Abstract
Increasing evidence has implicated gangliosides, sialic acid–containing cell surface glycosphingolipids, in the biological and clinical behavior of many types of human tumors. Gangliosides are overexpressed and actively shed by tumor cells, can bind to normal cells in the tumor microenvironment, and have a number of biological properties that could conceivably alter tumor–host interactions to influence the survival of the malignant cells that carry these molecules. One major area of investigation is the modulation of cell signaling by gangliosides. Published studies have demonstrated modulation of growth factor signaling through the epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), Trk family, and insulin receptors. Studies conducted over the past 10 y have demonstrated either inhibition or enhancement of signaling by gangliosides, depending on cell type, ganglioside species, and experimental conditions. Of particular concern are conflicting studies that demonstrate opposite effects of gangliosides on the same growth factor receptor. This chapter discusses a methodological approach to addressing this apparent conflict.
Overview
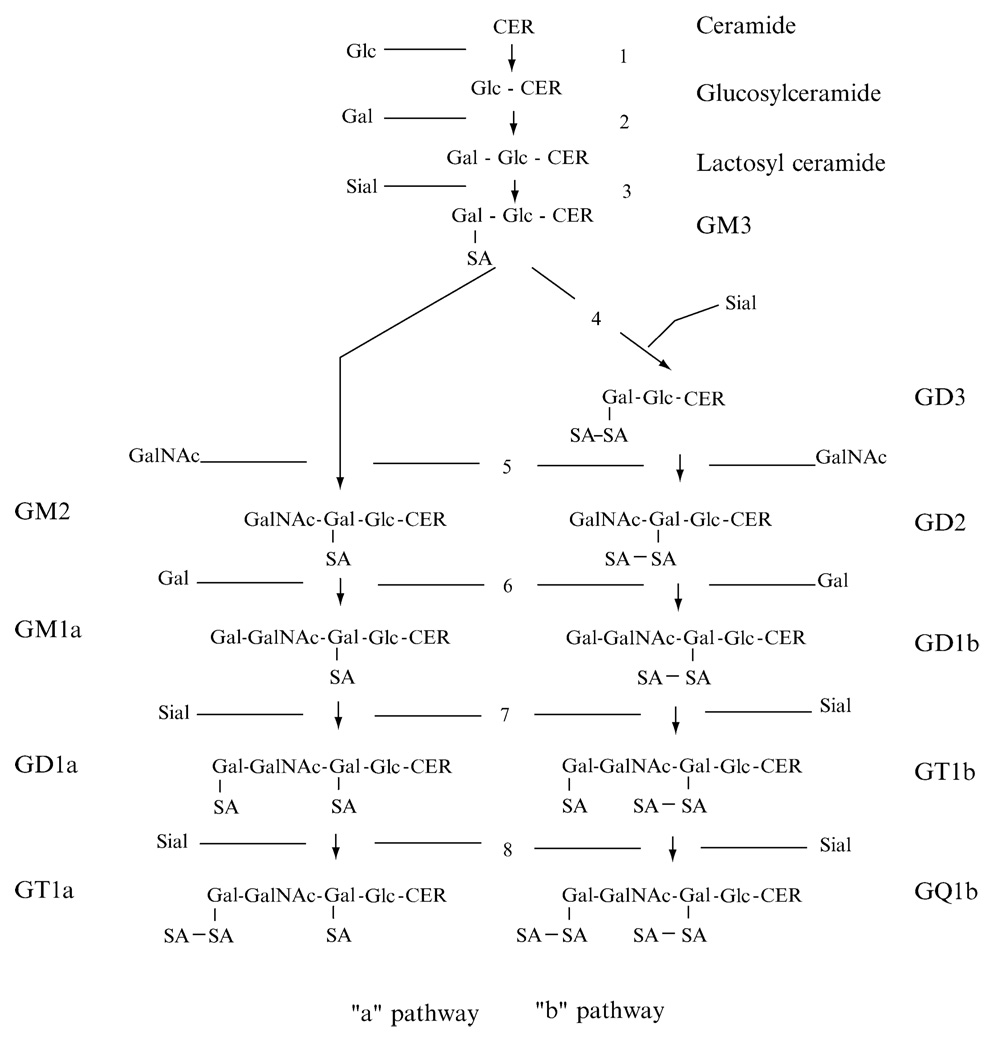
Ganglioside molecules consist of a sialic acid–containing carbohydrate portion and a hydrophobic lipid backbone (ceramide) embedded in the outer leaflet of the cell membrane (Ledeen and Yu, 1982). Individual carbohydrate species can be classified according to ganglioside biosynthetic pathways. Their biosynthesis, in a sequential order of glycosylations, occurs by two main pathways, designated “a” (GM2,GM1a,GD1a) and “b” (GD3,GD2,GD1b, GT1b, GQ1b), from a common precursor (GM3) derived from lactosylceramide (van Echten and Sandhoff, 1993) (Fig. 1). Each ganglioside is structurally more complex than its precursor molecule, and the stepwise addition of monosaccharide or sialic acid residues by specific membrane-bound glycosyltransferases in the Golgi apparatus is catalyzed by the same glycosyltransferases in both pathways. Gangliosides represent a diverse group of molecules, and marked structural differences in carbohydrate and ceramide structures distinguish the ganglioside complement of many tumors from that of normal tissue (Hakomori, 1996). This structural heterogeneity has implications for immunological activity, as evidenced, for example, by studies demonstrating differential immunosuppressive effects of ceramide subspecies of tumor gangliosides (Ladisch et al., 1994). Furthermore, gangliosides are overexpressed by tumor cells and shed into the tumor microenvironment. Tumor gangliosides can both influence the behavior of the tumor cells that produce them and can alter biological functions of target cells to which shed gangliosides bind.
FIG. 1.
Schematic representation of the major pathways of ganglioside biosynthesis. GM3, derived from lactosylceramide, is the common precursor for both “a” and “b” pathway gangliosides. Each ganglioside consists of a ceramide backbone (CER) and a carbohydrate chain (glc, glucose; gal, galactose; GalNAc, N-acetylgalactosamine) containing one or more sialic acid (SA, sialic acid) residues. Parallel steps in both pathways are catalyzed by the same glycosyltransferases: (1) glucosylceramide synthase; (2) lactosylceramide synthase; (3) GM3 synthase; (4)GD3 synthase; (5)GM2/GD2 synthase; (6)GD1b/GM1a synthase; (7)GT1b/GD1a synthase; (8) GQ1b/GT1a synthase.
Gangliosides, which exist in glycosphingolipid-enriched domains (Hakomori et al., 1998), have several important biological properties, including potent immunosuppressive activity (Bergelson et al., 1989;Grayson and Ladisch, 1992; Ladisch et al., 1983; Lu and Sharom, 1996), proangiogenic activity (Alessandri et al., 1987; Manfredi et al., 1999; Zeng et al., 2000), and enhancement of growth factor–mediated fibroblast and vascular endothelial cell proliferation (Lang et al., 2001; Li et al., 2001; Rusnati et al., 2002). These findings have in turn led to interest in the role of gangliosides in the modulation of cell signaling, resulting in studies spanning a wide range of growth factors, cell types, ganglioside species, and experimental conditions. A number of growth factor receptors, including receptors for epidermal growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), and insulin are known to be influenced by gangliosides. This diversity has, not surprisingly, led to equally wide-ranging results, including reports delineating opposing effects of gangliosides on the same receptor-ligand pair. Inhibitory effects include inhibition of EGFR phosphorylation by GM3 (Alves et al., 2002; Bremer et al., 1986; Miljan et al., 2002; Mirkin et al., 2002; Rebbaa et al., 1996; Suarez Pestana et al., 1997) and inhibition of PDGFR phosphorylation by GM1, GM2, GD1a, and GT1b (Farooqui et al., 1999; Hynds et al., 1995). In contrast, enhancement of growth factor receptor phosphorylation has been demonstrated for GD1a and GM1 in a number of systems including EGFR (Li et al., 2000, 2001; Liu et al., 2004), and FGF (Rusnati et al., 2002). The body of evidence suggests that gangliosides exert their effects on cell surface receptors by not just one but multiple mechanisms of action (Miljan and Bremer, 2002); however, divergent opinions exist as to whether ganglioside effects are in general stimulatory or inhibitory. We have previously shown that when normal human fibroblasts are preincubated with gangliosides, followed by removal of unbound gangliosides, enhanced proliferation is observed in response to EGF, PDGF, and bFGF, whereas when gangliosides are present in the culture medium during growth factor stimulation, an overall inhibitory effect on cell proliferation is observed (Li et al., 2000). Similar results have been obtained in Chinese hamster ovary (CHO) cells fibroblast growth factor-2 (FGF2) (Rusnati et al., 2002). In general, a fundamental distinction may exist between the activity of soluble gangliosides present in the cellular milieu as opposed to gangliosides bound to cell membranes.
The inconsistencies in current methodological approaches to the study of ganglioside modulation of signal transduction have resulted in the comparison of studies that assess the effects of soluble gangliosides with those that assess the effects of membrane-bound gangliosides. In addition, this divergence in experimental approach has raised the question of how to best reflect the physiological conditions of the cellular microenvironment in in vitro experimental systems. For example, in a compilation of 11 studies investigating the role of exogenously added gangliosides in modulation of signaling through the EGF receptor, 4 different gangliosides were studied in 8 different cell lines, with growth factor concentrations ranging from 1–100 ng/ml and ganglioside concentrations ranging from 5–1000 µM (Table I). In addition, the serum content of the cell culture medium and use of cell washing to remove unbound ganglioside varied among the 11 studies, with 4 studies (Li et al., 2000, 2001, Liu et al., 2004; Suarez Pestana et al., 1997)using low serum concentrations (≤2%). In eight of the studies (Alves et al., 2002; Bremer et al., 1986; Meuillet et al., 2000; Miljan et al., 2002; Mirkin et al., 2002; Rebbaa et al., 1996; Sottocornola et al., 2003; Suarez Pestana et al., 1997) gangliosides inhibited signaling through the EGF receptor, in three studies (Bremer et al., 1986; Mirkin et al., 2002; Sottocornola et al., 2003) no ganglioside effect could be demonstrated, and in four studies (Li et al., 2000, 2001; Liu et al., 2004; Miljan et al., 2002) signaling was enhanced. Although the cell lines and the gangliosides used in these studies varied, a review of the methods used reveals striking differences in the experimental conditions used. Specifically, among the six studies in which inhibition of EGF receptor phosphorylation was observed, gangliosides were added at concentrations in excess of 100 µM, and gangliosides were present in the culture medium throughout the experiment without removal of unbound molecules. In addition, in only one of the six studies (Suarez Pestana et al., 1997), phosphorylation studies were carried out in the media containing less than 5% serum. Finally, the six studies demonstrating inhibition of EGF phosphorylation used EGF concentrations of at least 10 ng/ml. In contrast, in three of four (Li et al., 2000, 2001; Liu et al., 2004) studies in which addition of exogenous gangliosides resulted in an enhancement of EGF phosphorylation, ganglioside concentrations ranged from 10–50 µM, unbound gangliosides were removed by washing before stimulation with EGF, studies were conducted under low serum (≤2%) concentrations, and receptor stimulation was induced using low EGF concentrations (2 ng/ml).
TABLE I.
Studies examining the effect of exogenous addition of gangliosides on signaling through the EGFR
| Ganglioside | Concentration | Cell line/cell | Modulation |
|---|---|---|---|
| GM1 | 100 µM | A431 | Positive (Miljan et al., 2002) |
| 125~1000 µM | KB; NBL-W | No effect (Bremer et al., 1986; Mirkin et al., 2002) |
|
| GM3 | 5~1000 µM | A431; NBL-W; H125; A1S; HC11 |
Negative (Alves et al., 2002; Bremer et al., 1986; Meuillet et al., 2000; Miljan et al., 2002; Mirkin et al., 2002; Rebbaa et al., 1996; Sottocornola et al., 2003; Suarez Pestana et al., 1997) |
| 125 µM | MG1361 | No affect (Sottocornola et al., 2003) |
|
| GD1a | 5~1000 µM | NBL-W | Negative (Mirkin et al., 2002) |
| 5~100 | A431; NHDF | Positive (Li et al., 2000, 2001, 2004; Miljan et al., 2002) |
|
| GT1b | 5~1000 µM | NBL-W | Negative (Mirkin et al.2002) |
| 100 µM | A431 | Positive (Miljan et al. 2002) |
To more fully understand the role of gangliosides in modulating cell signaling, one methodological approach is the development of in vitro systems that mimic the in vivo situation to the maximum extent possible. This general approach underlies efforts to study the effects of gangliosides on signaling in cells that have been engineered to produce the ganglioside of interest by transfection (Nishio et al., 2005; Zurita et al., 2001). Practically, however, these systems are more difficult to develop, and systems using the exogenous addition of gangliosides will continue to be the primary venue for the study of ganglioside modulation of cell signaling. The physiological relevance of the results obtained in this latter system is directly related to the extent to which the milieu in which gangliosides exert their effects in vivo is replicated in vitro.
The characteristics of gangliosides, their interaction with cell membranes, and the conditions existing in the cellular microenvironment are important to consider in designing a physiologically relevant in vitro system. First, the cellular microenvironment consists of tumor cells and target cells in close proximity with little or no free interstitial proteins. Second, gangliosides are present in low concentration on the membranes of normal cells. The membrane content of tumor cells is generally much higher, and active shedding of gangliosides results in their cell-to-cell transfer followed by binding to the cell membrane. Third, gangliosides of differing carbohydrate structures are known to have differential effects on a number of cellular functions including growth factor signaling (Miljan et al., 2002; Mirkin et al., 2002). Two other characteristics of gangliosides are important when considering in vitro systems using their exogenous addition. First, it is known that high ganglioside concentrations (in excess of 100 µM) behave as detergents, resulting in cell membrane disruption. Second, because gangliosides are known to bind to serumproteins in serum-containing systems, the effective concentrations of free unbound ganglioside is not accurately known. Finally, in experimental systems in which gangliosides and receptor ligands are present concomitantly, dissection of the mechanism of ganglioside interaction with the receptor-ligand pair is not possible.
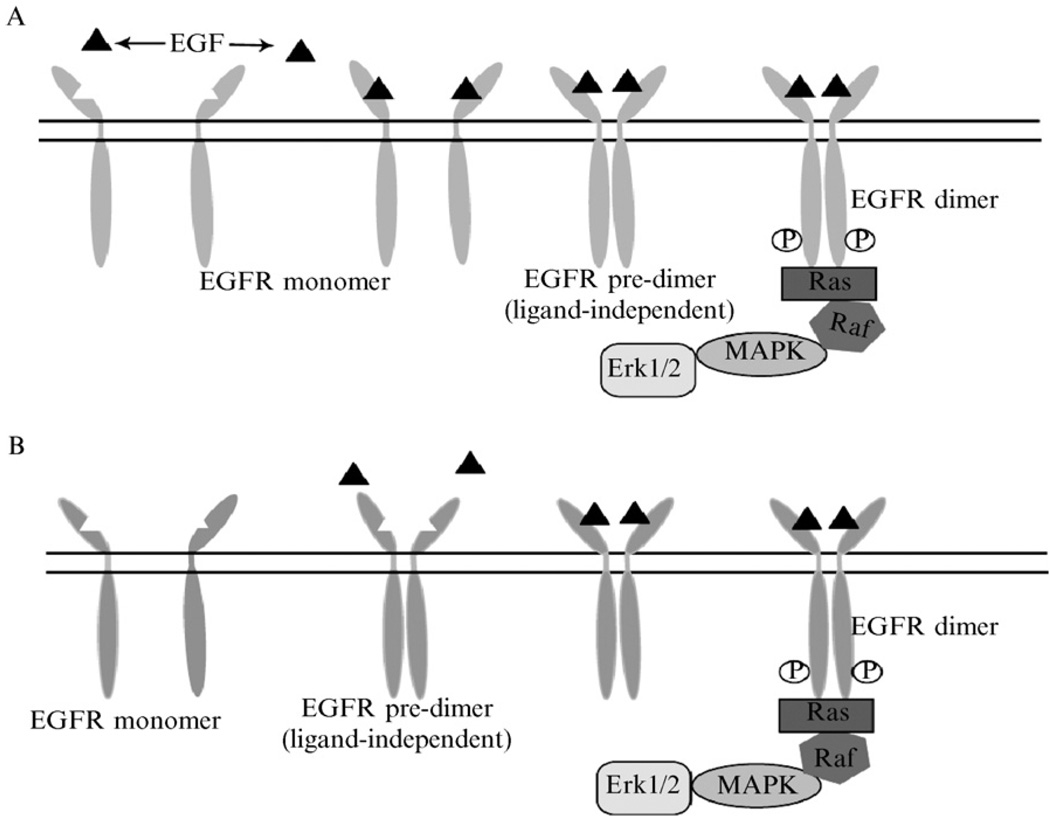
The interaction of gangliosides with EGFR provides an excellent example not only of the association of ganglioside structure with activity but also of the multiple points in the process of signal transduction that can be modulated by gangliosides. The EGFR is actually a family of four receptors each consisting of an extracellular domain, a transmembrane domain, and a tyrosine kinase cytoplasmic domain. It is believed that the receptors undergo both ligand-dependent and ligand-independent predimer formation. Unbound receptors reside in lipid rafts until ligand stimulation. On binding of EGFR predimers to ligand, dimerization and exit from the raft domain is followed by autophosphorylation and subsequently downstream signaling (Fig. 2). As membrane-associated molecules, gangliosides can act to alter signal transduction by modulating one or more of the steps that occur before, during, or after ligand binding. Identification of the precise steps that are influenced by gangliosides requires strict adherence to rigorous methods, which are designed to closely mimic the in vivo physiological state and permit assessment of ganglioside interaction at discreet steps in the signal transduction process.
FIG. 2.
The EGF–EGFR interaction at the cell membrane. Predimer formation is both ligand (EGF, ▲)-dependent and ligand-independent.
Taking the characteristics of both gangliosides and the cellular microenvironment together, an in vitro system that is designed to test the effects of membrane-associated gangliosides in an in vitro system that mimics the in vivo environment could be envisioned as using low concentrations of gangliosides in a low serum or serum-free milieu, the removal of excess unbound ganglioside before ligand exposure, and the use of low ligand (growth factor) concentrations. We have developed and successfully used a method for measuring EGFR phosphorylation and dimerization using low concentrations of serum, growth factor, and ganglioside, which more closely mimics the in vitro microenvironment.
Methods
Cell Culture
Normal human dermal fibroblasts (NHDF) purchased from Clonetics (San Diego, CA) are cultured in fibroblast complete growth medium, FGM-2 (Clonetics) that contains 2% fetal bovine serum and 0.5 ml each of insulin, hFGF, and GA1000 per 500 ml. The culture medium is changed every 3 days. All experiments will be performed using subconfluent cultures of passages 3–10 (if the storage cells are used for signaling study, the cell are cultured for at least one passage, and then seeded for the signaling study). Cell viability is assessed by trypan blue dye exclusion. For serum-free culture, fibroblast-basal medium (FBM) is used.
Preparation of Cell Lysate
1–2×105NHDF are seeded per 100-mm dish or perwell in 6-well plates in FGM-2. On reaching subconfluence, the cells are washed with FBM twice and then incubated with gangliosides in FBM for 6 or 18 (low concentration available) h. Then the culture medium are removed, the cells are washed twice with FBM to remove unbound ganglioside, and exposed to 2 ng/mlEGF in FBM for 5 min at 37° (Iyer et al., 1999). Then the cells are immediately washed twice with ice-cold phosphate-buffered saline (PBS) and lysed for 20 min in lysis buffer, 1 ml/100-mm dish, or 300 µl/6-well culture plate. The lysis buffer contains 20 mMTris, pH 7.5, 150 mMNaCl, 1 mMEDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mMNa3VO4, 1 µg/ml leupeptin, and 1 mMphenylmethylsulfonyl flouride (PMSF). The lysates are transferred to microcentrifuge tubes, sonicated briefly on ice, and centrifuged at 10,000g for 10 min at 4°. The supernatant is used for the kinase assays. Proteins are quantified by the Lowry method, using bovine albumin as a standard (Lowry et al., 1951).
Cell Preparation for the EGFR Inhibitor Assay
To test for the specificity of the effect of ganglioside preincubation on receptor autophosphorylation, the EGFR inhibitor, AG1478, is used. NHDF will be exposed to ganglioside GD1a in FBM for 18 h. During the last 3 h, 1 µM AG1478 or DMSO is added (Jo et al., 2000). Then the cells are exposed to 2 ng/ml EGF for 5 min, harvested, and the cell lysate are prepared as previously.
EGFR Autophosphorylation Assay
To preclear nonspecific binding, about 200 µl of cell lysate (>100 µg total protein) is mixed with 50 µl of washed protein G-Sepharose agarose bead slurry (50 µl packed beads), stirred for 2 h at 4° and microcentrifuged at 10,000g for 5 sec. The supernatant is transferred to a new Microfuge tube, mixed with 4 µg of sheep polyclonal IgG anti-EGFR antibody, and incubated overnight with gentle stirring at 4°. The immune complexes are recovered by adding 50 µl of the Protein G-Sepharose agarose bead slurry, gently rocking the mixture for 2 h at 4°, and microcentrifuging at 14,000g for 5 sec. When the supernatant is removed, the beads are washed three times with ice-cold lysis buffer, resuspended in 50 µl 2× SDS sample buffer, boiled for 5 min, and microcentrifuged; 20 µl of each supernatant (µ40 µg) protein is loaded onto a 7.5% SDS-polyacrylamide gel. EGFR autophosphorylation is detected by Western blot using an anti-phosphotyrosine antibody p-Tyr (PY99) (Li et al., 2001), and total EGFR will be detected by an anti-EGFR antibody.
Western Blotting
The samples are transferred to a polyvinylidene fluoride (PVDF) membrane (the PVDF membrane must be activated by methanol for 2 min, and then transferred to transfer buffer balanced for >5min before sample transfer), incubated in 25 ml of blocking buffer for 1 h at room temperature, and then incubated with primary antibody (rabbit polyclonal IgG; 1:1000 dilution) with gentle agitation overnight at 4°. After three washes, the membrane will be incubated in the medium containing horseradish peroxidase–conjugated anti-rabbit antibody (1:2000). Proteins will be detected by chemiluminescence and compared with standard proteins of different molecular weights.
EGFR Tyrosine Kinase Activity
EGFR tyrosine kinase activity is measured as the phosphorylation of an EGFR substrate peptide, using a tyrosine kinase assay kit (Calbiochem). The EGFR are immunoprecipitated from the cell lysate (~200 µg protein). Half of the immunoprecipitated protein (10 µl) is transferred to a Microfuge tube that contains 20 µl protein tyrosine kinase (PTK) reaction mix, containing 30 µM ATP, 50 µM substrate peptide, 1 µCi [32P]ATP, and 4 µl 10× PTK reaction buffer, consisting of 200 mM magnesium chloride, 10 mM manganese chloride, 2 mM EGTA, 80 mM beta-glycerophosphate, and 80 mM imidazole hydrochloride, pH7.3. The mixture is incubated at 30° for 10 min with agitation. The reaction will be stopped by placing the tubes on ice, followed by centrifugation at 10,000g at 4° for 5 sec. The supernatant will be transferred to a new tube containing 10 µl stop solution mix (8 M guanidine hydrochloride) and briefly centrifuged; 8 µl of avidin solution is added to the supernatants, and the samples will be incubated for 5 min at room temperature; 50 µl of wash solution and 20 µl of the reaction samples are transferred into the reservoirs of centrifugal ultrafiltration units, centrifuged for 5 min at 14,000g, and washed three times with 100 µl of wash solution. The washed filters will be transferred to scintillation vials, scintillation cocktail is added, and radioactivity is quantified. Net cpm of [32P] incorporated into the substrate peptide is calculated by subtracting the nonspecific binding of [32P]ATP from the total cpm.
Phospho-p44/42 MAP Kinase Assays
Phosphorylation of p44/42 MAP kinase is determined by Western blot, using the phospho-p44/42 MAP kinase antibody. A MAP kinase antibody is used to detect the total MAP kinase in each sample. Equal volumes of lysate and sample buffer are mixed, boiled for 5 min, microcentrifuged for 2 min, and then subjected to SDS-PAGE electrophoresis (12% gel) and Western blotting.
Plasma Membrane Separation and In Vitro Assay of EGFR Autophosphorylation
NHDF are incubated with GD1a in FBM for 18 h. The plasma membranes are separated as previously described (Smart et al., 1995). After aspirating the medium, the cells are washed twice with the ice-cold buffer A (0.25Msucrose, 1 mMEDTA, 20 mMTricine, pH 7.8), scraped into 3 ml buffer A, centrifuged at 1000g for 5 min, resuspended in 1 ml buffer A, and homogenized in a 2-mlWheaton Tissue grinder with 20 strokes. The cell homogenates are centrifuged at 1000g for 10 min. The pellets are resuspended in 1 ml buffer A, homogenized and centrifuged at 1000g for 10 min. The two supernatants are combined, layered on 30% Percoll in buffer A, ultracentrifuged at 84,000g for 30 min, and the visible bands are collected. These plasma membrane fractions are washed three times with HNG buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid], 150 mM NaCl, 10% glycerol, pH 7.5), and the protein content is quantified. The resuspended pellets are assayed for EGFR activity (following).
In Vitro EGFR Activity Assays
Fifteen microliters protein of plasma membrane preparation (about 10 µg protein) is mixed, on ice, with 15 µl autophosphorylation buffer (HNG buffer containing 40 µMATP, 30mMMnCl2, and 400mMNa3VO4). EGF is added to a final concentration of 2 ng/ml. Then the mixture is incubated at 37° for 10 min with gentle agitation. The reaction is stopped by adding 30 µl of 2× lysis buffer, and the sample is stored on ice for 30 min. The samples are immunoprecipitated with the EGFR antibody (sheep polyclonal IgG); and the p-EGFR, total EGFR, and p-tyr kinase activities are tested as before.
Plasma Membrane Binding of GD1a
To assess 14C–GD1a binding, cells are cultured in FBM with 3.5 × 105 cpm 14C–GD1a/well in a 24-well plate for 18 h. Then the medium is removed, and the cells are washed twice with PBS, trypsinized, and harvested. The cell membranes are separated, and membrane-bound radioactivity is quantified by scintillation counting.
125I-EGF Binding
Binding of 125I-EGF to whole cells is assessed by modified Scatchard for 18-h analysis (Bremer et al., 1984). Three parallel sets of 104 NHDF are grown for 24 h in a 96-well plate in FGM-2, washed twice with FBM, and incubated with 0, 5, or 10 µM GD1a in FBM for 18 h. One set of cells is to be washed and incubated at 4° for 2 h with 0.5–20 ng/ml 125I-labeled human recombinant EGF in FBM, without cold EGF, to determine the total binding. The second set, used to test for nonspecific binding, is treated as earlier, but with the addition of 300 ng/ml unlabeled EGF. After washing, 50 µl of lysis buffer is added to each well, the samples will be lysed on ice for 30 min, and the cpm of 125I-EGF in 20-µl aliquots are counted. Specific binding is calculated by subtracting the nonspecific binding of 125I-EGF from the total binding. The third set of cells will be trypsinized and the cell number determined. The binding curves and Scatchard analysis of the data is performed using GraphPad Prism 3.03 software.
Assessment of EGFR Dimerization and Ligand-Independent Dimerization
NHDF cells are cultured in 100 × 20-mm culture dishes and preincubated with GD1a in FBM for 18 h, washed, treated with (for dimerization) or without (for ligand-independent dimerization) 2 ng EGF/ml in FBM for 5 min, and washed twice with ice-cold phosphate-buffered saline (PBS); 3 ml of PBS containing 1 mg/ml bis(sulfosuccinimidylpropionate)suberate (BS3) is added, and the cells are incubated on ice for 30 min, washed twice with ice-cold PBS, and lysed in 1 ml lysis buffer for 20 min on ice (because of BS3 sensitivity to moisture, it must be prepared before adding to the cell system). Total EGFR is immunoprecipitated with the EGFR antibody; 500 µg of lysate protein is loaded onto a 7.5% SDS-PAGE, and dimerization of the EGFR is detected by Western blotting using an anti-EGFR antibody.
Measurement of Effects of Cellular Ganglioside Enrichment on Cell Proliferation
Fibroblasts are seeded at 5× 103 cells/well in 96-well plates (area = 0.32 cm2; Corning Glass) FGM containing 2% FBS. In selected assays, fibroblasts are seeded at 3 × 104 cells/well in the larger 24-well plates (area = 1.9 cm2; Corning Glass). The medium is replaced with fresh medium ± gangliosides on the following day. After an 18-h incubation, the culture medium is removed, and the cells are starved overnight in serum-free FBM. This medium is to be replaced with serum-free medium ± EGF, and the cells are further cultured for 24 h. In some experiments, the starvation period is omitted, and after incubation of the cells with gangliosides for 18 h, the culture medium is replaced directly with serum-free medium ± EGF, and the cells are further cultured for 24 h. During the final 3 h, the cells were pulsed with 50 µl of [3H]thymidine (5 µCi/ml) and harvested by a cell harvester. [3H]Thymidine uptake is determined by β-scintillation counting. In selected experiments, [3H]thymidine incorporation into trichloroacetic acid-insoluble material is also determined as described previously. Under these same conditions, in parallel experiments, cell counts of the 24-well plates were performed after trypsinization of the cells. The data are expressed as the mean ± SD of duplicate or triplicate cultures.
Results and Conclusion
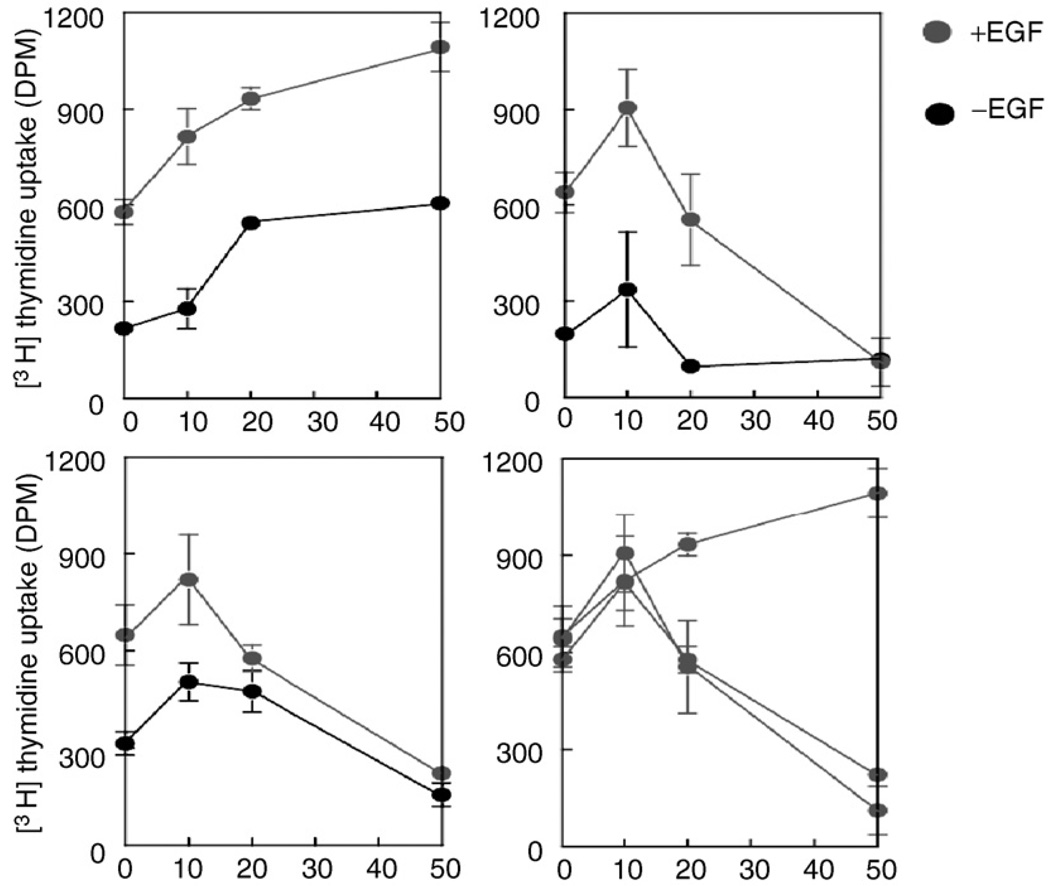
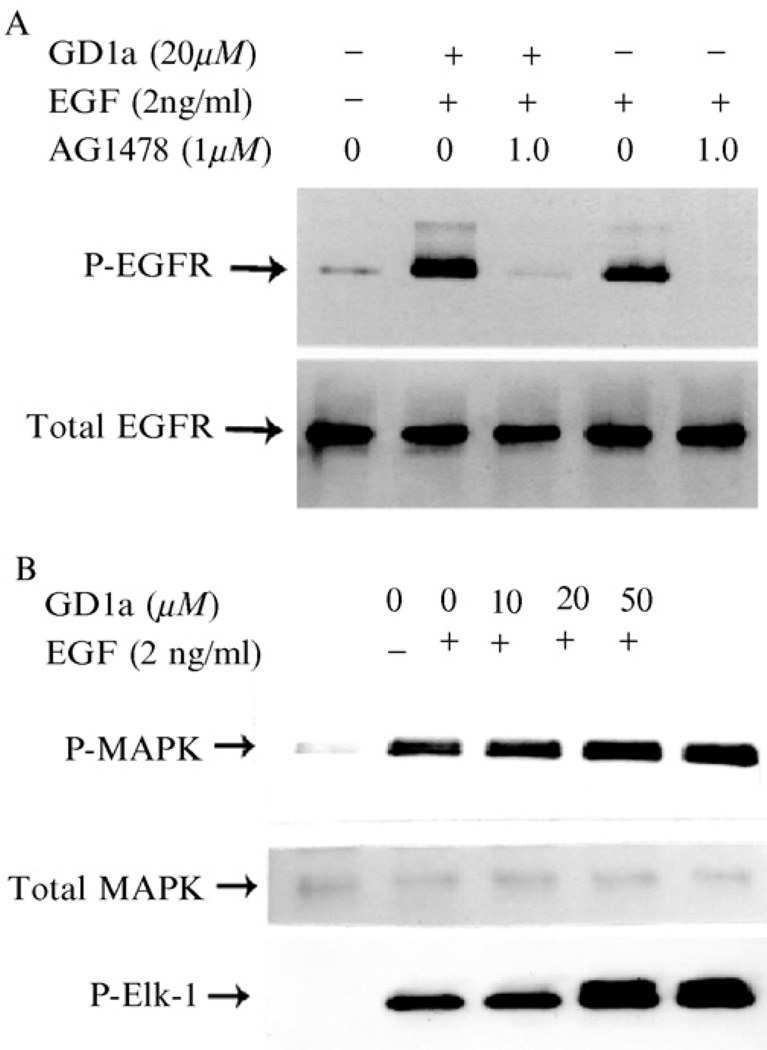
As we have described previously, gangliosides may act on cell membrane to modulate growth factor–induced proliferation by influencing important membrane-associated events, including receptor dimerization. We hypothesize that the current controversy as to the exact nature of the effect of gangliosides on cell signaling may be clarified by the adoption of a standard methodological approach that as closely as possible mimics the in vivo physiological environment. Using the methods outlined previously, we have demonstrated that preincubation of HDF with ganglioside (GD1a, GM3, or GD3) followed by washout of unbound ganglioside before ligand (EGF) stimulation results in enhancement of fibroblast proliferation (Fig. 3). In contrast, cell proliferation was inhibited when gangliosides were not removed from the culture media before ligand addition. Furthermore, we have demonstrated enhancement of signaling through the EGF receptor in the presence of GD1a, including receptor autophosphorylation, as well as activation of downstream molecules Ras and MAPK (Fig. 4). Finally, lending some insight into the possible mechanism of action of gangliosides in enhancement of signaling through EGFR, we have demonstrated that GD1a enhances EGFR dimerization (and hence the effective number of high-affinity receptors) both in the presence and in the absence of ligand (Fig. 5).
FIG. 3.
Effect of ganglioside GD1a concentration, and of its presence during incubation with EGF, on cell proliferation. The effect of ganglioside GD1a on cell proliferation was evaluated under the following three condition: (1) preincubation of cells with gangliosidesGD1a (Panel A); (ii) continuous incubation of cells with ganglioside GD1a (Panel B); and (iii) ganglioside preincubation, starvation without ganglioside presence, and co-incubation of the cells with ganglioside + EGF (Panel C). NHDF cells were seeded at 5 × 103 cells/well in a 96-well plate in FGM-2. The medium was replaced with fresh medium ± ganglioside GD1a on the following day. After an 18 hours, the medium was removed, and the cells were cultured in serum-free medium (FBM) overnight to starved the cells and were then replaced with serum-free medium ± EGF (2ng/ml). The cells were further cultured for 24 hours, and cell proliferation was measured by [3H]thymidine uptake (A). In (B), ganglioside GD1a was present in the culture medium throughout the experiment (i.e., during the preincubation, the starvation period, and the incubation of the cells with EGF). In (C) the cells were preincubated with ganglioside GD1a for 18 h and then starved overnight in the absence of ganglioside. GangliosideGD1awas added back to the culture medium during the incubation with EGF. (D) Composite of (A–C), showing the effect of ganglioside GD1a on EGF-induced cell proliferation. Each value is the mean ± SD of duplicated culture. Reproduced from Li et al. (2000), with permission.
FIG. 4.
Ganglioside GD1a preincubation enhances EGF-induced EGFR and MAPK phosphorylation. In (A) NHDF were cultured in FGM-2 to subconfluence, washed twice, and incubated with GD1a in FBM for 18 h. The inhibitor AG1478 (1 µM) was added to the culture system for the final 3 h of the incubation. The cells were then exposed to EGF (2 ng/ml) in FBM for 5 min, washed with ice-cold PBS, and lysed; 200 µl cell lysate containing 100 µg total protein was immunoprecipitated using an anti-EGFR sheep polyclonal IgG. The immune complex was subjected to SDS-PAGE and Western blot. EGFR phosphorylation was detected by an anti-phosphotyrosine antibody. Total EGFR was detected using an anti-EGFR sheep polyclonal IgG. (B) After the cell density reached 70% of confluence, the cells were incubated with ganglioside GD1a in FGM with 2% FBS for 18 h after removal of the culture medium by aspiration, the cells were washed twice and starved in serum free FBM overnight. The cells were then cultured in serum-free medium ± EGF (2 ng/ml) in for 5 min, immediately washed with ice-cold PBS, and lysed; 20 µg of the cell lysate was subjected to SDS-PAGE and Western blot, using either the phospho-P44/42 MAP kinase or the P44/42 MAP kinase antibody. In addition, the MAP kinase activity was determined by measuring Elk-1 phosphorylation. In these experiments, the cell lysate containing 200 µg of total protein for each sample was immunoprecipitated using a specific anti-phospho–p44/42 MAP kinase antibody. The kinase assay was performed using Elk-1 as the substrate. One third of the total product was subjected to SDS-PAGE and Western blot to visualize the phosphorylated Elk-1 bands, using anti-phospho-Elk-1 antibody. Reproduced from Liu et al. (2004) (A) and Li et al. (2001) (B), with permission.
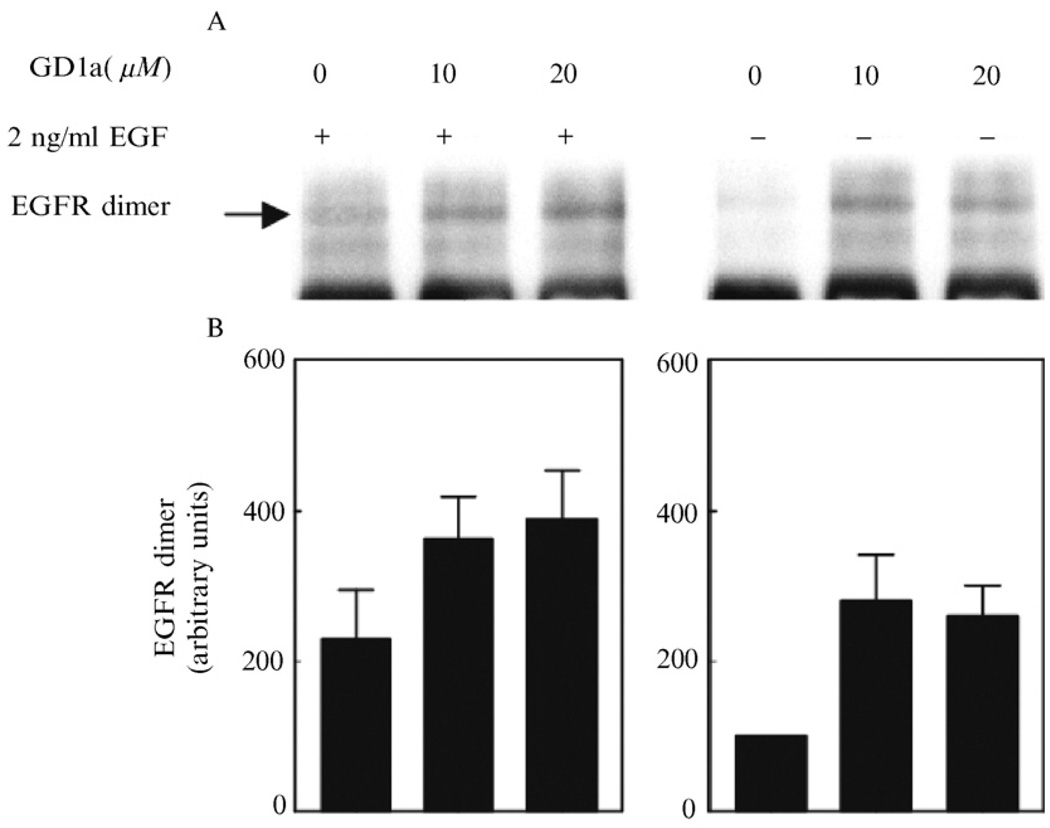
FIG. 5.
Effects of GD1a pretreatment on EGFR dimerization. Subconfluent NHDF cells were washed, preincubated with GD1a, and treated with EGF as in Fig. 2A. Then, the cells were washed twice with ice-cold phosphate-buffered saline (PBS) and incubated with 3 ml of PBS containing 1 mg/ml BS3 for 30 min on ice, washed again, and lysed in 1 ml lysis buffer for 20 min. Immunoprecipitation and detection of EGFR dimers were performed as described in “Materials and Methods.” The relative optical densities of the EGFR dimer bands, representing the composite (mean ± SD) results of five separate experiments, are shown below the Western blot. (A) Ganglioside preincubation followed by EGF exposure; (B) ganglioside incubation only (no EGF exposure). Reproduced from Liu et al. (2004), with permission.
We have demonstrated an enhancement of signaling through the EGFR by the ganglioside GD1a, which stands in apparent contrast to previously published studies that demonstrate an overall inhibitory effect of gangliosides on signaling through EGFR (Alves et al., 2002; Bremer et al., 1986; Meuillet et al., 2000; Miljan et al., 2002; Mirkin et al., 2002; Rebbaa et al., 1996; Sottocornola et al., 2003; Suarez Pestana et al., 1997) and other growth factor receptors (Farooqui et al., 1999; Hynds et al., 1995). Although it is certainly true that different gangliosides may have differing effects on signaling through the same receptor, we believe that the observed differences may be attributable in part to critical differences in methodological approaches. High concentrations of gangliosides and the continuous presence of ganglioside (including concomitant presence of ganglioside and receptor ligand), the presence of serum proteins, and the use of high ligand (growth factor) concentrations may create a number of biological phenomena that one would not expect to find in the in vivo state. Specifically, high ganglioside concentrations that are present even during growth factor stimulation may not only intercalate into the membrane but may also bind to ligand, preventing binding to its receptor. Serum proteins present in the experimental milieu may nonspecifically bind gangliosides reducing their availability for membrane intercalation (and will, at the very least, prevent accurate knowledge of the exact ganglioside concentration). Finally, use of high ligand (growth factor) concentrations will maximally stimulate signaling, preventing detection of the subtle changes in signaling that are more likely present in the in vivo state.
Acknowledgments
This work was supported in part by the National Institutes of Health Grant R01 CA61010 (S. L.) and R01 CA106532 (K. K.) and by The Children’s Cancer Foundation, Baltimore, MD (K. K. and S. L.)
References
- Alessandri G, Filippeschi S, Sinibaldi P, Mornet F, Passera P, Spreafico F, Cappa PM, Gullino PM. Influence of gangliosides on primary and metastatic neoplastic growth in human and murine cells. Cancer Res. 1987;47:4243–4247. [PubMed] [Google Scholar]
- Alves F, Borchers U, Keim H, Fortte R, Olschimke J, Vogel WF, Halfter H, Tietze LF. Inhibition of EGF-mediated receptor activity and cell proliferation by HK1-ceramide, a stable analog of the ganglioside GM3-lactone. Glycobiology. 2002;12:517–522. doi: 10.1093/glycob/cwf058. [DOI] [PubMed] [Google Scholar]
- Bergelson LD, Dyatlovitskaya EV, Klyuchareva TE, Kryukova EV, Lemenovskaya AF, Matveeva VA, Sinitsyna EV. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur. J. Immunol. 1989;19:1979–1983. doi: 10.1002/eji.1830191102. [DOI] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S, Bowen-Pope DF, Raines E, Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J. Biol. Chem. 1984;259:6818–6825. [PubMed] [Google Scholar]
- Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- Farooqui T, Kelley T, Coggeshall KM, Rampersaud AA, Yates AJ. GM1 inhibits early signaling events mediated by PDGF receptor in cultured human glioma cells. Anticancer Res. 1999;19:5007–5013. [PubMed] [Google Scholar]
- Grayson G, Ladisch S. Immunosuppression by human gangliosides. II.Carbohydrate structure and inhibition of human NK activity. Cell Immunol. 1992;139:18–29. doi: 10.1016/0008-8749(92)90096-8. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco) lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- Hakomori S, Handa K, Iwabuchi K, Yamamura S, Prinetti A. New insights in glycosphingolipid function: “glycosignaling domain,” a cell surface assembly of glycosphingolipids with signal transducer molecules involved in cell adhesion coupled with signaling. Glycobiology. 1998;8:xi–xix. doi: 10.1093/oxfordjournals.glycob.a018822. [DOI] [PubMed] [Google Scholar]
- Hynds DL, Summers M, Van Brocklyn J, O’Dorisio MS, Yates AJ. Gangliosides inhibit platelet-derived growth factor-stimulated growth, receptor phosphorylation, and dimerization in neuroblastoma SH-SY5Y cells. J. Neurochem. 1995;65:2251–2258. doi: 10.1046/j.1471-4159.1995.65052251.x. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr., Boguski MS, Lashkari D, Shalon D, Botstein D, Brown PO. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- Jo M, Stolz DB, Esplen JE, Dorko K, Michalopoulos GK, Strom SC. Cross-talk between epidermal growth factor receptor and c-Met signal pathways in transformed cells. J. Biol. Chem. 2000;275:8806–8811. doi: 10.1074/jbc.275.12.8806. [DOI] [PubMed] [Google Scholar]
- Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983;43:3808–3813. [PubMed] [Google Scholar]
- Ladisch S, Li R, Olson E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc. Natl. Acad. Sci. USA. 1994;91(5):1974–1978. doi: 10.1073/pnas.91.5.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Z, Guerrera M, Li R, Ladisch S. Ganglioside GD1a enhances VEGF-induced endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 2001;282:1031–1037. doi: 10.1006/bbrc.2001.4630. [DOI] [PubMed] [Google Scholar]
- Ledeen RW, Yu RK. Gangliosides: Structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Li R, Liu Y, Ladisch S. Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J. Biol. Chem. 2001;276:42782–42792. doi: 10.1074/jbc.M101481200. [DOI] [PubMed] [Google Scholar]
- Li R, Manela J, Kong Y, Ladisch S. Cellular gangliosides promote growth factor-induced proliferation of fibroblasts. J. Biol. Chem. 2000;275:34213–34223. doi: 10.1074/jbc.M906368199. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li R, Ladisch S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J. Biol. Chem. 2004;279:36481–36489. doi: 10.1074/jbc.M402880200. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lu P, Sharom FJ. Immunosuppression by YAC-1 lymphoma: Role of shed gangliosides. Cell. Immunol. 1996;173:22–32. doi: 10.1006/cimm.1996.0248. [DOI] [PubMed] [Google Scholar]
- Manfredi MG, Lim S, Claffey KP, Seyfried TN. Gangliosides influence angiogenesis in an experimental mouse brain tumor. Cancer Res. 1999;59:5392–5397. [PubMed] [Google Scholar]
- Meuillet EJ, Mania-Farnell B, George D, Inokuchi JI, Bremer EG. Modulation of EGF receptor activity by changes in the GM3 content in a human epidermoid carcinoma cell line, A431. Exp. Cell Res. 2000;256:74–82. doi: 10.1006/excr.1999.4509. [DOI] [PubMed] [Google Scholar]
- Miljan EA, Bremer EG. Regulation of growth factor receptors by gangliosides. Sci, STKE. 2002;2002:RE15. doi: 10.1126/stke.2002.160.re15. [DOI] [PubMed] [Google Scholar]
- Miljan EA, Meuillet EJ, Mania-Farnell B, George D, Yamamoto H, Simon HG, Bremer EG. Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J. Biol. Chem. 2002;277:10108–11013. doi: 10.1074/jbc.M111669200. [DOI] [PubMed] [Google Scholar]
- Mirkin BL, Clark SH, Zhang C. Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1A and GT1B. Cell Prolif. 2002;35:105–115. doi: 10.1046/j.1365-2184.2002.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M, Tajima O, Furukawa K, Urano T, Furukawa K. Over-expression of GM1 enhances cell proliferation with epidermal growth factor without affecting the receptor localization in the microdomain in PC12 cells. Int. J. Oncol. 2005;26:191–199. [PubMed] [Google Scholar]
- Rebbaa A, Hurh J, Yamamoto H, Kersey DS, Bremer EG. Ganglioside GM3 inhibition of EGF receptor mediated signal transduction. Glycobiology. 1996;6:399–406. doi: 10.1093/glycob/6.4.399. [DOI] [PubMed] [Google Scholar]
- Rusnati M, Urbinati C, Tanghetti E, Dell’Era P, Lortat-Jacob H, Presta M. Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA. 2002;99:4367–4372. doi: 10.1073/pnas.072651899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocornola E, Berra B, Colombo I. GM3 content modulates the EGF-activated p185c-neu levels, but not those of the constitutively activated oncoprotein p185neu. Biochim. Biophys. Acta. 2003;1635:55–66. doi: 10.1016/j.bbalip.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Suarez Pestana E, Greiser U, Sanchez B, Fernandez LE, Lage A, Perez R, Bohmer FD. Growth inhibition of human lung adenocarcinoma cells by antibodies against epidermal growth factor receptor and by ganglioside GM3: Involvement of receptor-directed protein tyrosine phosphatase(s) Br. J. Cancer. 1997;75:213–220. doi: 10.1038/bjc.1997.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Echten G, Sandhoff K. Ganglioside metabolism. Enzymology, topology, and regulation. J. Biol. Chem. 1993;268:5341–5344. [PubMed] [Google Scholar]
- Zeng G, Gao L, Birkle S, Yu RK. Suppression of ganglioside GD3 expression in a rat F-11 tumor cell line reduces tumor growth, angiogenesis, and vascular endothelial growth factor production. Cancer Res. 2000;60:6670–6676. [PubMed] [Google Scholar]
- Zurita AR, Maccioni HJ, Daniotti JL. Modulation of epidermal growth factor receptor phosphorylation by endogenously expressed gangliosides. Biochem. J. 2001;355:465–472. doi: 10.1042/0264-6021:3550465. [DOI] [PMC free article] [PubMed] [Google Scholar]