Abstract
The isomerization of glucose into fructose is a large-scale reaction for the production of high-fructose corn syrup (HFCS; reaction performed by enzyme catalysts) and recently is being considered as an intermediate step in the possible route of biomass to fuels and chemicals. Here, it is shown that a large-pore zeolite that contains tin (Sn-Beta) is able to isomerize glucose to fructose in aqueous media with high activity and selectivity. Specifically, a 10% (wt/wt) glucose solution containing a catalytic amount of Sn-Beta (1∶50 Sn:glucose molar ratio) gives product yields of approximately 46% (wt/wt) glucose, 31% (wt/wt) fructose, and 9% (wt/wt) mannose after 30 min and 12 min of reaction at 383 K and 413 K, respectively. This reactivity is achieved also when a 45 wt% glucose solution is used. The properties of the large-pore zeolite greatly influence the reaction behavior because the reaction does not proceed with a medium-pore zeolite, and the isomerization activity is considerably lower when the metal centers are incorporated in ordered mesoporous silica (MCM-41). The Sn-Beta catalyst can be used for multiple cycles, and the reaction stops when the solid is removed, clearly indicating that the catalysis is occurring heterogeneously. Most importantly, the Sn-Beta catalyst is able to perform the isomerization reaction in highly acidic, aqueous environments with equivalent activity and product distribution as in media without added acid. This enables Sn-Beta to couple isomerizations with other acid-catalyzed reactions, including hydrolysis/isomerization or isomerization/dehydration reaction sequences [starch to fructose and glucose to 5-hydroxymethylfurfural (HMF) demonstrated here].
Keywords: glucose isomerization, heterogeneous catalysis
The isomerization of sugars is a key reaction used in various relevant industrial processes. For instance, the conversion of glucose into fructose for the production of high-fructose corn syrups (HFCS) has become the largest immobilized biocatalytic process worldwide. HFCS have reached a global production exceeding 8 × 106 tons/year (in the United States alone, per capita consumption of HFCS reached 37.8 lbs/year in 2008) (1–3). In addition, the recent drive to use biomass as an alternative to petroleum for the production of fuels and chemical intermediates has triggered a renewed interest in carbohydrate chemistry. In this respect, glucose isomerization is a crucial step in the efficient production of valuable chemical intermediates, such as 5-hydroxymethylfurfural (HMF) and levulinic acid, from biomass; however, a heterogeneous isomerization catalyst (biological or inorganic) that can easily integrate glucose isomerization with the transformation of fructose into these intermediates is lacking (4, 5). Here, we present highly active heterogeneous inorganic catalysts for the isomerization of glucose that resemble the performance of enzymatic catalysts by generating remarkably high-fructose yields at glucose conversions near the reaction equilibrium. Furthermore, unlike enzymatic catalysts, these materials maintain high activity over multiple cycles, can be easily regenerated with a mild calcination process, and work over a wide range of temperatures. Importantly, these inorganic catalysts can operate effectively in a highly acidic environment, making them attractive candidates for sequential or one-pot acid-catalyzed reaction sequences, including those required in various important biomass conversion schemes.
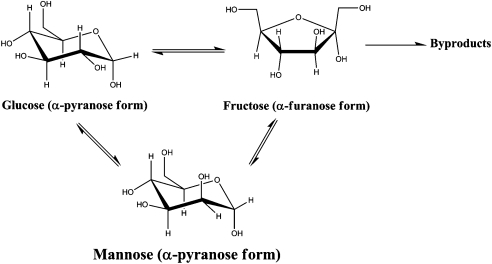
The isomerization of glucose to fructose can be performed under mild conditions using either biological or chemical catalysts (see Scheme 1). This reaction is slightly endothermic (ΔH = 3 kJ/mol) and reversible (Keq ∼ 1 at 298 K), which means that the maximum attainable degree of conversion of glucose to fructose is governed by the thermodynamic equilibrium between both sugars at the reaction temperature (Fig. S1) (6). The preferred industrial isomerization method involves the use of an immobilized enzyme (xylose isomerase) at 333 K that generates an equilibrium mixture of 42% (wt/wt) fructose, 50% (wt/wt) glucose, and 8% (wt/wt) other saccharides (1). Although fructose yields are high, this enzymatic process has various drawbacks that include: (i) the need for various prereaction purification processes to remove impurities from the feed that strongly inhibit enzyme activity, e.g., Ca2+ ions present from the previous starch liquefaction/saccharification step must be removed to levels < 1 ppm, (ii) the use of buffered solutions to maintain an optimal pH between 7.0 and 8.0 (Na2CO3) and to activate the enzyme (MgSO4) that requires postreaction ion-exchange procedures, (iii) an optimal operating temperature of 333 K to maximize both product yield and enzyme lifetime that precludes faster reaction rates that could be attained at more elevated temperatures, and (iv) higher operating costs resulting from the periodic replacement of the catalyst bed due to the irreversible decay in activity suffered by the enzyme over time. As compared to biological catalysis, chemical catalysis employing inexpensive inorganic materials to isomerize sugars could offer attractive advantages, including operation over a wider range of temperatures and longer lifetimes, faster reaction rates that could give shorter reactor residence times, and a higher resistance to impurities. It has been shown that glucose undergoes isomerization in the presence of base catalysts at temperatures ranging from 298 to 423 K; unfortunately, monosaccharides are unstable in alkaline media and readily degrade into numerous byproducts at temperatures above 313 K (2, 7–10). Thus, base catalysts typically generate fructose yields < 10% (high-fructose selectivities [> 90%] are only afforded at low glucose conversions [< 10%]), thereby making them unlikely candidates for use in large-scale glucose processing.
Scheme 1.
Schematic representation of the glucose isomerization reaction pathways catalyzed by either biological or chemical catalysts.
The catalysts presented in this paper feature tin (Sn) or titanium (Ti) metal centers that can act as solid acids in aqueous media when incorporated in the framework of a siliceous microporous material. We hypothesized that large-pore zeolites containing these types of acid centers would be active in the isomerization of aldoses, such as glucose, while preventing sugar degradation reactions usually encountered in base-catalyzed processes. We based this hypothesis on evidence suggesting the existence of strong interactions between these types of metal centers and hydroxyl/carbonyl moieties that are present in aldoses. Indeed, recent reports by Corma et al. have shown that Sn-Beta zeolites are highly active in the Meerwein–Ponndorf–Verley (MPV) reduction of carbonyl compounds, whereby a hydride transfer occurs from the hydroxyl group of an alcohol to the carbonyl group of a ketone (11–13). Similarly, Hayashi and Sasaki (14) and Christensen et al. (15) have shown that Sn-containing materials catalyze the conversion of trioses, e.g., glyceraldehyde and dihydroxyacetone, into alkyl lactates by way of a Lewis-acid-mediated isomerization/esterification reaction sequence in the presence of alcohols.
Results and Discussion
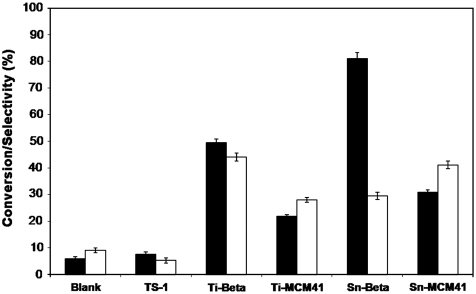
Initially, a series of silica materials containing either Sn or Ti metal centers were screened for their activity in the glucose isomerization reaction. Specifically, Sn and Ti metal centers were incorporated into the framework of a large-pore zeolite (Beta), Ti was incorporated into a medium-pore zeolite (TS-1), and Sn and Ti were incorporated into an ordered mesoporous silica (MCM-41). Sn-Beta and Ti-Beta showed the highest glucose isomerization activity, reaching glucose conversions above 50% in 90 min at 413 K (Fig. 1). In contrast, Sn-MCM41 and Ti-MCM41 only revealed moderate activity, and TS-1 was virtually inactive under the same reaction conditions (Fig. 1). Reactivity differences between TS-1 and Ti-Beta indicate that glucose molecules are able to diffuse into the pores of the Beta zeolite (ca. 0.8 nm pore diameter), but not into the pores of TS-1 (0.5–0.6 nm pore diameter). Note that glucose conversion values beyond the theoretical thermodynamic equilibrium are due to fructose degradation reactions observed at this high temperature after long reaction times, requiring further glucose conversion to reestablish the thermodynamic equilibrium.
Fig. 1.
Glucose isomerization reaction catalyzed by various metal-containing solids (Glucose conversion, Black; Fructose selectivity, White). Reaction conditions: 10% (wt/wt) glucose in water, 413 K, 90 min, and 1∶50 metal:glucose molar ratio. Conversion is defined as moles of glucose consumed divided by initial glucose moles. Selectivity is defined as moles of fructose divided by moles of glucose consumed.
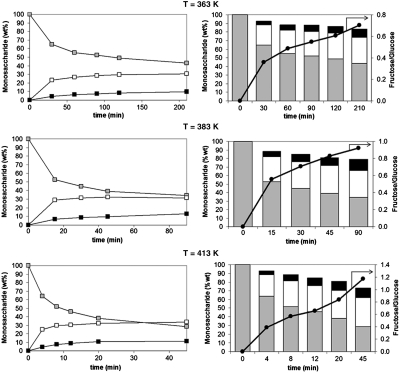
Further reaction studies with the two most active materials showed that the Sn-Beta catalyst isomerizes glucose with superior performance when compared to Ti-Beta. Specifically, a 10% (wt/wt) glucose solution containing a catalytic amount of Sn-Beta (1∶50 Sn:glucose molar ratio) generated product yields of approximately 46% (wt/wt) glucose, 31% (w/w) fructose, and 9% (wt/wt) mannose after 30 min and 12 min at 383 K and 413 K, respectively. At the 383 K, the Ti-Beta catalyst achieved much lower glucose conversions, even when the reaction was allowed to proceed for longer periods of time (Table 1, entries 4 and 7). The onset of auto-catalyzed degradation reactions decreased total saccharide yields with increased reaction times (Fig. 2). Varying the reaction temperature did not appear to have an impact on the total amount of sugar lost at a given glucose conversion value, thus suggesting that the degradation reaction pathway has an apparent activation barrier similar to that of the isomerization reaction. Importantly, the Sn-Beta catalyst can be used with more concentrated glucose solutions like those employed in large-scale conversion. For example, a product distribution of 46% (wt/wt) glucose, 29% (wt/wt) fructose, and 8% (wt/wt) mannose was obtained after reacting a 45 wt% glucose solution containing a catalytic amount of Sn-Beta (1∶225 Sn:glucose molar ratio) for 60 min at 383 K (Table 1, entries 4 and 10). This result approximates those obtained in the industrial enzymatic process, and to our knowledge, represents the highest fructose yield obtained from a highly concentrated glucose solution using an inorganic catalyst (1, 2, 8, 10).
Table 1.
Results for the isomerization of glucose in water
| Yield (wt/wt% ) | |||||||
| Entry | Catalyst | Temperature (K) | Time (min) | Glucose | Fructose | Mannose | Total saccharides |
| 1 | None | 383 | 90 | 97 | 0 | 0 | 97 |
| 2 | None | 413 | 90 | 95 | 1 | 0 | 95 |
| 3 | HCl (pH = 2) | 383 | 90 | 98 | 0 | 0 | 98 |
| 4 | Sn-Beta | 383 | 30 | 45 | 32 | 9 | 86 |
| 5 | Sn-Beta | 413 | 12 | 46 | 30 | 9 | 85 |
| 6 | Sn-Beta/HCl (pH = 2) | 383 | 30 | 44 | 33 | 9 | 86 |
| 7 | Ti-Beta | 383 | 90 | 74 | 14 | 5† | 93 |
| 8 | SnO2 | 383 | 60 | 96 | 0 | 0 | 96 |
| 9 | SnCl4·5H2O | 383 | 60 | 90 | 0 | 0 | 90 |
| 10* | Sn-Beta | 383 | 60 | 46 | 29 | 8 | 83 |
Reactions were performed with a 10 wt% glucose solution, using the corresponding amount of catalyst to maintain a 1∶50 metal:glucose molar ratio.
*Reaction of a 45 wt% glucose solution, using a 225∶1 glucose:Sn molar ratio.
†An unidentified sugar, rather than mannose, was obtained with Ti-Beta. The reported 5 wt/wt% yield was calculated using the response factor associated with hexoses.
Fig. 2.
Glucose isomerization reaction profiles and product distributions as a function of time at 363 K, 383 K, and 413 K for Glucose (Gray), Fructose (White), and Mannose (Black) using Sn-Beta as a catalyst. Reaction conditions: 10% (wt/wt) glucose in water and 1∶50 Sn:glucose molar ratio. Error bars for the reaction profile plots on the left panel are not visible because they are smaller than the data-point icons.
To test the stability of the Sn-Beta catalyst, two types of studies were performed. In the first study, the reusability of the catalyst was tested by performing four consecutive isomerization cycles at 383 K for 30 min each. After each cycle, the catalyst was filtered and washed with water before adding a fresh glucose solution. As seen from the data listed in Table 2, after three reaction cycles, the catalyst maintained its initial activity and product distribution. After the third cycle, the catalyst was calcined in air at 813 K before performing one last cycle. The cycle 4 results show that the catalyst again maintained its original activity and product distribution thereby confirming that it is able to withstand a typical zeolite regeneration process. A second test was designed to probe for the presence of homogeneous catalysis due metal species leached into the solution. Specifically, an isomerization cycle was initiated with the Sn-Beta catalyst at 383 K for 12 min. The catalyst was then removed by filtration while the solution was still hot in order to avoid any possible readsorption of leached species during cool-down, and the filtrate was allowed to react at 383 K for a further 30 min. The reaction results show that in the presence of catalyst, glucose isomerization proceeded as expected (product yields of 57% (wt/wt) glucose, 27% (wt/wt) fructose, and 6% (wt/wt) mannose). However, the reaction did not continue once the catalyst was removed, indicating that no homogenous catalysis occurred by leached metal ions (Table 2 entries 5a and 5b). The results from these two tests indicate that Sn-Beta is heterogeneously catalyzing the isomerization reaction and can be used for multiple reaction cycles.
Table 2.
Results for catalyst stability studies
| Yield (wt/wt%) | |||||
| Entry | Catalyst | Time (min) | Glucose | Fructose | Mannose |
| Study 1: Catalyst reusability | |||||
| 1 | Sn-Beta Cycle 1 | 30 | 41 | 32 | 9 |
| 2 | Sn-Beta Cycle 2 | 30 | 45 | 30 | 8 |
| 3 | Sn-Beta Cycle 3 | 30 | 47 | 29 | 7 |
| 4 | Sn-Beta Cycle 4 (calcination) | 30 | 46 | 30 | 8 |
| Study 2: Testing for leached species | |||||
| 5a | Sn-Beta | 12 | 57 | 27 | 6 |
| 5b | None (catalyst removed by filtration) | 30 | 57 | 27 | 6 |
Reactions were performed at 383 K using a 10 wt% glucose solution with a 1∶50 Sn:glucose molar ratio (except for entry 5b). For Study 1, after each cycle, the catalyst was washed with water and fresh glucose solution was subsequently added to start a new cycle. In Cycle 4, the catalyst recovered from Cycle 3 was calcined in air at 813 K for 3 h using a temperature ramp rate of 2 K/ min. For Study 2, the reaction with Sn-Beta was allowed to proceed for 12 min (entry 5a). Then, the catalyst was filtered hot from the solution, and the filtrate was reacted for an additional 30 min (entry 5b).
Remarkably, Sn-Beta is able to perform the isomerization reaction in a highly acidic environment. No differences in activity or product distribution were observed for reactions using Sn-Beta in an acidic 10% (wt/wt) glucose solution (pH = 2, HCl), when compared to the reaction performed without HCl (Table 1, entries 4 and 6). Indeed, the ability to isomerize sugars in an acidic solution is essential to overcome some of the main bottlenecks encountered during base catalysis, some of which include the neutralization of active sites by acidic byproducts and the low stability of sugars in alkaline environments. Furthermore, working at low pH values opens up exciting opportunities to couple upstream and downstream acid-catalyzed reactions, e.g., hydrolysis or dehydration, with the glucose isomerization reaction without the need to use additional unit operations. For example, in the production of HFCS, the starch hydrolysis step (typically performed in a separate set of reactors using either acid catalysts or a combination of enzymes) required prior to the isomerization step could be combined with the glucose isomerization step (1). Similarly, in the production of HMF, the base-catalyzed isomerization step required to convert glucose into fructose before performing the acid-catalyzed dehydration step could be combined in a single reactor to obtain higher product yields more efficiently. Proof-of-concept experiments using Sn-Beta in an acidic environment show promising results for hydrolysis/isomerization and isomerization/dehydration reaction sequences. Specifically, hydrolysis of a 10% (wt/wt) starch solution using HCl (pH = 1) at 413 K for 90 min generated a 87% (wt/wt) glucose and 13% (wt/wt) starch solution that was then isomerized by adding a catalytic amount of Sn-Beta (1∶50 Sn:glucose molar ratio) to the acidic solution and heating for an additional 12 min at 413 K to obtain a product distribution consisting of 13% (wt/wt) starch, 39% (wt/wt) glucose, 23% (wt/wt) fructose, and 7% (wt/wt) mannose (Table 3, entries 1a and 1b). Also, a 10% (wt/wt) glucose solution reacted in the presence of HCl (pH = 1) and Sn-Beta together for 120 min at 413 K generated an HMF yield of 11% (wt/wt) in addition to 18% (wt/wt) fructose and 2% (wt/wt) mannose (Table 3, entry 4). These data can be compared to the dehydration results obtained from reacting fructose in aqueous HCl (24% (wt/wt) HMF yield, 85% conversion; Table 3 entry 3) and from dehydrating glucose under similar aqueous HCl reaction conditions (< 1% (wt/wt) HMF yield, 9% conversion; Table 3 entry 2). Thus, while further optimizations of these reaction sequences are necessary to find the best suited pH values to perform the acid-catalyzed conversions while minimizing possible silica dissolution that could be encountered at pH values less than 2 (currently underway in our group) (16), the results show that Sn-Beta is an attractive candidate for one-pot reaction sequences requiring catalytic couplings of isomerization and other acid-catalyzed reactions.
Table 3.
Results for Sn-catalyzed glucose reactions in water under acidic conditions at 413 K
| Yield (wt/wt%) | ||||||||
| Entry | Feed solution | Catalyst | Time (min) | Starch | Glucose | Fructose | Mannose | HMF |
| 1a | 10 wt% Starch | HCl (pH = 1) | 90 | 13 | 87 | 0 | 0 | 0 |
| 1b | Postreaction mixture of entry 1a | Sn-Beta+HCl (pH = 1) | 12 | 13 | 39 | 23 | 7 | 0 |
| 2 | 10 wt% Glucose | HCl (pH = 1) | 120 | - | 91 | 0 | 0 | 1 |
| 3 | 10 wt% Fructose | HCl (pH = 1) | 120 | - | 0 | 15 | 0 | 24 |
| 4 | 10 wt% Glucose | Sn-Beta+HCl (pH = 1) | 120 | - | 28 | 18 | 2 | 11 |
For entries 1b and 4, a 1∶50 Sn:glucose molar ratio was used.
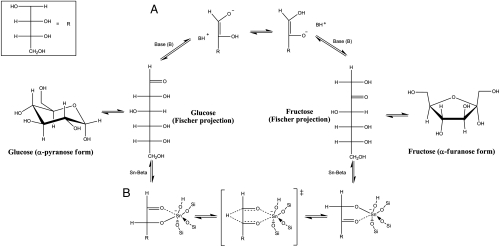
The active sites for the isomerization reaction in Sn-Beta are Sn atoms incorporated into the framework of the zeolite. Neither SnCl4·5H2O nor SnO2 showed isomerization activity (Table 1, entries 8 and 9), and UV data for Sn-Beta did not reveal absorption bands corresponding to extraframework Sn (Fig. S2). However, the mechanism by which the formal transfer of a hydrogen atom from the C-2 to C-1 positions of the α-hydroxy aldehyde to form the corresponding α-hydroxy ketone remains unproven. Studies using deuterated solvents have shown that enzymes and bases perform the aldose/ketose transformation through a mechanism involving a cis 1,2-enediol intermediate, whereby the bonding electron pair at C-2 moves through the carbon skeleton to C-1 (Scheme 2A) (17, 18). Kinetic studies of isomerization reactions report that certain acids and metals are able to transfer the hydrogen directly through a hydride shift between C-2 and C-1 (Scheme 2B) (19). Corma et al. showed that Sn incorporated in the framework of zeolite Beta is a good catalyst for the MPV reaction between alcohols and ketones that requires adequate Lewis acidity in the catalyst to polarize the carbonyl group in the ketone while also coordinating both the alcohol and the ketone to facilitate a hydride shift between them (12, 13). It is therefore plausible that Sn in zeolite Beta performs the isomerization reaction following an intramolecular hydride shift mechanism between the carbonyl-containing C-1 and the hydroxyl-bearing C-2 of glucose by way of a 5-member complex (Scheme 2B). Important factors in the Sn-Beta isomerization of glucose in water include the role of the solvent, the confinement and polarity effects within the micropores of the zeolite, and the impact of the coordination state of the Sn atom in the framework as either partially hydrolyzed framework Sn centers (-Si - O - )3Sn - OH or fully framework coordinated Sn atoms Sn(-Si - O - )4; these factors are currently the subject of great interest and additional studies within our group.
Scheme 2.
Schematic representation of the glucose isomerization mechanisms by way of (A) base-catalyzed and (B) metal-catalyzed (hypothesized) reaction pathways.
Materials and Methods
Synthesis of Sn-Beta.
Sn-Beta zeolite was prepared as follows: 7.57 g of tetraethylammonium hydroxide solution [Sigma-Aldrich, 35% (wt/wt) in water] was diluted with 15 g of water. Next, 7.011 g of tetraethylorthosilicate [Sigma-Aldrich, 98% (wt/wt)] and 0.121 g of tin (IV) chloride pentahydrate [Sigma-Aldrich, 98% (wt/wt)] were added to the solution. The mixture was stirred until complete hydrolysis of the tetraethylorthosilicate and then allowed to reach the desired water ratio by complete evaporation of ethanol and some water. Finally, 0.690 g of HF solution [Mallinckrodt, 52% (wt/wt)] was added, resulting in a thick gel. The gel composition was SiO2/0.01 SnCl4/0.55 TEAOH/0.54 HF/7.52 H2O. This gel was transferred to a Teflon-lined stainless steel autoclave and heated at 413 K for 40 d. The solids were recovered by filtration, extensively washed with water, and dried at 373 K overnight. The solid was calcined at 853 K for 6 h to remove the organic content located in the crystalline material.
X-ray diffraction confirmed that the solid material has the Beta zeolite topology (Fig. S3). The UV-vis diffuse reflectance spectrum of the calcined sample shows the presence of a unique band at ∼200–250 nm that can be assigned to Sn tetrahedrally coordinated into the zeolite framework (Fig. S2). Scanning electron microscopy (SEM) (Fig. S4) shows the Sn-Beta crystals are several microns in size and energy dispersive x-ray spectroscopy (EDS) measurements for the Sn-Beta sample show an atomic ratio Si : Sn of 96∶1 (Table S1).
Detailed descriptions of procedures followed to synthesize the rest of the materials cited in the manuscript can be found in SI Appendix.
Catalytic Test.
Isomerization experiments were carried out in 10 ml thick-walled glass reactors (VWR) heated in a temperature-controlled oil bath placed on top of a digital stirring hotplate (Fisher Scientific). In a typical experiment, 1.5 g of an aqueous solution composed of 10% (wt/wt) glucose and the corresponding catalyst amount to achieve a 1∶50 metal:glucose molar ratio were added to the reactor and sealed (elemental analyses for all the samples are provided in Table S1). The reactor was placed in the oil bath and removed at specific times. The reaction was stopped by cooling the reactor in an ice bath, and small aliquots were taken for analysis. Sample analyses were performed by means of high performance liquid chromatography (HPLC) using a Agilent 1200 system (Agilent Technologies Corp.) equipped with PDA UV (320 nm) and evaporative light-scattering (ELS) detectors. Glucose, fructose, and mannose concentrations were monitored with a Biorad Aminex HPX87C (300 × 7.8) column (Phenomenex), using ultrapure water (pH = 7) as the mobile phase at a flow rate of 0.60 ml/ min and a column temperature of 353 K.
Supplementary Material
Acknowledgments.
This work was financially supported as part of the Catalysis Center for Energy Innovation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC00010004. M.M. acknowledges the Fundación Ramón Areces Postdoctoral Research Fellowship Program for financial support.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1002358107/DCSupplemental.
References
- 1.Bhosale S, Rao M, Deshpande V. Molecular and industrial aspects of glucose isomerase. Microbiol Rev. 1996;60(2):280–300. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lecomte J, Finiels A, Moreau C. Kinetic study of the isomerization of glucose into fructose in the presence of anion-modified hydrotalcites. Starch-Starke. 2002;54(2):75–79. [Google Scholar]
- 3.USDA. Sugar and Sweeteners: Market Outlook. 2010. in http://www.ers.usda.gov/Briefing/Sugar/marketoutlook.htm.
- 4.Zhao H, Holladay JE, Brown H, Zhang ZC. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science. 2007;316(5831):1597–1600. doi: 10.1126/science.1141199. [DOI] [PubMed] [Google Scholar]
- 5.Roman-Leshkov Y, Barrett CJ, Liu ZY, Dumesic JA. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature. 2007;447(7147):982–985. doi: 10.1038/nature05923. [DOI] [PubMed] [Google Scholar]
- 6.Tewari Y. Thermodynamics of industrially-important, enzyme-catalyzed reactions. Appl Biochem Biotech. 1990;23(3):187–203. doi: 10.1007/BF02942054. [DOI] [PubMed] [Google Scholar]
- 7.Yang BY, Montgomery R. Alkaline degradation of glucose: Effect of initial concentration of reactants. Carbohyd Res. 1996;280(1):27–45. [Google Scholar]
- 8.De Wit G, Kieboom APG, van Bekkum H. Enolisation and isomerisation of monosaccharides in aqueous, alkaline solution. Carbohyd Res. 1979;74(1):157–175. [Google Scholar]
- 9.Girisuta B, Janssen LPBM, Heeres HJ. Green chemical: A kinetic study on the conversion of glucose to levulinic acid. Chem Eng Res Des. 2006;84(5):339–349. [Google Scholar]
- 10.Kooyman C, Vellenga K, De Wilt HGJ. The isomerization of d-glucose into d-fructose in aqueous alkaline solutions. Carbohyd Res. 1977;54(1):33–44. [Google Scholar]
- 11.Corma A, Garcia H. Lewis acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem Rev. 2003;103(11):4307–4366. doi: 10.1021/cr030680z. [DOI] [PubMed] [Google Scholar]
- 12.Corma A, Nemeth LT, Renz M, Valencia S. Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer–Villiger oxidations. Nature. 2001;412(6845):423–425. doi: 10.1038/35086546. [DOI] [PubMed] [Google Scholar]
- 13.Corma A, Domine ME, Nemeth L, Valencia S. Al-free Sn-Beta zeolite as a catalyst for the selective reduction of carbonyl compounds (Meerwein–Ponndorf–Verley reaction) J Am Chem Soc. 2002;124(13):3194–3195. doi: 10.1021/ja012297m. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi Y, Sasaki Y. Tin-catalyzed conversion of trioses to alkyl lactates in alcohol solution. Chem Commun. 2005;21:2716–2718. doi: 10.1039/b501964h. [DOI] [PubMed] [Google Scholar]
- 15.Taarning E, et al. Zeolite-Catalyzed Isomerization of Triose Sugars. ChemSusChem. 2009;2(7):625–627. doi: 10.1002/cssc.200900099. [DOI] [PubMed] [Google Scholar]
- 16.Iler RK. The Chemistry of Silica. 1st Ed. New York: John Wiley & Sons Inc; 1979. p. 42. [Google Scholar]
- 17.Nagorski RW, Richard JP. Mechanistic imperatives for aldose–ketose isomerization in water: Specific, general base- and metal ion-catalyzed isomerization of glyceraldehyde with proton and hydride transfer. J Am Chem Soc. 2001;123(5):794–802. doi: 10.1021/ja003433a. [DOI] [PubMed] [Google Scholar]
- 18.Rose IA. Chemistry of proton abstraction by glycolytic enzymes (aldolase, isomerases and pyruvate kinase) Philos T R Soc B. 1981;293(1063):131–143. doi: 10.1098/rstb.1981.0067. [DOI] [PubMed] [Google Scholar]
- 19.Collyer CA, Blow DM. Observations of reaction intermediates and the mechanism of aldose-ketose interconversion by D-xylose isomerase. Proc Natl Acad Sci USA. 1990;87(4):1362–1366. doi: 10.1073/pnas.87.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.