Abstract
Objective
To assess relations between midpregnancy vaginal defensin levels, a component of the host innate immune response, bacterial vaginosis and risk of preterm delivery. These relations are compared across race groups because previous studies have repeatedly shown that the prevalence of bacterial vaginosis and the risk of preterm delivery are greater in African-American women compared to that in White women.
Methods
Data are from a prospective study that enrolled pregnant women from 52 clinics in five Michigan communities. In the study subcohort, defensins (human neutrophil peptides 1, 2 and 3) and bacterial vaginosis (Nugent criteria) were measured in vaginal fluid collected at enrollment (15th -27th week of pregnancy) from 1,031 non-Hispanic White and African-American women (787 term, 244 preterm). Preterm deliveries were categorized by clinical circumstances; ie, spontaneous and medically indicated.
Results
Among African Americans, vaginal human neutrophil peptides 1-3 levels greater than or equal to the median were associated with bacterial vaginosis and specifically with spontaneous preterm delivery only (adjusted odds ratio = 2.3, 95% CI 1.2, 4.3). Once African-American women were stratified by human neutrophil peptide 1-3 levels, bacterial vaginosis added nothing to the prediction of spontaneous preterm delivery risk. None of the above associations were observed in non-Hispanic Whites.
Conclusions
The relations between human neutrophil peptide 1-3 levels, bacterial vaginosis, and preterm delivery vary by race group. In African Americans, midpregnancy human neutrophil peptide 1-3 levels were more informative to preterm delivery risk than was bacterial vaginosis, suggesting an important role for host response. In addition, elevated human neutrophil peptide 1-3 levels may be a marker for particular high-risk vaginal milieus that are not distinguished by the current bacterial vaginosis Nugent scoring system.
INTRODUCTION
Genital tract infection/inflammation is considered one cause of preterm delivery (PTD), especially early PTD (1-3). Bacterial vaginosis, characterized by a decreased concentration of lactobacilli and an overgrowth of a diverse community of bacteria (4, 5), has been associated with upper genital tract infection/inflammation and with PTD (6-9). The higher prevalence of bacterial vaginosis in African Americans compared with non-Hispanic Whites (10-12) has lead some to hypothesize that bacterial vaginosis might be related to racial disparities in PTD risk (13). Unfortunately, randomized clinical trials using antimicrobial agents to prevent PTD have yielded primarily disappointing results (14-21), and there remains a limited understanding of the complex interplay between bacterial overgrowth and host immune response in the vagina and in gestational tissues.
As part of the vaginal tract’s innate immune system, mucosal epithelial cells and inflammatory cells produce antimicrobial peptides. Antimicrobial peptides include lactoferrin, lysozyme, defensins, and cathelicidins (22-26), which can be produced constitutively or induced by infection. Antimicrobial peptides of the defensin class belong to two structurally distinct groups in humans, α-defensins and β-defensins (22). Of the six α-defensins identified in humans, HNPs 1,2,3 and 4 are produced by neutrophils (24, 27). Elevations in amniotic fluid HNPs 1, 2 and 3 were found in pregnant women with preterm labor and subclinical intra-uterine infection (28). High HNP 2 levels detected in vaginal fluid collected at 24-29 weeks of pregnancy were linked to an increased risk of spontaneous PTD at less than 32 weeks (29).
Building on previous studies, this study evaluated HNPs 1, 2 and 3 in vaginal fluid sampled earlier in pregnancy, at 15-27 weeks. The goal was to assess relations between vaginal HNP 1-3 levels, bacterial vaginosis, and risk of PTD and compare the findings across two race groups, non-Hispanic Whites and African Americans.
MATERIALS AND METHODS
This study included participants from the Pregnancy Outcomes and Community Health (POUCH) Study (30), which enrolled women in their 15th -27th week of pregnancy from 52 clinics in five Michigan communities from 1998 to 2004. Eligibility criteria included maternal age >14 years, English-speaking, a singleton pregnancy with no known congenital or chromosomal abnormalities, no pre-pregnancy diabetes, and screening at 15th -22nd weeks’ gestation for maternal serum alpha-fetoprotein (MSAFP), a biomarker of particular interest in the POUCH Study. Eligible women were randomly sampled into the study and those with unexplained high MSAFP (> 2 multiples of the median) were over-sampled (7% of cohort). Of the 3,038 women enrolled, 19 were lost to follow-up, leaving 3,019 (99.4 %) in the cohort. Study protocols were approved by institutional review boards at Michigan State University and eight hospitals in the five communities. All participants provided written consent prior to enrollment. Comparisons of POUCH study women with aggregate birth certificate data specific to the five study communities showed that the POUCH sample was very similar to community mothers on most factors measured, including age, parity, education levels, the proportions of women with Medicaid insurance, PTD, previous stillbirth, previous preterm infant, and previous low birth weight infant. The one exception, the percentage of African Americans over 30 years of age, was 14% in the POUCH study and 21% in the birth certificate data (30).
A sub-cohort was selected to maximize resources. The sub-cohort included all women who delivered preterm, all women with unexplained high MSAFP levels, and a random sample of women who delivered at term with normal MSAFP, with an over-sampling of African Americans in this latter category. In the POUCH study sub-cohort (N=1,371), 692 (50.5%) women reported their race/ethnicity as non-Hispanic White, 579 (42.2%) as African American, and 100 (7.3%) as another racial/ethnic group. Because of the small number of participants and the broad racial/ethnic mix, the “other” group was excluded from the following analyses. In addition, 235 (18%) non-Hispanic White and African-American women declined vaginal fluid sampling and 5 had samples with insufficient volume, leaving a sub-cohort sample of 1,031 women for the current analyses (787 term, 244 preterm).
At enrollment, study participants met with a study nurse and provided information about demographics, current pregnancy, reproductive history, health behaviors such as smoking, and psychosocial factors. Vaginal fluid samples were collected via a fetal fibronectin specimen collection kit (Adeza International, Sunnyvale, CA, USA). After placement of a vaginal speculum, vaginal fluid was collected from the posterior fornix with a sterile Dacron swab. The swab was placed in 1 ml extraction buffer and refrigerated (4°C) for at least 24 hours. After the refrigeration period, buffer was expressed from the swab, the specimen was filtered using a 10.25mm × 4” serum filter (Fisherbrand Serum Filter System, Fisher Scientific, Pittsburgh, PA, USA), divided into 0.5 ml aliquots, and stored at −80°C.
Vaginal fluid specimens were later thawed and 50 μl aliquots were removed, refrozen, and shipped to the University of Alabama at Birmingham for assay. An ELISA kit measured total HNP level which included HNP 1, 2 and 3 (HyCult Biotechnology, The Netherlands) and was reported out from the assay as one value. This approach has been used in other studies, including one that assessed defensin levels and presence of sexually transmitted pathogens in urethral lavages (31). Thirteen women had a vaginal HNP 1-3 level below the lower limit of detection and these values were imputed as half the detection level.
At the time of vaginal fluid sampling, study nurses also obtained vaginal smears. Smears were Gram-stained and evaluated for bacterial vaginosis by a microbiologist using the Nugent scoring system (5). Scores ranged from 0 to 10, with bacterial vaginosis status categorized as negative (0-3), intermediate (4-6), and positive (7-10).
Information on three major vaginal infections, Chlamydia, Gonorrhea, and Trichomonas, was ascertained in two ways. At enrollment, just prior to vaginal fluid sampling, women were asked about diagnoses of these vaginal infections for two different periods, the year before the pregnancy and during pregnancy. Prenatal and labor and delivery records were abstracted and women were classified as being culture positive or negative for any of the three infections. Culture results from the pregnancy period were available in 99% of women. In addition, the maternal interview asked women to report antibiotic use from the beginning of the pregnancy up through the time of enrollment.
Preterm delivery was defined as birth before 37 completed weeks’ gestation. Gestational age was determined by the last menstrual period (LMP) or by ultrasound data when the LMP-derived gestational age differed from the ultrasound estimate by at least two weeks. Based on information abstracted from labor and delivery medical records, PTD was divided into two groups: 1) Spontaneous PTD included women with preterm labor defined as regular contractions that led to cervical change (≥2 cm dilatation), or spontaneous premature rupture of membranes; and 2) medically indicated PTD included women who had labor induced or who were delivered by C-section before either preterm labor or premature rupture of membranes.
The overall analytic goals were first to assess relations between vaginal HNP 1-3 levels and maternal characteristics including bacterial vaginosis, and second to examine associations between vaginal HNP 1-3 levels and risk of PTD. All analyses incorporated sampling weights to account for the sampling schemes used to construct the cohort and sub-cohort, with preterms and African-American women weighted to their original proportion in the cohort, and unexplained MSAFP weighted to the original proportion in the population. The proportional sampling weights remove any bias introduced by oversampling at risk groups into the sub-cohort. For the comparison of mean vaginal HNP 1-3 levels between African Americans and non-Hispanic Whites, HNP 1-3 levels were transformed to the natural log scale and analyzed using SAS Surveymeans and Surveyreg procedures (32).
HNP 1-3 levels were dichotomized using the median value (high ≥ median, low < median) from the distribution of HNP 1-3 levels in women who delivered at term and had normal MSAFP levels. The median was selected because effects were similar for women in the upper third and fourth quartile of HNP 1-3 levels. Race-specific analyses were performed to evaluate the bacterial vaginosis Nugent score and other maternal characteristics (age, education level, smoking, Medicaid Insurance status, week of pregnancy at study enrollment, and parity) in relation to high and low HNP 1-3 levels (SAS surveyfreq). The Rao-Scott chi-square test, used in complex survey designs (33), was applied to test for statistically significant associations. Maternal characteristics associated with HNP 1-3 levels at p<0.10 were considered potential confounders to be included in final models.
Polytomous logistic regression models (34) (SAS surveylogistic) tested associations between covariates (high/low HNP 1-3 levels, bacterial vaginosis, and race) and a three-level outcome variable: Term (referent group), spontaneous PTD, and medically indicated PTD. Results showed a statistically significant three-way interaction between race, HNP 1-3 levels, and bacterial vaginosis status. This led to the development of race-specific models to calculate unadjusted and adjusted odds ratios for the associations between HNP 1-3 levels and PTD subtypes. Models with a four-level outcome; i.e., same as above but spontaneous PTD subdivided into < 32 weeks and 32-36 weeks, explored the specificity of the relation between HNP 1-3 levels and timing of spontaneous PTD. Beta estimates from race-specific models were used to calculate probabilities of each PTD subtype for six groups defined by bacterial vaginosis (normal, intermediate, high) and HNP 1-3 (high, low) status and results were displayed in graphs.
RESULTS
Maternal characteristics are presented without sampling weights to describe the sub-cohort and with sampling weights to compare characteristics across race groups (Table 1). Analyses of weighted data showed that, compared with non-Hispanic Whites, African-American women were younger, had fewer years of education, were more likely to be insured by Medicaid, smoked less if still smoking at enrollment, and were more likely to enroll in the study before 20 weeks’ gestation. In addition PTD risk was significantly greater in African-American women (14%) compared to that in non-Hispanic White women (10%). With the exception of the 13 women with undetectable levels, participants’ HNP1-3 levels ranged from 2.0 to 90,340 ng/ml (weighted median = 2,661 ng/ml; weighted mean = 7,398 ng/ml). The weighted mean log HNP1-3 level was significantly higher in non-Hispanic Whites compared to that in African Americans.
Table 1.
Maternal characteristics of 1031 non-Hispanic White and African American POUCH study subcohort women with vaginal fluid analyses
| Maternal Characteristics | Non-Hispanic White (n=569) |
African American (n=462) |
||||
|---|---|---|---|---|---|---|
| No. | Sample (%) |
Weighted (%)‡ |
No. | Sample (%) |
Weighted (%)‡ |
|
| Age (years) † | ||||||
| <20 | 51 | (9) | (9) | 126 | (27) | (27) |
| 20-29 | 319 | (56) | (57) | 272 | (59) | (60) |
| ≥30 | 199 | (35) | (34) | 64 | (14) | (13) |
| Education † | ||||||
| <12 years (age<20) | 30 | (5) | (5) | 90 | (20) | (20) |
| <12 years (age≥20) | 33 | (6) | (6) | 88 | (19) | (19) |
| 12 years | 166 | (29) | (27) | 139 | (30) | (31) |
| > 12 years | 340 | (60) | (62) | 145 | (31) | (30) |
| Smoking † | ||||||
| No smoke during pregnancy | 404 | (71) | (73) | 325 | (71) | (69) |
| Stopped before enrollment | 62 | (11) | (10) | 38 | (8) | (9) |
| Smoked <1/2 pack/day at enrollment | 56 | (10) | (9) | 84 | (18) | (19) |
| Smoked ≥ 1/2 pack/day at enrollment | 47 | (8) | (8) | 15 | (3) | (3) |
| Medicaid Insured * † | ||||||
| No | 359 | (63) | (65) | 74 | (16) | (16) |
| Yes | 209 | (37) | (35) | 388 | (84) | (84) |
| Week of Pregnancy At Enrollment † | ||||||
| <20 weeks | 85 | (15) | (14) | 91 | (20) | (20) |
| 20-24 weeks | 414 | (73) | (75) | 319 | (69) | (69) |
| 25-27 weeks | 70 | (12) | (11) | 52 | (11) | (11) |
| Parity * | ||||||
| 0 live birth | 231 | (41) | (39) | 192 | (42) | (42) |
| ≥1 live birth | 337 | (59) | (61) | 270 | (58) | (58) |
| Pregnancy Outcome † | ||||||
| Term | 412 | (72) | (90) | 375 | (81) | (86) |
| Preterm | ||||||
| Medically Indicated | 49 | (9) | (3) | 28 | (6) | (5) |
| Spontaneous | 108 | (19) | (7) | 59 | (13) | (9) |
| Bacterial Vaginosis † | ||||||
| Negative | 482 | (85) | (85) | 318 | (69) | (68) |
| Intermediate | 46 | (8) | (9) | 54 | (12) | (12) |
| Positive | 41 | (7) | (6) | 90 | (19) | (20) |
| Vaginal HNP1-3 ng/ml (defensins) ‡ | ||||||
| Median (Interquartile Range) | 2725 (6939) | 2167 (6433) | ||||
| Mean (SD) | 7414 (593) | 7355 (646) | ||||
| ln(HNP1-3) Mean (SD)† | 7.68 (0.1) | 7.33 (0.1) | ||||
Data missing for 1 woman
p<0.05 for comparisons between non-Hispanic White and African-American using weighted data
Weighted for the subcohort sampling scheme; approximates distribution in original cohort
In non-Hispanic Whites two factors were associated with low HNP1-3 levels, maternal age over 29 years and bacterial vaginosis, though the inverse relation with bacterial vaginosis was not statistically significant (Table 2). Among African Americans, samples collected earlier in pregnancy had lower HNP1-3 levels. The bacterial vaginosis positive group had the largest percentage of women with high HNP1-3 levels (57%), followed by the bacterial vaginosis intermediate group (53%), and the bacterial vaginosis negative group (43%).
Table 2.
Race-specific relations between maternal characteristics and vaginal HNP1-3 levels
| Maternal Characteristics | Non-Hispanic White (n=569) |
African American (n=462) |
||
|---|---|---|---|---|
| Low HNP1-3 (n=272) |
High HNP1-3 (n=297) |
Low HNP1-3 (n=242) |
High HNP1-3 (n=220) |
|
| %‡ | %‡ | %‡ | %‡ | |
| Age (years | ||||
| <20 | 49 | 51 | 51 | 49 |
| 20-29 | 41 | 59 | 51 | 49 |
| ≥30 | 54† | 46 | 64 | 36 |
| Education | ||||
| <12 years (age<20 | 58 | 42 | 57 | 43 |
| <12 years (age≥20 | 52 | 48 | 52 | 48 |
| 12 years | 48 | 52 | 49 | 51 |
| >12 years | 44 | 56 | 55 | 45 |
| Smoking | ||||
| No smoking during pregnancy | 44 | 56 | 51 | 49 |
| Stopped before enrollment | 42 | 58 | 59 | 41 |
| Smoked <1/2 pack/day at enrollment | 58 | 42 | 55 | 45 |
| Smoked ≥ 1/2 pack/day at enrollment | 61 | 39 | 67 | 33 |
| Medicaid Insured * | ||||
| No | 44 | 56 | 60 | 40 |
| Yes | 50 | 50 | 52 | 48 |
| Week of Pregnancy at Enrollment | ||||
| <20 weeks | 47 | 53 | 67† | 33 |
| 20-24 weeks | 46 | 54 | 50 | 50 |
| 25-27 weeks | 49 | 51 | 46 | 54 |
| Parity * | ||||
| 0 live birth | 49 | 51 | 50 | 50 |
| ≥1 live birth | 44 | 56 | 55 | 45 |
| Bacterial Vaginosis | ||||
| Negative | 44 | 56 | 57 | 43 |
| Intermediate | 56 | 44 | 47 | 53 |
| Positive | 58 | 42 | 43 | 57† |
| STD Positive Culture in Pregnancy § | ||||
| No | 46 | 54 | 55 | 45 |
| Yes | 40 | 60 | 45 | 55 |
Data missing for 1 woman
p<0.05 for high versus low vaginal HNP1-3 within race/ethnic group
Weighted for the sub-cohort sampling scheme; approximates distribution in original cohort
Information abstracted from medical records; Data missing for 10 women
High vaginal HNP1-3 levels at mid-pregnancy were associated with spontaneous PTD, unadjusted odds ratio = 2.2 (95% CI 1.2, 4.0), but not with medically indicated PTD in African-American women (Table 3). These results remained virtually unchanged after adjusting for maternal age, week of pregnancy at sampling, bacterial vaginosis status, and culture results for Chlamydia, Gonorrhea, and Trichomonas ascertained from medical records. Inclusion of other covariates such as maternal self-report of vaginal infections in the year prior to the pregnancy, vaginal infections during pregnancy, and antibiotic use during pregnancy (up through the time of vaginal fluid sampling for HNP1-3 assay and bacterial vaginosis evaluation) did not alter the results. In analogous models with non-Hispanic Whites, vaginal HNP1-3 levels were unrelated to PTD. After further dividing spontaneous PTD by weeks of gestation, high HNP1-3 levels were linked to deliveries at < 32 weeks and at 32-36 weeks in African Americans. There was some evidence that mid-pregnancy vaginal HNP1-3 levels might also be increased in non-Hispanic whites who later delivered spontaneously at < 32 weeks, but statistical power was limited.
Table 3.
Race-specific odds ratios (OR) and adjusted odds ratios (AOR) for high (≥median) versus low (<median) vaginal HNP1-3 levels in mid-pregnancy and risk of preterm delivery.
| Non-Hispanic White (N=569) |
African American (N=462) |
|||||
|---|---|---|---|---|---|---|
| OR (95% CI) |
AOR(95% CI)* |
AOR(95% CI)† |
OR(95% CI) |
AOR(95% CI)* |
AOR(95% CI)† |
|
| Term | Ref | Ref | Ref | Ref | Ref | Ref |
| Medically Indicated | ||||||
|
Preterm Delivery
(<37 weeks) |
0.8 (0.4, 1.5) | 0.8 (0.4, 1.4) | 0.8 (0.4, 1.4) | 0.7 (0.3, 1.5) | 0.7 (0.3, 1.7) | 0.7 (0.3, 1.8) |
| Spontaneous | ||||||
|
Preterm Delivery
(<37 weeks) |
0.8 (0.5, 1.3) | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.3) | 2.2 (1.2, 4.0) | 2.4 (1.3, 4.4) | 2.3 (1.2, 4.3) |
| 32-36 weeks | 0.8 (0.5, 1.2) | 0.8 (0.5, 1.3) | 0.8 (0.5, 1.3) | 2.3 (1.2, 4.3) | 2.4 (1.2, 4.7) | 2.3 (1.1, 4.7) |
| <32 weeks | 2.2 (0.4, 13.4) | 1.9 (0.3, 13.1) | 1.8 (0.2, 13.2) | 2.0 (0.6, 7.5) | 2.3 (0.6, 9.2) | 2.3 (0.6, 9.2) |
Adjusted for maternal age, week of pregnancy at study enrollment and bacterial vaginosis status
Adjusted for maternal age, week of pregnancy at study enrollment, bacterial vaginosis status, and culture results for sexual transmitted diseases during pregnancy (negative/positive)
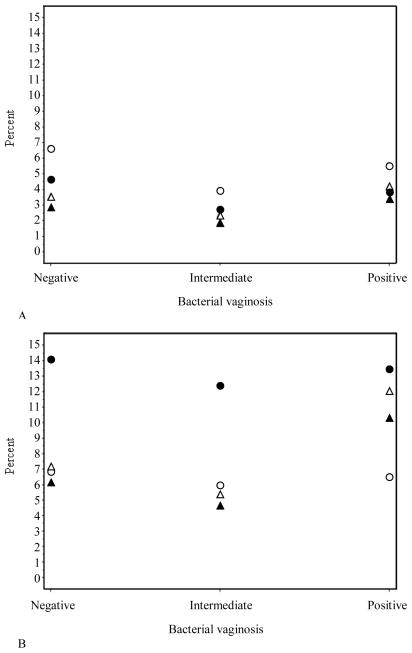
The relation between HNP1-3 and PTD was also considered within the context of bacterial vaginosis. Because the sub-cohort was sampled from a prospective cohort study, weighted analyses permitted calculations of the probabilities (risks) of each PTD subtype given HNP1-3 and bacterial vaginosis status at mid-pregnancy (Figure 1). In both race groups, bacterial vaginosis status and HNP1-3 levels were unrelated to the risk of medically indicated PTD. The risk of spontaneous PTD was significantly associated with high HNP1-3 levels in African Americans, and this association persisted regardless of bacterial vaginosis status. There was no link between spontaneous PTD and vaginal HNP1-3 levels in non-Hispanic Whites among any of the bacterial vaginosis groups.
Figure 1.
Probability of preterm delivery by bacterial vaginosis status among 4 groups; groups defined by vaginal HNP 1-3 levels (high/low) and race (non-Hispanic White/African American).
A-Medically indicated preterm delivery, B-Spontaneous preterm delivery
● ● ● African American, high HNP 1-3 level ▴ ▴ ▴ Non-Hispanic White, high HNP 1-3 level ○ ○ ○ African American, low HNP 1-3 level ▵ ▵ ▵ Non-Hispanic White, low HNP 1-3 level
DISCUSSION
We found that in African-American women, vaginal HNP1-3 levels ≥ median were associated with bacterial vaginosis and with spontaneous PTD. Once African-American women were stratified by vaginal HNP1-3 levels, bacterial vaginosis added nothing to the prediction of spontaneous PTD risk. None of the above associations were observed in non-Hispanic Whites.
The one other cohort study on vaginal defensin levels, bacterial vaginosis and pregnancy outcome reported that elevated defensins were linked to intermediate bacterial vaginosis but not to positive bacterial vaginosis, and to PTD at <32 weeks’ gestation but not to all PTD at < 37 weeks (29, 35). The sample in this study was described as 44% Black and 56% nonblack, similar to our sample, but unlike our results, they found no evidence of effect modification by race. They measured HNP 2, and the interval from vaginal fluid sampling (24th -29th week) to early delivery (<32 weeks) was short. We measured HNP1-3, and vaginal samples were collected earlier in pregnancy (15th -27th week; about 90% before 25 weeks), indicating that higher vaginal defensin levels may not be just a marker of impending labor.
The functional or pathological significance of high defensin levels in vaginal fluid during pregnancy is unclear. The vagina is routinely exposed to a variety of microbes that may elicit neutrophil activation and defensin production, which could be protective or contribute to the risk of PTD. Expression of defensins in the vaginal tract could be constitutive, inducible by infectious/inflammatory stimuli, or both (31, 36). HNP 1-3 levels appear to be induced in urethral lavages of men infected with Neisseria gonorrhoeae and Chlamydia (31) and defensins have been shown to be inducible by proinflammatory cytokines (37). In Simhan et al.’s study, vaginal fluid defensins were positively associated with levels of IL-8 (38).
To explain the positive link between vaginal HNP 1-3 levels and bacterial vaginosis in African Americans but not in non-Hispanic Whites, we considered confounding due to other vaginal infections such as Trichomonas vaginalis (38, 39) but adjustment for sexually transmitted diseases (culture results in pregnancy) did not change our findings. Effect modification by race group might be due to differences in host innate immune response to bacterial vaginosis. Alternatively, because bacterial vaginosis is broadly defined, higher HNP 1-3 levels might signify the presence of specific bacterial vaginosis-related bacteria which are more prevalent in African-American bacterial vaginosis cases than in non-Hispanic White bacterial vaginosis cases, such as mobiluncus species (40).
When we divided PTD by gestational weeks statistical power was limited, but results suggested that high vaginal HNP 1-3 levels were associated with spontaneous PTD < 32 weeks in both non-Hispanic whites and African Americans. Among African Americans the association persisted for the 33-36 week spontaneous PTD as well. These findings could be interpreted as evidence that the pathway to spontaneous PTD marked by elevated HNP 1-3 is of similar importance in the two race groups for the earliest deliveries and continues to be important for the later spontaneous PTDs in African Americans. Our HNP 1-3 level cutoff for ‘high,’ ≥ median, was based on the distribution of HNP 1-3 levels in both non-Hispanic White and African-American women. When we tried using race-specific medians, only 5% of non-Hispanic White and 4% of African-American women were assigned to a different category, and our results and conclusions were unchanged.
A major strength of our study was the composition of the cohort, which consisted of women sampled from 52 clinics and from a wide range of socioeconomic backgrounds. In addition, vaginal HNP 1-3 levels were measured at mid-pregnancy, providing evidence for the early appearance of an inflammatory process and a maternal immune response linked to PTD. The use of less invasive methods (vaginal fluid sampling) for detecting a maternal inflammatory response offers greater clinical applications. We also separated medically indicated PTD from spontaneous PTD, thereby demonstrating specificity and biologic coherence in our findings. A limitation of this study was the single, simultaneous measurement of bacterial vaginosis status and HNP 1-3 levels, precluding establishment of a temporal relation between bacterial vaginosis and elevated HNP 1-3 levels.
The observed effect modification by race group in the relations between bacterial vaginosis status, HNP 1-3 levels, and PTD is a new finding and requires additional studies to explore underlying explanations for these results. It may be informative to compare the vaginal microbial ecology across different race groups in relation to vaginal HNP 1-3 levels. In addition, elevated HNP 1-3 levels may be a marker for particular high-risk vaginal milieus that are not distinguished by the current bacterial vaginosis Nugent scoring system.
Acknowledgments
Supported by the National Institute of Child Health and Human Development grant number R01 HD034543, National Institute of Nursing Research (Renewal NIH POUCH) grant number R01 HD34543, March of Dimes Foundation (Perinatal Epidemiological Research Initiative Program) Grants 20-FY98-0697 through 20-FY04-37, Thrasher Research Foundation grant 02816-7 and Centers for Disease Control and Prevention grant U01 DP000143-01.
Footnotes
Presented at the Society for Pediatric & Perinatal Epidemiology meeting, Boston, Massachusetts, June 19, 2007.
Financial Disclosure: The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 2.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–8. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–26. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 5.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333:1737–42. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 7.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189:139–47. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 8.Peipert JF, Montagno AB, Cooper AS, Sung CJ. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–7. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 9.Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100:456–63. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 10.Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–20. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg RL, Klebanoff MA, Nugent R, Krohn MA, Hillier S, Andrews WW. Bacterial colonization of the vagina during pregnancy in four ethnic groups. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1996;174:1618–21. doi: 10.1016/s0002-9378(96)70617-8. [DOI] [PubMed] [Google Scholar]
- 12.Rajamanoharan S, Low N, Jones SB, Pozniak AL. Bacterial vaginosis, ethnicity, and the use of genital cleaning agents: a case control study. Sex Transm Dis. 1999;26:404–9. doi: 10.1097/00007435-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88:233–8. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin-positive women. Obstet Gynecol. 2003;101:847–55. doi: 10.1016/s0029-7844(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 15.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg RL, Mwatha A, Read JS, Adeniyi-Jones S, Sinkala M, Msmanga G, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol. 2006;194:650–61. doi: 10.1016/j.ajog.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Guaschino S, Ricci E, Franchi M, Frate GD, Tibaldi C, Santo DD, et al. Treatment of asymptomatic bacterial vaginosis to prevent pre-term delivery: a randomised trial. Eur J Obstet Gynecol Reprod Biol. 2003;110:149–52. doi: 10.1016/s0301-2115(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 18.Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med. 1995;333:1732–6. doi: 10.1056/NEJM199512283332603. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487–93. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 20.Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol. 2003;188:752–8. doi: 10.1067/mob.2003.167. [DOI] [PubMed] [Google Scholar]
- 21.McDonald H, Brocklehurst P, Parsons J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database of Systematic Reviews. 2005;(1) doi: 10.1002/14651858.CD000262.pub2. CD000262. [DOI] [PubMed] [Google Scholar]
- 22.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1(3):141–50. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 24.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, et al. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–58. [PMC free article] [PubMed] [Google Scholar]
- 25.Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB, Jr., Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 27.Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–9. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27:513–8. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 29.Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, et al. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol. 2003;101:862–8. doi: 10.1016/s0029-7844(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 30.Holzman C, Bullen B, Fisher R, Paneth N, Reuss L, Group PS. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):136–58. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 31.Porter E, Yang H, Yavagal S, Preza GC, Murillo O, Lima H, et al. Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun. 2005;73:4823–33. doi: 10.1128/IAI.73.8.4823-4833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SAS Institute . Base SAS 9.1.3 procedures guide. 2nd ed SAS Pub.; Cary, NC: 2006. [Google Scholar]
- 33.Rao JNK, Scott AJ. The Analysis of Categorical-Data from Complex Sample-Surveys - Chi-Squared Tests for Goodness of Fit and Independence in 2-Way Tables. J Am Stat Assoc. 1981;76:221–30. [Google Scholar]
- 34.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd edition John Wiley & Sons; New York (NY): 2000. [Google Scholar]
- 35.Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, et al. Bacterial vaginosis and vaginal fluid defensins during pregnancy. Am J Obstet Gynecol. 2002;187:1267–71. doi: 10.1067/mob.2002.126989. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Xu Z, Peng L, Fang X, Yin X, Xu N, et al. Recent advances in the research and development of human defensins. Peptides. 2006;27:931–40. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Holst N, Oian P, Aune B, Jenssen TG, Burhol PG. Increased plasma levels of vasoactive intestinal polypeptide in pre-eclampsia. Br J Obstet Gynaecol. 1991;98:803–6. doi: 10.1111/j.1471-0528.1991.tb13486.x. [DOI] [PubMed] [Google Scholar]
- 38.Simhan HN, Anderson BL, Krohn MA, Heine RP, de Tejada B Martinez, Landers DV, et al. Host immune consequences of asymptomatic Trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol. 2007;196:59, e1–5. doi: 10.1016/j.ajog.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 39.Cotch MF, Pastorek JG, 2nd, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Pereira L, Culhane J, McCollum K, Agnew K, Nyirjesy P. Variation in microbiologic profiles among pregnant women with bacterial vaginosis. Am J Obstet Gynecol. 2005;193:746–51. doi: 10.1016/j.ajog.2005.01.069. [DOI] [PubMed] [Google Scholar]