Abstract
4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N(4,6-dimethyl-2-pyrimidiny)benzene sulphonamide and its derivatives were evaluated for antiviral activity against Pathogenic viruses such as Hepatitis C Virus and SARS-CoV in Vero and Huh 5-2 cells, respectively. The 5-fluoro derivative inhibited the HCV RNA synthesis at 6 μg/ml, without toxicity at a concentration up to 42 μg/ml in Huh 5-2 cells. Among the compounds tested SPIII-5F exhibits the 45% maximum protection against replication of SARS-CoV in Vero cells.
Keywords: Isatin, HCV, SARS-CoV, vero cells, huh 5-2 cells
Isatin (2,3-dioxoindole), a versatile lead molecule for potential bioactive agents, and its derivatives were reported to posses anticancer1, antibacterial activities2–4. Methisazone (N-methylisatin-β-thiosemicarbazone) was one of the first clinically used synthetic antiviral agent5. Isatin derivative were reported for antiviral activity against a verity of pathogens viruses6 and N,N-disubstitutedthiosemicarbazone derivative of isatin were tested for inhibition of HIV-1 replication7. Previously we reported synthesis of novel isatin derivatives and evaluated antiviral activity against HIV-1 and HIV-2 in MT-4 cells8. Significant antiviral activity was observed with these compounds against HIV-1 replication9.
In view of the broad spectrum activities of isatin derivatives, we aimed at evaluating the antiviral activity of some novel 4-1[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N(4,6-dimethyl-2-pyrimidiny)-benzenesulphonamide and its derivatives (fig. 1) against pathogenic viruses such as hepatitis C virus (HCV) in human hepatoblastoma cells (Huh 5-2 cells) and Severe Acute Respiratory Syndrome corona virus (SARS-CoV) in Vero cultures.
Fig. 1.
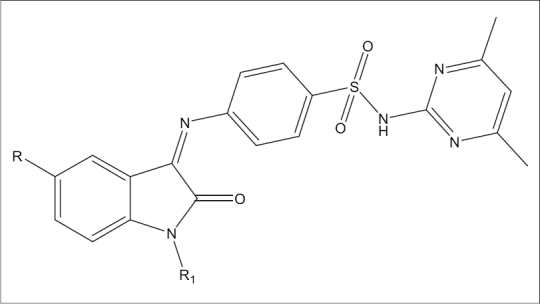
N-(4,6-dimethylpyridin-2-yl)-4-(2-oxo-1,2-dihydroindol-3- ylideneamino) benzenesulfonamide and its derivatives. R and R1 for SPIII-5Br are Br and H, for SPIII-5Cl are Cl and H, for SPIII-5F are F and H, for SPIII-5H are H and H, for SPIII-Me are CH3 and H and for SPIII-NA are H and COCH3, respectively.
4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N(4,6-dimethyl-2-pyrimidiny)benzene sulphonamide and its derivatives (fig. 1) were prepared by condensing the isatin and its derivatives (5-chloro, 5-bromo, 5-flouro, 5-methyl and N-acetyl) with sulphadimidine in the presence of glacial acetic acid9.
In the method adopted for antiviral activity against SARS-CoV in vero cells10. Vero E6 cells in 96-well tissue culture plates were used confluent. Culture medium was removed and 100 μL of minimum essential medium supplemented with 2% fetal bovine serum containing an appropriate concentration of antiviral compound was added. Inside a biosafety laboratory-3,25 μL of a SARS-CoV virus solution added. Five concentrations were tested for cytotoxicity of the antiviral compounds. After an incubation period of three days at 37° in 5% CO2, the inhibition of the cytopathic effect (CPE) by the compounds was measured in a spectrophotometer (at 492) by the reduction by cellular dehydrogenase of the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrzolium(MTS) dye (Cell titer 96 Aqueous One Solution kit, promega) (20 μL MTS for 3h at 37°) in to a water soluble coloured formazan product. The antiviral activity and cytotoxcity of the test compounds are presented in Table 1.
TABLE 1.
ANTIVIRAL ACTIVITY OF ISATIN DERIVATIVE AGAINST SARS-COV IN VERO E6 CELLS
| Compound code | EC50a (μg/ml) | CC50b (μg/ml) | Maximum protection (%) (at 125 μg/ml) |
|---|---|---|---|
| 5CI-IS-AC | >125 | >125 | 0 |
| SPIII-5H | >125 | >125 | 22 |
| SPIII-5Cl | >125 | >125 | 10 |
| SPIII-5Br | >125 | >125 | 2 |
| SPIII-5F | >125 | >125 | 45 |
| SPIII-5Me | >125 | >125 | 12 |
| SPIII-NA | >125 | >125 | 12 |
50% effective concentration required to reduce virus-induced cytopathicity by 50%.
50% cytotoxic concentration required to reduce host cell viability by 50%
Replication assay undertaken with Huh-5-2 cells11–13 [a cell line with a persistent HCV replication 1389luc-ubi-neo/NS3-3/5.1; replication with firefly luciferase-lubquitin-neomycine phosphotransferase fusion protein EMCV-IRES drivan NS3-5B HCV polyprotein] was cultured in RPMI medium 2 mM glutamine, 1× non essential amino acid (Life Technologies, DC); 100 IU/ml penicillin and 100 μg/ml streptomycin and 250 μg/ml G418 (Geneticin, Life Technologies Washington DC). Cells were seeded at a density of 7000 cells per well in 96 well view plate TM (Packard, CA) in medium containing the same compounds as described above, except for G418. Cells were allowed to adhere and proliferate for 24 h. At that time, culture was removed and serial dilution of test compounds were added in culture medium lacking G418. Interferon alfa 2a (500 IU) was added as a positive control. Plates were further incubated at 37° and 5% CO2 for 72 h replication of HCV replicon in Huh-5 cells results in luciferase activity in the cells. Luciferase activity was measured by adding 50 μl of 1 × Gloysis buffer (Promega) for 15 min of followed by adding 50 μl Steady-Glo Luciferase assay reagent (promega). Luciferase activity was measured with luminometer and signal in each individual well was expressed as a percentage of the untreated culture. Parallel culture of Huh 5-2 cells, seeded at a density of 7000 cells/well of classical 96-well cell culture plates (Becton-Dicknson) were treated in a similar fashion except that no Glo-lysis buffer or Stady-Glo Luciferase reagent was added. Instead the density of the cluture was measured by means of the MTS method (Promega). The antiviral activity and cytotoxicity of the test compounds are prepared in Table 2.
TABLE 2.
ANTI-HCV ACTIVITY OF ISATIN DERIVATIVES
| Compound code | EC50 (μg/ml) HCV RNA* | CC50 (μg/ml) Cell growth* | SI |
|---|---|---|---|
| 5CI-IS-AC | >50 | 50 | 0 |
| SPIII-5H | 19 | >50 | >2 |
| SPIII-5CI | >50 | >50 | 0 |
| SPIII-5BR | 17 | >50 | >3 |
| SPIII-5F | 6 | 42 | 7 |
| SPIII-5Me | >50 | >50 | 0 |
| SPIII-NA | >50 | >50 | 0 |
% untreted control. Interferon alfa-2b at 10.000 units/well reduced the signal in the viral RNA (luciferase) assay to background levels; without any cytostatic activity
From the antiviral and cytotoxicity assay it was observed that the compounds 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N(4,6-dimethyl-2-pyrimidiny)benzene sulphonamide (SPIII-5H) and bromo derivative (SPIII-Br) inhibits HCV RNA synthesis at the EC50 of 17 and 19 μg/ml, respectively while its CC50 for cell growth was 42 μg/ml in Huh 5-2 cells. The isatin lead molecule 5Cl-IS-AC did not inhibit the HCV RNA synthesis (EC50 and CC50 more than 50 μg/ml) and the replication of SARS-CoV (maximum protection 0%). SPIII derivative showed 2-45% maximum protection against the replication of SARS-CoV in Vero cells and compound SPIII 5F exhibited 45% maximum protection against the replication of acutely infected SARS-CoV in Vero cell.
The present study was aimed at investigating some novel isatin derivative for antiviral activities against HCV and SARS-CoV to identify potential bioactive agent in the series. From the results of biological activities it appeared that some of the derivatives showed antiviral activity against HCV virus in Huh 5-3 cells.SPIII-5H and bromo derivatives inhibited the synthesis of HCV RNA, but only at a relatively high concentration (17 and 19 μl/ml).
In the present study, the test compound SPIII-5F inhibited the HCV RNA synthesis in Huh 5-2 Cells (SI=7) and 45% maximum protection against the replication against the replication of acutely infected SARS-CoV in Vero cells (Table 1 and 2). Isatin lead molecule (5CI-IS-AC) did not inhibit the HCV RNA synthesis and replication of SARS-CoV. Presence of sulphonamide side chain in the 3rd position was essential for antiviral activity (SPIII-5H, 5Br and 5F). Further modification in the series may help in optimizing antiHCV activity.
Footnotes
Selvam, et al.: In Vitro Antiviral Activity of Isatin Derivatives
REFERENCES
- 1.Popp FD. Synthesis of potential anti-neoplastic agents, XX: Compounds related to the 3-o-nitrophenylhydrazone of isatin. J Pharm Sci. 1969;12:182–4. doi: 10.1021/jm00301a054. [DOI] [PubMed] [Google Scholar]
- 2.Varma RS, Nobles WL. Synthesis and Antiviral and Antibacterial Activity of Certain N-Dialkylaminomethylisatin β-Thiosemicarbazones. J Med Chem. 1967;10:972–4. doi: 10.1021/jm00317a061. [DOI] [PubMed] [Google Scholar]
- 3.Pandeya SN, Sriram D, De Clercq E, Pannecouque C, Witvrouw M. Anti-HIV activity of some Mannich bases of isatin derivatives. Indian J Pharm Sci. 1998;60:207–12. doi: 10.1159/000007182. [DOI] [PubMed] [Google Scholar]
- 4.Pandeya SN, Sriram D, Nath G, De Clercq E. Synthesis, antibacterial, antifungal and anti-HIV activities of Norfloxacin Mannich bases. Eur J Med Chem. 2000;35:249–55. doi: 10.1016/s0223-5234(00)00125-2. [DOI] [PubMed] [Google Scholar]
- 5.Bauer DJ, Sadler PW. The structure-activity relationships of the antiviral chemotherapeutic activity of isatin β-thiosemicarbazone. Br Pharm Chemother. 1960;15:101–10. doi: 10.1111/j.1476-5381.1960.tb01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf ME. Antiviral agents: Burger Medicinal Chemistry. 4th ed., Part-II. New York: John Wiley and Sons; 1979. p. 553. [Google Scholar]
- 7.Teitz Y, Ronen D, Vansover A, Stematsky T, Rigg JL. Inhibition of human immunodeficiency virus by N-methylisatin-β-4':4' diethylthiosemicarbazone and N-allyl isatin-β-4':4' diallylthiosemicarbazone. Antiviral Res. 1994;24:305–14. doi: 10.1016/0166-3542(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 8.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols DJ, Herdewijin P, et al. Rapid and automated tetrazolium based colourimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–21. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 9.Selvam P, Chandramohan M, De Clercq E, Pannecouque C, Witrouw M. Synthesis and anti-HIV activity of 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene) amino]-N (4, 6-dimethyl-2-pyrimidinyl)-benzenesulphonamide and its derivatives. Eur J Pharm Sci. 2001;14:313–6. doi: 10.1016/s0928-0987(01)00197-x. [DOI] [PubMed] [Google Scholar]
- 10.Keyaerts E, vijgen L, Maes P, Neyts J, Ranst MV. In vitro inhibition of SARS-CoV Virus by Chloroquine, Biochem Biophys Res Commun. 2004;323:264–7. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartenschalager R. Hepatitis C virus replicons: Potential role for drug development. Nature Rev Drug Disc. 2002;1:911–6. doi: 10.1038/nrd942. [DOI] [PubMed] [Google Scholar]
- 12.Pietschman T, Lohmann V, Rutter G, Kurpanek K, Bartenschlager R. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J Virol. 2001;75:1252–64. doi: 10.1128/JVI.75.3.1252-1264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lohmann V, Korner F, Koch J, Herian V, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis virus RNAs in a hepatoma cell line. Science. 1999;285:110–3. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]


