Abstract
Purpose
Obesity, physical inactivity and altered estrogen metabolism play an integrated role contributing to the disease risk profiles of postmenopausal women. These same risk factors also affect modulation of the autonomic nervous system (ANS).
Methods
We examined 332 postmenopausal, overweight, previously sedentary women (Mean ± SD; Age, 57.6 ± 6.3 y; Wt, 84.3 ± 11.9 kg; BMI, 31.7 ± 3.7 kg/m2) participating in a 6-month, moderate intensity, aerobic exercise training intervention to determine the relationship between heart rate variability (HRV) derived autonomic function and fasting insulin. We analyzed quartiles of change in time and frequency domain indices of ANS activity and changes in insulin for between and within group differences using ANCOVA and Tukey post-hoc tests adjusted for age, ethnicity, randomization group, change in fitness, and change in weight.
Results
We observed at baseline that insulin was positively correlated with body anthropometry (body weight, r2 = 0.34; BMI, r2 = 0.39; waist circumference, r2 = 0.29; all, P < 0.001), and inversely associated with rMSSD (r2= −0.12) and SDNN (r2 = −0.18; all, P < 0.01). After the intervention, changes in rMSSD (r2 = −0.21, P<0.002) and SDNN r2 −0.19, P<0.0001) were inversely correlated to insulin change. Further ANCOVA analysis revealed that rMSSD and SDNN were both significant (P<0.0001); however, only rMSSD exhibited a step-wise pattern of improvement when quartiles of rMSSD were compared to corresponding insulin reductions: Q1 (referent group, 8.41 ± 3.2 uIU/ml), Q2, (−3.30 ± −3.2 uIU/ml), Q3 (−5.66 ± −3.2 uIU/ml; P < 0.02), and Q4 (−9.60 ± −3.2 uIU/ml; P<0.006).
Conclusion
Our study shows that changes in autonomic function are associated with changes in insulin and that exercise training may influence this relationship in postmenopausal women.
Keywords: heart rate variability, autonomic balance, exercise training
Introduction
Menopause represents a transitory period of physiologic changes, whereby women move from a low to a higher metabolic disease risk profile. Central to this transition is the role of estrogen metabolism and its influence on the health of the autonomic nervous system (ANS). Estrogen metabolism is associated with disease protection in women prior to menopause (1). Research indicates that premenopausal women have higher ANS function than postmenopausal women and that estrogen influences the ANS both centrally and peripherally by suppressing sympathetic tone and elevating parasympathetic tone (2). Part of the higher prevalence of risk associated with postmenopausal status is the observation that continued aging, as well as lifestyle behaviors such as diet, physical inactivity, and obesity affect the ANS, cardiovascular disease mortality, insulin resistance and diabetes (3–5). Taken collectively, fluctuations in endogenous hormones, coupled with lifestyle behaviors, play a major role in the age-related ANS function of postmenopausal women (3). Several studies demonstrate a strong relationship between reduced vagal tone, insulin and glucose concentration, and the prevalence of type II diabetes (6)(7, 8).
The examination of mechanisms surrounding the ANS and insulin is difficult and involves elaborate feedback and feed forward mechanisms. Several studies have shown that the ANS is responsive to alterations in insulin via the use of glucose clamp techniques (9, 10). However, one could argue that this is not “physiologic,” per se, as the insulin is given intravenously rather than produced via the pancreas. In essence, the ANS is modulated via feedback mechanisms. We propose that pancreatic function is more likely regulated via feed forward mechanisms and that alterations in the ANS/insulin relationship may be a function of aging and menopause status.
Accordingly, the balance between parasympathetic and sympathetic nervous system activity influences pancreatic function where noradrenaline released from sympathetic nerves and adrenaline secreted from the adrenal glands inhibits pancreatic B cells and excite A cells (11). Whereas B cells are crowded with β2 receptors, A cells have a high concentration of α2 receptors. When noradrenaline is released from sympathetic nerves, it excites A cells by acting on α1-receptors. Thus, it is conceivable that an ANS imbalance resulting in reduced sympathetic nervous system activity as premenopausal women have more robust ANS function than postmenopausal women (3, 12). It can be further hypothesized that lifestyle behaviors, such as exercise, may promote a beneficial effect on pancreatic function insulin secretion via improvements in the autonomic balance. Clinically, ANS balance can be measured non-invasively via HRV, where reduced HRV is associated with poor survival in individuals with CHD, and higher risk for cardiovascular disease, type 2diabetes and insulin resistance (3–5, 7, 13).
We recently demonstrated that exercise training positively increases maximal exercise capacity in previously sedentary postmenopausal women exercising at 50% (4 kilocalories per kilogram of body weight per week, KKW), 100% (8 KKW), and 150% (12 KKW) of the NIH Consensus Panel physical activity recommendation on cardiorespiratory fitness in a dose-dependent manner (14). In a recent ancillary report from the DREW cohort, we demonstrated a dose dependent increase in all parasympathetically derived time and frequency domain measurements (14). Carnethon et al have also shown that an improvement in autonomic in the lifestyle modification arm of the Diabetes Prevention Program, inclusive of physical activity, was inversely associated with the development of diabetes independent of weight change (8). Due to the close relationship between menopause, physical inactivity, obesity and ANS function, we hypothesize that changes in parasympathetic activity may favorably affect fasting insulin concentration independently of fitness and obesity.
Materials and Methods
Study Design
The Dose-Response to Exercise in postmenopausal Women trial (DREW) is a randomized, single-center, dose-response exercise training trial in sedentary, overweight or obese postmenopausal women with elevated blood pressure (1). The study was originally reviewed annually by The Cooper Institute and subsequently approved by the Pennington Biomedical Research Centers IRB for the continued analysis and publication of pertinent research findings. Prior to participation, all participants signed a written informed consent document outlining the procedures involved in the DREW study. The primary outcomes for DREW study included peak aerobic capacity and resting blood pressure. We recently published a complete description of the DREW design, methods, and primary outcomes (15, 16).
Participants
In brief, DREW study participants were sedentary (exercising < than 20 minutes; <3 d/wk; < 8000 steps/d assessed over the course of 1 week), overweight or obese (BMI; 25.0 to 43.0 kg/m2), and had a systolic blood pressure of 120.0 to 159.9 mm Hg. We excluded women who had a history of stroke, myocardial infarction, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. Following baseline testing participants were randomized into the respective treatment groups. After an initial run-in period, we randomized 464 postmenopausal women (45–75 y) to 1 of the 3 exercise training groups or a non-exercise control for a 6-month intervention period. Cardiovascular exercise training consisted of having women expend 4, 8, or 12 kcal/kg/week KKW. The non-exercise training group was instructed to maintain their current level of activity during the trial period. Exercising women participated in 3–4 supervised exercise sessions per week on a semi-recumbent cycle ergometer and treadmill at a heart rate associated with 50% of each woman's peak VO2 (15). Mean adherence to the prescribed exercise training was 92% with no significant differences in adherence across treatment groups (16).
Indices of Insulin Function
During the baseline and the follow-up visit, we collected standard fasting (12 hr) blood chemistries inclusive of insulin and glucose. As a tertiary analysis, we also calculated the homeostatic model assessment for insulin resistance (HOMA-IR) model as a surrogate marker of insulin resistance (17).
Heart Rate Variability
Participants reported for HRV testing in the mornings between 6:30 and 11:00 following a 12-hour fast, having abstained from consuming caffeine-containing products and alcoholic beverages for 12 hours and heavy exercise for 48 hours. During HRV testing, participants rested quietly in the supine position for 25 minutes in a semi-dark room with a temperature between 22–23°C. Participants controlled their respiration rate by breathing with a metronome at a fixed rate of 12 breaths per minute (0.2 Hz). Beat-to-beat measurements of R-R intervals were collected during the entire period. The R-R interval measurements were conducted at the same time (± 1 hour) of the day for each participant during baseline and post-test assessment periods.
We used an IBM-compatible PC equipped with a program for signal processing and HRV analysis (Polar Precision Performance SW 3.02, Polar Electro OY, Finland). Two-channel ECG signal was detected by a Polar Heart Rate Monitor and transmitted online to a PC through a Polar chest strap and Polar Advantage Interface receiver. The QRS timing accuracy of Polar Advantage Interface is fixed to 1 ms.
We used a computer program to label each QRS complex, and the resulting signal was passed through a filter that eliminated ectopic beats and artifacts. Additionally, an R-R interval tachogram was displayed for manual editing and areas of ectopy or artifacts were identified and removed. Each edited R-R interval was replaced with an average value of the surrounding beats. Segments containing >15% of edited R-R intervals were interpreted as premature beats and were excluded from data analysis (18). These segments accounted for <2% of edited 10-minute intervals in every subject. HRV was quantified from the last 5 minutes of R-R interval recording and this portion of our recordings was used to calculate time and frequency domain indices of HRV.
For the purposes of this report, we focused our primary analysis on the time domain indices rMSSD and SDNN to determine parasympathetic and mixed signaling activity, respectively. We choose to focus on rMSSD because rMSSD represents the most commonly used measure of interval differences and is a robust measure of parasympathetic modulation during short (i.e., 5-min) recordings. As HRV data is often presented for both time and frequency domain indices, we have included as a secondary analysis the frequency domain. We calculated time domain indices directly from the R-R intervals of each assessment period. To examine parasympathetic modulation, we calculated the square root of the mean of the sum of the squares of differences between adjacent R-R intervals (rMSSD). The use of rMSSD is considered to be a stable measure of parasympathetic modulations in heart rate (19). We also calculated the standard deviation of all R-R intervals (SDNN), which reflects all the cyclic components responsible for variability in the period of recording and reflective of both sympathetic and parasympathetic modulation, although parasympathetic tone predominates in the resting state. We obtained the frequency domain measurements by analyzing the power spectrum quantified in three frequency bands. We reported the normalized high frequency power (HFPLn; 0.15 – 0.40 Hz), low frequency power (LFPLn; 0.04 – 0.15 Hz), very low frequency power (VLFPLn; 0.0033 – 0.04 Hz), and total frequency power (PTPLn; 0.00 – 0.40 Hz). While HF is mediated by variations in the parasympathetic activity, the mechanisms underlying the LF are still inconclusive (20–22). Changes in HRV were calculated as the difference between HRV prior the exercise training intervention and HRV post trial.
Statistical Analysis
Descriptive baseline characteristics were tabulated as mean (SD) or as percentages (Table 1). Spearman correlation coefficients were calculated to examine pair-wise associations between variables for both baseline values and change values (Table 2). We adjusted all our outcomes among the randomization groups for select specified covariates including baseline HRV, race, and use of antidepressant medication. Between-group differences at baseline and follow-up were examined using Chi-square tests. To examine the dose-response association between changes in HRV and changes in insulin, we examined quartiles of change in rMSSD and SDNN as the independent variables with changes in insulin concentration as the dependent variable. Between group differences were tested using ANCOVA with adjustment for age, ethnicity, randomization group, change in fitness and change in weight. Within group differences were tested using Tukey studentized range adjustment. Results are presented as adjusted least-squares means. All analyses were performed using SAS version 9.1 (Cary, NC) and all reported P values are two-sided (P<0.05).
TABLE 1.
Baseline Characteristics of DREW participants.
| Total Cohort (N=332) |
Control (n=78) |
4 KKW (n=110) |
8 KKW (n=68) |
12 KKW (n=76) |
||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, mean (SD) y | 57.6 (6.3) | 57.5 (5.8) | 58.2 (6.3) | 57.6 (6.6) | 56.6 (6.4) | |
| Ethnicity (%) | ||||||
| Caucasian | 68 | 69 | 67 | 65 | 72 | |
| African American | 26 | 23 | 27 | 28 | 26 | |
| Hispanic or Other | 5 | 8 | 5 | 7 | 1 | |
| Anthropometry | ||||||
| Weight (kg) | 84.3 (11.9) | 85.2 (12.1) | 83.3 (11.2) | 85.6 (13.0) | 83.6 (11.5) | |
| BMI (kg/m2) | 31.7 (3.7) | 32.0 (3.8) | 31.4 (3.7) | 32.3 (3.9) | 31.3 (3.6) | |
| Waist circumference (cm) | 100.99 (11.4) | 102.44 (11.6) | 100.19 (10.8) | 102.87 (12.0) | 98.94 (11.1) | |
| Cardiovascular | ||||||
| VO2 (L/min) | 1.31 (0.2) | 1.33 (0.3) | 1.29 (0.2) | 1.29 (0.2) | 1.32 (0.2) | |
| Blood pressure (mmHg) | ||||||
| Systolic | 139.3 (13.1) | 142.5 (12.5) | 138.3 (12.9) | 139.0 (13.8) | 137.9 (12.8) | |
| Diastolic | 80.8 (8.8) | 80.8 (7.9) | 80.2 (9.4) | 80.7 (8.9) | 81.6 (8.7) | |
| Hematology* | ||||||
| LDL-C (mg/dL) | 117.56 (26.2) | 118.16 (26.5) | 114.35 (27.6) | 117.31 (26.3) | 121.84 (24.5) | |
| HDL-C (mg/dL) | 57.44 (14.2) | 57.14 (14.5) | 58.30 (14.2) | 57.15 (15.1) | 57.78 (13.2) | |
| Glucose | 95.24 (9.2) | 96.65 (10.7) | 93.76 (8.9) | 95.35 (8.6) | 95.84 (8.4) | |
| Triglycerides | 130.78 (66.9) | 135.74 (73.5) | 129.98 (60.1) | 128.93 (61.7) | 128.49 (74.3) | |
| Insulin | 74.5 (43.7) | 76.35 (52.1) | 72.01 (38.50) | 77.78 (43.6) | 73.29 (42.2) | |
| HOMA-IR | 2.98 (1.9) | 3.13 (2.4) | 2.84 (1.7) | 3.10 (1.8) | 2.93 (1.80) | |
| Time Domain Indices of Autonomic Function | ||||||
| rMSSD (ms) | 23.12 (11.9) | 23.09 (11.1) | 24.01 (12.6) | 22.71 (11.0) | 22.17 (12.7) | |
| SDNN (ms) | 32.88 (11.8) | 32.61 (10.9) | 34.15 (11.8) | 33.57 (13.7) | 30.78 (11.0) | |
| Frequency Domain Indices of Autonomic Function | ||||||
| LFPln (ms2) | 5.28 (0.9) | 5.30 (0.9) | 5.41 (0.9) | 5.27 (1.0) | 5.09 (0.9) | |
| VLFPln (ms2) | 4.60 (0.8) | 4.63 (0.8) | 4.63 (0.8) | 4.60 (1.0) | 4.53 (0.8) | |
| HFPln (ms2) | 5.14 (1.1) | 5.22 (0.9) | 5.19 (1.1) | 5.02 (1.3) | 5.06 (1.0) | |
| TotalPln (ms2) | 6.26 (0.9) | 6.28 (0.8) | 6.35 (0.9) | 6.26 (0.9) | 6.11 (0.8) | |
| Medication history (%) | ||||||
| Antihypertensive | 30 | 24 | 25 | 36 | 33 | |
| Thyroid | 16 | 18 | 10 | 16 | 20 | |
| Antidepressant | 21 | 19 | 21 | 21 | 22 | |
| Hyperlipidemic | 16 | 17 | 20 | 15 | 12 | |
| HRT | 48 | 50 | 47 | 46 | 49 | |
| Smoking (%) | ||||||
| Never | 57 | 54 | 61 | 60 | 53 | |
| Past | 37 | 41 | 35 | 35 | 39 | |
| Current | 5 | 5 | 5 | 4 | 8 | |
Abbreviations: LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; HRT, hormone replacement therapy
To convert LDL-C and HDL-C to mmol/L multiply by 0.0259
Table 2.
Relationships between baseline participant characteristics and fasting glucose, insulin, HOMA-IR and triglycerides.
| Glucose | Insulin | HOMA IR | Triglycerides | ||
|---|---|---|---|---|---|
| Age | r2 | 0.10 f | −0.07 | −0.05 | −0.001 |
| Anthropometry | |||||
| Weight | r2 | 0.13 | 0.34 a | 0.34 a | 0.01 |
| BMI | r2 | 0.14 c | 0.39 a | 0.39 a | 0.09 |
| Waist cm | r2 | 0.24 a | 0.29 a | 0.31 a | 0.12 e |
| Blood Chemistry | |||||
| LDL | r2 | 0.08 | 0.05 | 0.06 | 0.07 |
| HDL | r2 | −0.21 a | −0.33 a | −0.34 a | −0.41 a |
| CRP | r2 | 0.03 | 0.14 c | 0.13 c | 0.18 a |
| Cardiorespiratory Capacity and Hemodynamic Function | |||||
| VO2max | r2 | 0.07 | 0.14 c | 0.15 c | −0.02 |
| SBP | r2 | 0.02 | −0.06 | −0.06 | 0.06 |
| DBP | r2 | −0.12 e | 0.04 | 0.02 | −0.01 |
| Time Domain Indices of HRV | |||||
| rMSSD | r2 | −0.07 | −0.12 e | −0.12 e | −0.17 b |
| SDNN | r2 | −0.08 | −0.18 c | −0.13 c | −0.17 b |
| Frequency Domain Indices of HRV | |||||
| LFPLn | r2 | −0.09 | −0.09 | −0.1 | −0.14b |
| VLFPLn | r2 | −0.04 | −0.14 | −0.13c | −0.16c |
| HFPLn | r2 | −0.05 | −0.12 e | −0.12 e | −0.14b |
| TotalPLn | r2 | −0.11e | −0.13d | −0.13d | −0.17b |
Statistical notations: (a) P<0.0001; (b) P<0.002, (c) P<0.01; (d) P<0.02; (e) P<0.03; (f) P<0.06
RESULTS
Overall, we randomized 464 participants into the DREW study. Of these individuals, we obtained fasting insulin measurements and usable HRV data on 332 individuals (Mean ± SD; Age, 57.6 ± 6.3 y; Wt, 84.3 ± 11.9 kg; BMI, 31.7 ± 3.7 kg/m2). As previously reported, the DREW study was a well-controlled trial, completed under laboratory conditions, using the extensive monitoring of exercise energy expenditure, heart rate, and steps taken outside of the structured exercise prescription. Similar to previous reports, we observed a dose response improvement in VO2max that was unaccompanied by a change in body weight following 6-months of exercise training (16, 23). Also similar to our first report, we observed significant time effect for resting HR (P<0.0001), but not for treatment. The baseline characteristics of our current DREW analysis are presented in Table 1 and a CONSORT diagram details the study inclusion and exclusions criteria in Figure 1.
Figure 1.
CONSORT overview of DREW heart rate variability and insulin analysis.
At baseline, we observed that insulin was significantly and positively related to body weight, BMI, and waist circumference (all, P < 0.001; Table 2). We also observed that insulin concentration was significantly and negatively associated with time domain indices of parasympathetic modulation (rMSSD; P < 0.03) and mixed ANS signaling (SDNN; P<0.01, Table 2), as well as the log normalized frequency domain indices for HF (P<0.002) and Total power (P<0.03). After 6-m of exercise, changes in body weight were positively associated with changes in insulin (r2 = 0.18, P < 0.002). Further, both the time domain indices of rMSSD (r2 = −0.21; P < 0.002) and SDNN (r2 = −0.21; P<0.0001), as well as the frequency domain indices HF (r2 = −0.21; P<0.002) and total power (r2 = −0.21; P<0.002) were negatively associated with changes in insulin concentration (Table 3).
Table 3.
Relationships between change in baseline characteristics and change in fasting glucose, insulin, HOMA IR and triglycerides following 6-months of exercise intervention
| Glucose | Insulin | HOMA IR | Weight | VO2max | ||
|---|---|---|---|---|---|---|
| Anthropometry and Blood Chemistry | ||||||
| Weight | r2 | 0.10 f | 0.18 b | 0.19 b | 0.03 | |
| Waist cm | r2 | 0.18 b | 0.07 | 0.11 c | 0.32 b | −0.02 |
| Triglycerides | r2 | 0.06 | 0.09 | 0.10 f | 0.10 | 0.04 |
| CRP | r2 | 0.12 e | −0.01 | 0.02 | 0.11 c | −0.06 |
| Cardiorespiratory Capacity | ||||||
| VO2max | r2 | −0.02 | −0.01 | −0.01 | 0.03 | |
| Time Domain Indices of HRV | ||||||
| rMSSD | r2 | −0.14 c | −0.21 b | −0.21 b | −0.09 | 0.01 |
| SDNN | r2 | −0.08 | −0.21 a | −0.19 a | −0.13 d | 0.02 |
| Frequency Domain Indices of HRV | ||||||
| LFPLn | r2 | −0.02 | −0.1 | −0.08 | −0.08 | 0.03 |
| VLFPLn | r2 | −0.01 | −0.08 | −0.08 | −0.15c | 0.04 |
| HFPLn | r2 | −0.17b | −0.21b | −0.21b | −0.08 | 0.01 |
| TotalPLn | r2 | −0.11e | −0.17b | −0.17b | −0.09 | 0.02 |
Statistical notations: (a) P<0.0001; (b) P<0.002, (c) P<0.01; (d) P<0.02; (e) P<0.03; (f) P<0.06
When we examined quartiles of change for rMSSD (Fig. 2a) and SDNN (Fig. 2b), we observed a significant main ANCOVA statistical effect for the corresponding reduction in insulin (P < 0.0001). For rMSSD, our post-hoc comparisons showed that the corresponding reduction in insulin (vs. Q1 as referent) occurred in a dose-dependent, step-wise fashion and was significant for Q3 (P<0.02) and Q4 (P< 0.006). Although this pattern for rMSSD changes with insulin remained consistent for HOMA-IR (Table 4), we did not observe significant changes in glucose. Post-hoc comparisons of SDNN data showed that the corresponding reduction in insulin (vs. Q1, referent) was significant for Q3 (P < 0.05).
Figure 2.
Data [Mean (SD)] represent main ANCOVA statistical effects (all, P< 0.0001) among DREW participants adjusted for age, ethnicity, randomization group, antidepressant medication, change in fitness and change in weight for quartiles of change in time domain indices of HRV vs. corresponding changes in insulin concentration. Panel A represents post-hoc comparisons of change in insulin relative to quartiles of change in parasympathetic tone denoted by rMSSD, where Q3 (P < 0.02a) and Q4 (P < 0.006b) are significantly different from Q1 (i.e., referent). Panel B represents post-hoc comparisons of change in insulin relative to quartiles of change in mixed signaling tone denoted by SDNN, where Q3 (P < 0.02c) and is significantly different from Q1 (i.e., referent).
Table 4.
Data represent quartiles of change in rMSSD, SDNN, and corresponding changes in glucose and Homa-IR.
| rMSSD | ||||
|---|---|---|---|---|
| Q1: < −2.4 | Q2: −2.4 – < 2.5 | Q3: 2.5 – < 8.0 | Q4: > 8.1 | |
| Glucose (mg/dL) | −0.27 ± 0.9 | 0.73 ± 0.9 | −1.89 ± 0.9 | −2.39 ± 0.9 |
| Homa-IR a | 0.33 ± 0.1 | −0.12 ± 0.1 | −0.28 ± 0.1 b | −0.44 ± 0.1 b |
| SDNN | ||||
| Q1: < −2.9 | Q2: −2.9 – < 2.2 | Q3: 2.2 – < 8.0 | Q4: > 8.0 | |
| Glucose (mg/dL) | −0.99 ± 0.9 | −0.11 ± 0.9 | −1.6 ± 0.9 | −1.05 ± 0.9 |
| Homa-IR | 0.20 ± 0.1 | 0.11 ± 0.1 | −0.63 ± 0.1 | −0.19 ± 0.1 |
| High Frequency | ||||
| Q1: < −56.3 |
Q2: −56.3 – <39.8 |
Q3: 39.9 – 165.8 |
Q4: > 165.8 | |
| Glucose (mg/dL) a | −0.28 ± 0.9 | 0.72 ±0.9 | −2.48 ± 0.9 b | −1.77 ± 0.9 b |
| Homa-IR a | 0.12 ±0.1 | 0.08 ±0.1 | −0.42 ±0.1 b | −0.29 ± 0.1 b |
| Total Power | ||||
| Q1: < −2.9 | Q2: −2.9 – < 2.2 | Q3: 2.2 – < 8.0 | Q4: > 8.0 | |
| Glucose (mg/dL) | −1.04 ± 0.9 | −0.40 ± 0.9 | −0.75 ± 0.9 | −1.61 ± 0.9 |
| Homa-IR a | 0.16 ± 0.1 | 0.04 ± 0.1 | −0.49 ± 0.1 b | −0.24 ± 0.1 |
Data (mean ± SE)
Significant main (ANCOVA) statistical effect adjusted for age, ethnicity, randomization group, change in fitness and change in weight
Significant Tukey post-hoc statistical effect compared to the referent group (Q1; p<0.05)
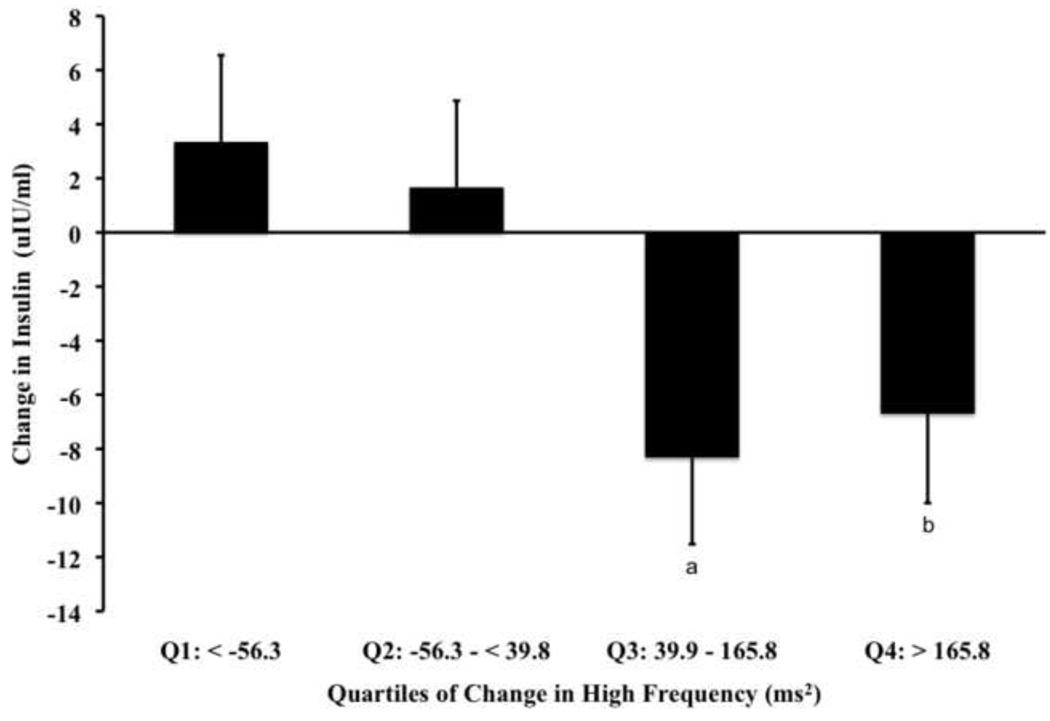
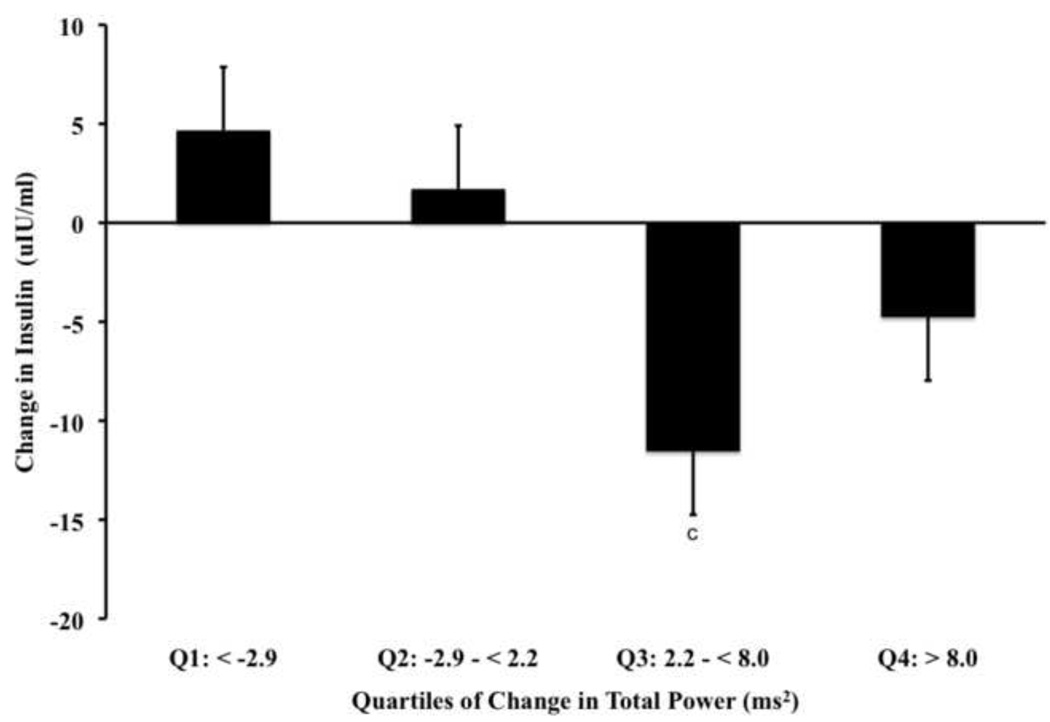
When examining quartiles of change for HF (Fig. 3a, P<0.0008) and Total Power (Fig. 3b, P<0.0001), we observed a significant main ANCOVA statistical effect for the corresponding reductions in insulin. For HF, our post-hoc comparisons of showed that the reduction in insulin was significant for Q3 (P<0.009) and Q4 group (P<0.04). However, the effect was not dose related but rather, exhibited a plateau after Q3. When examining quartiles of HOMA-IR, we also observed that like rMSSD, the HF component of HRV showed the change in Q3 and Q4 to be significantly greater than Q1 (Table 4). Differing from rMSSD is the observation that Q3 and Q4 were also different than Q1 when examining glucose. When examining Total Power, our post-hoc analysis showed Q3 to be significantly lower than Q1 (P<0.004).
Figure 3.
Data [Mean (SD)] represent main ANCOVA statistical effects (all, P< 0.0008) among DREW participants adjusted for age, ethnicity, randomization group, antidepressant medication, change in fitness and change in weight for quartiles of change in frequency domain indices of HRV. Panel A represents post-hoc comparisons of change in insulin relative to quartiles of change in parasympathetic tone denoted by HF, where Q3 (P < 0.009a) and Q4 (P < 0.04b) are significantly different from Q1 (i.e., referent). Panel B represents post-hoc comparisons of change in insulin relative to quartiles of change in mixed signaling tone denoted by Total Power, where Q3 (P<0.004c) is significantly different from Q1 (i.e., referent).
DISCUSSION
The primary finding of our investigation shows that an improvement in parasympathetic modulation is associated with a reduction in insulin concentration independent of changes in aerobic capacity in postmenopausal women. These findings are clinically important as previous research shows that impairment of the ANS is associated with cardiovascular disease mortality, the prevalence of insulin resistance and diabetes and cancer (3–5, 7, 13, 24). Cardiovascular disease and cancer continue to be the two leading comorbidities in postmenopausal women (25). As such, menopause represents a transitional period in a woman’s health that is partially mediated by gonadal sex steroids that are in turn modulated by the ANS. The ANS is also linked to a constellation of mechanisms influencing a woman’s health including the physiology surrounding glucose regulation.
Parasympathetic modulation shifts to a lower range with normal aging (26, 27). Although parasympathetic modulation is generally higher in women than men, aging reduces the difference between genders whereby changes in HRV begin approximately at menopause (28, 29). These changes to the ANS have both central and peripheral implications (12, 30). In our current study, we observed a distinct independent relationship between increased parasympathetic modulation and a corresponding reduction in insulin concentration in previously sedentary, moderately hypertensive, and overweight women participating after a 6-month exercise intervention. Although the change we observed in insulin was not directly related to changes in fitness, participation in cardiorespiratory exercise training does appear to modulate HRV indices.
Overall, cardiorespiratory fitness is associated with enhanced HRV in endurance-exercise training young and older men (31, 32). This relationship is not as clear in older women where some studies show no difference in HRV, while others report an improvement in women exhibiting higher physical activity levels (33, 34). Literature describing the use of exercise training to modulate HRV in women is limited and difficult to interpret due to variances in study protocol, participant age, study length, small sample sizes and the exercise training intensity used. For example, We reported an improvement in HRV accompanying an 8-week exercise training intervention period (37). Ito et al also observed a significant improvement in HRV indices following 8 weeks of exercise training in mildly obese younger women (45.9 ± 4 y) (36), while Stein et al observed an improvement in HRV for older women (66 ± 4 y) exercising at 70% of VO2max. However, Davy et al reported that 12 weeks of aerobic exercise was not associated with improvement in HRV in eight postmenopausal women (35). Stemming from these observations, our findings of a reduction in insulin may be a beneficial “side effect” due to changes in autonomic balance due that is associated with exercise participation.
Autonomic balance plays an important role in women’s health as parasympathetic modulation governs sympathetic over-stimulation of the neural input influencing pancreatic function. Overall, the ANS modulates pancreatic function by coordinating islet cell cross-talk involved in maintaining glucose homeostasis within a normal physiological range (11). Key to this regulation is that when noradrenaline is released from sympathetic nerves, it excites pancreatic A cells by acting at the α1-receptors level. Thus, an excessive release of noradrenaline could provoke an increase in glucagon secretion resulting in over-excitation of insulin secretion from pancreatic B cells. It is also conceivable that an imbalance in the ANS resulting in a reduction in parasympathetic tone may create a “relative hyperinsulinemic state” due to a comparatively higher sympathetic nervous system activity and increased glucagon output. Lifestyle behaviors, such as exercise, may promote “corrective” effect on insulin secretion.
Our current report demonstrates a distinct dose relationship between an increase in rMSSD and a step-wise decrease in insulin (Fig. 2a). We also observed a similar change in the HF frequency domain analysis vs. insulin for the Q3 and Q4 groups. The HF component of the frequency domain analysis is also associated with parasympathetic activity. Thus the pattern of association between the time and frequency domain measures is similar, though the time domain component of our analysis demonstrated a more robust, dose dependent relationship, while the HF component demonstrates a plateau effect.
It is difficult to interpret the influence of the sympathetic nervous system as HRV indices describing sympathetic activity represent mixed sympathetic and parasympathetic signaling. Indeed, we did not observe a significant relationship between quartiles of change in SDNN and insulin (Fig. 2b). A similar pattern was also observed for total power within the frequency domain; however, the relationship insulin and changes in total power appeared to exhibit a plateau in effect, rather than one that was dose related. Given that rMSSD reflects solely changes in parasympathetic activity, it is conceivable that HRV indices such as SDNN are not sensitive enough to ascertain sympathetic neural influences related to overall glucose metabolism.
Although we did not observe a reduction in fasting glucose, we did observe a significant improvement in insulin resistance as determined by HOMA-IR. Insulin resistance is of clinical importance as postmenopausal status is associated with a 60% increase in risk for developing the metabolic syndrome that emerges with estrogen deficiency and changes in lifestyle behaviors inclusive of physical inactivity (38, 39). Insulin resistance is also associated with several adverse clinical outcomes and is a central component of the metabolic disturbances associated with the metabolic syndrome (41). In our cohort, women at baseline on average presented with two out of three qualifying features for metabolic syndrome; specifically, elevated waist circumferences and elevated blood pressure (40). Given the underlying etiology surrounding the progression of metabolic syndrome, the transition from pre- to post-menopause represents “new risk profile” that evolves more rapidly (42). Further, an improvement in parasympathetic tone due to lifestyle changes may attenuate the likelihood of postmenopausal women to develop metabolic syndrome in the future.
The primary strengths of the DREW study is that it is an efficacy study, using a large cohort and a well controlled exercise dose where all exercise was completed in the laboratory using extensive monitoring of exercise energy expenditure, heart rate, and steps taken outside of the structured exercise prescription. All HRV indices were performed under standardized resting conditions at the same time of the day and paced for breathing frequency. The study participants had excellent exercise adherence and a low dropout rate. Findings from our post-hoc analysis of the DREW study are restricted to sedentary, overweight or obese postmenopausal women at moderate risk for cardiovascular disease. This report is a secondary outcome report and the study participants were not recruited based on elevated insulin concentrations. Nonetheless, our study sample is a group that is likely to benefit from exercise training and the group we studied represents a sizeable proportion of US women in the age range of 45 to 75 years (25). Furthermore, physical activity is common standard-of-care recommendation for all women throughout their life span (43).
Our current findings suggest that that insulin is associated with parasympathetic nervous system activity in postmenopausal women. Although we cannot discount an improvement in sympathetic modulation, the mixed signaling indices of HRV collected at rest prohibits us from substantiating this premise. In light of the fact that parasympathetic modulation is encompassed within these mixed signaling parameters, the overall effect of an improvement in HRV status may be more reflective of an improvement in overall ANS balance. We further hypothesize that insulin concentration may improve from a “relative hyperinsulinemic state” that occurs due to comparatively higher sympathetic nervous system activity when autonomic balance is changed due to lower parasympathetic modulation. Our findings confirm those of observational studies that have reported an association between autonomic function and markers of insulin and glucose metabolism (6, 7, 44). Further study with more detailed measures of specific components of function are needed in order to determine whether the changes are primarily associated with parasympathetic vs. sympathetic tone.
Acknowledgment
We wish to extend our gratitude to the DREW staff and participants for their outstanding contributions to the successful completion of this study.
Funding/Support: This work was supported by grant HL66262 from the National Institutes of Health. We also thank Life Fitness for providing exercise equipment.
Role of Sponsor: The funding sponsor had no role in the design, protocol development, or in the conduct of the trial, data collection, data analysis, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration. clinicaltrials.gov Identifier NCT 00011193
References
- 1.Physical activity and cardiovascular health. NIH consensus development panel on physical activity and cardiovascular health. JAMA. 1996 Jul 17;276(3):241–246. [PubMed] [Google Scholar]
- 2.McCabe PM, Porges SW, Carter CS. Heart period variability during estrogen exposure and withdrawal in female rats. Physiol Behav. 1981 Mar;26(3):535–538. doi: 10.1016/0031-9384(81)90184-0. [DOI] [PubMed] [Google Scholar]
- 3.Brockbank CL, Chatterjee F, Bruce SA, Woledge RC. Heart rate and its variability change after the menopause. Exp Physiol. 2000 May;85(3):327–330. [PubMed] [Google Scholar]
- 4.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, et al. The Framingham Heart Study. Reduced heart rate variability and mortality risk in an elderly cohort. Circulation. 1994 Aug;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 5.Carnethon MR, Anthony MS, Cascio WE, Folsom AR, Rautaharju PM, Liao D, et al. The Atherosclerosis risk in communities study. Prospective association between hormone replacement therapy, heart rate, and heart rate variability. J Clin Epidemiol. 2003 Jun;56(6):565–571. doi: 10.1016/s0895-4356(03)00008-8. [DOI] [PubMed] [Google Scholar]
- 6.Liao D, Cai J, Brancati FL, Folsom A, Barnes RW, Tyroler HA, et al. Association of vagal tone with serum insulin, glucose, and diabetes mellitus--The ARIC Study. Diabetes Res Clin Pract. 1995 Dec;30(3):211–221. doi: 10.1016/0168-8227(95)01190-0. [DOI] [PubMed] [Google Scholar]
- 7.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation. 2003 May 6;107(17):2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 8.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006 Apr;29(4):914–919. doi: 10.2337/diacare.29.04.06.dc05-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen T, Vauhkonen IK, Niskanen LK, Hartikainen JE, Lansimies EA, Uusitupa MI, et al. Power spectral analysis of heart rate variability during hyperinsulinemia in nondiabetic offspring of type 2 diabetic patients: evidence for possible early autonomic dysfunction in insulin-resistant subjects. Diabetes. 1999 Jun;48(6):1295–1299. doi: 10.2337/diabetes.48.6.1295. [DOI] [PubMed] [Google Scholar]
- 10.Schachinger H, Port J, Brody S, Linder L, Wilhelm FH, Huber PR, et al. Increased high-frequency heart rate variability during insulin-induced hypoglycaemia in healthy humans. Clin Sci (Lond) 2004 Jun;106(6):583–588. doi: 10.1042/CS20030337. [DOI] [PubMed] [Google Scholar]
- 11.Samols E, Stagner JI, Ewart RB, Marks V. The order of islet microvascular cellular perfusion is B----A----D in the perfused rat pancreas. J Clin Invest. 1988 Jul;82(1):350–353. doi: 10.1172/JCI113593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechin F, van der Dijs B. Central nervous system circuitry involved in the hyperinsulinism syndrome. Neuroendocrinology. 2006;84(4):222–234. doi: 10.1159/000098005. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, et al. The Framingham Heart Study. Impact of reduced heart rate variability on risk for cardiac events. Circulation. 1996 Dec 1;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 14.Earnest CP, Lavie CJ, Blair SN, Church TS. Heart rate variability characteristics in sedentary postmenopausal women following six months of exercise training: the DREW study. PLoS ONE. 2008;3(6):e2288. doi: 10.1371/journal.pone.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, et al. Dose Response to Exercise in Women aged 45–75 yr (DREW): design and rationale. Med Sci Sports Exerc. 2004 Feb;36(2):336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 16.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: A randomized controlled trial. JAMA. 2007 May 16;297(19):2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 18.Huikuri HV, Seppanen T, Koistinen MJ, Airaksinen J, Ikaheimo MJ, Castellanos A, et al. Abnormalities in beat-to-beat dynamics of heart rate before the spontaneous onset of life-threatening ventricular tachyarrhythmias in patients with prior myocardial infarction. Circulation. 1996 May 15;93(10):1836–1844. doi: 10.1161/01.cir.93.10.1836. [DOI] [PubMed] [Google Scholar]
- 19.Marek M, Bigger J, Camm A, Kleiger R, Malliani A, Moss J, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354–381. [PubMed] [Google Scholar]
- 20.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981 Jul 10;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 21.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985 Jan;248(1 Pt 2):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 22.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986 Aug;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 23.Church TS, Martin CK, Thompson AM, Earnest CP, Mikus CR, Blair SN. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS One. 2009;4(2):e4515. doi: 10.1371/journal.pone.0004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd DB. Insulin and cancer. Integr Cancer Ther. 2003 Dec;2(4):315–329. doi: 10.1177/1534735403259152. [DOI] [PubMed] [Google Scholar]
- 25.Bethesda, MD: NIH Office of Research on Women’s Health, and Giovanni Lorenzini Medical Science Foundation; International Position Paper on Women’s Health and Menopause: A Comprehensive Approach. 2002 Contract No.: No. 02–3284.
- 26.Fluckiger L, Boivin JM, Quilliot D, Jeandel C, Zannad F. Differential effects of aging on heart rate variability and blood pressure variability. J Gerontol A Biol Sci Med Sci. 1999 May;54(5):B219–B224. doi: 10.1093/gerona/54.5.b219. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz JB, Gibb WJ, Tran T. Aging effects on heart rate variation. J Gerontol. 1991 May;46(3):M99–M106. doi: 10.1093/geronj/46.3.m99. [DOI] [PubMed] [Google Scholar]
- 28.Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, et al. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J Appl Physiol. 2001 Dec;91(6):2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- 29.Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999 Dec;277(6 Pt 2):H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- 30.Du XJ, Riemersma RA, Dart AM. Cardiovascular protection by oestrogen is partly mediated through modulation of autonomic nervous function. Cardiovasc Res. 1995 Aug;30(2):161–165. [PubMed] [Google Scholar]
- 31.Levy WC, Cerqueira MD, Harp GD, Johannessen KA, Abrass IB, Schwartz RS, et al. Effect of endurance exercise training on heart rate variability at rest in healthy young and older men. Am J Cardiol. 1998 Nov 15;82(10):1236–1241. doi: 10.1016/s0002-9149(98)00611-0. [DOI] [PubMed] [Google Scholar]
- 32.Yataco AR, Fleisher LA, Katzel LI. Heart rate variability and cardiovascular fitness in senior athletes. Am J Cardiol. 1997 Nov 15;80(10):1389–1391. doi: 10.1016/s0002-9149(97)00697-8. [DOI] [PubMed] [Google Scholar]
- 33.Reland S, Ville NS, Wong S, Senhadji L, Carre F. Does the level of chronic physical activity alter heart rate variability in healthy older women? Clin Sci (Lond) 2004 Jul;107(1):29–35. doi: 10.1042/CS20030405. [DOI] [PubMed] [Google Scholar]
- 34.Davy KP, Miniclier NL, Taylor JA, Stevenson ET, Seals DR. Elevated heart rate variability in physically active postmenopausal women: a cardioprotective effect? Am J Physiol. 1996 Aug;271(2 Pt 2):H455–H460. doi: 10.1152/ajpheart.1996.271.2.H455. [DOI] [PubMed] [Google Scholar]
- 35.Davy KP, Willis WL, Seals DR. Influence of exercise training on heart rate variability in post-menopausal women with elevated arterial blood pressure. Clin Physiol. 1997 Jan;17(1):31–40. doi: 10.1046/j.1365-2281.1997.01010.x. [DOI] [PubMed] [Google Scholar]
- 36.Ito H, Ohshima A, Tsuzuki M, Ohto N, Yanagawa M, Maruyama T, et al. Effects of increased physical activity and mild calorie restriction on heart rate variability in obese women. Jpn Heart J. 2001 Jul;42(4):459–469. doi: 10.1536/jhj.42.459. [DOI] [PubMed] [Google Scholar]
- 37.Jurca R, Church TS, Morss GM, Jordan AN, Earnest CP. Eight weeks of moderate-intensity exercise training increases heart rate variability in sedentary postmenopausal women. Am Heart J. 2004 May;147(5):e21. doi: 10.1016/j.ahj.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Knopp RH. Risk factors for coronary artery disease in women. Am J Cardiol. 2002 Jun 20;89(12A):28E–34E. doi: 10.1016/s0002-9149(02)02409-8. discussion E-5E. [DOI] [PubMed] [Google Scholar]
- 39.Paul S, Smith L. The metabolic syndrome in women: a growing problem for cardiac risk. J Cardiovasc Nurs. 2005 Nov–Dec;20(6):427–432. doi: 10.1097/00005082-200511000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004 Feb 3;109(4):551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 42.Rossi R, Grimaldi T, Origliani G, Fantini G, Coppi F, Modena MG. Menopause and cardiovascular risk. Pathophysiol Haemost Thromb. 2002 Sep–Dec;32(5–6):325–328. doi: 10.1159/000073591. [DOI] [PubMed] [Google Scholar]
- 43.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical Activity and Public Health in Older Adults. Recommendation From the American College of Sports Medicine and the American Heart Association. Circulation. 2007 Aug 1; doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 44.Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, et al. The Framingham Heart Study. Association of hyperglycemia with reduced heart rate variability. Am J Cardiol. 2000 Aug 1;86(3):309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]