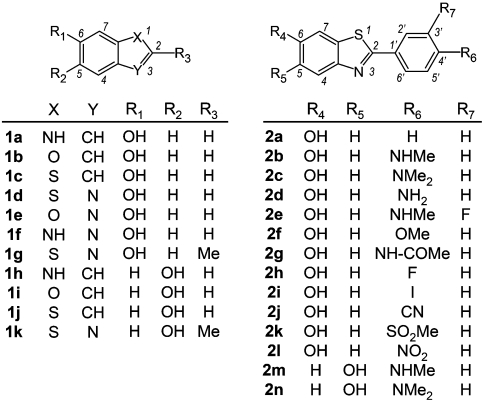
Abstract
This work focuses on the development of specific substrates for estrogen sulfotransferase (SULT1E1) to produce molecular imaging probes for this enzyme. SULT1E1 is a key enzyme in estrogen homeostasis, playing a central role in the prevention and development of human disease. In vitro sulfation assays showed alkyl and aryl substitutions to a fused heterocyclic system modeled after β-naphthol (βN), based on compounds that interact with the estrogen receptor, rendered several molecules with enhanced specificity for SULT1E1 over SULT1A1*1, SULT1A1*2, SULT1A3, and SULT2A1. Several 6-hydroxy-2-arylbenzothiazoles tested demonstrated excellent affinity—Vmax/Km ratios—and specificity for SULT1E1. Km values ranged from 0.12–2.36 μM. A strong correlation was observed between polarity of the 4′-sustituent on the 2-aryl moiety (Hammett σp) and the log(Vmax/Km) (r = 0.964). Substrate sensitivity is influenced by the acidity of the 6-phenolic group demonstrated by correlating its 1H NMR chemical shift (δOH) with the log(Vmax/Km) (r = 0.963). Acidity is mediated by the electron withdrawing capacity of the 4′-substituent outlined by the correlation of the C-2 13C NMR chemical shift (δC2) with the log(Vmax/Km) (r = 0.987). 2-[4-(Methylamino)phenyl]-6-hydroxybenzothiazole (2b) was radiolabeled with carbon-11 (11C-(2b)) and used in vivo for microPET scanning and tissue metabolite identification. High PET signal was paralleled with the presence of radiolabeled 11C-(2b)-6-O-sulfate and the SULT1E1 protein detected by western blot. Because this and other members of this family presenting specificity for SULT1E1 can be labeled with carbon-11 or fluorine-18, in vivo assays of SULT1E1 functional activity are now feasible in humans.
Keywords: molecular imaging probes, positron emission tomography, Pittsburgh Compound B, PIB, 18F-Flutemetamol
Cytosolic sulfotransferases (SULT enzymes; EC 2.8.2) are Phase II metabolic enzymes that mediate sulfation from a donor (3′-phosphoadenosine 5′-phosphosulfate, PAPS) to a variety of endogenous and exogenous substrates containing aryl alcohol, alkyl alcohol, hydroxylamino, or amino groups (Fig 1A) (1, 2). SULTs play an important role in the homeostasis of the body in two ways. First, by sulfoconjugating drugs and xenobiotic compounds for their removal via hepatobilliary and urinary systems; second, by sulfoconjugating and deactivating endogenous active substances [e.g., dopamine (DA), thyroid hormones, and estrogens], therefore, participating in their regulation both systemically and locally in different organs. In mammalian species, SULTs are found throughout the body in the gut, liver, kidneys, adrenal and thyroid glands, lungs, reproductive organs, breast tissue, brain, and blood (1, 3). More than 65 distinct SULT enzymes, spanning 33 isoforms, have been identified and characterized, 11 of which are human sulfotransferases (hSULTs) (2) found in three families: SULT1, SULT2, and SULT4. Although human SULTs sulfoconjugate multiple classes of hydroxylated molecules, they show increased affinity for specific endogenous substrates, for example, SULT1A3 for DA, SULT1B1 for thyroid hormones, SULT1E1 for estrogens, and SULT2A and SULT2B for hydroxysteroids.
Fig. 1.
(A) Interplay of estrogen sulfation and estrogen receptor (ER). Tissue levels of E2 are regulated by SULT1E1 and STS, thus, modulating E2 interaction with the estrogen receptor (ER). (B): Active site of human SULT1E1 with E2 (Green) bound in the active site. Amino acid residues that form the binding pocket are show in Beige (oxygen, Red; nitrogen, Blue; sulfur, Red; and free water molecures, Cyan) [Modified with permission from (25).© National Institute of Environmental Health Sciences, 2003].
Three hSULTs can metabolize estrogens: SULT1A1 (EC2.8.2.1), SULT1E1 (EC2.8.2.4), and SULT2A1 (EC2.8.2.2) (1), but only SULT1E1 possesses sufficient affinity for sulfoconjugation of 17β-estradiol (E2) [Km = 5 nM (4)] to allow its inactivation at a concentration range that competes with binding to estrogen receptors (ER) [KD = 1 nM (4)]. E2 is released from its inactive 3-O-sulfated (E2S) form by steroid sulfatase (STS) (Fig. 1A), an enzyme also widely distributed throughout the body (3).
E2 and other estrogens interact with target tissues (or immune cell types) primarily via estrogen receptors (ERα and ERβ). They have tissue dependent effects, for example, contributing to bone homeostasis (5), cardiovascular system protection (6), and regulation of inflammation in autoimmune, neurodegenerative, and other diseases (7). Therefore, estrogen sensitivity depends on both the concentration of estrogen receptors as well as SULT1E1/STS regulation of the free estrogen in these tissues (8, 9). This role of SULT1E1 (estrogen sulfotransferase or EST) as a “molecular switch” (8) is evident in estrogen sensitive cancers such as breast and endometrial cancer. In hormone responsive cancers, an increase in E2 (10), in part resulting from down regulation or loss of SULT1E1, potently stimulates tumor growth. Alternatively, presence of SULT1E1 in the absence of STS leads to lower recurrence rate and longer survival (10–13). Several high-impact neurodegenerative diseases are also being examined for interactions with E2 and other estrogen analogs based on the assumed interaction of the estrogens with the ER (14, 15), however, estrogen sulfating enzymes (e.g., SULT1E1) are rarely, if ever, invoked in these experiments.
In this work a family of SULT1E1 substrates were synthesized and investigated for sensitivity and specificity by examining their ability to act as substrates with four other common sulfotransferases: SULT1A1*1, SULT1A1*2, SULT1A3, and SULT2A1. It is of great interest to assess the in vivo status of SULT1E1 in estrogen target tissues and to determine changes in SULT1E1 expression as a result of inflammation, cancer, and neurodegeneration. Molecular imaging with PET using optimum SULT1E1 specific substrates radiolabeled with a short-lived positron emitting radioisotope such as carbon-11 (t1/2 = 20.4 min) or fluorine-18 (t1/2 = 109.8 min) would offer a sensitive tool for this assessment. Identification of patient populations in which SULT1E1 expression is elevated or absent would help define unique adjuvant cancer therapies symbiotic to estrogen therapies currently in use. Considering the antiinflammatory role of estrogen and the role of SULT1E1 in regulating estrogen levels (7), it can also be anticipated that identification of SULT1E1 in patients with neurodegenerative diseases would have significant diagnostic value and potential therapeutic connotations.
Results
Enzyme Assay Validation.
Control reactions were used to confirm the activity and identity of the commercially purchased enzymes. p-Nitrophenol (PNP) and SULT1A1*1 or SULT1A1*2, E2 and SULT1E1, dehydroepiandrosterone (DHEA) and SULT2A1, and DA and SULT1A3 gave Km values of 0.1 μM, 0.8 μM, 0.006 μM, 0.8 μM, and 2.1 μM, respectively, in agreement with previously published values (4, 16).
Fused heterocyclic substrates.
None of the fused heterocyclic substrates (1a–1k) assayed (Scheme 1 and Table S1) provided a preference for SULT1E1 over either SULT1A1 isoform with Km values in the μM range (5.91–177 μM). However, they showed significantly lower affinity for SULT1A3 (Km values higher than 150 μM) or no activity for SULT2A1. Heterocyclic moieties (1) accepted as substrates by SULT1E1 include: benzofurans (1b and 1i), benzothiophenes (1c and 1j), benzoxazole (1e), and benzothiazoles (1d, 1g, and 1k). Heterocycles with a proton on the ring nitrogen (e.g., indoles 1a and 1h and benzoimidazole 1f) are not tolerated well because only negligible sulfation activity was observed. All heterocycles 1 were efficient substrates for SULT1A1*1 and SULT1A1*2, Km values in the μM or sub-μM range (0.023–6.62 μM), yet when compared with βN (Km = 0.03 μM and 0.17 μM, resp.) all benzothiazoles, benzoxazole 1e and benzimidazole 1f showed one to two orders of magnitude higher Km values (0.42–2.75 μM and 2.08–19.3 μM, resp.). Methyl (1g) or phenyl substitution (2a) at carbon-2 of the 6-hydroxybenzothiazole ring lead to 7- and 60-fold increases in affinity (lower Km) for SULT1E1, resp., over the parent compound 1d. However, these compounds have parallel Km effects with both SULT1A1 isoforms detracting from any possible specificity trend.
2-Aryl 6-(and 5-)hydroxybenzothiazoles.
The effect of 4′-substitution was examined in enzyme assays with 2-phenyl-6-(and 5-)hydroxybenzothiazoles (Scheme 1, Table 1) against SULT1E1, SULT1A1*1, SULT1A1*2, SULT1A3, and SULT2A1 enzymes. With the 6-hydroxybenzothiazole derivatives (2a–2d, 2f–2l), there was a correlation between the polarity of the 4′-substitution on the 2-phenyl ring (Hammett σp) and SULT1E1 substrate sensitivity (Vmax/Km) (Fig. 2A) (17). This is due to the electron-donating or withdrawing effects of the 4′-substituents on the acidity of the 6-arylhydroxyl moiety. To corroborate this interpretation, the 1H NMR chemical shift of the hydroxyl group (δOH) is also tightly correlated with the log(Vmax/Km) kinetic parameter (Fig. 2C). Furthermore, there is an analogous correlation of the 13C NMR chemical shift of C-2 (δC2) in these benzothiazoles with the log(Vmax/Km) (Fig 2B). Exceptions to these correlations included compounds with large 4′-substituents, such as sulfone (2k) or acetamide (2g), which have lower Vmax/Km values (Table 1) than expected from their electronic character.
Scheme 1.
Table 1.
Michaelis–Menten kinetic parameters for 2-aryl substituted hydroxybenzothiazoles
| SULT1E1 |
SULT1A1*1 |
|||||
| Km | Vmax | Vmax/Km | Km | Vmax | Vmax/Km | |
| 2a | 0.99 ± 0.03 | 3.18 ± 0.87 | 3.22 ± 0.9 | 0.018 ± 0.006 | 4.97 ± 0.57 | 275 ± 97 |
| 2b | 1.42 ± 0.12 | 1.99 ± 0.07 | 1.40 ± 0.13 | * | * | * |
| 2c | 1.42 ± 0.32 | 2.18 ± 0.08 | 1.54 ± 0.35 | * | * | * |
| 2d | 2.11 ± 0.20 | 2.88 ± 0.14 | 1.36 ± 0.15 | * | * | * |
| 2e | 1.00 ± 0.06 | 1.98 ± 0.05 | 1.97 ± 0.08 | * | * | * |
| 2f | 0.90 ± 0.11 | 1.97 ± 0.08 | 2.19 ± 0.26 | * | * | * |
| 2g | 2.36 ± 0.14 | 2.04 ± 0.04 | 0.87 ± 0.05 | 0.025 ± 0.005 | 0.88 ± 0.05 | 36 ± 7 |
| 2h | 0.56 ± 0.09 | 4.08 ± 0.25 | 7.29 ± 1.25 | 0.029 ± 0.008 | 0.91 ± 0.09 | 31 ± 9 |
| 2i | 0.52 ± 0.05 | 2.95 ± 0.08 | 5.64 ± 0.56 | 0.01 ± 0.003 | 0.67 ± 0.05 | 67 ± 21 |
| 2j | 0.25 ± 0.02 | 4.33 ± 0.12 | 17.1 ± 1.4 | * | * | * |
| 2k | 1.32 ± 0.08 | 3.57 ± 0.06 | 2.69 ± 0.16 | * | * | * |
| 2l | 0.12 ± 0.02 | 3.33 ± 0.19 | 27.75 ± 4.89 | 0.058 ± 0.019 | 1.24 ± 0.09 | 21 ± 7 |
| 2m | 0.77 ± 0.08 | 1.74 ± 0.05 | 2.26 ± 0.24 | † | † | † |
| 2n | 0.43 ± 0.10 | 1.05 ± 0.08 | 2.44 ± 0.60 | † | † | † |
SULT1A1*2 assay with compound 2a yielded Km = 0.045 ± 0.01 and Vmax = 5.25 ± 0.55 (Vmax/Km 117 ± 23); sulfate formation was seen for compounds 2m and 2n, however, kinetic parameters could not be reliably measured. All 2-aryl substituted hydroxybenzothiazoles were assayed against SULT1A3 and SULT2A1 with no detectable activity. Compound 2b was additionally assayed against the SULT1B1 enzyme from 20 nM to 3 μM with no detectable activity. All experiments were performed in triplicate and all values are expressed as mean ± s.d. Units: Km as μM, Vmax as nmol/ min /mg, andVmax/Km as (nmol/ min /mg)/μM.
*No detectable activity.
†Activity was seen, however kinetic parameters could not be reliably measured.
Fig. 2.
(A) Linear correlation between log(Vmax/Km) and Hammett σp (r = 0.964) for 2-arylsubstituted-6-hydroxybenzothiazole derivatives. Compounds 2g (4′ = NHCOCH3) and 2k (SO2-CH3) are apparent outliers due to possible steric hindrance of the large 4′-substituents affecting binding with SULT1E1. (B) Linear correlation between δC2 and the log(Vmax/Km) (r = 0.987). Compounds 2g and 2k are apparent outliers due to steric hindrance. (C) 6-Hydroxy proton chemical shifts (δOH) and the log(Vmax/Km) (r = 0.963). Compounds 2g and 2k are outliers due to steric hindrance. All numbers represent the average of triplicate values.
All 4′-substituted 6-hydroxybenzothiazoles 2b-n, had no reactivity with SULT1A3, SULT2A1, or SULT1A1*2 and only acetamido (2g), fluoro (2h), iodo (2i), and nitro (2l) derivatives showed reactivity with SULT1A1*1, the most common SULT1A1 allozyme. The 5-hydroxybenzothiazoles 2m and 2n displayed similar Km for SULT1E1 as the 6-hydroxybenzothiazole counterparts (2b and 2c); but surprisingly they showed good affinities for SULT1A1*1 and SULT1A1*2. Benzothiazoles that were nonreactive with both SULT1A1 allozymes (2b–2f, 2j–2k) were tested from low nanomolar to low picomolar concentrations to confirm that these are not high-affinity substrates undergoing substrate inhibition, a hallmark of sulfotransferase enzymes (1, 18).
In vivo data.
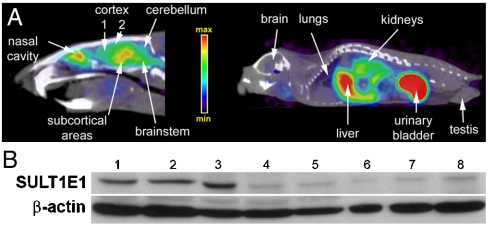
Representative microPET images of rat (brain) and mouse (whole body) obtained after IV administration with 11C-(2b) are shown in Fig. 3. There was very little variability in uptake and spatial distribution observed between animals within the species. SUVR values for rat brain were normalized to the cerebellum. The majority of the SULT1E1 activity was observed in the frontal cortical area (SUVR 1.77 ± 0.35), superior cortical areas (1.72 ± 0.28), subcortical areas (2.39 ± 0.45), and brain stem (1.58 ± 0.18). Areas of high 11C-(2b) retention also corresponded with areas showing high proportions of sulfated probe (11C-(2b)-6-O-sulfate) and confirmed presence of the SULT1E1 protein by western blot (Fig. 3 and Table 2). Peripheral organs in mice showed more variability in probe retention, although the spatial distribution was essentially identical between animals. SUVR values were normalized by a 3D isocontour region of interest covering the whole mouse. SUVR values (n = 3) included 2.2 ± 0.6 (liver), 8.5 ± 2.5 (gall bladder), 0.37 ± 0.13 (lung), 0.17 ± 0.05 (brain), and 1.8 ± 0.4 (kidney). Organs presenting prominently high expression of SULT1E1 were the liver and kidney, in agreement with published reports (1, 3). TLC autoradiographs showed a single polar metabolite in all tissue samples tested. This polar metabolite had the same Rf value as that of enzymatically produced 35S-(2b)-6-O-sulfate. Its desulfation could be achieved by treatment with the steroid sulfatase enzyme (STS) resulting in regeneration of the parent 11C-(2b). This polar metabolite remained essentially unaffected by the glucuronidase enzyme (GLU) consistent with a sulfate structure. Occasionally, rat plasma at late time points (30 min after IV injection) showed a second, more polar minor metabolite that is consistent with 11C-(2b)-6-O-glucuronidate. This second metabolite was not found in mouse plasma or any other specimen tested.
Fig. 3.
(Top) Representative microPET scans in rat brain (Top, Left) and whole-body mice (Top, Right) with 11C-(2b) co-registered with microCT images. The animals were scanned in a dynamic mode for 25 min. (Bottom): Western blot data obtained from the same tissues. Lane 1: 80 μg frontal cortical area (Cortex 1); Lane 2: 80 μg superior cortical areas (Cortex 2); Lane 3: 80 μg subcortical areas; Lane 4: 80 μg cerebellum; Lane 5: 80 μg brain stem; Lane 6: 50 μg testis; Lane 7 100 μg testis; and Lane 8: 150 μg testis
Table 2.
Quantitative microPET and ground tissue metabolite data.
| RATS (n = 3) | MICE (n = 7) | ||||
| Brain region | SUVR* | 11C-(2b)-6-O-sulfate (% of total activity) | Organ | SUVR† | 11C-(2b)-6-O-sulfate (% of total activity) |
| Cortex1‡ | 1.77 ± 0.35 | 57.4 ± 4.4 | Brain | 0.17 ± 0.05 | 3.1 ± 8.2 |
| Cortex2§ | 1.72 ± 0.28 | 65.6 ± 7.7 | Lungs | 0.37 ± 0.13 | 72.9 ± 18.9 |
| Subcortical | 2.39 ± 0.45 | 64.8 ± 1.8 | Liver | 2.2 ± 0.6 | 82.7 ± 5.1 |
| Cerebellum | 1 | 34.7 ± 4.3 | Kidney | 1.8 ± 0.4 | 82.2 ± 11.5 |
| Brainstem | 1.58 ± 0.18 | 35.5 ± 4.7 | Testis | ¶ | 58.9 ± 25.3 |
| Plasma | — | 66.3 ± 6.2∥ | Plasma | — | 78.5 ± 6.8 |
*Normalized to cerebellum.
†Normalized to whole body.
‡Frontal cortex.
§Superior cortical regions.
¶Testis could not be measured within the field of view.
∥A minor, more polar component (7 ± 10%) consistent with 11C-(2b)-6-O-glucuronidate was occasionally found only in rat plasma.
Discussion
Therapeutic agents such as raloxifene and 4-hydroxytamoxifen (Scheme 2) (19), as well as naturally occurring dietary flavonoids, are substrates or inhibitors of sulfotransferases (SULT1A1 and SULT1E1) to varying degrees (16, 20). Until now, no family of structurally related compounds has been reported to provide substrate preference for SULT1E1 as is seen with estrogens. 2-Aryl-6-hydroxybenzothiazoles 2b-2f, 2j-2k, several of which have been recently reported in connection with amyloid Aβ aggregate detection in the brain of Alzheimer’s disease patients (21, 22) or as possessing breast cancer cell cytotoxicity (23), are shown here to be highly specific substrates for SULT1E1. These compounds have afforded a unique opportunity to examine the effect of changing substituent electronic properties on the specificity of SULT1E1. This was previously examined with SULT1A1 (18) but has remained elusive for SULT1E1.
Scheme 2.
Earlier literature reports (24) identified βN (Scheme 2) as one of the smallest aromatic substrates for SULT1E1, although it shows substantial preference for SULT1A1 (Km values, Table S1). Substitutions on ring-A (the ring having the hydroxy group) serve to decrease catalytic efficiency or convert a substrate into a SULT1E1 inhibitor (18, 25). Introduction of an ethyl chain at position 6 (ring-B) on the βN substructure results in a 5-fold increase in affinity (24) pointing to ring-B modification and elongation as a favorable structural modification for increased SULT1E1 activity.
One structural element that is a substrate for both SULT1E1 and SULT1A1 is the 2-phenyl-6-hydroxybenzothiophene core component of raloxifene (Scheme 2), an estrogen receptor (ER) antagonist used as a chemotherapeutic agent (19). Therefore, 5- and 6-hydroxy substituted fused heterocyclic phenols 1a-1k were tested first to examine the extent to which the atomic constituents of ring-B would affect the affinity of the resulting fused heterocyclic phenols for SULT1E1 and other SULTs (1A1*1, 1A1*2, 1A3, 2A1) (Table S1). Comparison of Km(SULT1E1)/Km(SULT1A1) isoform ratios for all compounds 1 with affinity for SULT1E1 clearly shows that only benzothiazoles 1d, 1g, and 1k have Km that ratios are comparable or lower (146, 5.2, and 18.5 for 1A1*1; 29, 1.2, and 2.7 for 1A1*2, resp.) than that of βN (219 and 34.8 for 1A1*1 and *2, resp.); all other compounds 1 had Km(1E1)/Km(1A1) ratios above 95. Therefore, benzothiazoles were further explored for SULT1E1 substrate specificity and other compounds 1, that is, indoles, benzofurans, benzothiophenes, benzoxazoles, and benzimidazoles were excluded from further examination.
It is notable that all 4′-substituted 2-aryl 6-hydroxybenzothiazoles 2b-2l lacked substrate affinity for SULT1A1*2, SULT1A3, and SULT2A1. Additionally, excluding acetamido (2g), fluoro (2h), iodo (2i), and nitro derivatives (2l), they also presented a very limited affinity for the SULT1A1*1 allozyme. Thus, 2-aryl 6-hydroxybenzothiazoles with 4′-electron withdrawing substituents (2j, 2k) and all compounds with electron-donating substituents (2b-2d, 2f), in addition to the structurally related compound 2e, exhibited complete specificity for SULT1E1 (Table 1).
Enzyme sensitivity (based on Vmax/Km) of the analogs 2a-2d and 2f-2l with SULT1E1 was strongly correlated with the electronegativity of the 4′-substituent. Electron withdrawing groups (higher Hammett σp) provided better kinetics (higher Vmax/Km) with the 4′-nitro substitution (2l) being most efficient (Fig. 2A, r = 0.964). Both Km and Vmax were affected by the electronegativity of the 4′-substitution on the 2-aryl group.
Because δOH represents the extent of the dissociation of the hydroxyl group, or the pKa of the phenol, the transference of the sulfate group from the cofactor PAPS to the substrate is facilitated by electronic delocalization of the phenolic oxygen electron pair (Fig. 2C, r = 0.963) through C-2 (Fig. 2B, r = 0.987) mediated by the 4′-substituent. The correlation between the polarity of 4′-substituents (Hammett σp) and kinetic parameters for SULT1A1 has previously been shown for simple phenol derivatives (26); the rate limiting step was said to be the nucleophilic attack of the phenoxide ion onto the sulfate group of PAPS.
X-ray crystallographic analysis of hSULT1E1 co-crystallyzed with E2 and PAP reveals a hydrophobic pocket with E2’s aromatic ring inserted in the active site through a “gate” consisting of two phenylalanine residues (F80 and F141, Fig. 1B). These features control the orientation of the substrate and position the phenolic 3-OH group in the proximity of the catalytic histidine residue (H107) (25, 27). 4′-Substitutions to the 2-aryl-6-hydroxybenzothiazole appear to provide the necessary anchoring elements, in addition to hydrophobic interactions within the binding pocket, for a catalytically competent reaction. This adds credence to the possibility that the 4′-substituent may be interacting with amino acid residues in the outer regions of the substrate binding pocket. It is known from crystal structure determinations that differences in the outer region of the substrate binding pocket, both at the amino acid level and at secondary structural levels, are responsible for endogenous substrate specificity among different SULTs. For example, Asn87 is in a small, two-strand β-sheet that forms a hydrogen bond with the 17-OH group of E2 on SULT1E1, Glu146 and Glu89, which form hydrogen bonds with the amino group of DA on SULT1A3, or two loops that close over the SULT1A1 substrate binding pocket (25, 28).
The apparent lack of substrate affinity for SULT1A1 displayed by 6-hydroxybenzothiazoles 2b-f and2j,k as opposed to high substrate affinity for SULT1A1 displayed by compounds 2a, 2g-i, and 2l is somewhat surprising. Based on crystallographic analysis of SULT1A1 co-crystallized with PAP and PNP (29) or E2 (30), it is evident that the SULT1A1 substrate binding pocket can undergo significant plastic deformations to accommodate a variety of structurally different substrates. The outer loops, 146–154 and 84–90, of SULT1A1 that cover the substrate binding site with PNP would assume a more open conformation to accommodate the larger E2, albeit as a weak substrate. When bound to the binding pocket of SULT1A1, 2-aryl substituted 6-hydroxybenzothiazoles (2) may also have the 4′-substituents interacting with other aminoacid residues in the pocket as suggested with SULT1E1. Thus, based on their ability to fit optimally into the SULT1A1 binding site, these 6-hydroxybenzothiazoles (2) would either assume a catalytically competent binding orientation or be forced into a catalytically noncompetent binding orientation as seen in the case of E2. E2 binding to SULT1A1 in a catalytically noncompetent orientation results in a dead-end complex hypothesized as the cause of the substrate inhibition at higher concentrations (30).
The two exceptions to the Vmax/Km versus Hammet σp trend (Fig. 2) are compounds having sterically demanding groups, for example, acetamide (2g) and methylsulfone (2k) (Table 1). This suggests that the acidity of the phenolic group is not the sole determinant of substrate efficiency. The poor SULT1E1 substrate ability of 2g and 2k can be rationalized on the basis that these substituents produce significant steric hindrance to fit optimally in the binding pocket of SULT1E1 (27).
In contrast to 6-hydroxy derivatives, 5-hydroxybenzothiazoles are substrates for both SULT1A1 allozymes at very low substrate concentrations, effectively removing the SULT1E1 specificity observed with many of the 6-hydroxy counterparts (Table 1). This SULT1A1 interaction of 5-hydroxybenzothiazoles is remarkable considering that the 6-hydroxy counterparts are not SULT1A1 substrates. It is not possible, based on the data available, to determine the exact reasons for this disparity, but NMR data (Table S2) provides a clue by showing significantly different π-electronic configurations of 5-hydroxy compounds (e.g., δC6 and δC2) as compared with that of the 6-hydroxy counterparts indicating different electronic interactions with specific amino acid residues in the binding pocket of SULT1A1, transforming unproductive binding (as is the case with the 6-hydroxy counterparts) into a binding configuration that would position the 5-OH group into a catalytically competent orientation.
To establish a preliminary demonstration of the ability of 6-hydroxy benzothiazoles to act as in vivo SULT1E1 probes, microPET imaging was performed in rodents with 11C-(2b) (Fig. 3). The SULT1E1-mediated accumulation of substrates [e.g., 11C-(2b)] is based on the principle of metabolic trapping of 11C-(2b)-6-O-sulfate analogous to the use of 2-deoxy-2-[18F]fluoro-D-glucose (2-FDG), whose affinity for hexokinase permits tissue formation of its 6-phosphate (31). In agreement with this premise, biochemical analysis on all mice scanned has demonstrated significant levels of 11C-(2b)-6-O-sulfate in organs with high PET signal (Table 2). Radioactivity accumulation, as observed with microPET in mice, is also consistent with areas known to have SULT1E1 expression, especially in the kidney and liver (1) (Fig. 3). Similar observations were made with the regional distribution of brain activity upon intravenous administration of 11C-(2b) in rats, also demonstrating its capacity to be sulfated in the brain in vivo because no 11C-(2b)-6-O-sulfate of peripheral origin is expected to cross the blood brain barrier (32). Although the low affinity glucuronidation pathway (33) could also be involved, the high affinity sulfation pathway seems to be predominant. This is demonstrated by the presence of 11C-(2b)-6-O-sulfate as the main radiolabeled component in both brain and peripheral tissues.
In summary, this work presents a unique systematic attempt to design, synthesize, and characterize specific SULT1E1 substrates. The successful in vivo utilization of 11C-(2b) also provides direct evidence of the feasibility to determine local tissue measures of SULT1E1 activity in intact animals. Added significance of this finding is provided by the fact that ex vivo determination of SULT1E1 activity is highly problematic due to the post mortem inactivation of cytosolic sulfotransferases (32, 34).
The use of SULT1E1 specific PET molecular imaging probes has exciting implications for the biochemical characterization of major diseases where inflammation is known to play significant roles. Two specific SULT1E1 substrates presented in this work, 2-[4-(methylamino)phenyl]-6-hydroxybenzothiazole (2b, Pittsburgh Compound B or PIB) and 2-[3-fluoro-4-(methylamino)phenyl]-6-hydroxybenzothiazole (2e, 18F-flutemetamol or 18F-GE067) have been radiolabeled with carbon-11 and fluorine-18, resp., (35, 36) and used for brain imaging Aβ aggregates in Alzheimer’s disease, in vitro as well as in vivo (21, 22). Similarly, and based on the results of this work, it would be expected that other PET probes proposed for amyloid plaque detection in humans having the 6-hydroxybenzothiazole, for example, 2-[6-(methylamino)pyridin-3-yl]-6-hydroxybenzothiazole (37), and the 6-hydroxybenzoxazole skeletons (38) would also be good substrates for SULT1E1. The newly discovered SULT1E1 substrate activity for the family of compounds presented in this investigation may have important implications regarding the previous interpretation of their in vivo behavior as imaging agents considering that SULT1E1 is widely distributed in humans and its presence has been also demonstrated in the human brain (3). Further work to this end is warranted.
Materials and Methods
Materials.
βN, PNP, E2, DHEA, DA, dithiothreitol (DTT), 2-methyl-5-hydroxy-benzothiazole (1k) Mgcl2, and cytosolic extracts of sf-9 cells infected with a baculovirus containing SULT cDNAs (SULT1A1*2, SULT1A3, SULT1E1, SULT2A1) were purchased from Sigma. 5-Hydroxyindole (1h) was purchased from Alfa-Aesar and 6-hydroxy-indole (1a) from Acros. E. coli cell lysates containing SULT1A1*1 were purchased from Cypex. [35S]-3′-Phosphoadenosine 5′-phosphosulfate (PAPS) (1–3 Ci/mmol) was purchased from Perkin Elmer. Normal phase, aluminum backed TLC plates were purchased from Whatman.
Chemistry.
All synthesis methods are described in SI Text and outlined in Schemes S1–S3.
In Vitro Enzymatic Assays.
Assays performed using each SULT expressed in sf-9 cytosolic extracts (SULT1A1*2, SULT1A3, SULT1E1, and SULT2A1) or E. Coli extracts (SULT1A1*1) that were used as provided without additional purification. Initially, compounds were screened using a wide range of concentrations (10 nM–25 μM). Next, kinetic parameters were determined (19). reactions included the substrate initially dissolved in DMSO (final concentration 0.2%), 50 mM Tris-HCl, pH 7.4, 0.1% BSA, 7.5 mM DTT, and 1 μM [35S]-PAPS (39) in a 50 μL volume. SULT1E1 and SULT2A1 reactions were supplemented with 7 mM MgCl2; the DA control reaction with SULT1A3 included 1 mM pargyline to inhibit endogenous monoamine oxidase activity from the sf-9 cells. Control reactions contained only the solvent vehicle. Reactions were carried out at 37 °C for 15 min then were terminated with 50 μL of ice cold MeOH and placing at -80 °C. Twenty μL aliquots were spotted on silica gel TLC plates followed by development in 1-BuOH:HOAc:water (8∶1∶1 by volume) allowing separation of sulfated products (Rf = 0.66 ± 0.05) from unreacted PAPS (Rf = 0) (40). TLC plates were optimally exposed on phosphor imaging plates and read by using a Fuji BAS-5000 digital autoradiography plate reader. Spots were localized; the TLC plates scraped into liquid scintillation vials and counted using MP - Ecolite+ LS fluid and a Packard Tricarb 2300TR LSC for 5 min per vial. Reactions were monitored for linearity with time, enzyme concentration, and the amount of each substrate used to ensure the enzyme was the limiting factor.
Data Analysis.
Michaelis–Menten kinetics.
The sulfate transfer mechanism (18) is assumed to follow Michaelis–Menten kinetics:
 |
[1] |
The curve was fitted to Eq. 1, and Km and Vmax values were calculated by using GraphPad PRISM4 statistical analysis software. The electron withdrawing capacity of the 4′-substituent in the 2-aryl moiety of the benzothiazoles was correlated with Vmax/Km.
MicroPET imaging.
All animal experiments were performed under the strict guidelines of the University of California, Los Angeles Animal Research Committee. Male Sprague–Dawley rats (n = 3, age 1 yr, weight 360 g ± 40) and male Balb/c mice (n = 7, age 3 mo, weight 29 g ± 2) (Charles River) were anesthetized via 2% isoflurane (IsoFlo; Abbot Laboratories) in 100% oxygen in an induction box equipped with an inductive heating pad. Animals were weighed and transferred to an imaging chamber equipped with a nose cone for gas delivery and inductive heater (41, 42).
MicroPET scans were acquired with a Focus 220 microPET scanner (Concorde Microsystems, Inc.). Animals were injected with 1.5 mCi (for rats) or 200 μCi (for mice) of 11C-(2b) at the start of the scan that was performed for 25 min when the time activity curves (TAC) in all organs of interest ceased to show rapid changes (43) (Figs. S1B and S2B). Images were reconstructed using filtered back projection and a ramp filter. Images were corrected for attenuation by using microCT (42). At the termination of the experiment, 1 mL of whole blood was drawn and animals were euthanized via intra venous pentobarbital overdose. Blood samples were centrifuged for 5 min to separate blood plasma. The rat brain was divided in half sagitally and then into five regions (Fig. 3). Mouse tissues were harvested and divided into two equal pieces. Half of all tissues were snap frozen in liquid nitrogen and stored at -80 °C for western blot analysis; the other half was weighed and counted for radioactivity prior to ground tissue metabolite analysis.
Determination of radioactive probe sulfate content in tissue and plasma.
Harvested tissues were homogenized on ice in cold acetonitrile (1 mL/g) to precipitate proteins by using an automated homogenizer (Biospec Products inc.; Bartlesville, OK) for 60 sec. Samples were transferred to microcentrifuge tubes and centrifuged for 2 min at 10,000 g. Ten μL aliquots of the supernatant were carefully spotted onto reverse phase (C-18) TLC plates and developed with MeOH:H2O:HOAc (80∶15∶5) allowing separation of the primary metabolite [11C-(2b)-6-O-sulfate; Rf = 0.71 ± .05) and secondary metabolite Rf = 0.78 ± 0.03] from the precursor, 11C-(2b) (Rf = 0.47 ± .06) (Figs. S1A, S2A, and S3). TLC plates were exposed on phosphor imaging plates and read as above. Spots were localized and the percentage of metabolized probe was quantified by using Fuji Multi-Gauge 3.0 software.
Polar metabolite identification.
Harvested tissues were homogenized on ice in cold PBS (1 mL/g) by using an automated homogenizer then centrifuged as above. One hundred μL aliquots of supernatants were added to 1,000 units of steroid sulfatase (STS, EC3.1.6.2) or glucuronidase (GLU, EC3.2.1.31) and placed at 37 °C for 30 min. Next, samples were diluted 1∶2 with acetonitrile and 20 μL aliquots of each sample were spotted on reverse phase TLC plates and developed as indicated above.
Western blotting.
Tissues were homogenized 60 s on ice in 10 mM Tris buffer (pH 7.4) with 1 mM EDTA and Roche complete protease inhibitor cocktail (1 mL/g), centrifuged at 100,000 × g for 1 h, and then concentration was determined by Bradford assay (BioRad). Protein was resolved on precast Tris-HCl 12% gels (BioRad), transferred to PVDF membranes, and probed with anti-SULT1E1 mouse monoclonal antibodies (1∶100, Biovision) visualized by chemiluminesence with HRP-conjugated ani-mouse antibodies (Vector). Testis tissue was used as a positive control because of its high expression of SULT1E1 (3, 8).
Statistical methods.
All results are shown as mean ± s.d.
Supplementary Material
Acknowledgments.
We thank L. Petersen (National Institute of Health) for a previously published figure and G. Timbol, A. Smid, and E. Basarah for technical assistance. We acknowledge financial support from the National Institutes of Health (Grant P01AG025831) and the Korean Research Foundation (G. Keum, MOEHRDl KRF-2006-611-C00004). J.R.B. gratefully acknowledges the support of the Elizabeth and Thomas Plott Chair Endowment in Gerontology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914904107/DCSupplemental.
References
- 1.Falany C. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11(4):206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard RL. Human cytosolic sulfotransferases. In: Pacifici GM, Coughtrie MWH, editors. Boca Raton, FL: Taylor and Francis Group, LLC; 2005. pp. 1–8. [Google Scholar]
- 3.Miki Y, et al. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87(12):5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Varmalova O, Vargas FM, Falany CN, Leyh TS. Sulfuryl transfer: The catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273(18):10888–10892. doi: 10.1074/jbc.273.18.10888. [DOI] [PubMed] [Google Scholar]
- 5.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 6.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–95. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 8.Song WC. Biochemistry and reproductive biology of estrogen sulfotransferase. Ann NY Acad Sci. 2001;948:43–50. doi: 10.1111/j.1749-6632.2001.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 9.Hobkirk R. Steroid sulfotransferase and steroid sulfate sulfatases: Characteristics and biological roles. Can J Biochem Cell Biol. 1985;63:1127–1144. doi: 10.1139/o85-141. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualini JR, Cortes-Prieto J, Chetrite G, Talbi M, Ruiz A. Concentrations of estrone, estradiol and their sulfates, and evaluation of sulfatase and aromatase activities in patients with breast fibroadenoma. Int J Cancer. 1997;70(6):639–643. doi: 10.1002/(sici)1097-0215(19970317)70:6<639::aid-ijc2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res. 2003;63(11):2762–2770. [PubMed] [Google Scholar]
- 12.Utsunomiya H, et al. Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin Cancer Res. 2004;10:5850–5856. doi: 10.1158/1078-0432.CCR-04-0040. [DOI] [PubMed] [Google Scholar]
- 13.Sato R, et al. Steroid sulfatase and estrogen sulfotransferase in colon carcinoma: Regulators of intratumoral estrogen concentrations and potent prognostic sactors. Cancer Res. 2009;69:914–922. doi: 10.1158/0008-5472.CAN-08-0906. [DOI] [PubMed] [Google Scholar]
- 14.Sicotte NL, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 15.Baum LW. Sex, hormones, and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama T, et al. Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug Metab Pharmacok. 2002;17(3):221–228. doi: 10.2133/dmpk.17.221. [DOI] [PubMed] [Google Scholar]
- 17.Gordon AJ, Ford RA. New York, NY: John Wiley & Sons; The chemist’s companion: A handbook of practical data, techniques, and references; pp. 45–47. [Google Scholar]
- 18.Duffel MW, Jakoby WB. On the mechanism of aryl sulfotransferase. J Biol Chem. 1981;256:11123–11127. [PubMed] [Google Scholar]
- 19.Falany JL, Pilloff DE, Leyh TS, Falany CN. Sulfation of raloxifene and 4-Hydroxytamoxifen by human cytosolic sulfotransferases. Drug Metab Dispos. 2006;34:361–368. doi: 10.1124/dmd.105.006551. [DOI] [PubMed] [Google Scholar]
- 20.Ung D, Nagar S. Variable sulfation of dietary polyphenols by recombinant human sulfotransferase (SULT) 1A1 genetic variants and SULT1E1. Drug Metab Dispos. 2007;35:740–746. doi: 10.1124/dmd.106.013987. [DOI] [PubMed] [Google Scholar]
- 21.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 22.Nelissen N, et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. J Nucl Med. 2009;50:1251–1259. doi: 10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- 23.Shi DF, et al. Antitumor benzothiazoles. 3. synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in Vitro and in Vivo. J Med Chem. 1996;39:3375–3384. doi: 10.1021/jm9600959. [DOI] [PubMed] [Google Scholar]
- 24.Falany CN, Ström P, Swedmark S. Sulphation of o-desmethylnaproxen and related compounds by human cytosolic sulfotransferases. Br J Clin Pharmacol. 2005;60:632–640. doi: 10.1111/j.1365-2125.2005.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shevtsov S, Petrotchenko EV, Pedersen LC, Negishi M. Crystallographic analysis of a hydroxylated polychlorinated biphenyl (OH-PCB) bound to the catalytic estrogen binding site of human estrogen sulfotransferase. Environ Health Perspect. 2003;111:884–888. doi: 10.1289/ehp.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall AD, McPhie P, Jakoby WB. Redox control of aryl sulfotransferase specificity. Arch Biochem Biophys. 2000;382:95–104. doi: 10.1006/abbi.2000.2020. [DOI] [PubMed] [Google Scholar]
- 27.Petrotchenko EV, Doerflein ME, Kakuta Y, Pedersen LC, Negishi M. Substrate gating confers steroid specificity to estrogen sulfotransferase. J Biol Chem. 1999;274:30019–30022. doi: 10.1074/jbc.274.42.30019. [DOI] [PubMed] [Google Scholar]
- 28.Yoshinari K, Petrotchenko EV, Pedersen LC, Negishi M. Crystal structure based Studies of cytosolic sulfotransferases. J Biochem Mol Toxic. 2001;15:67–75. doi: 10.1002/jbt.1. [DOI] [PubMed] [Google Scholar]
- 29.Gamage NU, et al. The structure of a human carcinogen-converting enzyme, SULT1A1 structural and kinetic implications of substrate inhibition. J Biol Chem. 2003;278:7655–7662. doi: 10.1074/jbc.M207246200. [DOI] [PubMed] [Google Scholar]
- 30.Gamage NU, et al. The structure of human SULT1A1 crystallyzed with estradiol. An insight into active site plasticity and substrate inhibition with multi-ring substrates. J Biol Chem. 2005;280:41482–41486. doi: 10.1074/jbc.M508289200. [DOI] [PubMed] [Google Scholar]
- 31.Barrio JR. PET: Molecular Imaging and Its Biological Applications. In: Phelps ME, editor. New York, NY: Springer-Verlag New York, Inc.; p. 279. [Google Scholar]
- 32.Mathis CA, et al. Species-dependent metabolism of the amyloid imaging agent [C-11]PIB. J Nucl Med. 2004;45(Suppl):114P. [Google Scholar]
- 33.Raftoganis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000;27:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 34.Young WF, Jr, Okazaki H, Laws ER, Jr, Weinshilboum RM. Human brain phenol sulfotransferase: Biochemical properties and regional localization. J Neurochem. 1984;43:706–715. doi: 10.1111/j.1471-4159.1984.tb12790.x. [DOI] [PubMed] [Google Scholar]
- 35.Mathis CA, et al. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 36.Solbach C, Uebele M, Reischl G, Machulla HJ. Efficient radiosynthesis of carbon-11 labeled uncharged thioflavin T derivatives using [11C] methyl triflate for beta-amyloid imaging in Alzheimer’s disease with PET. Appl Radiat Isotopes. 2005;62:591–595. doi: 10.1016/j.apradiso.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Johnson AE, et al. AZD2184: A radioligand for sensitive detection of beta-amyloid deposits. J Neurochem. 2009;108:1177–1186. doi: 10.1111/j.1471-4159.2008.05861.x. [DOI] [PubMed] [Google Scholar]
- 38.Malmstroem J, et al. Preparation of benzoxazole derivatives for imaging amyloid deposits in patients. PCT Int Appl. 2007. WO2007149030A1.
- 39.Otake Y, Nolan AL, Walle UK, Walle T. Quercetin and resveratrol potently reduce estrogen sulfotransferase activity in normal human mammary epithelial cells. J Steroid Biochem Mol Biol. 2000;73:265–270. doi: 10.1016/s0960-0760(00)00073-x. [DOI] [PubMed] [Google Scholar]
- 40.Tremaine LM, Diamond GL, Quebbemann AJ. In Vivo Quantification of renal glucuronide and sulfate conjugation of 1-naphthol and p-nitrophenol in the rat. Biochem Pharmacol. 1984;33:419–427. doi: 10.1016/0006-2952(84)90235-1. [DOI] [PubMed] [Google Scholar]
- 41.Fueger BJ, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 42.Chow PL, Stout DB, Komisopoulou E, Chatziioannou AF. A method of image registration for small animal, multi-modality imaging. Phys Med Biol. 2006;51:379–390. doi: 10.1088/0031-9155/51/2/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopresti BJ, et al. Simplified quantification of Pittsburgh compound β-amyloid imaging PET studies: A comparative analysis. J Nucl Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.