Abstract
A high-performance liquid chromatographic method for the estimation of berberine in the stem of Tinospora cordifolia (Willd.) Miers. ex Hook.f. and Thoms. and Tinospora sinensis (Lour.) Merrill is described. The dried stems of T. cordifolia and T. sinensis were defatted with petroleum ether (60-80°). The marc was dried and further extracted with methanol. The concentration of berberine in methanol extract was determined using a C-18 reverse phase column with a mobile phase of acetonitrile:water (10:90 v/v) at a flow rate of 0.6 ml/min and with UV detection at 266 nm. TLC and HPLC comparison of both the species revealed significant variation in the chemical constitution of the two species. This observation becomes important in the context of the use of T. sinensis in place of the genuine drug T. cordifolia.
Keywords: Tinospora cordifolia, Tinospora sinensis, berberine, HPLC
T. cordifolia (Family: Menispermaceae), known as Amrita (Guduchi) in Sanskrit is a widely used plant in folk and Ayurvedic systems of medicine. The term Amrita meaning divine nectar is attributed to this drug in recognition of its capacity to impart youthfulness, vitality, and longevity to the consumer1. Drug consists of the dried stem with bark intact. It is widely used in folk and Ayurvedic systems of medicine for its general tonic, anticancer2, antiulcer3, antipyretic4, antihepatitis5, immunomodulatory6, antioxidant7, hypoglycaemic8, antineoplastic, cardiotonic, antibacterial, antimicrobial, antileishmanial, antiinflammatory, antiarthritic, analgesic and diuretic9,14 properties. The drug is reported to possess 20% of the analgesic effect of sodium salicylate10,12. The plant is used in Ayurvedic Rasayanas to improve the immune system and the body resistance against infections11. Amrita is a constituent of several preparations like Amritarishtam, Dhanvantaram tailam, Cheriya rasnadi kashayam and Valiya marmagulika1. T. sinensis (Fam: Menispermaceae) is used almost in the same way as T. cordifolia12. However, practitioners consider T. cordifolia as the genuine source for Amrita.
Sesquiterpene tinocordifolin15, sesquiterpene glycoside tinocordifolioside16, an immunologically active arabinogalactan17, phytoecdysones18 viz., ecdysterone and makisterone, Alkaloids19,20 viz., berberine and magnoflorine are the major chemical compounds isolated from the stem of T. cordifolia. Magnoflorine, berberine, tinosporicide, menispermacide, palmatine, (+)− malabarolide and tinosinen I are the major chemical compounds isolated from the stem of T. sinensis21–23.
A reverse phase HPLC method has been developed to quantitatively estimate the berberine content in the stem of T. cordifolia and T. sinensis. Berberine (B1) is an isoquinoline alkaloid reported to have antimalarial, antipyretic, antimicrobial, antibacterial, antitumour and antiprotozoal (Leishmania) activities13,14. An attempt has also been made to chromatographically compare the methanolic extracts of both the species.
The stem of T. cordifolia and T. sinensis were collected from the Herb Garden, Arya Vaidya Sala, Kottakkal, Kerala State, India in July 2006 and voucher specimens were deposited at the Herbarium, Centre for Medicinal Plants Research, Kottakkal, Kerala (Voucher No. 02202, 01363, 02426, and 03378). The stem was dried in the shade and coarsely powdered. Powdered material of T. cordifolia and T. sinensis (5 g) were defatted with 100 ml petroleum ether in a Soxhlet extractor for 12 h. The marc was air-dried and was further extracted with 50 ml methanol in a Soxhlet extractor for 12 h at 60°. The extract was filtered and concentrated to dryness under reduced pressure below 60° using a rotary flash evaporator. Different concentrations of the residue were prepared in methanol and used for HPLC analysis to quantify the berberine content.
Solvents used were of HPLC grade (E. Merck). Test solutions were filtered through 0.20 μm nylon-6,6 membrane before injection. All analyses were run in triplicate and averaged. The standard berberine used was purchased from Fluka Chemicals, Switzerland. TLC was done on pre- coated silica gel 60 F254 plates (E. Merck) of uniform thickness of 0.2 mm.
A Shimadzu (Kyoto, Japan) HPLC system consisted of LC-10AT VP pump, SPD-M10A VP photodiode array detector, CLASS-VP 6.12 SP5 integration software and a Rheodyne injection valve fitted with a 20 μl injection loop, was used for the analysis. Baseline resolution of B1 was obtained at 25 ± 2° using a Phenomenex Luna C-18 column (250 × 4.6 mm i.d; 5 μm) and an isocratic solvent system consisting of acetonitrile-water in the ratio 10:90 (v/v). The mobile phase was passed through 0.45 μm PVDF filter, degassed before use. The flow rate was kept constant at 0.6 ml/min and the detection was at 266 nm. For calibration, standard solutions of B1 were prepared at concentrations of 1, 5, 10, 20, 40, 50, 80, 100, 200, 400, 800 and 1000 μg/ml using methanol as solvent. The standard solution was injected in triplicate and the average detector response was measured. The stem extracts were assayed in triplicate and peak areas corresponding to B1 were compared with the calibration curve and amount of B1 was determined.
The validated parameters were specificity, linearity, precision and accuracy according to the ICH guidelines24. Inter-day reproducibility was verified by analyzing six different concentrations of B1, each injected four times, and determining the relative standard deviation (RSD%). The intra-day reproducibility was evaluated by the analysis of B1 at two different concentrations (80 and 100 μg/ml), four times a day on seven consecutive days and determining the RSD%. For recovery studies, 500 μl of methanol solution containing 100 μg ml of B1 was added to 500 μl of three methanol extract solutions (10, 20, 40 mg/ml). Recovery was calculated by comparing the resulting peak area with the peak obtained from equal concentrations of B1 and expressed as percentage of this ratio.
Thin layer and HPLC analyses were carried out to compare the crude methanolic extracts of T. cordifolia and T. sinensis. Thin layer chromatography was performed on precoated silica gel 60 F254 TLC plates (E. Merck) of uniform thickness of 0.2 mm. The chromatogram was developed up to 80 mm under chamber saturation conditions with chloroform-methanol, 70:20 (v/v) in a twin trough chamber. For HPLC analysis the conditions mentioned above were maintained.
In view of the potential therapeutic importance of the drug Amrita, and considering the degree of adulteration/substitution of the raw materials, a simple HPLC method was developed and validated in order to quantify berberine (B1). Satisfactory retention times and good resolution of B1 was achieved using reverse phase C-18 column eluted with acetonitrile-water (10:90 v/v) at a flow rate of 0.6 ml/min. A sharp and symmetric peak for B1 was obtained, with good baseline resolution and minimal tailing, thus facilitating the accurate measurement of peak area. The HPLC analysis was carried out in isocratic conditions and a retention time of 8.65 min was obtained for standard berberine. Typical HPLC chromatograms of B1 and methanol extracts of the stem of T. cordifolia and T. sinensis are shown in figs. 1–3.
Fig. 1.
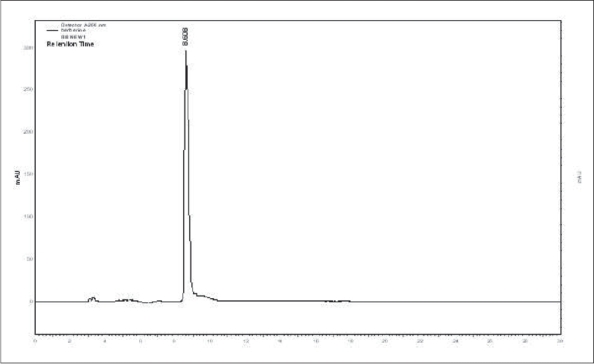
HPLC chromatogram of standard berberine. Berberine peak at the retetion time 8.6 min detected at a wavelength of 266 nm.
Fig. 3.
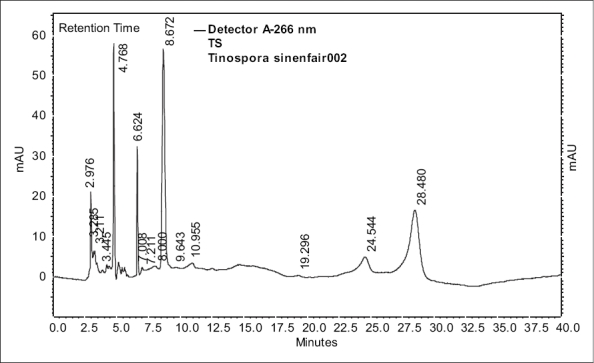
HPLC chromatogram of methanol extract of T. sinensis. Peak at the retention time 8.6 min correspond to berberine.
Fig. 2.
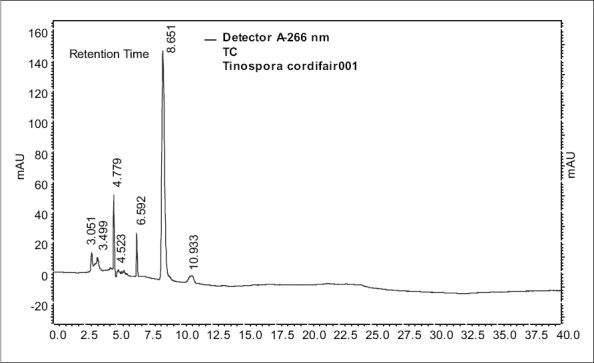
HPLC chromatogram of methanol extract of T. cordifolia. Peak at the retention time 8.6 min correspond to berberine.
The calibration curve for B1 was found to be linear over the range 0.1 to 0.01 mg/ml (r2 = 0.98). The linear regression equation for the calibration curve was y = 123512x -78787, where y is the peak area ratio of B1 and x is the concentration of B1 (μg/ml). The average recovery was 99.10±0.32%, while the inter-day and intra-day reproducibility were found to be 3.5% and 4.7%, respectively. The concentration of B1 in the stem of T. cordifolia and T. sinensis (dry weight basis) was found to be 0.3192% and 0.0967% (w/w), respectively (Table 1).
TABLE 1.
CONCENTRATION OF BERBERINE IN DIFFERENT SAMPLES
| Plant drug | Concentration of berberine (%) in the samplesa | |||
|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Average Conc. (%)b | |
| T. cordifolia | 0.3196 | 0.3188 | 0.3191 | 0.3192 |
| T. sinensis | 0.0965 | 0.0971 | 0.0966 | 0.0967 |
Methanol extract of the defatted plant drug
concentration of berberine in the dried plant drugs
Comparative chromatographic studies of the methanol extract of the two species revealed the presence of two additional compounds in T. sinensis. These two compounds were isolated by column chromatography followed by preparative TLC. HPLC analysis gave peaks at 24.54 and 28.48 min, in the methanol extract of T. sinensis. Preliminary studies (UV, FTIR, TLC and derivatisation) indicate that these two compounds possess a steroid nucleus. Detailed characterization studies of these compounds are under progress.
Ayurvedic practitioners of Kerala use both T. coridifolia and T. sinensis as Amrita. Present study revealed that T. cordifolia and T. sinensis show differences in chemical constituents. The berberine content of the two species show marked variation, T. cordifolia having three times higher berberine concentration. On the other hand T. sinensis contain certain compounds that do not occur in T. cordifolia. Whether they are of any therapeutic significance is not known and hence need to be investigated. Such chemical variation highlights the need for pharmacological standardization and validation. The analytical conditions presented here are applicable with respect to the identification and quantification of B1 and for the quality checking of raw drugs to distinguish the two species conveniently.
Acknowledgments
We are grateful to Kerala State Council for Science Technology and Environment (KSCSTE), Govt. of Kerala for providing financial assistance to carry out this work.
Footnotes
Srinivasan, et al.: HPLC Estimation of berberine in Tinospora species
REFERENCES
- 1.Sivarajan VV, Balachandran I. Ayurvedic Drugs and their Plant Sources. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd; 1999. [Google Scholar]
- 2.Mathew S, Kuttan G. Immunomodulatory and anti-tumour activities of T. cordifolia. Fitoterapia. 1999;70:35–43. [Google Scholar]
- 3.Bairy KL, Roopa K, Malini S, Rao CM. Protective effect of T. cordifolia on experimentally induced gastric ulcers in rats. J Nat Remedies. 2002;2:49–53. [Google Scholar]
- 4.Kumar A, Srivastava S. Study of antipyretic activity of Guduchi Sachitra. Ayurveda. 1995;48:289–91. [Google Scholar]
- 5.Prakash S, Rai NP. Role of T. cordifolia (Willd) Miers (Guduchi) in the treatment of infective hepatitis. J Res Ayurveda and Siddha. 1996;17:58–68. [Google Scholar]
- 6.Manjrekar PN, Jolly CI, Narayanan S. Comparative studies of the immunomodulatory activity of T.cordifolia and T.sinensis. Fitoterapia. 2000;71:254–7. doi: 10.1016/s0367-326x(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 7.Prince PSM, Menon VP. Antioxidant action of T.cordifolia root extract in alloxan diabetic rats. Phytother Res. 2001;15:213–8. doi: 10.1002/ptr.707. [DOI] [PubMed] [Google Scholar]
- 8.Prince PSM, Menon VP. Hypoglycaemic and other related action of T.cordifolia roots in alloxan diabetic rats. J Ethnopharmacol. 2000;70:9–15. doi: 10.1016/s0378-8741(99)00136-1. [DOI] [PubMed] [Google Scholar]
- 9.Sharma PC, Yelne MB, Dennis TJ, editors. Database on Medicinal Plants Used in Ayurveda. Vol.3. New Delhi: CCRAS; 2001. [Google Scholar]
- 10.Rajul B, Bhatt JV. Phagocytic coefficient as a measure for evaluating plant antibiotic. Indian J Pharm. 1953;15:309. [PubMed] [Google Scholar]
- 11.Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and medicinal properties of T.cordifolia (Guduchi) Indian J Pharmacol. 2003;35:83–91. [Google Scholar]
- 12.Chadha YR, editor. The Wealth of India-Raw Materials. Vol. X. New Delhi: CSIR; 1976. [Google Scholar]
- 13.Budavari S, editor. The Merck Index. 13th ed. Whitehouse Station, NJ: Merck and Co Inc; 2001. [Google Scholar]
- 14.Patani A, editor. Indian Herbal Pharmacopoeia. Revised New ed. Mumbai: Indian Drug Manufactures Association; 2002. [Google Scholar]
- 15.Maurya R, Handa SS. Tinocordifolin, a sesquiterpene from T.cordifolia. Phytochemistry. 1998;49:1343–6. [Google Scholar]
- 16.Maurya R, Dhar KL, Handa SS. A sesquiterepene glycoside from T.cordifolia. Phytochemistry. 1997;44:749–50. [Google Scholar]
- 17.Chintalwar G, Jain A, Sipahimalani AT, Banerji A, Sumariwalla P, Ramakrishnan R, et al. An immunologically active arabinogalactan from T.cordifolia. Phytochemistry. 1999;52:1089–93. doi: 10.1016/s0031-9422(99)00386-6. [DOI] [PubMed] [Google Scholar]
- 18.Pradhan P, Gangan VD, Sipahimalani AT, Banerji A. Two phytoecdysones from T. cordifolia: Structural assignments by 2D NMR spectroscopy. Indian J Chem Sec B. 1997;36:958–62. [Google Scholar]
- 19.Pachaly P, Schneider C. Alkaloids from Tinospora cordifolia. Arch Pharm (Weinheim Ger) 1981;314:251–6. [Google Scholar]
- 20.Bisset NG, Nwaiwu J. Quaternary alkaloids of Tinospora species. Planta Medica. 1983;48:275–9. doi: 10.1055/s-2007-969933. [DOI] [PubMed] [Google Scholar]
- 21.Rastogi RP, Mehrotra BN, editors. Compendium of Indian Medicinal Plants. Vol. 3. PID, New Delhi: CSIR; 1993. [Google Scholar]
- 22.Rastogi RP, Mehrotra BN, editors. Compendium of Indian Medicinal Plants. Vol. 4. PID, New Delhi: CSIR; 1995. [Google Scholar]
- 23.Rastogi RP, Mehrotra BN, editors. Compendium of Indian Medicinal Plants. Vol. 5. PID, New Delhi: CSIR; 1998. [Google Scholar]
- 24.Swartz ME, Krull IS. Analytical method development and validation. NY, USA: Marcel Dekker Inc; 1997. [Google Scholar]


