Abstract
Topoisomerase IIIα (topo IIIα), a member of the conserved Type IA subfamily of topoisomerases, is required for the cell proliferation in mitotic tissues, but has a lesser effect on DNA endoreplication. The top3α gene encodes two forms of protein by utilizing alternative translation initiation sites: one (short form) with the nuclear localization signal only, exclusively localized in the nuclei, and the other (long form), retaining a mitochondrial import sequence at the N-terminus and the nuclear localization sequence at the C-terminus, localized primarily in the mitochondria, though with a small portion in the nuclei. Both forms of topo IIIα can rescue the viability of null mutants of top3α. No apparent defect is associated with the flies rescued by the long form; short-form-rescued flies (referred to as M1L), however, exhibit defects in fertilities. M1L females are sterile. They can lay eggs but with mitochondrial DNA (mtDNA) copy number and ATP content decreased by 20- and 2- to 3-fold, respectively, and they fail to hatch. Of the newly eclosed M1L males, 33% are completely sterile, whereas the rest have residual fertilities that are quickly lost in 6 days. The fertility loss of M1L males is caused by the disruption of the individualization complex and a progressive loss of germ-line stem cells. This study implicates topo IIIα in the maintenance of mtDNA and male germ-line stem cells, and thus is a causative candidate for genetic disorders associated with mtDNA depletion.
Keywords: mtDNA depletion, DNA replication, DNA segregation, topoisomerase
Intertwining of DNA duplex provides an elegant means to store and transmit genetic information. However, because of this bihelical structure, the transaction of genetic information leads to DNA entanglement such as supercoiling, knotting, and catenation. DNA topoisomerases are nature’s solution for resolving the topological problems associated with DNA metabolism. Based on structure and mechanism, there are two main groups of these enzymes (1, 2). Type I topoisomerases can reversibly cleave one DNA strand at a time, and type II enzymes are able to generate transient double strand breaks and transport another DNA segment through the reversible breaks. There are additional subtypes for each group. Bacterial topo I/III and eukaryotic topo III belong to type IA enzymes, and they work via a mechanism of strand passage through an enzyme-bridged single strand break. There are two isozymes of topo III, α and β, in metazoans: topo IIIα is essential for viability, and topo IIIβ is not (3, 4). Eucaryotic topo I is classified as type IB enzymes, and it forms an evolutionarily distinct class when compared with type IA enzymes, presumably a descendent of site-specific recombinases in bacteria (5). These enzymes work by generating a strand break where protein is linked to the 3′-phosphoryl end, allowing the free 5′ end to undergo a restrained rotation around the nonscissile strand (6). Type IB can thus serve as an efficient swivel to remove supercoiling accumulated during replication or transcription. Whereas type IA enzymes can also function as a swivel, their strand passage activity can promote reactions as diverse as segregating replicated chromosomes and regulating the formation/resolution of recombination intermediates (7). Members in type II enzymes are grouped into A and B subtypes, and they are closely related both in structure and mechanism. These enzymes are able to alter DNA supercoiling, but have essential functions in the segregation of daughter chromosomes. Most organisms have at least one or two members of each type of topoisomerases. The presence and function of topoisomerases in organelles like mitochondria and chloroplast, not as thoroughly investigated as their nuclear counterpart, remain to be elucidated.
Mitochondria have a number of critical functions beyond a source for metabolic energy, including processing of key metabolites and the control of programmed cell death (8). Abnormal mitochondria and mitochondrial dysfunction are known to be associated with a number of clinically important human diseases (9). Whereas the nuclear genome encodes all of the functions involved in the intermediate metabolism and biogenesis of mitochondria, the mitochondrial genome encodes part of the mitochondrial protein synthesis machinery, and more importantly, the core peptides for oxidative phosphorylation. Thus, mutations affecting the stable maintenance and transmission of mitochondrial genome can disrupt oxidative phosphorylation and ATP production.
The complexity of mitochondrial genomes varies greatly, with those from fungi and plants having the most complexity. Animal mitochondrial DNA (mtDNA) is a small genome, and typically exists as a circular, covalently closed molecule ranging from 15 to 20 kb (10). The known nuclear genes that are essential for mitochondrial DNA replication include DNA polymerase gamma, polymerase gamma accessory protein, DNA helicase, mitochondrial transcriptional factor A, and RNA polymerase serving as a primase [(11, 12); reviewed in (13)]. Interestingly, whether any DNA topoisomerases have a critical function in mitochondrial DNA replication remains to be elucidated. For DNA replication in kinetoplast, a mitochodrion-like organelle in trypanosome, both topoisomerase II (topo II) and a type IA topoisomerase are known to have essential functions (14, 15). The picture is less clear for animal cells. There exists a dedicated mitochondrial type IB enzyme in mammalian cells (16). However, genetic studies suggest that it is dispensable for mitochondrial DNA maintenance (17). Biochemical analysis using subcellular fractionation provides evidence for the presence of a truncated form of topo IIβ in bovine heart (18). There is also clear evidence to demonstrate the presence of a mitochondia-targeting form of a type IA enzyme using an alternate translational initiation of top3α gene in human cells (19). However, whether these two enzymes are essential for mitochondrial DNA maintenance remains to be determined.
Drosophila topo IIIα has a short, unconserved sequence at the amino terminus of the protein, which contains a putative mitochondrial import sequence, similar to its mammalian counterpart (4). This paper demonstrates that the amino-terminal sequence has the predicted function in targeting topo IIIα to mitochondria. Furthermore, we took the advantage of the genetic tools we developed for studying Drosophila topo IIIα, and showed that both the maintenance of mitochondrial DNA and mitochondrial functions depend on the presence of topo IIIα. Lastly, we described the phenotypes associated with mitochondrial dysfunction, including the female sterility and the loss of germ-line stem cells of males, as a result from topo IIIα deprivation.
Results
topo IIIα Is Localized in Both Nuclei and Mitochondria.
The amino-terminal sequence of eukaryotic topo IIIα contains a mitochondial targeting signal, and human topo IIIα contains this sequence can localize to the mitochondria in the cultured cells (19). The N-terminal sequence of metazoan topo IIIα, including that of Drosophila, also contains such a mitochondrial import sequence (4, 19). Drosophila oogenesis and spermatogenesis are favorable systems to examine the mitochondrial subcellular localization and its functional importance. We analyzed the subcellular localization of the endogenous Drosophila topo IIIα protein during gametogenesis.
During the spermatogenesis (20), germ-line stem cells located at the apical tip of the coiled testis undergo mitotic cell division to give rise to spermatogonial cells, followed by four additional cycles of mitosis, each producing 16 primary spermatocytes that are interconnected as a result of incomplete cytokinesis, and forming a 16-cell cyst surrounded by two somatic cyst cells. Following a growth stage where the cyst increases 25-fold in size, the 16 primary spermatocytes in the cyst undergo meiosis, resulting in 64 haploid spermatids. By the onion stage of early spermatid differentiation, the mitochondria in each spermatid have aggregated to form the mitochondrial derivative, nebenkern. Each spermatid contains a single nucleus paired with a nebenkern. In the later stages of spermatogenesis, the spermatids go through elongation, nuclei condensation, and individualization, during which the syncytial spermatid bundle is resolved into 64 separate sperm cells. Finally, the sperms curl up, the cyst ruptures, and the mature sperms move into the seminal vesicle for storage.
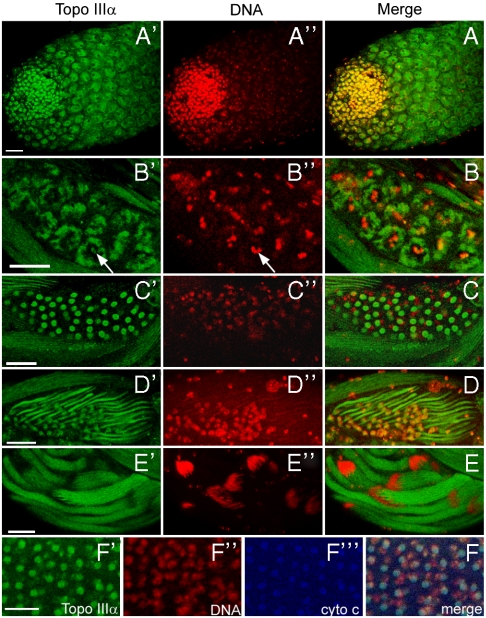
We dissected the testes and examined the expression patterns of topo IIIα by immunostaining with an affinity-purified polyclonal antibody. topo IIIα was detected in both nuclei and cytoplasm of spermatogonia and spermatocytes, with a localization mainly in the nuclei (Fig. 1A). In contrast, during the metaphase of meiosis I, topo IIIα was prominent in the mitochondria of the spermatocytes, with detectable staining on the highly condensed chromatin (Fig. 1 B and B′ and Arrow) surrounded by the mitochondria. topo IIIα expression persists in the mitochondria in the onion and elongation stages of spermatids (Fig. 1 C and D). At these stages, mitochondria (nebenkerns) show a characteristic position of neighboring a nucleus. The localization of topo IIIα in the mitochondria was further confirmed by triple staining with a mitochondria marker cytochrome c (Fig. 1F). Following the elongation process, while topo IIIα was still present in the elongated mitochondrial derivative, it was not detectable in the condensed, needle-shaped nuclei bundles (Fig. 1E). We also examined the topo IIIα expression pattern during oogenesis. Similarly, it localized in both nuclei and mitochondria, with a predominant presence in nuclei. Collectively, topo IIIα is localized in both nuclei and mitochondria during gametogensis.
Fig. 1.
The topo IIIα is localized in both mitochondria and nuclei during spermatogenesis. (A) The apical tip of an adult testis. (B) A cyst of 16 spermacytes in the metaphase meiosis I, which is featured by the highly condensed chromatin and clustered mitochondria surrounding the equator spindle. (C) A cyst of onion stage spermatids. (D) Elongating spermatids at comet stage. (E) Elongated spermatids. A–E are the merged double staining of topo IIIα (A′–E′), and DNA (A′′–E′′). (F) Merged triple staining of a cyst of onion stage spermatids with staining for topo IIIα, DNA, and cytochrome c (F′, F′′, and F′′′, respectively). Abundant topo IIIα staining is colocalized with a mitochondrial marker, cytochrome c. Scale bars: 25 μm.
The Amino Terminus of topo IIIα Contains a Mitochondrial Import Sequence.
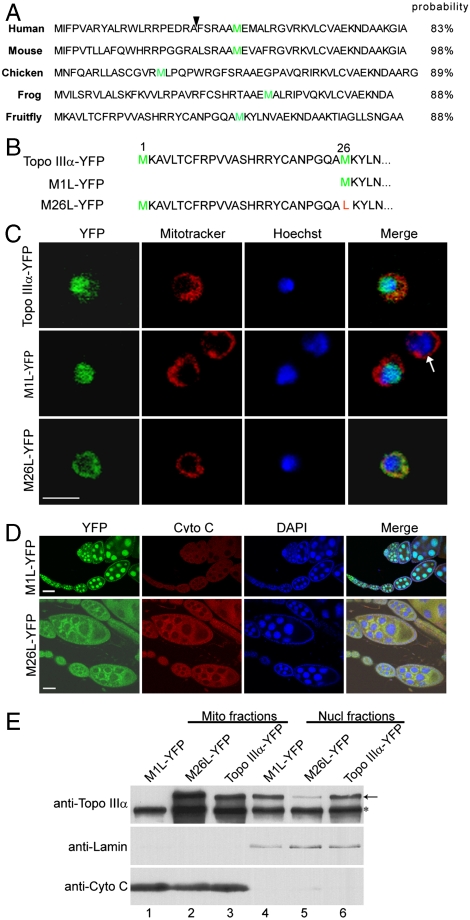
Drosophila, human, and mouse topo IIIα all have a putative, unconserved mitochondrial import sequence at the N terminus of the protein, sandwiched between two tandem initiating methionines (4, 19). topo IIIα of metazoans is thus characterized with the presence of dual subcellular localization sequences: a mitochondrial import sequence at the N-terminus, and a nuclear localization signal in the C-terminal domain following the catalytic core domain. Analysis using the mitochondrial prediction algorithm (21) gives import probabilities higher than 83% for these mitochondrial localization sequences (Fig. 2A). To investigate the role of the putative mitochondrial import sequence in the localization of Drosophila topo IIIα, we altered the translational initiation by changing either the first or second translational initiation condon from AUG to UUG. We first generated a wild type top3α genomic transgene, top3α-YFP, with an in-frame YFP fusion at the carboxyl terminus of topo IIIα (Fig. 2B and Fig. 3A). M1L-YFP and M26L-YFP were generated based on top3α-YFP (Fig. 2B and SI Materials and Methods), and their DNA sequences were identical to top3α-YFP except for the first or second ATG, which were changed to TTG, respectively.
Fig. 2.
The unconserved amino (N) termini of topo IIIα contain mitochondrial import signals. (A) N-terminal sequences of topo IIIα of human, mouse, chicken, frog, and fruit fly. The mitochondrial import probabilities were obtained by using the algorithm developed by Claros and Vincens (21). Arrowhead indicates the mitochondrial endopeptidase cleavage sites (19). The second methionines are colored green. (B) The N-termini of proteins encoded by the wildtype and mutant transgenes of Drosophila. Methionines are colored green and the mutated amino acid is marked red. For M1L-YFP transgene, the first AUG was mutated to UUG, therefore the translation would start exclusively from the second AUG. (C) Confocal images of live S2 cells transfected with the plasmid DNA containing a transgene of topo IIIα-YFP, M1L-YFP, or M26L-YFP. Arrow indicates an S2 cell not transfected with transgenic vector. Scale bar, 10 μm. (D) Confocal images of fixed ovarioles of M1L-YFP and M26L-YFP transgenic females. Scale bar, 25 μm. (E) Subcellular fractionation and immunoblots. Mitochondria fractions (Lanes 1–3) and nuclei fractions (Lanes 4–6) were separated as described in Materials and Methods, and were subjected to SDS-PAGE, followed by Western blotting with antibodies against topo IIIα, lamin (nuclear marker) and cytochrome c (mitochondrial marker). Asterisk indicates the endogenous topo IIIα and arrow marks YFP fusion products.
Fig. 3.
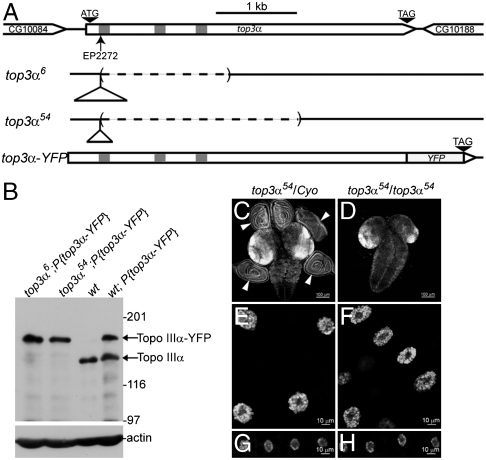
The top3α gene and its null mutant top3α6 and top3α54. (A) Genomic structure of top3α gene and its mutants top3α6 and top3α54. Open triangles represent the remnant inserts derived from P-element and the dashed lines indicate the deleted sequences. Introns are shown as gray bars. (B) Mutants top3α6 and top3α54 were verified as null mutants by Western blotting. Lysate of ovaries was subjected to SDS-PAGE and the transferred protein bands were probed with antibodies against topo IIIα, and actin (loading control). Upper bands correspond to topo IIIα-YFP fusion protein, encoded by the transgene top3α-YFP, and the lower bands to the endogenous topo IIIα. (C, D) Brains and attached imaginal discs (Arrowheads) were dissected from wandering larvae of control (C) and top3α54 mutant (D) visualized with DAPI staining. Salivary gland nuclei (E, F) and fat body nuclei (G, H) of wandering larvae from control (E, G) and top3α54 mutants (F, H) were stained with DAPI.
The protein encoded by topo IIIα-YFP transgene is localized in both nuclei and mitochondria, with vast majority in the nuclei (Fig. 2C). M1L-YFP, with the first AUG altered to UUG and initiating exclusively from the second AUG, encodes a protein deprived of the mitochondrial import sequence, but retaining the nuclear localization signal at the carboxyl terminal portion (Fig. 2B). As expected, the protein was exclusively localized in nuclei of cultured cells and ovarioles (Fig. 2 C and D).
With the second AUG was changed to UUG, the translation will be exclusively from the first AUG, giving rise to a protein with a mitochondrial import sequence at its N-terminus and a nuclear localization signal at its C-terminal portion (Fig. 2B). This protein is predominantly localized in mitochondria, with a detectable fraction in the nuclei (Fig. 2 C and D). This result thus suggests that the second AUG in the wildtype top3α is used as an alternative translation initiation codon to produce the major nuclear form of topo IIIα.
To further confirm the localization patterns of M1L-YFP and M26L-YFP, we isolated nuclei and mitochondria by sucrose gradient centrifugation. Western blotting with topo IIIα antibody showed that whereas M1L-YFP could be detected in nuclei only (Fig. 2E, Lane 1 vs. Lane 4), M26L-YFP was present in both mitochondria and nuclei, albeit with a lower level in nuclei (Fig. 2E, Lane 2 vs. Lane 5). These biochemical data are consistent with observations in the immunostaining. Altogether, these results demonstrate that the polypeptide between the first and second methionine of Drosophila topo IIIα (Fig. 2B) has a function in mitochondrial import.
Generation of top3α Null Mutants.
The top3α transgenes with differential targeting to either nucleus or mitochondrion provide a genetic tool to dissect its functions in these subcellular compartments. Our earlier experiments have identified a lethal top3α mutant, top3α191, demonstrating the essentiality of top3α in Drosophila development (4). Because top3α191 is a hypomorphic mutant, we will need to isolate a null mutation that can be used to investigate the distinct nuclear vs. mitochondrial function of topo IIIα. In the top3α null mutant with the transgenes having specific targeting preference, the only functional top3α would be that from the transgenes, thus allowing us to dissect its function in distinct subcellular compartments. The knock-out mutants were generated through imprecise excision after mobilizing a P-element in the fly strain top3αEP2272 (4). We isolated two imprecise excision alleles that are homozygous lethal, top3α6 and top3α54, both of which can be rescued by the genomic transgene of top3α, top3α-YFP (Fig. 3A and Table S1). Molecular mapping results indicate that at the original P-element insertion site, top3α6 and top3α54 have remnant insertions of 638 bp and 331 bp derived from the P-element, respectively. In addition, they also have a deletion of 1540 bp and 2437 bp from nucleotide 203 bp downstream of the first translational initiation codon (Fig. 3A). Western blotting confirmed that top3α6 and top3α54 are null mutants (Fig. 3B).
top3α Is Required for Cell Proliferation in Mitotic Cells.
Homozygotes of top3α6 and top3α54 could develop through third instar stage with a 9 day delay as compared to their heterozygous siblings and died at pupation stage. Because topoisomerases play important roles in DNA metabolism, we examined the tissues active in DNA replication from third instar larvae by DAPI staining. Imaginal discs are the only mitotically active tissues during late larval development. Compared with the brain tissue and imaginal discs of their heterozygous siblings (Fig. 3C), third instar larvae of top3α54 (or top3α6) mutants had about normal size brains, but surprisingly, a total absence of any imaginal discs (Fig. 3D). Therefore, top3α is required for proliferation of mitotic cells. They did have, however, salivary glands and fat bodies with nuclei size comparable to those of heterozygous siblings (Fig. 3 E vs. F and G vs. H). There is endoreplication in these tissues, a specialized DNA replication undergoing multiple rounds of DNA synthesis without cytokinesis, hence giving rise to polyploidy nuclei. Because of the potential presence of low levels of maternally stored topo IIIα, endoreplication either does not require topo IIIα or low levels of topo IIIα would suffice. Interestingly, the origin recognition complex components Orc1 and Orc2 are also essential for mitotic cell division, and the endoreplication was not affected in their null mutants (22).
topo IIIα Is Required for the Mitochondrial Genome Maintenance and Fertilities of Flies.
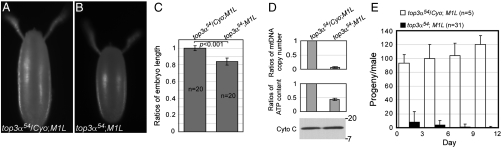
To explore the biological function of topo IIIα in mitochondrion or nucleus, we introduced the transgenes with a preference in organelle-targeting, M1L-YFP or M26L-YFP, into top3α null background. In such a background, transgenes were the only functional topo IIIα. Both M1L-YFP and M26L-YFP could fully rescue the viability of null mutant top3α54 and top3α6 (Table S1). M26L-YFP produces a protein with both mitochondrial and nuclear import sequence. Despite the greatly diminished amount of M26L-YFP imported into the nuclei (Fig. 2 C–E), it is sufficient to sustain the essential functions for viability and fertility. However, M1L-YFP produces topo IIIα without a mitochondrial import sequence and is exclusively localized in the nuclei (Fig. 2 C–E). M1L-YFP rescued flies (referred to as M1L) exhibit fertility defects in both sexes. M1L females are completely sterile. Compared with the heterozygous siblings, M1L females have no significant difference in egg yields during a 12 day period, but their eggs appear to be smaller as a whole, and 17% shorter in length (Fig. 4 A–C). They are also prone to collapse, indicative of defects in vitellogenesis (23, 24). The remaining intact embryos could not hatch, but could develop to different stages up to gastrulation. There is significant DNA fragmentation, suggesting severe defects in nuclear DNA replication. To examine the effect on mtDNA replication, we examined whether there was a change in mtDNA copy number in the 0–1 hr M1L embryos by quantitative PCR. The mtDNA copy number was decreased by 20-fold as a result of the removal of mitochondrial import signal of topo IIIα (Fig. 4D), suggesting that topo IIIα has a critical function in mitochondrial genome maintenance. Because mitochondria are the major source for cellular energy, we made measurements for ATP content in M1L embryos. ATP content of M1L was 2- to 3-fold lower than those of their heterozygous siblings (Fig. 4D). Interestingly, no apparent change in the level of cytochrome c, a nucleus-encoded protein, was detected in these embryos (Fig. 4D). These defects in mtDNA copy number and ATP content are not limited to embryos. Both ovaries and testes of M1L also have a lower ATP level (Fig. S1) and a decreased mtDNA content (Fig. S2) relative to their heterozygous siblings.
Fig. 4.
The topo IIIα is required for mtDNA genome maintenance and fertilities of flies. (A, B) Eggs laid by M1L females, which are shorter than their heterozygous siblings, cannot hatch. (C) Embryo length of 20 eggs for each genotype was measured with Qcapture Pro version 5.1 (Qimaging) and analyzed with Student’s t test. (D) 0–1 hr embryos laid by M1L females have lower mtDNA copy number and ATP content, although cytochrome c level is not affected. (E) Residual fertility of M1L males is lost after 6 day sperm depletion.
The fertility of M1L males is also severely impaired. Their fertility decreases drastically as the flies age. In the newly eclosed M1L males, 33% of them were completely sterile, and the rest of them had a fertility < 10% of their heterozygous siblings (Fig. 4E). To analyze the dependence of fertility on the fly age, we conducted sperm depletion experiments in a period of 12 days. M1L males completely lost fertility after 6 days, whereas heterozygous siblings maintained a steady level of fertility throughout 12 days (Fig. 4E).
Individualization Complexes of M1L Testes Are Disrupted During Spermatogenesis.
There are two major aspects in the fertility defects of M1L males: a severe defect for the newly eclosed males and its rapid deterioration over time. We first examined the spermatogenesis of newly eclosed males. The final stage of spermatogenesis requires the resolution of a spermatid cyst into separate gametes by packaging each of 64 elongated spermatids in its own plasma membrane, a process referred to as individualization (20). Individualization complex (IC) is an actin-rich structure that is assembled at the nuclear end of the cyst and can be clearly visualized by phalloidin-TRITC staining (Fig. S3 A, B, and Insets). In control testes, the ICs showed distinct triangular shape, and they moved in a coordinated fashion (Fig. S3 A and Inset). In M1L testes, the ICs were not aligned as in the control, but were scattered along the testes (Fig. S3 B and Inset), suggesting that they did not move in synchrony. The triangular shape of ICs was not affected, implicating that it could be the movement, not the formation of the ICs that had been impaired.
The fertility defect of M1L males is also reflected in their testes structure. As a whole, 3 day old M1L testes appeared to be 50% smaller in diameter than the control (Fig. S3 C and D, and quantified in Fig. S3E). Half of the testes had no motile sperm observed in the sperm storage organ seminal vesicles, and the rest had rare. The spermtid nuclei in the same cyst of a normal testis can elongate and condense to form a needle-shaped nuclear bundle (20) (Fig. S3 C and Inset). However, in the M1L testis, the condensed nuclei were scattered throughout the testis tube (Fig. S3 D and Inset).
topo IIIα Is Required for the Maintenance of Male Germ-Line Stem Cells (GSC).
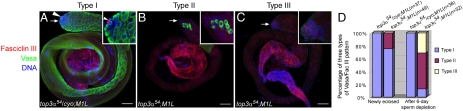
M1L males, though having residual fertilities, became completely sterile over a period of 6 days, while the fertilities of their heterozygous siblings remained unchanged (Fig. 4E). To pinpoint the cellular cause, we examined the testes from the males either newly eclosed or with an age of 6 day by triple immunostaing for Vasa, a marker of germ-line cells (25, 26), Fasciclin III, a marker of hub cells (27, 28), and DNA. The hub cells provide a niche necessary for the self-renewal of GSC. For the freshly eclosed males, control testes all had apparent Fasciclin in the hub cells (Fig. 5 A and Inset) and different stages of germ-line cells, which were laid out throughout the testes (Fig. 5 A and D). In contrast, 25% of M1L testes had no apparent Fasciclin signal and very few germ-line cells scattered along the testes (Fig. 5 B and D). At the age of 6 days, whereas the control testes had the same staining patter as the newly eclosed (Fig. 5D), the M1L testes exhibited apparent deterioration in spermatogenesis. Fifty-six percent of the testes showed a pattern of few scattered germ-line cells and no Fascilin signal for hub cells (Fig. 5 B and D). Furthermore, 31% of them were completely devoid of germ-line cells (Fig. 5 C and D). We did not detect cell death at the apical tip of testes from two-day old males by Tunel Assay, suggesting that GSC loss is a result of defects in cell proliferation. Altogether these data demonstrate that the functions of topo IIIα in mitochondria are indispensable for the maintenance of male GSC.
Fig. 5.
The functions of topo IIIα in mitochondria are required for male germ-line stem cell maintenance. Testes dissected from the control (A) and M1L (B and C) males after 6 day sperm depletion (SI Materials and Methods) were fixed and stained with antibody against Vasa (marker for germ-line cells), Fasciclin III (marker for hub cells, the niche of germ-line stem cells), and DAPI. Insets (A–C) highlight the tips, pointed by the arrows, of testes with higher magnification. Arrowhead in (A) indicates the hub cells marked by Fasciclin III. The Vasa/Fasciclin III immnunostaining patterns fell into three types: Type I (Panel A), with distinct Fasciclin III and Vasa signals, Type II (Panel B), without apparent Fasciclin III, but with germ-line cells scattered throughout the testis tube and Type III (Panel C), no germ-line cells present. (D) Quantification of three types of Fasciclin III/Vasa pattern for testes of males newly eclosed and after 6 day sperm depletion. Scale bars, 50 μm.
Discussion
We have demonstrated that Drosophila top3α is essential for cell proliferation, but not so stringently required for endoreplication, a specialized form of DNA replication with cells undergoing multiple round of DNA replication without cytokinesis. top3α encodes two forms of topo IIIα by using alternative translational initiation codons. One (long form) with a mitochondrial import sequence at the amino terminus and a nuclear localization signal at the carboxyl terminus of the protein, is predominantly localized in the mitochondria, with a detectable fraction in the nuclei. The other one (short form) without the mitochondrial import sequence, is present exclusively in the nuclei (Fig. 2). Both forms of topo IIIα can fully rescue the viabilities of top3α null mutants, suggesting the topo IIIα’s functions in nuclei are essential for viabilities. No apparent defect in fertility was observed for flies harboring the long form of topo IIIα. However, both males and females retaining only the short form of topo IIIα exhibit fertility defects, implying that topo IIIα’s functions in mitochondria are indispensable for the fertility of flies. The females are completely sterile, and the males have reduced fertility with the residual fertility lost over a period of 6 days due to disruption of ICs and the loss of germ-line stem cells. Moreover, deprivation of topo IIIα in mitochondria led to the loss of mtDNA, implying topo IIIα is required for the mitochondrial genome maintenance. The work presented in this paper thus clearly demonstrates the essential function of a topoisomerase in the metazoan mitochondria.
In the absence of topo IIIα in mitochondria, though the M1L flies can survive to adulthood, they have a 4- to 15-fold decrease in mtDNA copy number, and a lowered ATP content by 2- to 3-fold in ovaries or testes, indicating that topo IIIα plays a key role in mtDNA maintenance and function. Drosophila mtDNA is a circular molecule of about 20 kb. One expects its maintenance would require at least one member from each type of topoisomerases. Among all the topoisomerases present in Drosophila, topo IIIα (4) and topo IIIβ (29) (Type IA), topo I (30) (Type IB), and topo II (31) (Type II), only topo IIIα has been shown to be apparently imported into both the nuclei and the mitochondria. Although it has been reported that human nuclear genome encodes a mitochondria-specific Type 1B topoisomerase, Top1mt (16), which is not essential for viability (32), no such enzyme exists in Drosophila based on the genome sequence. The topo II immunostaining of the testes, a powerful tool to examine the mitochondrial localization of proteins, revealed that the sole Type II topoisomerase in Drosophila is exclusively localized in the nuclei, implying that Type II topoisomerase might be dispensable for the segregation of mtDNA. Mitochondrial import analysis predicts that topo I and topo II in human, Drosophila, and yeast have import probabilities lower than 5% (Table S2). Whereas topo IIIα is required for mtDNA maintenance and function in Drosophila, there is no report on the effect on mtDNA in yeast without top3. An interesting question is how mtDNA segregation is achieved if topo IIIα or topo III is the only topoisomerase in mitochondria in Drosophila or yeast. Several lines of experiments demonstrated that mtDNA replicates itself via a strand displacement mechanism (33). Specifically, replication of the leading strand initiates at the origin OH. Once the leading strand synthesis proceeds for two-thirds of the genome, the synthesis of the complementary strand initiates at the origin OL of the displaced strand. The daughter molecule containing the strand initiated at OL lags in completion and segregates before DNA is fully replicated (34). With this model of DNA replication, it is plausible that segregation of daughter chromosomes with extensive single-stranded regions can be accomplished by a type IA topoisomerase alone, without the participation of a Type II topoisomerase. Interestingly, earlier biochemical experiments showed that Escherichia coli topo III can efficiently decatenate the repicated plasmid DNA provided there exists single stranded regions in the daughter molecules (35).
The embryos laid by M1L females can develop to some extent with DNA fragmented, and die at the stage of early embryogenesis. The lethality could be caused by the defects in mtDNA content and deceased ATP level. Reduction of mitochondrial ATP production by 60% can impair G1-S phase transition in adult flies (36). Furthermore, the loss and mutations of mtDNA could trigger additional pathways that are deleterious to cells. Veatch et al. (37) recently reported that loss of mtDNA resulted in nuclear genome instability by inhibiting the production of proteins containing iron–sulfur clusters. Although mtDNA content is decreased by 4- to 15-fold, the M1L flies can survive to adults; however, their embryos cannot with a 20-fold decrease in mtDNA copy number. These results indicate that there may be a threshold of mtDNA copy number required for mitochondria’s cellular functions. Reduction of mtDNA copy number by < 70% is defined as mtDNA depletion, which will lead to human diseases (38). Many clinical cases with instability in mtDNA failed to reach a molecular diagnosis (39), and thus the causative agent for mtDNA depletion remains unknown. Absence of the mitochondria-targeting sequence in topo IIIα leads to a decrease of mtDNA copy number by at least 75%, suggesting that mistargeting topo IIIα can be a source of human disorders associated with mtDNA depletion. We have generated valuable tools to further dissect the mechanisms underlying the potential genetic disorder in mtDNA depletion due to topo IIIα deprivation in mitochondria.
Materials and Methods
See SI Materials and Methods for a detailed description of materials and methods.
Isolation of Nuclei and Mitochondria.
Dissected ovaries were homogenized in buffer containing 5 mM Hepes (pH 7.9), 0.3 M sucrose, 2 mM EDTA, 5 mM KCl. After differential centrifugation, both crude nuclei and mitochondria pellets were resuspended and purified by a linear sucrose gradient, and analyzed by Western blotting.
ATP Assay.
Ovaries and testes were dissected, ground, and boiled in Buffer D (5 mM Tris, pH8.0, 0.5 mM EDTA). ATP assay was performed with ATP Bioluminescent Assay Kit (Sigma) using a Beckman LS6500 scintillation counter.
Quantitative PCR.
0–1 hr embryos (dechorionated), ovaries of 3 day old females or testes were prepared for quantitative PCR using the primers listed in SI Text.
Supplementary Material
Acknowledgments.
We thank Larry Lee and Shane Chen for technical assistance. We also thank Dr. Jody Plank for providing topo IIIα antigen, and Lored Asllani for assistance in ATP measurement. This study was supported by National Institutes of Health Grant GM29006.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001855107/DCSupplemental.
References
- 1.Schoeffler AJ, Berger JM. DNA topoisomerases: Harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41(1):41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC. Cellular roles of DNA topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol. 2002;3(6):430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Wang JC. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc Natl Acad Sci USA. 1998;95(3):1010–1013. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plank JL, Chu SH, Pohlhaus JR, Wilson-Sali T, Hsieh TS. Drosophila melanogaster topoisomerase IIIalpha preferentially relaxes a positively or negatively supercoiled bubble substrate and is essential during development. J Biol Chem. 2005;280(5):3564–3573. doi: 10.1074/jbc.M411337200. [DOI] [PubMed] [Google Scholar]
- 5.Krogh BO, Shuman S. A poxvirus-like type IB topoisomerase family in bacteria. Proc Natl Acad Sci USA. 2002;99(4):1853–1858. doi: 10.1073/pnas.032613199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart L, Redinbo MR, Qiu X, Hol WG, Champoux JJ. A model for the mechanism of human topoisomerase I. Science. 1998;279(5356):1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 7.Plank J, Hsieh TS. Helicase-appended topoisomerases: new insight into the mechanism of directional strand transfer. J Biol Chem. 2009;284(45):30737–30741. doi: 10.1074/jbc.R109.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace DC, Fan W. The pathophysiology of mitochondrial disease as modeled in the mouse. Genes Dev. 2009;23(15):1714–1736. doi: 10.1101/gad.1784909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27(8):1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenleaf AL, Kelly JL, Lehman IR. Yeast RPO41 gene product is required for transcription and maintenance of the mitochondrial genome. Proc Natl Acad Sci USA. 1986;83(10):3391–3394. doi: 10.1073/pnas.83.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson NG, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18(3):231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 13.Copeland WC. Inherited mitochondrial diseases of DNA replication. Annu Rev Med. 2008;59:131–146. doi: 10.1146/annurev.med.59.053006.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay ME, Gluenz E, Gull K, Englund PT. A new function of Trypanosoma brucei mitochondrial topoisomerase II is to maintain kinetoplast DNA network topology. Mol Microbiol. 2008;70(6):1465–1476. doi: 10.1111/j.1365-2958.2008.06493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scocca JR, Shapiro TA. A mitochondrial topoisomerase IA essential for late theta structure resolution in African trypanosomes. Mol Microbiol. 2008;67(4):820–829. doi: 10.1111/j.1365-2958.2007.06087.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, et al. Human mitochondrial topoisomerase I. Proc Natl Acad Sci USA. 2001;98(19):10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Pommier Y. Mitochondrial topoisomerase I sites in the regulatory D-loop region of mitochondrial DNA. Biochemistry. 2008;47(43):11196–11203. doi: 10.1021/bi800774b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low RL, Orton S, Friedman DB. A truncated form of DNA topoisomerase IIbeta associates with the mtDNA genome in mammalian mitochondria. Eur J Biochem. 2003;270(20):4173–4186. doi: 10.1046/j.1432-1033.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Lyu YL, Wang JC. Dual localization of human DNA topoisomerase IIIalpha to mitochondria and nucleus. Proc Natl Acad Sci USA. 2002;99(19):12114–12119. doi: 10.1073/pnas.192449499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuller MT. Spermatogenesis. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Vol 1. Cold Spring Harbor, New York: Cold Spring Harbor Lab Press; 1993. pp. 71–147. [Google Scholar]
- 21.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241(3):779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Asano M. The origin recognition complex is dispensable for endoreplication in Drosophila. Proc Natl Acad Sci USA. 2008;105(34):12343–12348. doi: 10.1073/pnas.0805189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spradling AC. Developmental genetics of oogenesis. In: Bate M, Arias AM, editors. The Development of Drosophila melanogaster. Vol I. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1993. pp. 1–70. [Google Scholar]
- 24.Yan YL, Postlethwait JH. Vitellogenesis in Drosophila: Sequestration of a yolk polypeptide/invertase fusion protein into developing oocytes. Dev Biol. 1990;140(2):281–290. doi: 10.1016/0012-1606(90)90078-w. [DOI] [PubMed] [Google Scholar]
- 25.Hay B, Jan LY, Jan YN. A protein component of Drosophila polar granules is encoded by vasa and has extensive sequence similarity to ATP-dependent helicases. Cell. 1988;55(4):577–587. doi: 10.1016/0092-8674(88)90216-4. [DOI] [PubMed] [Google Scholar]
- 26.Lasko PF, Ashburner M. Posterior localization of vasa protein correlates with, but is not sufficient for, pole cell development. Genes Dev. 1990;4(6):905–921. doi: 10.1101/gad.4.6.905. [DOI] [PubMed] [Google Scholar]
- 27.Brower DL, Smith RJ, Wilcox M. Differentiation within the gonads of Drosophila revealed by immunofluorescence. J Embryol Exp Morphol. 1981;63:233–242. [PubMed] [Google Scholar]
- 28.Gonczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114(1):89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- 29.Wilson-Sali T, Hsieh TS. Generation of double-stranded breaks in hypernegatively supercoiled DNA by Drosophila topoisomerase IIIbeta, a type IA enzyme. J Biol Chem. 2002;277(30):26865–26871. doi: 10.1074/jbc.M204641200. [DOI] [PubMed] [Google Scholar]
- 30.Lee MP, Brown SD, Chen A, Hsieh TS. DNA topoisomerase I is essential in Drosophila melanogaster. Proc Natl Acad Sci USA. 1993;90(14):6656–6660. doi: 10.1073/pnas.90.14.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyckoff E, Hsieh TS. Functional expression of a Drosophila gene in yeast: Genetic complementation of DNA topoisomerase II. Proc Natl Acad Sci USA. 1988;85(17):6272–6276. doi: 10.1073/pnas.85.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Meng LH, Pommier Y. Mitochondrial topoisomerases and alternative splicing of the human TOP1mt gene. Biochimie. 2007;89(4):474–481. doi: 10.1016/j.biochi.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Brown TA, Cecconi C, Tkachuk AN, Bustamante C, Clayton DA. Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Genes Dev. 2005;19(20):2466–2476. doi: 10.1101/gad.1352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annu Rev Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- 35.DiGate RJ, Marians KJ. Identification of a potent decatenating enzyme from Escherichia coli. J Biol Chem. 1988;263(26):13366–13373. [PubMed] [Google Scholar]
- 36.Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell. 2005;9(6):843–854. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Veatch JR, McMurray MA, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron–sulfur cluster defect. Cell. 2009;137(7):1247–1258. doi: 10.1016/j.cell.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotig A, Poulton J. Genetic causes of mitochondrial DNA depletion in humans. Biochim Biophys Acta. 2009;1792(12):1103–1108. doi: 10.1016/j.bbadis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 39.Hudson G, et al. POLG1, C10ORF2, and ANT1 mutations are uncommon in sporadic progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Neurology. 2006;66(9):1439–1441. doi: 10.1212/01.wnl.0000210486.32196.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.