Abstract
Background
This study aimed to assess the relationship between IFN related adverse effects and HCV virologic response in HIV/HCV co-infected individuals treated with pegylated interferon and ribavirin.
Methods
We conducted two prospective, open-label trials treating HIV/HCV co-infected individuals with peg-IFN alpha-2b or alpha-2a and ribavirin for 48 weeks. Safety labs, HCV RNA, psychiatric and ophthalmologic evaluations were performed at baseline, and monthly until week 72.
Results
Responders were defined as those with HCV RNA decline of ≥ 2-log drop from baseline and non-responders were those who did not. Remarkably, of the 27 patients who (50%) developed psychiatric toxicities, twenty-six patients were responders, while only one of fourteen virologic non-responders experienced psychiatric toxicity. Other adverse effects such as anemia and ophthalmologic toxicities, were also more frequent in responders compared to non-responders. Decline in CD4+ T cell counts strongly correlated with HCV viral decline.
Conclusion
Our study demonstrate coupling of antiviral effect and occurrence of adverse events in HIV/HCV co-infected patients. These patients with IFN-related adverse effects need a multidisciplinary treatment approach, hence they are more likely to achieve SVR. Future studies are needed to evaluate the factors that predict the development of IFN-α dependent adverse events prior to therapy.
Keywords: HCV, HIV, Peg-Interferon alpha, adverse events, psychiatric toxicity
Co-infection with Hepatitis C [HCV] occurs in approximately one third of HIV-1 infected patients in the US 1. It has been shown that liver disease is more rapidly progressive in co-infected patients with liver fibrosis, cirrhosis, hepatocellular carcinoma and liver failure occurring earlier than in HCV mono-infected patients 2, 3. Introduction of antiretroviral therapy [ART], has resulted in decline of HIV related mortality; however HCV-related liver disease has become a leading cause of hospitalization and death 3, 4. The efficacy of pegylated interferon [peg-IFN] plus ribavirin as treatment for HCV infection in co-infected patients has been shown to be much lower than HCV mono-infected patients, particularly in those infected with genotype 1 5. HIV/HCV co-infected patients are also characterized by high rates of drug use and neurocognitive and psychiatric disorders rendering them potentially even more susceptible to the neuropsychiatric adverse events of interferon therapy 6, 7. Likewise, chronic HIV infection, use of antiretrovirals, prophylactic medications and malignancies may contribute to various cytopenias and make individuals more susceptible to hematologic toxicity from peg-IFN and ribavirin therapy 8, 9. It is also well established that maintenance of recommended doses of peg-IFN and ribavirin throughout treatment is crucial to the achievement of a successful outcome 10, 11. Many studies have suggested that there may be higher rates of toxicities in HIV/HCV co-infected patients 12–15, however, none have explored the possibility that there might be differences in IFN-α related adverse events in responders compared to non-responders. Since both antiviral response and adverse effects are mediated by IFN-α signaling of host cells 16, it would seem plausible that antiviral response and occurrence of severe adverse events are related to one another. In this regard, we investigated the relationship between IFN-α-related adverse effects and HCV virologic response in HIV/HCV co-infected individuals on therapy.
METHODS
Study Design
Two pilot, prospective, single center, open label trials were performed at the National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health from 2001 to 2008. Fifty-five HIV/HCV co-infected patients were treated with ribavirin daily (Rebetol, Schering-Plough, at 1000 mg dose <75kg, 1200 mg dose for >75kg) and peginterferon alpha-2b at 1.5ug/kg subcutaneously weekly (Peg-Intron, Schering-Plough) or peg interferon alpha-2a (Pegasys, Roche Laboratories) for 48 weeks and followed up for up to 24 weeks after completion of treatment. Treatment responders were defined as HCV viral RNA levels below level of detection (<615 IU/ml) or HCV RNA levels declining to greater than a 2-log drop from baseline by week 12. The responders include patients who achieved SVR and relapsers. Non-responders were individuals who never achieved greater than a 2-log decline in HCV RNA level by week 12. All patients gave written informed consent approved by the NIAID Institutional Review Board prior to enrollment in the studies.
Study Subjects Selection
HIV/HCV co-infected patients were eligible if they had a CD4 count >100 cells/mm3, absolute neutrophil count > 1000 cells/mm3, hemoglobin >10g/dL, HCV viral load > 2000 copies/ml, histologic evidence of chronic HCV infection. Exclusion criteria were advanced cirrhosis, severe liver decompensation, active and severe psychiatric disorders, active substance abuse or dependence (except nicotine), severe cardiopulmonary illness, renal disease, hemoglobinopathies, and retinopathies.
Laboratory Evaluations
Safety laboratory tests and immune profiles including CD4+ T cell counts were obtained at baseline and frequently during the entire study. G-CSF was initiated at 300 ug/week if ANC dropped below 750 cells/mm3 and titrated up based on response to maintain ANC>1000cells/mm3. erythropoetin was started at 40 ug/week when hemoglobin dropped below 10 g/dL and titrated to maintain hemoglobin above this level.
Psychiatric Evaluations
All patients underwent routine psychiatric interviews conducted by Board-certified psychiatrists from the National Institute of Mental Health. Patients were assessed for current and past psychiatric and substance abuse diagnoses. The majority of patients screened had a history of substance use. Patients who presented with active mood, anxiety, or psychotic symptoms were treated clinically prior to study enrollment. During the study, depression was evaluated by examination, standard diagnostic criteria (DSM-IV), and the Beck Depression Inventory (BDI). Patients who received at least 4 weeks of IFN ribavirin treatment were classified into two groups, those with and without psychiatric toxicity after initiation of therapy based on the criteria (Table 1). Study participation was discontinued for patients in whom severe psychiatric toxicity developed and could not be safely managed with ongoing IFN treatment.
Table 1.
Baseline demographics of patients Enrolled
| All patients (n = 55) |
Responders (n = 41) |
Non-responders (n =14) |
|
|---|---|---|---|
| Median age (SD) years | 47 | 46 | 49 |
| Male gender, n [%] | 48 (87%) | 37 | 11 |
| HCV Genotype, n [%] | |||
| Genotype 1 | 47 (85%) | 33 (80%) | 14 [100%] |
| Genotype 2 | 6 (11%) | 6 (15%) | 0 |
| Genotype 4 | 1 (2%) | 1 (2.5%) | 0 |
| Genotype 5 | 1 (2%) | 1 (2.5%) | 0 |
| Racial distribution, n [%] | |||
| White | 18 (33%) | 16 (39%) | 2 (14%) |
| Black | 32 (58%) | 20 (49%) | 12 (86%) |
| Hispanic | 4 (7%) | 4 (10%) | 0 |
| Other | 1 (2%) | 1 (2%) | 0 |
| Median baseline CD4 T cell count (cells/mm3) |
525 | 546 | 394 |
| Mean baseline CD4 T cell count (cells/mm3) |
558 | 587 | 473 |
| Median baseline HIV-RNA level (log 10 copies/ml) |
< 50 | <50 | <50 |
| Median baseline HCV-RNA levels (log 10 IU/ml) |
6.212 | 6.180 | 6.286 |
| Concomitant ART therapy [%] | 47 (85%) | 35 (85%) | 12 (85%) |
| Use of zidovudine at initiation of therapy | 10 | 10 | 0 |
Ophthalmology Evaluation
All patients underwent ophthalmologic evaluations including visual acuity, visual field testing (automated perimetry using the Field Analyzer Model 750 SITA-fast program; Carl Zeiss Meditec, Dublin, California, USA), color vision exam (using the Ishihara Test for color deficiency, plates 2–17) and indirect ophthalmoscopy were performed at baseline, at least every 3 months or when clinically indicated. Patients who developed cotton wool spots [CWS], cataracts or color vision abnormalities were monitored more frequently based on potential severity of lesions. Peg-IFN treatment was not stopped unless examination revealed more than ten CWS in either eye or deteriorating visual fields, loss of central acuity or any signs of optic neuropathy.
Statistical Analysis
Patients were classified into two groups, treatment responders (which included patients who relapsed after 48 weeks of therapy, but had HCV viral levels <615 IU/mL up until 48 weeks) and treatment non-responders. The IFN-related adverse events studied were anemia [or use of erythropoetin]; neutropenia [or use of G-CSF]; occurrence of psychiatric toxicities and ophthalmologic toxicities. T-tests and nonparametric tests were used to compare continuous outcomes between responders and non-responders, while chi-squared methods and Fisher’s exact test were used to compare categorical outcomes. The relationship between HCV viral load and CD4+ T cell counts was assessed in two ways. First, ordinary regression was used to summarize each patient’s slope relating HCV log viral load to log CD4 count. These per-patient slopes were then plotted to assess whether there was a consistent pattern across patients. A more sophisticated mixed model approach was used to take into account that different patients had different amounts of data. The mixed model incorporated an average intercept and slope across all patients (fixed effects), together with patient-specific intercepts and slopes (random effects)
RESULTS
Study Subjects
From 2001 to 2007, 55 HIV/HCV co-infected patients enrolled in the treatment studies. Baseline demographic data for patients including responders and non-responders are shown in Table 2. Both groups of patients received the same dose and statistically similar duration of therapy and were evaluated for pre-existing psychiatric and other conditions prior to therapy. The mean CD4 count was 558 (108 –1273); median HIV RNA was less than 50 copies/ml. A total of 10 patients discontinued the study before 48 weeks, 4 (40%) of whom were responders. Five of these patients (50%) discontinued by week 2 [day 5 to week 2] for financial, social and work issues that conflicted with the frequent demands of study participation. Five other patients discontinued between week 12 and 24. Three of these patients were responders, all of whom experienced psychiatric toxicity. One developed an acute psychotic episode requiring hospitalization while the other 2 had alcohol and substance abuse relapses that necessitated study discontinuation. The other 2 patients were non-responders and opted to come off the study by week 20 due to non-response to IFN-α. The duration of treatment for non-responders was not statistically different from that for responders (median 42 ± 13 weeks vs 48 ± 5.8 weeks; p>0.05).
Table 2.
Hematologic parameters in patients
| All patients (n =55) |
Responders (n=41) |
Non responders (n=14) |
|
|---|---|---|---|
| Median ANC values | 2310 | 2324 | 2223 |
| Mean ANC values (S.D) | 2517 ± 156 | 2558 ± 108 | 2546 ± 176 |
| Patients requiring G-CSF - requiring dose escalations |
24 (43.6%) 16 (66%) |
17(41.5%) 12 (71%) |
7 (50%) 4 (57%) |
| Median Hemoglobin value | 14.3 ± 0.8 | 14.3 ± 0.6 | 14.4 ± 1.1 |
| Patients requiring erythropoetin | 20 (36.4%) | 15 (36.6%) | 5(35.7%) |
| Use of zidovudine at initiation of therapy | 10 | 10 | 0 |
| Patients requiring both growth factors | 9 (16.3%) | 7(17%) | 2 (11.7%) |
Responders to peginterferon-α and ribavirin had significantly higher CD4+ T cell decline than non-responders
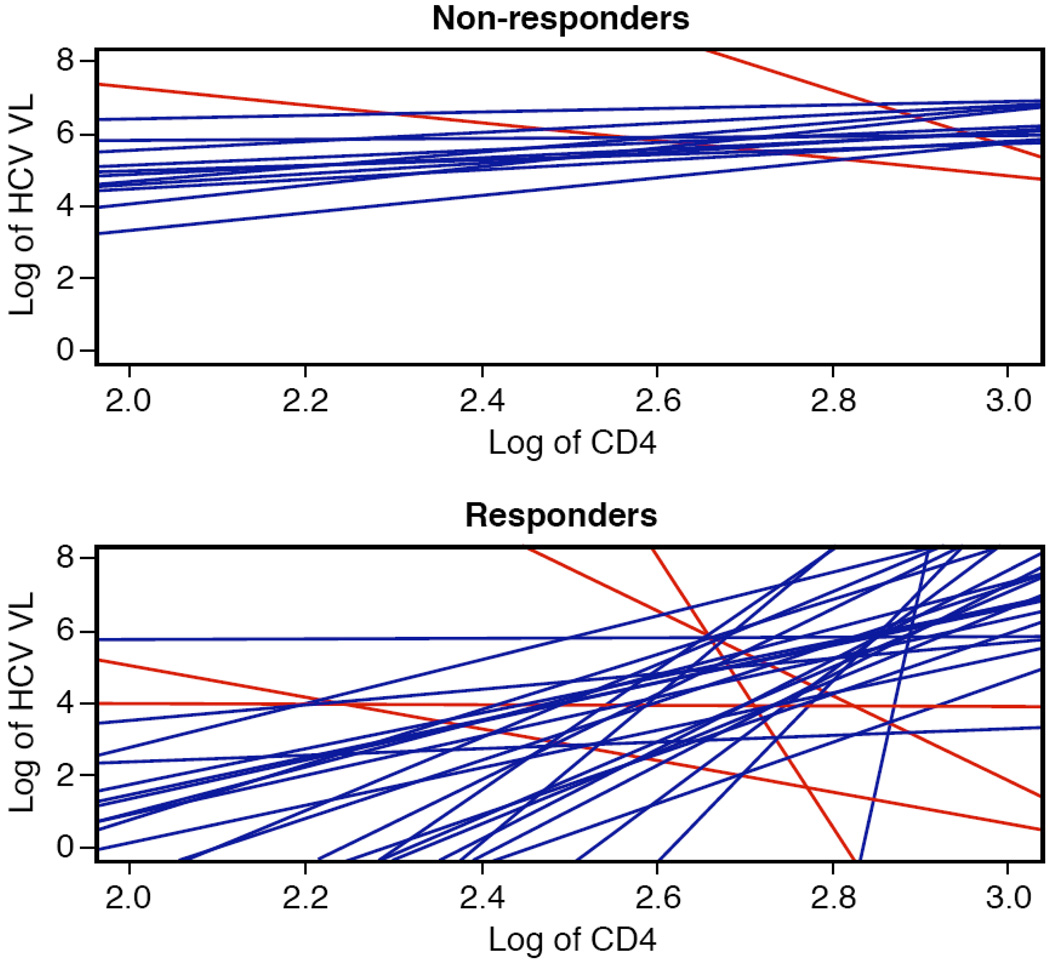
Two methods were used to determine whether CD4+ T cell count was associated with virologic response to peg-IFN and ribavirin therapy. Ordinary regression was used to compute, for each patient, a slope relating HCV log viral load to log CD4 count. These per-patient slopes, depicted in Figure 1, showed remarkable consistency across patients. The great majority of slopes were positive, indicating that HCV viral load and CD4 count tracked together; as one decreased, so did the other, and similarly for increases. A disadvantage of this type of analysis is that it does not take into account that different patients have different amounts of data. Therefore, a mixed model analysis was also used. This more sophisticated analysis addresses the same questions—is there a consistent trend across patients, and what is that trend?. T he result was a highly statistically significant positive slope for the relationship between HCV log viral load and log CD4+ T cell count (overall slope 1.12, p= 0.0001). This shows that CD4 counts tracked with HCV viral loads.
Figure 1.
Relationship between log of HCV viral load and log of CD4 count for each patient for non-responders (top panel) and responders (bottom panel). Each line summarizes the relationship between log HCV viral load and log CD4 count for one patient, and was estimated using ordinary regression. The great majority of patients’ lines had positive (blue) slopes, indicating that log viral load and CD4 counts tracked together. Only a few patients had negative (red) slopes.
IFN-α related psychiatric toxicities were more common in responders than non responders
Twenty-seven [50%] of 55 patients had psychiatric toxicities as defined by the criteria listed in the methods section. While 26 of 41 (63%) peg-IFN treatment responders experienced psychiatric toxicities, only 1 in 14 (7%) non-responders to peg-IFN developed such symptoms (p=0.009; Figure 2). The most common psychiatric adverse effects were anxiety, worsening depression, and other mood disturbances with associated sleep difficulties. Patients with a psychiatric history were no more likely to develop these symptoms than patients without a history of mental illness. Twenty-one of 27 (78%) patients with psychiatric toxicities required additions to or changes in psychiatric medications. The most commonly used antidepressants were mirtazapine (Remeron) and sertraline (Zoloft).
Figure 2.
Incidence of common adverse events in both responders and non-responders. Significantly higher percent of responders experienced a psychiatric adverse event than non-responders (p=0.009) (Figure 2A). Most responders and non-responders experienced neutropenia (Figure 2B) and anemia (Figure 2C), and both these events were not statistically significant (p>0.05 each). There were no significant differences in the incidence of ophthalmologic toxicities between the two groups (p>0.05) (Figure 2D), however the serious dose-limiting ophthalmic toxicities all occurred in subjects who were responding to IFN-α treatment (as described in the Results).
IFN-α related hematologic toxicities: neutropenia
Toxicity was defined as either an ANC level below 750 cells/mm3 and/or use of G-CSF to maintain ANC levels on treatment. Median ANC values were similar in both groups of responders and non-responders (p>0.05) (Table 2). Twenty-four patients [43.6%] experienced ANC decline to below 750 cells/mm3 requiring G-CSF therapy. Seventeen [70.8%] of whom were responders while seven [29%] were non-responders (p>0.05). Sixteen patients [66.6%] required dose escalations up to 480mcg and increased frequency up to thrice weekly, twelve [75%] of whom were responders (p>0.05). Overall, 17 [41.5%] of responders compared to 7 [50%] of non-responders developed neutropenia (p>0.05). Only one patient out of 24 with a baseline ANC > 2500 cells/mm3 required G-CSF whereas 23 out of 31 patients with a baseline ANC ≤ 2500 cells/mm3 did require G-CSF.
IFN-α related hematologic toxicities: anemia
Toxicity was defined as either an Hgb level below 10g/dL and/or use of erythropoetin to maintain Hgb levels on treatment. Twenty patients [36%] had at least one Hgb value less than 10g/dL requiring erythropoetin. Fifteen [75%] were responders while five [25%] were non-responders (p>0.05) (Table 3). Overall, 15 [36%] responders required erythropoetin compared to five [36%] non-responders. (p>0.05). At initiation of therapy, 10 patients were on zidovudine containing regimens and 3 (30%) were switched off due to concerns of anemia during treatment. The decline in Hgb was attributed to anti-HCV treatment; therefore other causes of anemia were not investigated unless there was no response to erythropoetin and/or clinical history was suggestive of further investigation. A total of thirty-two patients [58.2%] received either or both growth factors, while 9 required both factors out of whom seven [77.8%] were responders.
Table 3.
Abnormal ocular findings not IFN-α related.
| Abnormal ocular finding | Patients n=17 |
|---|---|
| Glaucoma | 4 |
| Non specific visual field change | 2 |
| Unchanged cataract * | 2 |
| Others | |
| - Ocular hypertension | 1 |
| - 20/40 OU with hx of sungazing | 1 |
| - Macular mottling | 1 |
| - Chalazion, eyelid lesion | 2 |
| - Amblyopia | 1 |
| - Peripheral retinal scars | 2 |
| - Optic neuritis | 1 |
development of unilateral cataract in a patient that did not progress while on therapy
IFN-α related ophthalmologic toxicities
A total of 38 of 55 (69%) patients had abnormal ocular findings, of whom twenty-one (38%) were judged to be IFN-α related. Seventeen [42%] responders had ophthalmologic toxicity compared to four [29%] non-responders. (p>0.05). There were three patients with serious ophthalmopathy (two with color vision changes and one with a sight threatening cotton wool spot involving the macula) of whom one had to be taken off study medications due to the severity of the event. All three patients had HCV viral load less than 615 IU/mL at the time of the adverse event. The major ophthalmologic adverse events attributable to IFN-α were color vision changes (2), bilateral cataracts (2) and cotton wool spots (17). Ocular pathologies that were not attributable to IFN-α are described in Table 4.
Table 4.
Prevalence of Interferon-related side effects
| Toxicity | All patients [%] |
Responders [%] |
Non responders [%] |
p-value |
|---|---|---|---|---|
| Psychiatric toxicities | 27 (50) | 26(63) | 1(8) | 0.009 |
| Ophthalmologic toxicities | 21(38) | 17 (41) | 4(29) | 0.528 |
| Anemia [Hgb<10] or use of Erythropoietin | 20 (36) | 15(36) | 5 (36) | 0.748 |
| Neutropenia [ANC<1000] or use of G-CSF | 24 (44) | 17(42) | 7 (50) | 0.756 |
Psychiatric and Ophthalmologic adverse events are defined as described in the Methods section.
IFN-α related multiple adverse events
A total of 28 patients (55%) had more than one of the above listed classes of toxicities, namely psychiatric, ophthalmologic, anemia and neutropenia. Twenty-three (56%) of these patients were responders compared to 5 (36%) of non-responders (p>0.05). A total of 10 patients had up to 3 classes of toxicities of whom 8 (80%) were responders. A total of 50 patients experienced at least one major class of IFN-α related adverse effects as shown. The major IFN-α related toxicities seen in this study population are summarized in Table 4.
Discussion
This study demonstrates that serious adverse events associated with IFN-α therapy are seen more commonly with patients who respond virologically. Specifically, the CD4+ T cell decline and psychiatric adverse effects are seen in virologic responders more frequently than in non-responders to IFN-α treatment. Frequent monitoring of CD4+ T cell counts and completion of 48 weeks of anti-HCV treatment for all patients including non-responders were critical in establishing the relationship between virologic response and development of serious adverse events due to IFN-α therapy.
Combination therapy for HCV with peg-IFN and ribavirin is associated with peripheral CD4+ T cell decline 17. Although there is a consistent decline in the absolute CD4+ T cell counts, there is no significant change in the CD4+ T cell percentage, and hence the mechanism and clinical relevance of this drop in CD4+ T cell count decline is unclear 17.. Interestingly, many patients with viral breakthroughs did have CD4+ T cell declines while they were responding with lower HCV viral loads initially, but had higher CD4+ T cell counts while they experienced breakthrough to combination therapy, suggesting a a direct effect of IFN signaling on the bone marrow resulting in a decreased release of cells.
Consistent with prior reports of dose-limiting toxicity seen with HCV/HIV co-infected individuals 5, we observed frequent psychiatric symptoms in patients treated with IFN. The principal challenge in characterizing this IFN-related toxicity is the assignment of psychiatric symptoms observed to the effects of medication as opposed to pre-existing mental conditions. In an effort to avoid type I error in suggesting causality, we established stringent criteria for emergent neuropsychiatric toxicity. An etiologic attribution of mental disturbance to IFN was determined on the basis of direct multidisciplinary evaluation of study subjects, including assessment by the psychiatrist who had seen them prior to IFN treatment.
Consistent with prior studies 18, 50% of patients enrolled experienced specific IFN-related psychiatric toxicities--an important finding in light of the fact that HIV/HCV co-infected patients are known to have high rates of pre-existing substance abuse, neurocognitive and psychiatric disorders 6. However, in contrast to prior studies 18–20, we we observed a strong relationship between viral response and treatment emergent psychiatric toxicity (63% of responders, compared to only 7% of non-responders), and not to baseline psychiatric morbidity.
Although our results stand in contrast to previous findings regarding the relationship between baseline mental health and risk of psychiatric toxicity from IFN, a correlation between emergent psychiatric symptoms with virologic response in IFN-α treated subjects has been reported 21. The therapeutic goal of administering IFN for hepatitis C is to induce a global immunologic expansion in the service of activating cellular processes that will aid the body in clearing HCV. That activation is dependent upon a cascade of immuno-endocrine interactions that are, in part, mediated by cytokines and other small molecules that can freely traverse the blood-brain barrier 22. Roles for such compounds in mediating neuropsychiatric symptoms have been hypothesized and investigated for a number of different disease states and their treatments, from cancer, to multiple sclerosis, to rheumatologic conditions 18–20, 23, 24. In the case of HCV, we suggest that when IFN is effective, one or more of the alterations in cytokine pools necessary for improved immune function may have corresponding negative effects on the brain. Although the profile of psychiatric symptomatology will vary on the basis of individual patients’ genes, environments, and relational patterns, most manifest signs in the realm of mood disturbance, presumably due to effect of the IFN on neural systems related to emotion, memory, and motivated behavior. Those who do not display such symptoms in some form may fail to experience specific aspects of immune system modulation, which are not only crucial for viral clearance, but also adversely affect mental functioning.
Even with the high frequency of psychiatric toxicity observed in this sample, it should be noted that only three patients [6% of all protocol subjects] had to discontinue the study due to psychiatric symptomatology, two of whom experienced substance abuse relapse. This observation reinforces that through extensive multidisciplinary clinical management, active psychiatric assessments and interventions most individuals can complete 48 weeks of HCV treatment with IFN. Similar to what has been observed with gefitinib 25, 26, the emergence of toxicity may, in this case, serve as an important predictor of positive treatment response 27. Furthermore, the presence of seemingly unrelated neuropsychiatric symptoms with IFN treatment for infectious hepatitis lends credence to hypothesized mechanisms of immune-mediated pathophysiology in mental illness 28. A novel approach in the management of HCV patients is developing molecular techniques to predict emergence of serious psychiatric adverse events during treatment with IFN-α 29, 30. Such a tool for screening and monitoring HCV/HIV co-infected individuals could help to optimize IFN-α treatment, enhance the SVR, and even elucidate genetic factors underlying the development of psychiatric symptoms.
Another significant dose-limiting adverse event observed with treatment with IFN- is development of hematologic toxicities such as anemia and neutropenia 31. I The need for G-CSF and erythroepoetin use in a majority of our patients to maintain their ANC counts and Hgb levels respectively within days after starting treatment in many cases made itdifficult to study their relationship between virologic response. The anemia observed was largely attributable to the effects of ribavirin rather than a direct result of an effect of IFN-α. Moreover, about half of our patients also developed neutropenia, prompting treatment of all patients G-CSF and a larger percentage of responders required dose escalations of G-CSF than non-responders. Our results confirm that anemia and neutropenia are common and can occur early in HCV treatment with about 60% of our patients requiring growth factors. In HCV mono-infected patients, early virologic suppression and maintenance of full doses of peg-IFN and ribavirin throughout treatment have been associated with higher SVR rates particularly in patients with genotype 1 infection 10, 11, which reiterates the need for use of hematopoietic factors in patients experiencing these side effects to avoid dose reductions..
Furthermore, more responders experienced ophthalmologic toxicity compared to non-responders. In our study, the two patients who developed severe color vision, requiring study discontinuation were both responding to IFN treatment and had HCV viral levels less than 615 IU/ml at the time of occurrence 32. Given these occasional dramatic ophthalmologic issues seen in responders, providers must follow patients closely for eye pathologies . The mechanisms of IFN-α induced ophthalmic toxicities are not completely understood. IFN-α may cause immune complexes to be deposited in the retinal vessels leading to capillary infarction. There is also some evidence that IFN-α may raise levels of complement 5a, which may result in infarction and lead to toxicity to ganglion cells 33. Several of our patients also had ocular pathologies that were observed in patients regardless of their virologic response (such as cotton wool spots). It is plausible that a different mechanism that are not mediated via the IFN-α-IFN-α receptor interactions result in these toxicities (such as infarcts of retinal vessels), which is not related to the antiviral mechanism of action of IFN-α.
Several demographic factors can influence the occurrence of adverse events 5. In this regard, there were no statistical differences in the baseline characteristics (race, age, liver disease staging) and treatment characteristics (type of IFN-α, duration of therapy, ribavirin dosing) between the two groups. Clinically, these findings suggest anticipation of serious adverse events among virologic responders and preemptive therapy for symptoms to enable successful completion of therapy and enhance the opportunity for achieving a cure for HCV infection in this difficult to treat population. Biologically, virologic response equates with development of adverse events emphasizing a role for refractoriness of immune cells to IFN-α as a significant mechanism of treatment failure.
Finally, this study underscores the importance of thorough interdisciplinary baseline evaluation, frequent monitoring for adverse events and prompt intervention, whenever symptoms threaten study completion. It also highlights the need for development of in vitro testing to predict and evaluate in vivo response to IFN-α. Such information would be prognostically informative and potentially invaluable in identifying virologic responders who need more frequent monitoring and supportive care during their long course of required therapy. Given the low rate of SVR, and lack of new treatment options available now to cure HCV among HIV co-infected patients, such an approach informed by clinical science is important to optimize therapeutic response in this population.
Acknowledgment
This research was supported in whole by the Intramural Research Program of the NIH, [National Institute of Allergy and Infectious Diseases, National Institute of Mental Health and the National Eye Institute].
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Conflict of Interest Statement
None of the authors have any conflicts of interest to report.
References
- 1.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clinical infectious diseases. 2002 Mar 15;34(6):831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 2.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clinical infectious diseases. 2001 Aug 15;33(4):562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Archives of internal medicine. 2006 Aug 14–28;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical infectious diseases. 2001 Feb 1;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Mallolas J, Laguno M. Pegylated IFN-alpha2b plus ribavirin for treatment-naive patients coinfected with HCV and HIV. Expert review of anti-infective therapy. 2008 Jun;6(3):281–289. doi: 10.1586/14787210.6.3.281. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Thomas DL. Perspectives on HIV/hepatitis C virus co-infection, illicit drug use and mental illness. AIDS (London, England) 2005 Oct;19 Suppl 3:S8–S12. doi: 10.1097/01.aids.0000192064.09281.48. [DOI] [PubMed] [Google Scholar]
- 7.Voigt E, Schulz C, Klausen G, et al. Pegylated interferon alpha-2b plus ribavirin for the treatment of chronic hepatitis C in HIV-coinfected patients. The Journal of infection. 2006 Jul;53(1):36–42. doi: 10.1016/j.jinf.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. The Medical clinics of North America. 1997 Mar;81(2):449–470. doi: 10.1016/s0025-7125(05)70526-5. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich DT, Spivak JL. Hematologic disorders associated with hepatitis C virus infection and their management. Clinical infectious diseases. 2003 Aug 15;37(4):533–541. doi: 10.1086/376971. [DOI] [PubMed] [Google Scholar]
- 10.Ferenci P, Fried MW, Shiffman ML, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirin. Journal of hepatology. 2005 Sep;43(3):425–433. doi: 10.1016/j.jhep.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Annals of internal medicine. 2004 Mar 2;140(5):346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. Jama. 2004 Dec 15;292(23):2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 13.Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. The New England journal of medicine. 2004 Jul 29;351(5):451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laguno M, Murillas J, Blanco JL, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS (London, England) 2004 Sep 3;18(13):F27–F36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 15.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. The New England journal of medicine. 2004 Jul 29;351(5):438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 16.Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Curr Med Chem. 2007;14(12):1279–1289. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- 17.Mauss S. Treatment of viral hepatitis in HIV-coinfected patients-adverse events and their management. Journal of hepatology. 2006;44(1 Suppl):S114–S118. doi: 10.1016/j.jhep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003 Mar–Apr;44(2):104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 19.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002 May;26(5):643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 20.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biological psychiatry. 2004 Dec 1;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Loftis JM, Socherman RE, Howell CD, et al. Association of interferon-alpha-induced depression and improved treatment response in patients with hepatitis C. Neurosci Lett. 2004 Jul 22;365(2):87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 22.Saris SC, Rosenberg SA, Friedman RB, Rubin JT, Barba D, Oldfield EH. Penetration of recombinant interleukin-2 across the blood-cerebrospinal fluid barrier. Journal of neurosurgery. 1988 Jul;69(1):29–34. doi: 10.3171/jns.1988.69.1.0029. [DOI] [PubMed] [Google Scholar]
- 23.Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient's initial affective state. The New England journal of medicine. 1999 Apr 29;340(17):1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- 24.Lerner DM, Stoudemire A, Rosenstein DL. Neuropsychiatric toxicity associated with cytokine therapies. Psychosomatics. 1999 Sep–Oct;40(5):428–435. doi: 10.1016/S0033-3182(99)71208-9. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed MK, Ramalingam S, Lin Y, Gooding W, Belani CP. Skin rash and good performance status predict improved survival with gefitinib in patients with advanced non-small cell lung cancer. Ann Oncol. 2005 May;16(5):780–785. doi: 10.1093/annonc/mdi157. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005 May;10(5):345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 27.Cuzick J, Sestak I, Cella D, Fallowfield L. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008 Oct 29; doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 28.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. 2002 Dec;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Borozan I, Feld J, et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005 May;128(5):1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Lempicki RA, Polis MA, Yang J, et al. Gene expression profiles in hepatitis C virus (HCV) and HIV coinfection: class prediction analyses before treatment predict the outcome of anti-HCV therapy among HIV-coinfected persons. The Journal of infectious diseases. 2006 Apr 15;193(8):1172–1177. doi: 10.1086/501365. [DOI] [PubMed] [Google Scholar]
- 31.Pau AK, McLaughlin MM, Hu Z, Agyemang AF, Polis MA, Kottilil S. Predictors for hematopoietic growth factors use in HIV/HCV-coinfected patients treated with peginterferon alfa 2b and ribavirin. AIDS patient care and STDs. 2006 Sep;20(9):612–619. doi: 10.1089/apc.2006.20.612. [DOI] [PubMed] [Google Scholar]
- 32.Farel C, Suzman DL, McLaughlin M, et al. Serious ophthalmic pathology compromising vision in HCV/HIV co-infected patients treated with peginterferon alpha-2b and ribavirin. AIDS (London, England) 2004 Sep 3;18(13):1805–1809. doi: 10.1097/00002030-200409030-00009. [DOI] [PubMed] [Google Scholar]
- 33.Sugano S, Suzuki T, Watanabe M, Ohe K, Ishii K, Okajima T. Retinal complications and plasma C5a levels during interferon alpha therapy for chronic hepatitis C. The American journal of gastroenterology. 1998 Dec;93(12):2441–2444. doi: 10.1111/j.1572-0241.1998.00701.x. [DOI] [PubMed] [Google Scholar]