531-5565(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 8.Holmes DJ, Flückiger R, Austad SN. Comparative biology of aging in birds: An update. Exp Gerontol. 2001;36:869–883. doi: 10.1016/s0531-5565(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 9.Cichon M, Sendecka J, Gustafsson L. Age-related decline in humoral immune function in collared flycatchers. J Evol Biol. 2003;16:1205–1210. doi: 10.1046/j.1420-9101.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Bize P, Devevey G, Monaghan P, Doligez B, Christe P. Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology. 2008;89:2584–2593. doi: 10.1890/07-1135.1. [DOI] [PubMed] [Google Scholar]
- 11.Angelier F, Weimerskirch H, Dano S, Chastel O. Age, experience and reproductive performance in a long-lived bird: A hormonal perspective. Behav Ecol Sociobiol. 2007;61:611–621. [Google Scholar]
- 12.Angelier F, Moe B-R, Weimerskirch H, Chastel O. Age-specific reproductive success in a long-lived bird: Do older parents resist stress better? J Anim Ecol. 2007;76:1181–1191. doi: 10.1111/j.1365-2656.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 13.Broggi J, et al. Sources of variation in winter basal metabolic rate in the great tit. Ecology. 2007;21:528–533. [Google Scholar]
- 14.Moe B, Angelier F, Bech C, Chastel O. Is basal metabolic rate influenced by age in a long-lived seabird, the snow petrel? J Exp Biol. 2007;210:3407–3414. doi: 10.1242/jeb.005090. [DOI] [PubMed] [Google Scholar]
- 15.Stephens DW, Brown JS, Ydenberg RC. Foraging: Behavior and Ecology. Chicago: Univ Chicago Press,; 2007. [Google Scholar]
- 16.Forslund P, Pärt T. Age and reproduction in birds: Hypotheses and tests. Trends Ecol Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- 17.Wunderle JM. Age-specific foraging proficiency in birds. Curr Ornithol. 1991;8:273–324. [Google Scholar]
- 18.Catry P, Phillips RA, Phalan B, Croxall JP. Senescence effects in an extremely long-lived bird, the grey-headed albatross, Thalassarche chrysostoma . Proc R Soc Lond B Biol Sci. 2006;273:1625–1630. doi: 10.1098/rspb.2006.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed TE, et al. Reproductive senescence in a long-lived seabird: Rates of decline in late-life performance are associated with varying costs of early reproduction. Am Nat. 2008;171:89–101. doi: 10.1086/524957. [DOI] [PubMed] [Google Scholar]
- 20.Weimerskirch H, Lallemand J, Martin J. Population sex ratio variation in a monogamous long-lived bird, the wandering albatross. J Anim Ecol. 2005;74:285–291. [Google Scholar]
- 21.Weimerskirch H, Bartle JA, Jouventin P, Stahl JC. Foraging ranges and partitioning of feeding zones in three species of southern albatrosses. Condor. 1988;90:214–219. [Google Scholar]
- 22.Weimerskirch H. Regulation of foraging trips and incubation routine in male and female wandering albatrosses. Oecologia. 1995;102:37–43. doi: 10.1007/BF00333308. [DOI] [PubMed] [Google Scholar]
- 23.Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP. Seasonal sexual segregation in two Thalassarche albatross species: Competitive exclusion, reproductive role special-ization or foraging niche divergence? Proc R Soc Lond B Biol Sci. 2004;271:1283–1291. doi: 10.1098/rspb.2004.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc R Soc Lond B Biol Sci. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaffer SA, Costa DP, Weimerskirch H. Behavioral factors affecting foraging effort of breeding wandering albatrosses. J Anim Ecol. 2001;70:864–874. [Google Scholar]
- 26.Bretagnolle V. Adaptive significance of seabird coloration: The case of Procellariiforms. Am Nat. 1993;142:141–173. doi: 10.1086/285532. [DOI] [PubMed] [Google Scholar]
- 27.Weimerskirch H, Lequette B, Jouventin P. Development and maturation of plumage in the wandering albatross Diomedea exulans . J Zool (Lond) 1989;219:411–421. [Google Scholar]
- 28.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 29.Angelier F, Shaffer SA, Weimerskirch H, Trouvé C, Chastel O. Corticosterone and foraging behavior in a pelagic seabird. Physiol Biochem Zool. 2007;80:283–292. doi: 10.1086/512585. [DOI] [PubMed] [Google Scholar]
- 30.Angelier F, et al. Corticosterone and foraging behavior in a diving seabird: The Adélie penguin, Pygoscelis adeliae . Gen Comp Endocrinol. 2008;156:134–144. doi: 10.1016/j.ygcen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Kitaysky AS, Piatt JF, Wingfield JC. Stress hormones link food availability and population processes in seabirds. Mar Ecol Prog Ser. 2007;352:245–258. [Google Scholar]
- 32.Matson KD, Cohen AA, Klasing KC, Ricklefs RE, Scheuerlein A. No simple answers for ecological immunology: Relationships among immune indices at the individual level break down at the species level in waterfowl. Proc R Soc Lond B Biol Sci. 2006;273:815–822. doi: 10.1098/rspb.2005.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackmer AL, et al. Exploring individual quality: Basal metabolic rate and reproductive performance in storm petrels. Behav Ecol. 2005;16:906–913. [Google Scholar]
- 34.Moe B, Rønning B, Verhulst S, Bech C. Metabolic aging in individual zebra finches. Biol Lett. 2009;5:86–89. doi: 10.1098/rsbl.2008.0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cote J, Arnoux E, Gaillard M, Sorci G, Faivre B. Age-dependent allocation of carotenoids to coloration versus antioxidant defences. J Exp Biol. 2010;213:271–277. doi: 10.1242/jeb.035188. [DOI] [PubMed] [Google Scholar]
- 36.Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 37.Nevitt GA, Losekoot M, Weimerskirch H. Evidence for olfactory search in wandering albatross, Diomedea exulans . Proc Natl Acad Sci USA. 2008;105:4576–4581. doi: 10.1073/pnas.0709047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- 39.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc Lond B Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara JM, Houston AI, Barta Z, Scheuerlein A, Fromhage L. Deterioration, death and the evolution of reproductive restraint in late life. Proc R Soc Lond B Biol Sci. 2009;276:4061–4066. doi: 10.1098/rspb.2009.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonduriansky R, Maklakov A, Zajitschek F, Brooks R. Sexual selection, sexual conflict and the evolution of aging and life span. Funct Ecol. 2008;22:443–453. [Google Scholar]
- 42.McElligott AG, Altwegg R, Hayden TJ. Age-specific survival and reproductive probabilities: Evidence for senescence in male fallow deer (Dama dama) Proc R Soc Lond B Biol Sci. 2002;269:1129–1137. doi: 10.1098/rspb.2002.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Møller AP, Fincher CL, Thornhill R. Why men have shorter lives than women: Effects of resource availability, infectious disease, and senescence. Am J Hum Biol. 2009;21:357–364. doi: 10.1002/ajhb.20879. [DOI] [PubMed] [Google Scholar]
- 44.Weimerskirch H. Reproductive effort in long-lived birds: Age-specific patterns of condition, reproduction and survival in the wandering albatross. Oikos. 1992;64:464–473. [Google Scholar]
- 45.Shaffer SA, Weimerskirch H, Costa DP. Functional significance of sexual dimorphism in wandering albatrosses, Diomedea exulans . Funct Ecol. 2001;15:203–210. [Google Scholar]
- 46.Loison A, Festa-Bianchet M, Gaillard JM, Jorgenson JT, Jullien JM. Age-specific survival in five populations of ungulates: Evidence of senescence. Ecology. 1999;80:2539–2554. [Google Scholar]
- 47.Mysterud A, Solberg EJ, Yoccoz NG. Ageing and reproductive effort in male moose under variable levels of intrasexual competition. J Anim Ecol. 2005;74:742–754. [Google Scholar]
- 48.Weimerskirch H, Gault A, Cherel Y. Prey distribution and patchiness: Factors in foraging success and efficiency of wandering albatrosses. Ecology. 2005;86:2611–2622. [Google Scholar]
- 49.MacNulty DR, et al. Predatory senescence in ageing wolves. Ecol Lett. 2009;12:1347–1356. doi: 10.1111/j.1461-0248.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 50.Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM. Measuring senescence in wild animal populations: Towards a longitudinal approach. Funct Ecol. 2008;22:393–406. [Google Scholar]
- 51.Weimerskirch H, Brothers N, Jouventin P. Population dynamics of wandering albatross Diomedea exulans and Amsterdam albatross D. amsterdamensis in the Indian Ocean and their relationships with long-line fisheries: Conservation implications. Biol Conserv. 1997;79:257–270. [Google Scholar]
- 52.Weimerskirch H, Doncaster CP, Cuenot-Chaillet F. Pelagic seabirds and the marine environment: Foraging patterns of wandering albatrosses in relation to prey availability and distribution. Proc R Soc Lond B Biol Sci. 1994;255:91–97. [Google Scholar]
- 53.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa DP. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc R Soc Lond B Biol Sci. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alonso-Álvarez C, Pérez-Rodríguez L, García JT, Viñuela J, Mateo R. Age and breeding effort as sources of individual variability in oxidative stress markers in a bird species. Physiol Bioch Zool. 2010;83(1):110–118. doi: 10.1086/605395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.