Abstract
The present study attempted to distinguish the independent contributions of the amygdala and hippocampus to fear expression. Rhesus monkeys (Macaca mulatta) with bilateral excitotoxic amygdala lesions (n=4), bilateral excitotoxic hippocampal lesions (n=8), and unoperated controls (n=9) were allowed to reach over a neutral junk object or fear-provoking stimulus (i.e., a rubber snake or a jumping rubber spider) to retrieve a food reward. Monkeys were exposed to each stimulus for 30 seconds. On each trial we recorded the monkey's latency to retrieve the food reward and scored monkeys' whole-body reactions to the object. Confirming previous work we found that, relative to controls, both operated groups showed shorter food-retrieval latencies and exhibited fewer defensive and more approach behaviors when exposed to the fear-provoking stimuli. However, only monkeys with amygdala lesions showed an abnormal, excessive visual interest in the snake and spider. By contrast, monkeys with hippocampal lesions displayed behaviors that were unrelated to the presence of the fear stimuli, thereby indicating a lack of interest in, and emotional reactivity towards, the snake and spider. These data show that the hippocampus and amygdala contribute independently to the overall expression of defensive responses.
Keywords: anxiety, snake, spider, emotion, monkey
Introduction
Both the amygdala and hippocampus play a prominent role in the generation and expression of defensive responses. In rats, for example, complete or partial lesions of the amygdala attenuate freezing behavior to stimuli (Blanchard and Blanchard, 1972a; LeDoux et al., 1988; LeDoux et al., 1990; Davis, 1992; Fanselow et al., 1994) or contexts (Davis, 1997; Maren and Fanselow, 1997; Amorapanth et al., 2000) that have been previously paired with footshock. In addition, lesions of the amygdala disrupt innate defensive responses to predator odor (Vazdarjanova et al., 2001; Takahashi et al., 2007). Although the hippocampus has a long-established role in spatial memory, recent studies have identified an essential role for the ventral hippocampus in the direct expression of defensive responses. Rats with ventral hippocampal lesions, for example, show reduced freezing to both context and discrete conditioned stimuli (Phillips and LeDoux, 1992; Richmond et al., 1999; Trivedi and Coover, 2004), display fewer defensive reactions during exposure to a live cat or cat odor (Kim et al., 1971; Blanchard and Blanchard, 1972b; Pentkowski et al., 2006), and show reduced expression of other unconditioned responses (Bannerman et al., 2002; Deacon et al., 2002). In rhesus monkeys, excitotoxic lesions of either the amygdala (Meunier et al., 1999; Kalin et al., 2004; Izquierdo et al., 2005) or hippocampus (Chudasama et al., 2008) disrupt the expression of innate defensive reactions (e.g., freezing, piloerection, eye/head aversion and avoidance) that normally accompany confrontation with real or artificial snakes and spiders (Nelson et al., 2003). Both structures receive visual sensory input from the inferotemporal and perirhinal cortex (Suzuki, 1996) and project to the orbital and medial prefrontal cortex (Ghashghaei and Barbas, 2001, 2002), regions that enable the appropriate selection of adaptive responses (Davidson and Irwin, 1999; Rushworth et al., 2004; Murray and Izquierdo, 2007). Furthermore, projections from the hippocampus distribute widely within the amygdala which in turn projects to brainstem nuclei involved in producing many of the autonomic correlates of fear and anxiety (Krettek and Price, 1978; Price and Amaral, 1981; Saunders et al., 1988).
We recently reported that monkeys with complete excitotoxic hippocampal lesions (Chudasama et al., 2008), like those with complete excitotoxic lesions of the amygdala (Izquierdo et al., 2005), were unable to generate appropriate defensive responses when exposed to a rubber snake and rubber spider. In the course of these investigations, however, it became apparent that the two experimental groups displayed different behaviors in response to the fear-provoking stimuli. To better characterize the effects of amygdala and hippocampus lesions on expression of defensive responses, we tested monkeys with amygdala lesions and their unoperated controls on a modified version of the original task that provided us the opportunity to observe the monkeys' responses to neutral and fear provoking objects for a longer period of time. This allowed us to directly compare the responses of monkeys with amygdala lesions to those of monkeys with hippocampal lesions that had been tested in the same manner. In addition, to capture the differences exhibited by the two experimental groups, we examined new clusters of behaviors with the expectation that a more fine-grained analysis of behavior might inform the independent contributions of these two regions.
Methods and Materials
Subjects
A total of 21 adult, male rhesus monkeys (Macaca mulatta), ranging in weight from 6.75 kg to 12.35 kg at the start of behavioral training, were used for this study. They were housed individually in temperature controlled rooms (76-80°F) under diurnal conditions (12 hr light/dark cycle). Eight monkeys with hippocampal lesions (Group H: H1-H8) and five unoperated controls (Group CON: CON1-CON5) were the same monkeys studied by Chudasama et al. (2008). Four monkeys with amygdala lesions (Group A: A1-A4), together with four concurrently tested unoperated controls (CON6-CON9) were the same monkeys studied by Izquierdo et al. (2005). Although the training histories of the groups with amygdala lesions and hippocampal lesions differed, each group had concurrently running controls. Importantly, before the present study, all groups had equivalent exposure to the snake and spider. Specifically, all monkeys had received five days training on the original version of the snake test, the results of which are reported elsewhere (Izquierdo et al., 2005; Chudasama et al., 2008). A subset of the data reported here for Group H has been presented previously (Chudasama et al., 2008). All monkeys were fed a controlled diet of primate chow (catalogue number 5038, PMI Feeds Inc., St. Louis, MO) supplemented with fresh fruit or vegetables. Water was available ad libitum. All procedures accorded with the Guide for the Care and Use of Laboratory Animals and were approved by the NIMH Animal Care and Use Committee.
Surgery
All lesions were performed under aseptic conditions. During surgery, monkeys received isotonic fluids via an intravenous drip, and heart and respiration rates, body temperature, blood pressure, and expired CO2 were monitored throughout the procedure. At the completion of surgery, the wound was closed in anatomical layers with Vicryl sutures. Pre- and postoperatively, monkeys received dexamethasone sodium phosphate (0.4 mg/kg i.m.) and an antibiotic (Cefazolin, 15 mg/kg i.m., or Di-Trim, 0.1mL/kg, 24% w/v solution i.m.) to reduce swelling and prevent infection, respectively. For three days after surgery, the monkeys received analgesic drugs consisting of Ketoprofen (10 to 15 mg/kg, i.m.), acetaminophen (40 mg/kg i.m.) or Banamine (flunixen meglumine, 5 mg/kg i.m.). Monkeys with amygdala lesions received ibuprofen (100 mg) for five additional days.
Excitotoxic hippocampal lesions
A detailed description of the surgical procedures for the hippocampal group is provided in Saksida et al. (2006) and Brasted et al. (2005). Monkeys were immobilized with a combination of medetomidine (0.1 mg/kg; Domitor, Pfizer) and butorphornal (0.3 mg/kg i.m.) and then anesthetized with isoflurane gas (1-3% to effect) and received injections of the excitotoxin N-methyl-d-aspartate (NMDA) bilaterally into the hippocampus in a single-stage surgery. For the lesion, a midline incision was made and the skin and galea were retracted to expose the cranium. The NMDA injections were made from two different stereotaxic approaches. First, a large bone flap (∼ 5 cm square) was removed and stored in sterile saline until closing. Using a standard dorsal approach (Murray and Mishkin, 1998), each monkey received 2-3 injections in the uncal portion of the hippocampus made via a 10 μl Hamilton syringe needle held in a Kopf electrode manipulator. At each site, 2.0 μl of NMDA (0.2 M) was injected at a rate of 0.2 μl/min. The injections were made in one hemisphere at a time. Second, a longitudinal approach was used to inject NMDA at intervals along a single needle track that extended through the rostrocaudal extent of the hippocampus (see Hampton et al., 2004). For this second set of injections, a craniotomy (∼1 cm) was made over the occipital cortex. A 25 μl Hamilton syringe needle was introduced through a slit in the dura and advanced to the most rostral injection site. NMDA was injected at 2 mm intervals throughout the length of the hippocampus. At each site, 2.0 μl (0.2 M) of NMDA was injected at a rate of 0.25 μl/min. After each injection, the needle was left in place for a further 3 minutes before being withdrawn to the next site. Seven to 10 injections per hemisphere were made via this approach. These second sets of injections were made in both hemispheres simultaneously using two manipulators, one mounted on each arm of the stereotaxic frame. The intended lesion was the dentate gyrus, cornu ammonis (CA) fields, and subicular complex. The intended extent and location of the lesion is provided in Figure 1.
Figure 1.
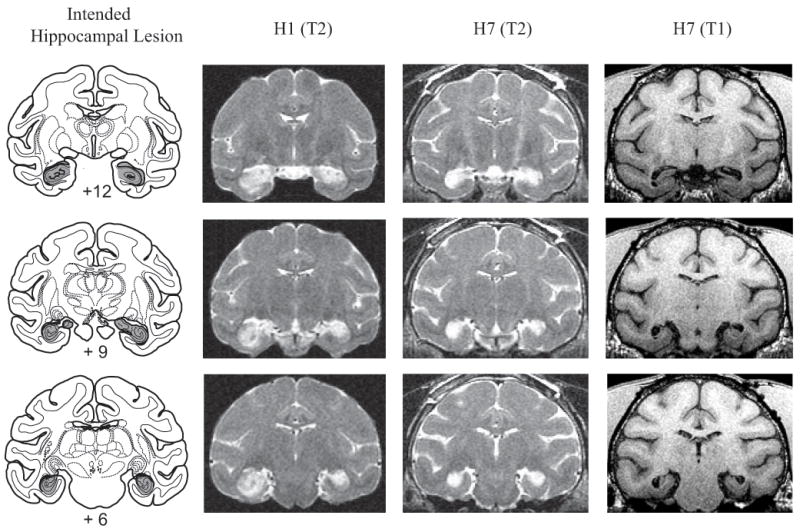
Left column shows coronal sections from a standard rhesus monkey brain depicting the intended hippocampal lesion (shaded region). Right columns show postoperative MR images for cases H1 and H7 at matching levels. T2-weighted MR images reveal the extent of white hypersignal, which reflects edema due to injections of excitotoxin and therefore the approximate site of the hippocampal lesions. T1-weighted MR images for monkey H7 shows gray matter – white matter contrast; note the marked shrinkage of the hippocampal formation bilaterally. Numerals indicate distance from interaural plane (0).
Excitotoxic amygdala lesions
A detailed description of the surgical procedure for the amygdala lesion is provided in Izquierdo et al. (2005). Anesthesia was induced with ketamine hydrochloride (10 mg/kg, i.m.) and maintained with isoflurane gas (1-3% to effect). Monkeys received injections of the excitotoxin ibotenic acid bilaterally into the amygdala in a two-stage surgery counterbalanced for hemisphere; Monkeys A2 and A4 received injections in the right hemisphere in the first operation, and monkeys A1 and A3 received injections in the left hemisphere first. The second stage surgery took place approximately 3.3 months after the first stage surgery.
Once the monkey was secure in the stereotaxic frame, a large bone flap (∼ 5 cm square) was removed and stored in sterile saline until closing. The sagittal sinus served as a landmark for the calculation of stereotaxic coordinates in the mediolateral dimension. Slits were cut in the dura to allow passage of the injection needle. Measures obtained from magnetic resonance (MR) images, acquired before the day of the surgery, were used to place injections of ibotenic acid stereotaxically throughout the amygdala in one hemisphere. At each site, 0.6-1.0μl (10-15 mg/ml) of ibotenic acid was injected at a rate of 0.2 μl/min. After each injection, the needle was left in place for a further 2-3 minutes before being advanced to the next site. Fifteen to 19 injections were made in each hemisphere. The intended lesion included the entire amygdala, the basolateral nuclear group as well as the central, medial and cortical nuclei. Figure 2 illustrates the extent and location of the intended amygdala lesion.
Figure 2.
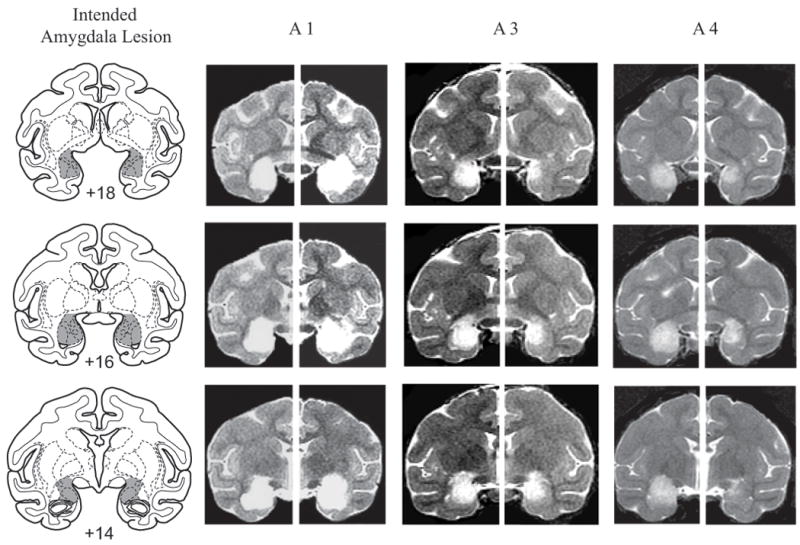
Left column shows coronal sections from a standard rhesus monkey brain depicting the intended amygdala lesion (shaded region). Right columns show postoperative T2-weighted MR images from matching levels in three monkeys in Group A (A1, A3, A4). T2-weighted MR images reveal the extent of white hypersignal, which reflects edema due to injections of excitotoxin and therefore the approximate site of the amygdala lesions. Numerals indicate distance from interaural plane (0).
Lesion assessment
Hippocampal lesions
A full assessment of the hippocampal lesions is provided in Chudasama et al. (2008). The extent of the lesions was assessed in two ways. The brains of four monkeys (H1-H4) underwent traditional histological processing. The lesions were then plotted onto a series of drawings of coronal sections from a standard rhesus monkey brain. The size of the lesion was estimated by expressing the extent of damage to the hippocampal formation (dentate gyrus, CA fields, subicular complex) relative to the volume of the same region in the standard. The NMDA injections produced cell loss in 45% of the hippocampal formation in this group.
For the four remaining monkeys in this group (H5-H8) we estimated the extent of the lesions by calculating the percent reduction in volume that followed NMDA injections. To this end we compared pre- and postoperative T1-weighted MRI scans (FSPGR, TE5.8, TR13.1, flip angle 30, NEX 8, 256 square matrix, FOV 100 mm, 1 mm slices). Using Scion Image software (Scion Corporation, Frederick, MD), the reduction in hippocampal volume was determined according to the methods of Málkóva et al. (2001). Monkeys H5-H8 sustained damage to 64% of the hippocampal formation. Table 1 shows the estimated percent damage (by volume) in monkeys H1-H8, and Figure 1 shows postoperative MR images from representative cases. In all cases, the lesion included the dentate gyrus and CA1–CA3 fields of the hippocampus. There was partial damage to the subicular complex as intended, but the perirhinal and entorhinal cortices were completely intact in both hemispheres.
Table 1. Estimated percent damage to hippocampal formation (H1-H8) or the amygdala (A1-A4).
| Monkey | Estimated % damage (by volume) | ||
|---|---|---|---|
| Left | Right | Mean | |
| H1 | 47.6 | 46.7 | 47.2 |
| H2 | 53.5 | 44.8 | 49.2 |
| H3 | 48.5 | 48.1 | 48.3 |
| H4 | 34.2 | 35.7 | 35.0 |
| H5 | 75.4 | 91.2 | 83.3 |
| H6 | 61.4 | 68.1 | 64.6 |
| H7 | 72.7 | 87.2 | 79.9 |
| H8 | 20.1 | 35.6 | 27.9 |
| A1 | 100 | 100 | 100 |
| A2 | - | 98.5 | 98.5 |
| A3 | 94.7 | 77.3 | 86.0 |
| A4 | 100 | 70.4 | 85.2 |
Left, left hemisphere; Right, right hemisphere
For monkeys H1-H4, % damage represents the extent of the lesion based on direct microscopic examination of Nissl-stained sections. For monkeys H5-H8 and A1-A4, the extent of the lesion was based on hippocampal volume reduction, which is directly related to % damage (see lesion assessment for details). In each of the cases H1-H8, the percent damage reflects the extent of lesion in the dentate gyrus, hippocampus proper, and subicular complex considered together. For A1-A4, the percent damage reflects damage to the basolateral nuclear group as well as the central, medial and cortical nuclei of the amygdala. Due to a problem in obtaining a post-operative MRI scan, only one hemisphere could be evaluated for monkey A2.
Amygdala lesions
For four monkeys with amygdala lesions (A1 – A4; participating in ongoing studies), the extent of amygdala damage was evaluated from T2-weighted MRI scans within 12 days of surgery. For each animal, the region of hypersignal evident in the T2-weighted MR scan was plotted onto a series of drawings of coronal sections from a standard rhesus monkey brain at 1mm intervals. The size of the lesion was estimated by comparing the extent of hypersignal in the amygdala relative to the volume of the same region in the standard expressed as a percentage. We estimated that monkeys A1-A4 sustained damage to 91.5% of the amygdala, bilaterally (range 85.2-100%). In monkey A1, the lesion encroached bilaterally into the anterior portions of the entorhinal cortex, hippocampus, ventral claustrum, substantia innominata and piriform cortex. The remaining monkeys sustained minor, unilateral damage to a subset of these areas. The mean percent damage to the amygdala for monkeys A1-A4 is presented in Table 1, and Figure 2 shows postoperative MR images from representative cases.
Apparatus
All testing was conducted in the manually operated, Wisconsin General Test Apparatus (WGTA). The WGTA comprised a test compartment lit by two 60-W light bulbs and a monkey compartment that was unlit during testing. An opaque screen separated the compartments during intertrial intervals, thereby preventing the animal from observing the preparation for the trial. In the test compartment, a clear Plexiglas box measuring 11.4 cm (width) × 71.1 cm (length) × 11.4 cm (height) was inserted. A hinge on the edge of the box nearest the experimenter enabled the experimenter to lift the Plexiglas and place objects inside the box.
Ten stimulus objects were used: eight neutral junk objects varying widely in size, shape and color, one grey/green colored rubber snake measuring 50.8 cm in length and approximately 2 cm in diameter, and one black, “hairy,” rubber spider which measured 10 cm (width) × 13.5 cm (length) × 2.5 cm (height) made to jump using an air bladder. In addition, another three junk objects were dedicated to pretraining. Food rewards consisted of ‘fruit snacks’ (Giant Food, Inc., Landover MD) or chocolate M&Ms, (Mars Candies, Hackettstown, NJ).
Monkeys' behavior during each session was recorded on videotape. The video recording set-up comprised: two video cassette recorders (VCRs, SR-TS1U), two color video monitors (TM-H1375SU) and two video CCTV cameras (TK-CJ20U) all from JVC Professional Product Company, Denver, CO. The VCRs were equipped with SMPTE time code generators (TG-50; Horita, Mission Viego, CA), which were synchronized at the beginning of each session. The dual camera set-up enabled the test sessions to be videotaped from two vantage points; camera #1 provided a view of the test compartment from above, and camera #2 provided a frontal view of the monkeys' reactions to stimuli in the Plexiglas box.
Behavioral testing procedure
Accommodation and pretraining
Monkeys accommodated to the video camera set-up, Plexiglas box and the WGTA on the very first session. For 20 trials, all monkeys successfully retrieved food rewards that were located on top of the Plexiglas box while the box was empty, and for a further 10 trials with one of the three pretraining objects placed inside the box.
Main task
Monkeys received one session comprising 10 trials; a different object was used on each trial. In eight trials, one of the neutral objects was placed on the surface of the test compartment in the center of the Plexiglas box. In the remaining two trials, the rubber snake or jumping rubber spider was placed in the center of the Plexiglas box. The spider was made to jump (using an air bladder) throughout the length of the trial at a rate of approximately one jump per second. The snake and spider trials occurred pseudorandomly in the sequence of 10 trials with the constraint that neither occurred on the first trial of the session. Trials were separated by a 20 sec ITI.
On each trial, the opaque screen that separated the monkey from the test compartment was raised. The monkey was free to reach over the object to retrieve the food reward. Each trial lasted 30 sec regardless of whether the food was retrieved. In a version of the task employed previously with these same monkeys (Izquierdo et al., 2005; Chudasama et al., 2008), each trial was terminated as soon as the food reward was taken. Because monkeys in the operated groups exhibited short food-retrieval latencies, we had only a limited amount of time in which to assess their whole body responses. The new procedure, using a fixed trial length of 30 sec, afforded a more precise analysis of the monkey's whole-body reactions to the objects.
Videotape analysis
The food retrieval latencies were derived from camera #1, which provided a top down view of the compartment, and were scored to the nearest millisecond. Time for the latency measure was initiated when the opaque screen was raised ∼ 15 cm above the test tray. This could be discerned in the videotape by a mark on the cage, visible in the view of camera #2, placed at the stated height. The response was considered complete when the monkey grasped the food reward just before it withdrew its arm. If no response was made within the trial limit of 30 sec, a score of 30 sec was recorded.
The frequency and duration of the behaviors expressed during each trial were derived from camera #2. Four main categories of behaviors were observed: approach, defense, visual interest and no-eye-contact. Table 2 provides a list of the constituent behaviors in each category. Approach and defense behaviors are standard measures in this and other laboratories (e.g., Kalin et al., 2004; Izquierdo et al., 2005; Machado and Bachevalier, 2007). Approach was scored if the monkey moved from the back to the front of the cage and stayed close to Plexiglas box. In some cases, the monkey proceeded to touch or handle the Plexiglas box in which the stimulus object was contained. Defense was scored when the monkey moved away from the stimulus and/or stayed in the back of the cage, froze, showed piloerection, averted his eyes or shifted his whole head to avoid eye contact with the object. We also created two new categories that emerged during data collection, intended to highlight the different responses of the two experimental groups. Visual interest behavior was scored when the monkey made direct eye contact with the object and engaged in an abnormal crouch-like posture in which the head was lowered to the level of the stimulus with hindquarters raised up or down. No-look-at behavior was scored when the monkey directed its gaze specifically toward something other than the stimulus, or looked around aimlessly indicating a lack of interest or emotional reactivity toward the object. It is important to note that no-look-at behavior is mutually exclusive from defensive behaviors (e.g., freezing, head aversion, eye aversion), in which monkeys actively avoid looking at the stimulus object.
Table 2. Behaviors analysed for each behavioral category.
| Behavior | Description |
|---|---|
| Approach | |
| Move/stay toward | Monkey moves from the back of the cage towards the stimulus inside the Perspex box or is standing/sitting in the front of the cage, close to the stimulus. |
| Touch | Monkey touches, displaces or handles the Perspex box in which the stimulus is contained with hands and/or feet. |
| Defense | |
| Freezing | Monkey is sitting, standing or hanging motionless for at least 3 seconds. |
| Eye/head aversion | Monkey avoids eye contact by glancing away from the stimulus or turns his whole face away from the stimulus. |
| Piloerection | Monkey's hair stands on end in particular on the nape of the neck. |
| Move/stay away | Monkey moves away from the stimulus inside the Perspex box or is standing/sitting at the back of the cage. The monkey may be climbing up in the back corner of the cage. |
| Visual Interest | Monkey examines the stimulus in detail as if scanning or surveying the stimulus. He makes direct eye contact with the stimulus and stares at the stimulus for a variable length of time. This visual examination is accompanied by an abnormal crouch-like posture in which the head is close to, and lowered to the level of the stimulus with hindquarters raised up or down. The monkey may glance at the stimulus while assuming an abnormal crouch-like posture (minimum 0.5 sec). |
| No-look-at | Monkey looks around aimlessly or is specifically directing its gaze towards something other than the stimulus. |
Two observers scored the videotaped behaviors during the neutral and fear object trials. One observer who was aware of the group assignment scored all monkeys. A subset of monkeys (two from each group) were viewed and scored by an independent observer naïve to the group assignments. Interrater reliability was then obtained.
Results
Food-retrieval latencies
Food-retrieval latencies for the snake and spider trial types did not differ significantly in the unoperated control monkeys [t(8) = 0.72; p>0.05]. Because intact monkeys exhibited qualitatively similar responses to the snake and spider, and these responses differed from those to the neutral objects, the food-retrieval latencies from the snake and spider trials were averaged to provide a single value (snake+spider trial type) for each monkey.
Figure 3 shows the mean food-retrieval latencies for the neutral object and snake + spider trial types. A 3 (group) × 2 (trial type) ANOVA of food-retrieval latencies revealed main effects of group [F(2, 18) = 22.09; p<0.0001] and trial type [F(1, 18) = 27.65; p<0.0001]. A significant group × trial type interaction was also obtained [F(2, 18) = 15.48; p<0.001]. Additional post hoc tests confirmed that whereas Group H and Group A displayed short food-retrieval latencies relative to Group CON during the snake +spider trials [Bonferroni, all p<0.001], they did not differ from each other [Bonferroni, p>0.05]. When exposed to neutral objects, Group H displayed short food-retrieval latencies relative to Group CON [Bonferroni, p<0.01; mean latencies (± SEM) in sec: Group H = 1.39 (± 0.06); Group CON = 3.35 (± 0.24)]. Group H, however, did not differ from Group A [Bonferroni, p>0.05].
Figure 3.
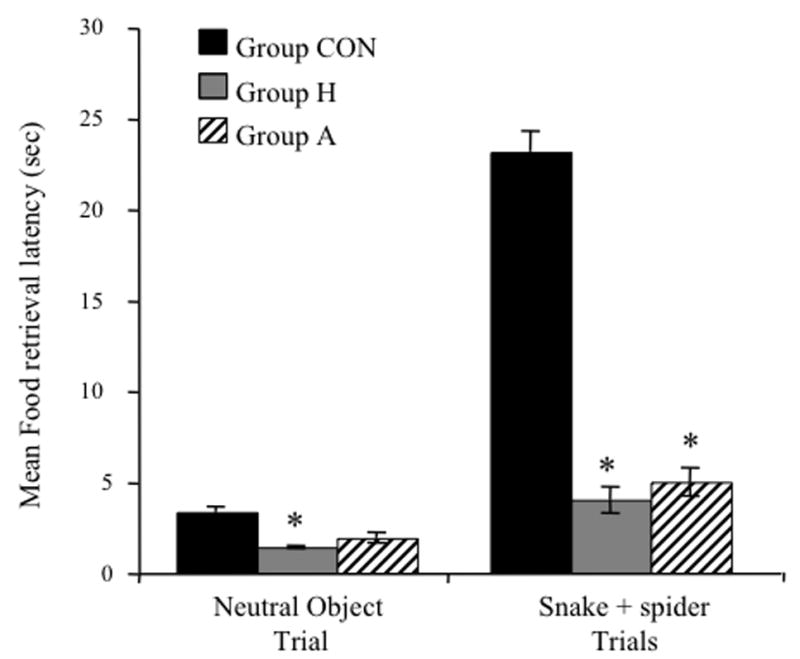
Mean food-retrieval latencies (± SEM) for CON, H and A groups during exposure to neutral and snake+spider trial types. [*significantly different from CON; p<0.001]
Behaviors during neutral object and snake+spider trials
Table 2 provides a description of the constituent behaviors scored for each behavioral category (i.e., approach, defense, visual interest and no-look-at). There was good interrater reliability between the two observers (all correlations for duration of behaviors >0.97; all p<0.0001; all correlations for frequency of behaviors >0.8, all p<0.05). Thus, the behaviors exhibited by the monkeys were reliably identified and distinguished from each other.
Defensive Behaviors
Figure 4 provides frames captured from our recorded videotape from two control monkeys (CON6 and CON9), one amygdalectomized monkey (A1) and one hippocampectomized monkey (H2) during a snake trial. Both controls show behaviors typical in response to the fake snake, including withdrawal from the snake, sitting in the rear of the cage, and avoidance of eye contact by diverting their gaze from the snake. A 3 (group) × 2 (trial type) ANOVA of the duration of defensive behaviors indicated a significant main effect of group [F(2, 18) = 7.48; p<0.005] and trial type [F(1, 18) = 16.95; p<0.001]. A group × trial type interaction was also obtained [F(2, 18) = 4.04; p<0.05]. Post hoc tests confirmed that, on neutral object trials, no group differences emerged. By contrast, on the snake+spider trials, both Group H and Group A showed a reduced duration of defensive behaviors relative to Group CON [Bonferroni all p<0.05; see Figure 4 and 5A] but did not differ from each other [Bonferroni p>0.05]. An identical pattern of results was found for frequency of defensive behaviors.
Figure 4.

Video frames of monkeys displaying defensive (monkeys CON 6 and CON 9, visual interest (monkey A1) and no-look-at behavior (monkey H2) in the presence of the rubber snake. Monkeys CON 6 and CON 9 exhibit representative defensive reactions; they withdraw to the rear of the cage, stay away from the snake, and avert their whole face away from the snake. Monkey A1 shows characteristic visual interest behavior. His head is lowered to the level of the Perspex box and gaze is directed at the snake. The snake occupies the whole length of the Perspex box. In the third frame, note monkey A1 staring at the snake in the far left of the Perspex box while adopting an abnormal posture with hindquarters down. In direct contrast to monkey A1, monkey H2 displays no-look-at behavior. He immediately retrieves the food, then sits close to the rubber snake but does not look at it; gaze is directed at something other than the snake, in this case, the camera and video cabinet.
Figure 5.
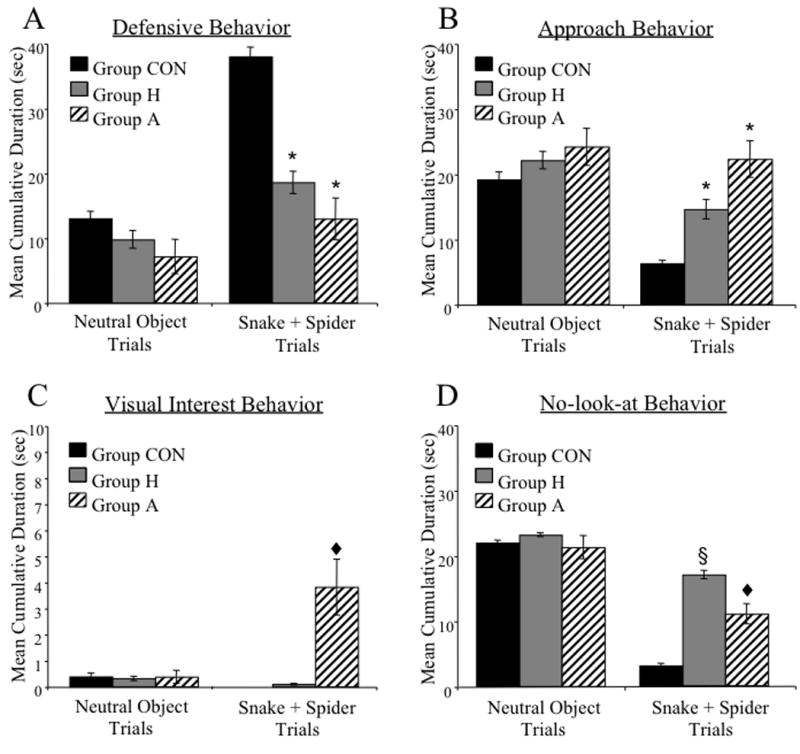
Group mean (± SEM) cumulative duration for defense (A), approach (B), visual interest (C) and no-look-at (D) behavior scored on neutral object and snake+spider trials. [*significantly different from Group CON p<0.01; ♥significantly different from Group CON and Group H p<0.001; §significantly different from Group CON and Group A].
Approach behaviors
A 3 (group) × 2 (trial type) ANOVA on the mean cumulative duration of approach behaviors revealed a significant main effect of group [F(2, 18) = 3.75; p<0.05] and trial type [F(1, 18) = 9.36; p<0.01]. Bonferroni post hoc analysis confirmed that monkeys in Group H and Group A spent more time engaging in approach behaviors during the snake + spider trials relative to monkeys in Group CON [all p<0.05; see Figures 4 and 5B]. The groups did not differ in duration of approach behavior during neutral object trials [F(2, 18) = 0.62; p>0.05]. Analysis of the frequency of approach behaviors yielded a significant main effect of trial type [F(1, 18) = 15.07; p<0.01] but no effect of group [F(2, 18) = 0.57; p>0.05] and no significant interaction of group and trial type.
Visual interest behavior
Figure 4 shows frames captured from video recordings of case A1 on the snake trial. In the first and third frames, A1 is displaying visual interest behavior, characterized by a crouching posture with head and eyes lowered directly to the level of the snake. In the middle frame A1 is reaching for the food reward. In this case the food retrieval has been delayed (beyond that typical for neutral object trials) by the excessive visual interest in the snake. The mean cumulative duration of visual interest behavior for neutral object and snake + spider trials is shown in Figure 5C. A 3 (group) × 2 (trial type) ANOVA on the cumulative duration of visual interest behavior resulted in a main effect of group [F(2, 18) = 7.96; p<0.01], a within subject effect of trial type [F(1, 18) = 8.62; p<0.01], and a significant group × trial type interaction [F(2, 18) = 11.93; p<0.001]. Post hoc tests confirmed that, when exposed to the snake+spider, monkeys in Group A engaged in visual interest behavior for longer periods of time relative to monkeys in either Group H or Group CON [Bonferroni, all p<0.001]. Group H and Group CON, however, did not significantly differ from each other in this behavior [Bonferroni, p>0.05]. Repeated-measures ANOVA of the frequency of visual interest behaviors revealed a similar pattern of results.
No-look-at behavior
An example of no-look-at behavior can be seen in Figure 4, which shows monkey H2 sitting in close proximity to the rubber snake but looking at something other than the rubber snake (e.g. video camera, VCR cabinet). A 3 (group) × 2 (trial type) ANOVA on the cumulative duration of no-look-at behavior revealed a significant main effect of group [F(2, 18) = 13.95; p<0.001], a significant main effect of trial type [F(1, 18) = 174.43; p<0.0001], and a significant interaction of group × trial type [F(2, 18) = 23.94; p<0.0001]. During the snake + spider trials, both operated groups exhibited significantly more no-look-at behavior relative to controls [Bonferroni, all p<0.05; see Figure 5D]. However, the duration of no-look-at behavior was significantly greater in monkeys in Group H compared with monkeys in Group A [Bonferroni, all p<0.05].
Discussion
The results of this study demonstrate, for the first time, the independent contributions of the amygdala and the hippocampus to marshalling defensive responses to specific, unlearned cues. There were three main findings. First, consistent with earlier reports from these and other groups of adult monkeys (Meunier et al., 1999; Kalin et al., 2004; Izquierdo et al., 2005; Chudasama et al., 2008), selective lesions of the amygdala or hippocampus altered monkeys' ability to generate normal emotional reactions to artificial snakes and spiders. The paucity of defensive responses, together with shorter latencies to retrieve food that was located in close proximity to the snake and spider, indicate a lack of fear in the experimental groups. Second, monkeys with selective amygdala lesions showed an abnormal, excessive visual interest in the snake and spider. Third, monkeys with hippocampal lesions exhibited a significant lack of interest and emotional reactivity towards the snake and spider. Whereas the first finding reconfirms the important role of the amygdala and the hippocampus in the normal expression of the fear response, the second and third indicate the two structures contribute in different ways.
Prior to the present study, all monkeys had been evaluated on several cognitive tasks. Importantly, monkeys in Group A and Group H acquired visual discrimination problems at the same rate as controls (Izquierdo and Murray, 2007; Chudasama et al., 2008). Therefore, it is highly unlikely that the aberrant responses of monkeys in the operated groups were due to altered visual perceptual abilities. In addition, amygdalectomized monkeys are capable of displaying emotional responses and social behaviors, albeit ones that are often inappropriate to the situation (Meunier et al., 1999; Amaral et al., 2003; Machado et al., 2008). As for the hippocampus, we note that monkeys in Group H (but not those in Group A) showed long food-retrieval latencies on their very first exposure to the snake (Chudasama et al., 2008), and produced facial expressions and movements that were mainly defensive (Chudasama and Murray, unpublished observations). These findings are consistent with a recent report by Machado et al. (2009), who found that monkeys with excitotoxic hippocampal lesions did not differ from unoperated controls in one-trial assessments of behavioral reactions to an artificial snake. Thus, it is unlikely that the operated groups were simply unable to produce appropriate emotional responses in the presence of the snake. Instead, the most parsimonious explanation of the data is that the deficit after amygdala lesions and hippocampal lesions is in linking cues with the appropriate set of defensive responses, as opposed to identifying the cues or expressing the responses per se.
The process underlying production of the defensive responses must include at least two components: 1) visual identification of the fear-provoking object; and 2) elicitation of the defensive response such as withdrawal, freezing, head aversion and eye aversion. In addition, processes of attention or arousal might influence the production of defensive behaviors. Novelty may also influence elicitation of defensive responses. The amygdala has been implicated in responding to novelty, usually to biologically salient stimuli such as foods, faces, and ambiguous or threatening novel stimuli (e.g., Mason et al., 2006). On the other hand, the hippocampus is required for detecting novel spatial arrays (e.g., Bachevalier and Nemanic, 2008). The visual interest behavior exhibited by monkeys with amygdala lesions included an abnormal crouch-like posture (i.e. hindquarters up or down), with the head located at the level of the stimulus, combined with direct eye contact with the object. Monkeys with amygdala lesions directed the bulk of visual interest behavior specifically to the threatening snake and spider objects. However, visual interest behavior was also occasionally observed towards the neutral objects. Indeed, all groups exhibited a small amount of visual interest behavior in response to neutral objects (see Table 3 and Figure 5C). The source of the excessive visual interest in objects that follows bilateral temporal lobectomy (Klüver and Bucy, 1939) as well as selective amygdala lesions (Meunier et al., 1999; present study) is unknown. The primate amygdala receives visual sensory inputs from inferior temporal cortex area TE and perirhinal cortex (Suzuki and Amaral, 1994; Stefanacci et al., 1996) and has strong anatomical links to the autonomic nervous system and brainstem regions responsible for generating freezing and piloerection, among other responses (LeDoux et al., 1988; Roberts et al., 2007). Thus, although many of the deficits that follow amygdala lesions may be understood as a disconnection of the sensory processing regions such as TE and perirhinal cortex from the parts of the amygdala known to trigger autonomic and behavioral responses of fear and flight (e.g. freezing, increases in heart rate and blood pressure), the intense visual interest behavior must be accounted for by a different mechanism. According to one influential model (Gray and McNaughton, 2000; McNaughton and Corr, 2004), the hippocampus signals the perceived intensity of threat, which could, in turn, influence other structures such as the perirhinal cortex, amygdala, and orbital prefrontal cortex. According to this view, the hippocampus is more involved with the assessment of the level of threat (also known as risk assessment) than with the expression of fear, and the reverse is true for the amygdala. If true, one possibility is that in the absence of the amygdala, the arousal signals generated by the septo-hippocampal system yield the excessive visual interest to fear stimuli. This speculative account requires the additional assumption that, in the context of a snake or spider, there is some innate, specialized capacity for generating the arousal in the first place.
Table 3. Duration of defense, approach, visual interest and no-look-at behavior observed in Group CON (CON1-CON 9), Group H (H1-H8) and Group A (A1-A4) during neutral and snake+spider trial.
| Monkey | Duration of behavior (seconds) | |||||||
|---|---|---|---|---|---|---|---|---|
| Neutral Trial | Snake+spider Trial | |||||||
| Defense | Approach | Visual Interest | No-look-at | Defense | Approach | Visual Interest | No-look-at | |
| CON1 | 16.5 | 17 | 0 | 17.5 | 32.25 | 7.25 | 0 | 0.25 |
| CON2 | 27.5 | 4.5 | 0 | 22 | 27.25 | 9.75 | 0 | 5.5 |
| CON3 | 23.5 | 7 | 0 | 23.5 | 39.25 | 3.25 | 0 | 2.25 |
| CON4 | 16.5 | 16 | 0 | 20.5 | 26.5 | 11.25 | 0 | 6.25 |
| CON5 | 1 | 29 | 0 | 26.5 | 61.5 | 0.25 | 0 | 0.25 |
| CON6 | 9.5 | 21.5 | 0 | 25 | 30.75 | 10.25 | 0 | 7.75 |
| CON7 | 3 | 30.5 | 0 | 19.5 | 33 | 6 | 0 | 1.75 |
| CON8 | 11.5 | 18.5 | 0.3 | 22 | 42 | 5 | 0 | 3 |
| CON9 | 8 | 28 | 0.5 | 21 | 49.5 | 2.75 | 0 | 0.25 |
| H1 | 0 | 30 | 0 | 27.5 | 6 | 25.75 | 0 | 21.75 |
| H2 | 4 | 29 | 0 | 23.5 | 11 | 21.5 | 0 | 21.75 |
| H3 | 13.5 | 22.5 | 0 | 23 | 14.75 | 16.5 | 0.75 | 18.5 |
| H4 | 1 | 29 | 0 | 24 | 1.25 | 30 | 0 | 17.75 |
| H5 | 28 | 3 | 0 | 22 | 34.25 | 2.75 | 0 | 10.25 |
| H6 | 14.5 | 18 | 1.5 | 20.5 | 23 | 10.25 | 0 | 15.75 |
| H7 | 14 | 17.5 | 0 | 22 | 27.25 | 5.25 | 0 | 10.5 |
| H8 | 3 | 27.5 | 1 | 22.5 | 31 | 4.5 | 0 | 19.25 |
| A1 | 4.5 | 29.5 | 1.5 | 18 | 9.5 | 23.75 | 7 | 9.25 |
| A2 | 2 | 28.5 | 0 | 26 | 21 | 10.5 | 2 | 17.75 |
| A3 | 3 | 27 | 0 | 15.5 | 0.75 | 30.25 | 6 | 9 |
| A4 | 19 | 11.5 | 0 | 25.5 | 20.5 | 24.5 | 0.25 | 7.75 |
The potential involvement of the hippocampus in modulating the perceived intensity of threat is supported by the finding that monkeys with hippocampal lesions display a striking lack of arousal or interest in the threatening stimuli. The lack of interest was evidenced by greater amounts of both approach behavior and no-look-at behavior exhibited by hippocampectomized monkeys relative to controls on snake+spider trials. Like the amygdalectomized monkeys, but unlike controls, monkeys with hippocampal lesions spent most of their time located in close proximity to the fear-provoking stimuli (i.e., approach behavior). Whereas controls exhibited freezing behavior and often actively avoided looking towards or at the snake or spider (e.g., head aversion, eye aversion), thereby obtaining high scores for defensive responses, monkeys in Group H directed their gaze indiscriminately on features such as the side or bottom of the cage, the wall, the video camera or some part of the test chamber. Except on their first ever exposure to the snake and spider, as mentioned earlier, monkeys with hippocampal lesions appeared equally uninterested in the snake, spider, and neutral objects.
Several lines of evidence suggest a role for the hippocampus in anxiety as distinct from fear, which is thought to depend on the amygdala (Davis and Shi, 1999; Gray and McNaughton, 2000; Bannerman et al., 2004) and our data are consistent with this theoretical framework. For example, hippocampal lesions in rats produce reductions in several behavioral indexes of anxiety including open arm avoidance in the elevated plus maze, social interaction, and novelty-induced reduction in eating (Bannerman et al., 2002; Kjelstrup et al., 2002). By comparison, amygdala lesions are without effect on such classical tests of anxiety (Treit and Menard, 1997; Kjelstrup et al., 2002) but animals with damage to either structure are insensitive to stimuli that normally evoke intense fear (Blanchard and Blanchard, 1972a, b; present study). According to this view, outlined in the previous paragraph (Gray and McNaughton, 2000; McNaughton and Corr, 2004), hippocampal and amygdala lesions produce the same behavioral effect of emotional blunting (i.e., increased approach and decreased avoidance), but for different reasons: the amygdala mediates the expression of the appropriate emotional response, and the hippocampus prepares the animal for effective escape by boosting arousal in the threatening context.
There is at least one more aspect of our data in need of explanation. Based on findings from functional imaging studies in humans, it has been suggested that the amygdala facilitates visual sensory or attentional processing of fear-producing stimuli (Morris et al., 1996; Pessoa et al., 2002; Phelps et al., 2006). Consistent with this interpretation, patients with amygdala damage fail to show enhanced attention to emotionally salient events (Anderson and Phelps, 2001), and patients with removals of the medial temporal lobe that include the amygdala fail to show the valence effect, i.e., they fail to show an enhancement of activity in visual neocortex when viewing facial expressions of emotion relative to neutral faces (Vuilleumier, 2005). The foregoing appears in direct contrast to the current results which suggest instead that amygdala damage in monkeys leads to “enhanced” attention to the threatening stimulus. The apparent discrepancy may be due to differences in neural processing of social stimuli versus potential predators, or in a lack of correspondence between looking behavior, on the one hand, and direction of attention to sensory processing, on the other hand.
In conclusion, in this study, we characterized the effects of amygdala and hippocampal lesions in monkeys on unlearned fear. Lesions of either structure prevented the normal expression of defensive, adaptive responses elicited by fear-provoking stimuli (i.e., fake snake and spider) confirming an important role for the amygdala and the hippocampus in the normal expression of the fear response. However, we also showed that the fear-provoking stimuli elicited an abnormal visual response in monkeys with amygdala lesions. This was in contrast with a general lack of interest and emotional reactivity exhibited by monkeys with hippocampal lesions when exposed to the threatening stimuli. Evidently, the hippocampus and amygdala contribute independently to the overall modulation of the emotional response, which presumably enables the animal to adapt to a threatening or negative item or context. Current evidence indicates it is the ventral portion of the hippocampus thought to be responsible for risk assessment in rodents. Future studies should examine whether the corresponding portion of the primate hippocampus, namely the rostral hippocampus, is essential for expression of snake fear. In addition, identification of the specific circuits that potentially signal fear and nonfear states (Herry et al., 2008; Likhtik et al., 2008) would be an important advance in delineating the selective roles of the amygdala and hippocampus in expression of conditioned and unconditioned fear.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health. YC is now at Department of Psychology, McGill University, 1205 Dr Penfield Avenue, Montreal, QC H3A 1B1. AI is now at Department of Psychology, California State University, 5151 State University Drive, Los Angeles, CA 90032.
Abbreviations
- H
Hippocampus
- A
Amygdala
- CON
unoperated controls
- NMDA
N-methyl-d-aspartate
- CA
Cornu Ammonis
- MRI
Magnetic resonance imaging
- WGTA
Wisconsin General Testing Apparatus
- CCTV
Closed Circuit Television
- SMPTE
Society of Motion Picture and Television Engineers
- ANOVA
Analysis of Variance
References
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, Prather M. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nature neuroscience. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Matthews P, Deacon RM, Rawlins JN. Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behavioral neuroscience. 2004;118:1033–1041. doi: 10.1037/0735-7044.118.5.1033. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of comparative and physiological psychology. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Effects of hippocampal lesions on the rat's reaction to a cat. Journal of comparative and physiological psychology. 1972;78:77–82. doi: 10.1037/h0032176. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Conditional motor learning in the nonspatial domain: effects of errorless learning and the contribution of the fornix to one-trial learning. Behavioral neuroscience. 2005;119:662–676. doi: 10.1037/0735-7044.119.3.662. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological psychiatry. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in cognitive sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual review of neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annals of the New York Academy of Sciences. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behavioral neuroscience. 2002;116:494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-D-aspartate antagonist DL-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behavioral neuroscience. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Neural interaction between the basal forebrain and functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 2001;103:593–614. doi: 10.1016/s0306-4522(00)00585-6. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of anxiety: An enquiry into functions of the septo-hippocampal system. Oxford University Press; Oxford: 2000. [Google Scholar]
- Hampton RR, Buckmaster CA, Anuszkiewicz-Lundgren D, Murray EA. Method for making selective lesions of the hippocampus in macaque monkeys using NMDA and a longitudinal surgical approach. Hippocampus. 2004;14:9–18. doi: 10.1002/hipo.10150. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Kim CC, Kim JK, Kim MS, Chang HK, Kim JY, Lee IG. Fear response and aggressive behavior of hippocampectomized house rats. Brain research. 1971;29:237–251. doi: 10.1016/0006-8993(71)90031-x. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of function of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. The Journal of comparative neurology. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. The European journal of neuroscience. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG. Bilateral neurotoxic amygdala lesions in rhesus monkeys (Macaca mulatta): consistent pattern of behavior across different social contexts. Behavioral neuroscience. 2008;122:251–266. doi: 10.1037/0735-7044.122.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of learning and memory. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys (Macaca mulatta): generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Malkova L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. The European journal of neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Annals of the New York Academy of Sciences. 2007;1121:273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH. Emotion. Vol. 3. Washington, D.C.: 2003. Individual differences in the responses of naive rhesus monkeys to snakes; pp. 3–11. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. The European journal of neuroscience. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral neuroscience. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Reekie Y, Braesicke K. Synergistic and regulatory effects of orbitofrontal cortex on amygdala-dependent appetitive behavior. Annals of the New York Academy of Sciences. 2007;1121:297–319. doi: 10.1196/annals.1401.019. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in cognitive sciences. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. No effect of hippocampal lesions on perirhinal cortex-dependent feature-ambiguous visual discriminations. Hippocampus. 2006;16:421–430. doi: 10.1002/hipo.20170. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: use for stereotactic neurosurgery. Experimental brain research. Experimentelle Hirnforschung. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. The Journal of comparative neurology. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. The Journal of comparative neurology. 1996;375:552–582. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. The Journal of comparative neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behavioral neuroscience. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Treit D, Menard J. Dissociations among the anxiolytic effects of septal, hippocampal, and amygdaloid lesions. Behavioral neuroscience. 1997;111:653–658. doi: 10.1037//0735-7044.111.3.653. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiology of learning and memory. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Cahill L, McGaugh JL. Disrupting basolateral amygdala function impairs unconditioned freezing and avoidance in rats. The European journal of neuroscience. 2001;14:709–718. doi: 10.1046/j.0953-816x.2001.01696.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends in cognitive sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]


