Abstract
Lipoma preferred partner (LPP) localizes to focal adhesions/dense bodies, is selectively expressed in smooth muscle cells (SMC) and enhances cell migration. SMCs cultured on denatured collagen or on a rigid substrate, up regulated expression of LPP, its partner palladin, tenascin C (TN-C), phosphorylated focal adhesion kinase (pFAK) and exhibited robust stress fibers. In an endothelial (EC) /SMC hemodynamic flow system, shear stress waveforms mimicking atheroprone flow, applied to the EC layer, significantly decreased expression of SMC LPP and palladin. They were also down regulated with TN-C, in an ApoE murine model of atherosclerosis and with oxidative stress but up regulated in an arterial injury model in response to upstream sequential changes in pFAK, Prx1 and TN-C. In conclusion, expression of LPP and palladin are modulated by a mix of mechanical cues, oxidative stress and substrate composition which translate into their up or down regulation in vessel wall injury and early atherogenesis.
Keywords: LPP, palladin, smooth muscle, injury, atherosclerosis
Introduction
Cell-extracellular matrix (ECM) adhesion is a fundamental process that is critically involved in embryonic development and numerous physiological and pathological processes including injury repair, progressive extracellular remodeling in chronic atherosclerotic, fibrotic and neurodegenerative diseases, and cancer. Cells and the ECM interaction occur at focal adhesions, where integrins cluster and bind to the matrix. Adhesion sites not only function as mechanical struts composed of cytoskeleton enriched scaffolds that transmit force from the outside of the cell to their interior and back again, but they also represent specialized subcellular signaling nodes composed of multiproteins that regulate cytoskeletal remodeling and cell movement through their immediate and long term actions (Gerthoffer and Gunst 2001). Currently more than 50 proteins have been reported to be associated with focal contacts and ECM adhesions. LPP (lipoma preferred partner) has been identified amongst a core of computationally identified SM specific genes from expressed sequence tag data (Nelander et al. 2003) and by protein identification studies (Gorenne et al. 2006). LPP and its recently identified partner palladin have been shown to be focal adhesion proteins highly expressed in smooth muscle cells (SMC) (Gorenne et al. 2006; Jin et al. 2007), yet their function and possible role in diseases of the vasculature is unknown .LPP, contains both proline rich and LIM domains (Petit et al. 1996), and is thought to function as an adaptor protein to anchor structural or regulatory proteins to focal adhesions and plasmalemmal dense bodies in SMCs and tissues respectively (Gorenne et al. 2006; Majesky 2006). As cell adhesion strength is an integral component of cell migration, it is not surprising that up or down regulation of LPP expression results in a increase or decrease, respectively, in cell migration (Gorenne et al. 2006). Its presence in every SM examined to date as well as its concentration (2–4µM) (Gorenne et al. 2003) are compatible with the possibility of LPP having a significant role in modulating the structure of the cytoskeleton, and in view of its relation to the plasma membrane it may serve as a general sensor of the state of the SMC cytoskeleton.
We have reported that the cytoskeleton protein, palladin (90–92KD isoform), interacts with LPP and unlike LPP is found in other tissues but is most highly expressed in SM tissue. Together, LPP and palladin play a significant role in SMC migration and spreading (Jin et al. 2007). Expression of both LPP and palladin, like SM alpha-actin (SMA), is increased by angiotensin II, regulated by actin dynamics, modulated by focal adhesion kinase (FAK) and the homeodomain protein, paired related homeobox gene 1 (Prx1), and appears in the neointimal of injured vessels (Jin et al. 2007). LPP was also found in a number of soft tissue tumors and in acute monoblastic leukemia (Petit et al. 1996; Rogalla et al. 2000; Daheron et al. 2001) and interacts with the tumor suppressor gene Scrib (Vervenne et al. 2008). Thus, the roles of LPP appear to be context dependent, serving a scaffolding role at the dense bodies in differentiated SM tissues and a migratory role in response to injury and environmental cues. In this study we address the complex nature of the environmental cues such as may occur with vessel injury or atherosclerosis, and show how they up or down regulate the expression of LPP and palladin and lead to cytoskeletal remodeling.
Material and Methods
Cell lines and treatment
Human iliac vein vascular smooth muscle cells (HIVS-125) were maintained as described previously (Gorenne et al. 2003). The use of rat aortic SMCs (R518) was as previously described (Hautmann et al. 1997). For some experiments, cells were starved overnight, and treated with 1µM of PDBu (Sigma, MO) for 1h and 30ng/ml of PDGF-BB for 30min (Millipore, MA). For H2O2 treatment, subconfluent cells were treated with 100µM of H2O2 (Sigma, MO). For oxLDL treatment, cells were treated with 50ng/ml of oxLDL (Kalan biomedical LLC, MD).
Preparation of native and denatured collagen gels
Collagen gels were prepared as previous described (Wren et al. 1986; Jones et al. 1997). Briefly, the native collagen gel was prepared by mixing a 3.0 mg/ml solution of bovine dermal type I collagen (Purecol TM, Inamed biomaterial, MA), 0.1 M NaOH, and 10× Ca2+ and Mg2+ -free Dulbecco’s phosphate buffered saline (DPBS) in an 8:1:1 volume ratio at 4°C for a final collagen concentration of 2.48 mg/ml. Aliquots of collagen were added to each well of 6 well culture plates for immunoblotting or on the glass bottom of 35mm culture dishs for immunostaining. Fibrillogenesis was initiated overnight in a humid 5% CO2 environment at 37°C. The heat denatured collagen was prepared by boiling 2.48 mg/ml of type I collagen for 60 min with 0.02 M acetic acid, neutralized with 0.1 M NaOH, and then air-dried to the bottom of each dish. Before use, the gels were rinsed three times with DMEM/F12 containing 0.1% BSA. Sub-confluent R518 cells were switched to DMEM/F12 containing 2% serum for 24 h and plated at a density of 1× 105/ ml on collagen substrates in DMEM/F12 containing 2% serum. Cells were cultured in medium containing 0.1% BSA and 0.1% FCS 6 h after plating for additional 24 h. For Western blotting, these cells were dispersed with 0.025% Trypsin-EDTA and 20mg/ml collagenase I (Sigma, MO), counted, and an equal amount of cells were lysed and loaded.
Preparation of polyacrylamide substrate
Polyacrylamide (PA) gels were prepared as previously described (Pelham and Wang 1997). The coverslip was flamed in a bunsen burner, soaked in 0.1 N NaOH, and air dried. The coverslips were aminosilanized with 3-aminopropultrimethoxysilane (Sigma, MO), and then immersed in a solution of 0.5% glutaraldehyde (Polysciences, IL) in PBS for 30min. After extensive washing, 50 µl of an acrylamide/bis-acrylamide mixture, containing 10% acrylamide and bis concentrations range from 0.26 to 0.03%, was then placed on the coverslip and covered with another coverslip. After polymerization, the gel was rinsed with 200 mM Hepes buffer (pH 8.5). The PA gels were chemical cross linked by exposure of sulfosuccinimidyl 6 (4 -azido-2 -nitrophenyl-amino) hexanoate ( Pierce, IL) in 50 mM Hepes buffer on the surface to UV light, followed by coating with a 0.2-mg/ml solution of type I collagen overnight at 4°C. Before plating cells, the gel was soaked in culture medium at 37°C for 1h.
Co-culture hemodynamic flow
The human endothelial cell (EC) and human SMC co-culture condition was previously described (Blackman et al. 2002; Hastings et al. 2007). Briefly, the transwell membranes (polycarbonate, 10µm thickness and 0.4µm pore diameter, Corning) were coated with 0.1% gelatin on the top and bottom surfaces. The membrane was inverted, and SMCs were plated at a density of 10,000 cells/cm 2 for 2 hrs. The transwell was then turned back into the holding well for 48 hours and incubated in M199 containing 2% FBS. ECs were then plated on the top surface of the membrane at a density of 80,000 cells/cm 2 under the same media condition for additional 24 hours. Hemodynamic flow patterns were derived from magnetic resonance imaging (MRI) of human common carotid artery and internal carotid sinus to directly apply atheroprotective and atheroprone shear stress patterns in vitro, respectively (Gelfand et al. 2006; Hastings et al. 2007). The two hemodynamic flow conditions were run for 24 hrs in parallel for each of EC/SMC subpopulations.
Rat aortic injury model and experimental atherosclerosis
Vascular injury was performed in Sprague Dawley rats (7–9w) as previous described (Jin et al. 2007). At 0, 3, 7 and14 days after injury, the aortic arteries were removed, fixed and embedded with paraffin for staining. Four rats were used for each of the time points, three sections were used for each antibody staining. Homozygous ApoE −/− and C57BL/6 male mice (The Jackson Laboratory, Bar Harbor, Me) were used as controls. The aortas were harvested at week 32. The mice were perfusion fixed via the heart with 4% paraformaldehyde for 30 minutes. The aorta was then removed and immersed in 4% paraformaldehyde overnight and embedded in paraffin for staining.
Immunohistochemistry
Paraffin embedded arterial sections (5µm thick) were treated and stained as previous described (Gorenne et al. 2003). Primary antibodies were:rabbit anti-LP olyclonal antibody (1: 500 dilution, Immunoglobe, Germany); rabbit anti-palladin polyclonal antibody (1:1500 dilution) (Jin et al. 2007); rabbit anti-pFAK polyclonal antibody (1:100 dilution, Sigma, MO); anti-SMA antibody (clone 1A4, 1:1000, Sigma,MO); rabbit TN-C polyclonal antibody (1: 100 dilution, Millipore, MA); and rabbit polyclonal Prx1 antibody (1:70 dilution) (Jin et al. 2007).
Indirect Immunofluorescence
Cultured cells were fixed with 4% paraformaldehyde and permeabilized with 0.03% Triton x-100 in PBS rinsed and blocked for 1 h in PBS containing 3% BSA and 1 mM Na azide and then incubated with the following: rabbit anti-LPP polyclonal antibody (1:200; ImmunoGlobe, Germany); anti-vinculin monoclonal antibody (1:500, Sigma,MO); anti-Tenascin C polyclonal antibody (1:100); anti-pFAK polyclonal antibody (Sigma, MO); and anti-palladin polyclonal antibody (1:500, a generous gift from Dr. Carol Otey, University of North Carolina at Chapel Hill). Antibodies were diluted in blocking solution for 2 h at RT or overnight at 4°C. Secondary antibodies were Alexa 594 conjugated goat anti-rabbit IgG (1:1000; Molecular Probes) and Alexa488 goat anti-mouse IgG (1:1000) in blocking buffer; Jackson ImmunoResearch, West Grove, PA). For actin labeling cells were labeled for 1 h with Alexa 488 conjugated-phalloidin (1:1,000; Molecular Probes). Cells were washed four times for 5 min with PBS before being mounted with Aqua Poly/Mount (Polysciences, IL). Confocal images were obtained and quantified on an Olympus FV300 microscope. Analysis comparing immunofluorescence of the multiple treatments in a given experiment was carried out under identical conditions of laser power and light collection. Quantitation of fluorescence in the nucleus verses cytoplasm was measured using Olympus Fluoview software. The cell area or nucleus area is defined by drawing the outline of the structure. The ratio of the average signal intensity of the nucleus compared to the average total cell signal intensity was compared in cells with different treatments to evaluate LPP translocation to the nucleus. Measurements were made in a total of 50 cells for each condition.
Western blot
Western blotting was carried as previously described (Gorenne et al. 2006; Jin et al. 2007). The results were analyzed on an ODYSSEY infrared imaging system.
Results
LPP and palladin are recruited to podosomes in SMCs
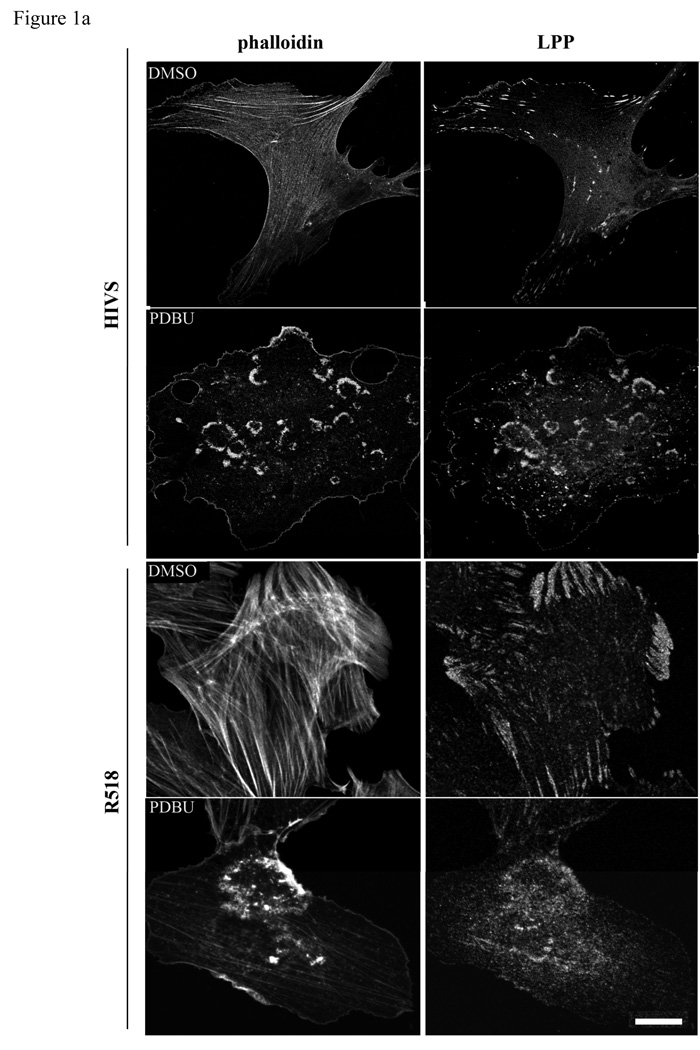
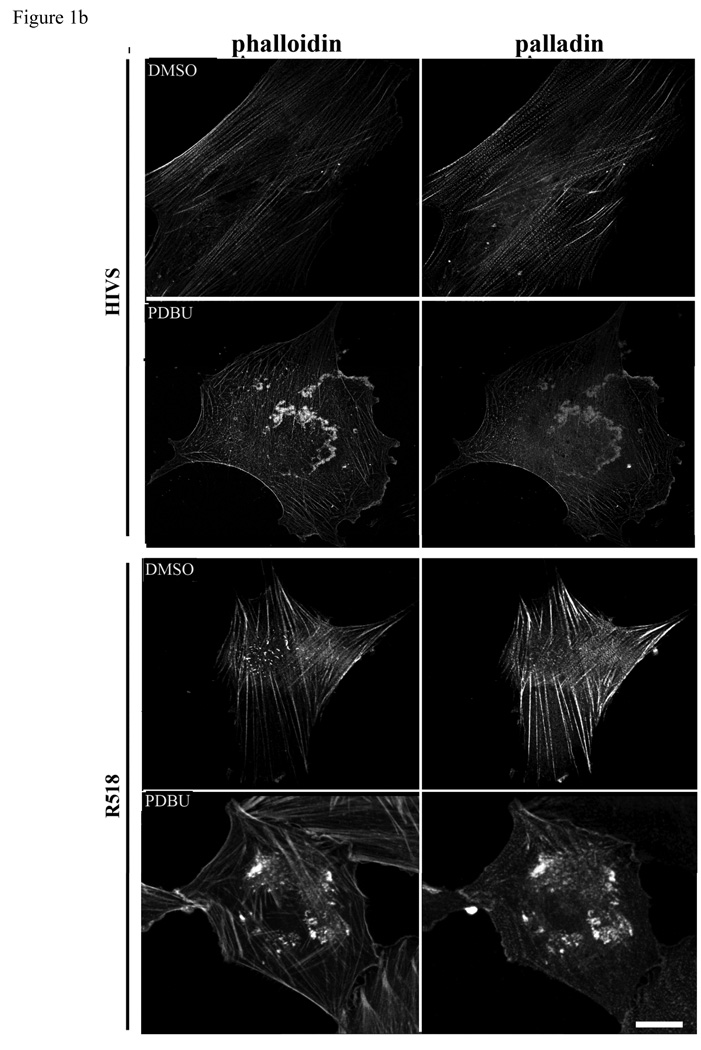
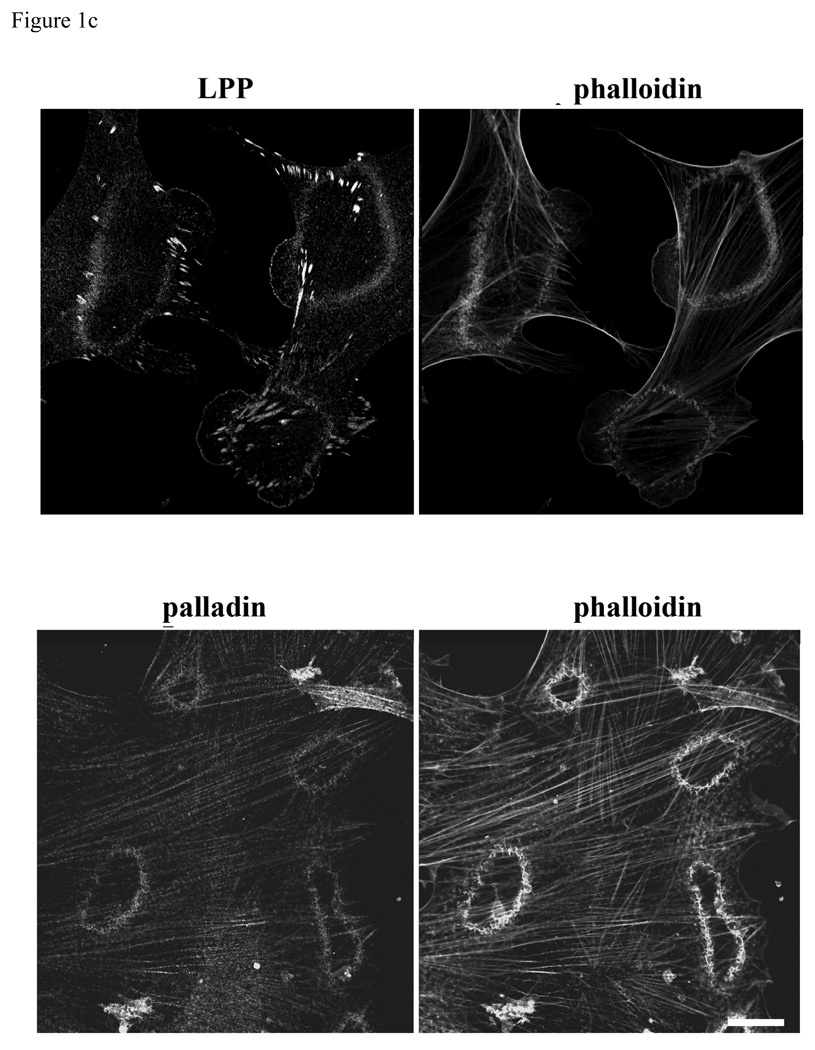
Podosomes are highly dynamic actin based structures commonly found in motile and invasive cells such as macrophages, osteoblasts and vascular SMCs (Hai et al. 2002; Linder and Aepfelbacher 2003). Recent reports have shown that podosomes are involved in the invasion of SMCs in vascular diseases such as atherosclerosis and restenosis (Lener et al. 2006). Podosomes, transient adhesive structures, which form on the ventral cell surface, are composed of actin, signaling molecules and actin associated proteins such as vinculin, α-actinin, talin, and nonerythroid spectin surrounding the actin filament core (Eves et al. 2006). The LPP binding partner palladin has been shown to play a role in phorbol ester (PDBu) induced podosome formation in A7r5 cells (Goicoechea et al. 2006). In this study, we confirm that 1µm PDBu induces podosome formation and show LPP (figure1a) as well as palladin (figure1b) recruitment to these structures in HIVS SMCs and rat aortic SMCs. Like podosomes, dorsal ruffles are also actin based motile structures that contain palladin (Goicoechea et al. 2006). PDGF-BB stimulation of quiescent cells typically results in a transient increase in dorsal membrane ruffles that precedes motility and we find both LPP and palladin are also recruited to these structures (figure 1c). This finding of LPP and its partner palladin enriched in these highly motile structures of variable size and shape extend and strengthen our previous observations showing their ability to enhance cell migration (Gorenne et al. 2006; Jin et al. 2007) and raising the possibility that they could play a role in SMC migration during formation of the neointima or atherosclerotic lesions as well as vasculogenesis.
Figure 1.
a,b: LPP and palladin immunolocalize to PDBu-induced podosomes. R518 and HIVS SMCs were plated on coverslips and treated with 1µm of PDBu for 1h. Cells were fixed and co-labeled with Alexa fluor 488-conjugated phalloidin, anti LPP polyclonal antibody (1a) and polyclonal anti-palladin polyclonal antibody (1b). 1c) LPP and palladin colocalize with actin in PDGF-BB induced dorsal ruffles. HIVS cells were treated with 30ng/ml of PDGFBB for 30min and fixed for immunofluorescence staining. Cells showing are representative cells of the population. Scare bar, 50 µm
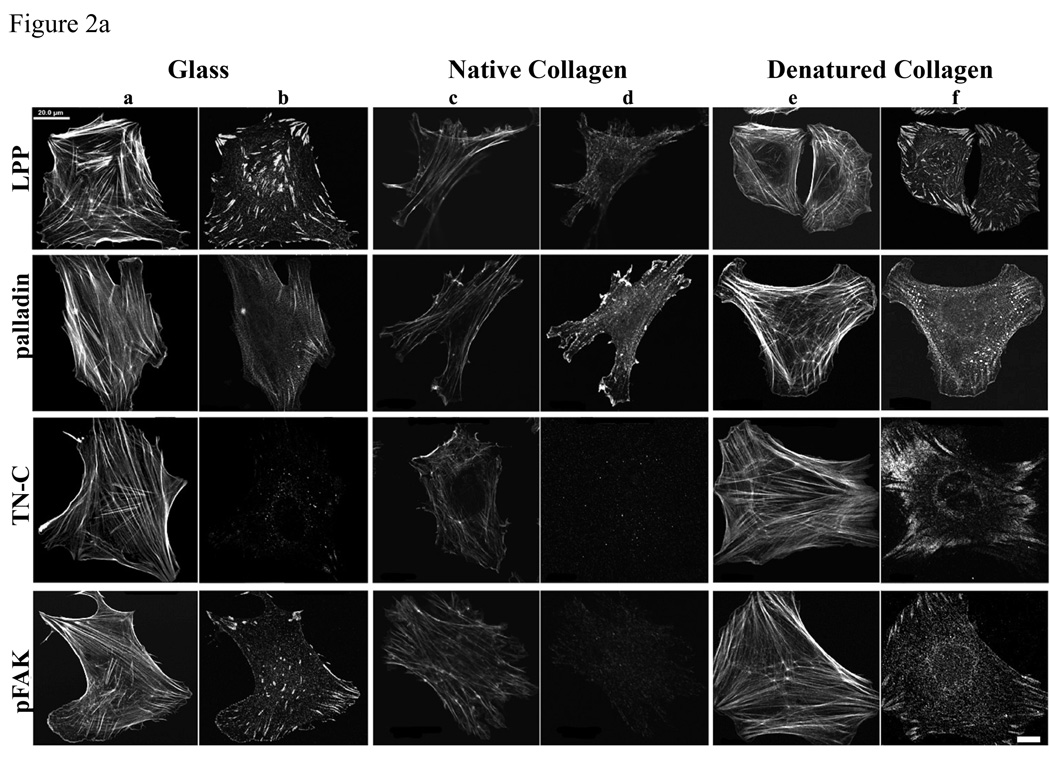
Denatured type I collagen upregulates LPP and palladin expression in SMCs
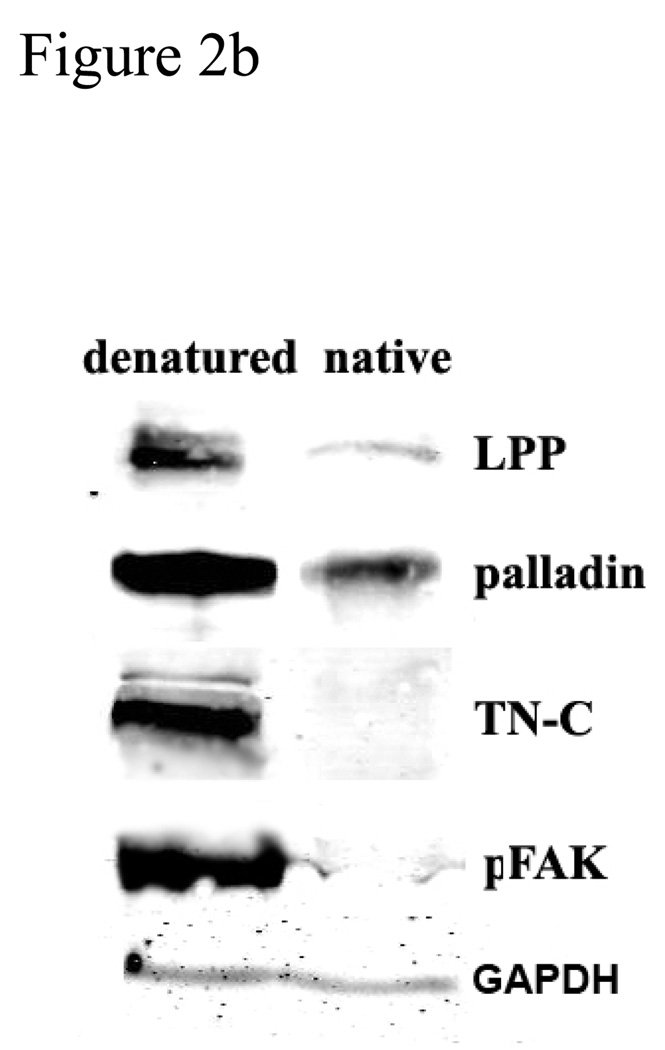
ECM protein components regulate SMC differentiation and proliferation via cell surface receptors. The use of denatured collagen as a substrate is thought to simulate its role in vessel injury where it activates cell surface receptors and contributes to SMC differentiation and proliferation (Jones 2007). SMCs show different morphologies on denatured and native collagen gels. R518 cells on denatured collagen gel are well spread (figure 2a, columns e and f), compared to the native collagen gels (figure 2a, column c and d) where the cells are smaller and less spread, with a stellate morphology, less focal adhesions and stress fibers. Focal adhesions are identified by immunolabeling for LPP, palladin and phospho-FAK. Stress fibers are detected with fluorescence conjugated phalloidin (figure 2a, column a, c,and e). As shown in figure 2a, the number of focal adhesion sites and actin stress fibers are markedly increased in cells cultured on denatured collagen, compared to native collagen gels. The expression of LPP and palladin is significantly induced by denatured collagen gels, for equal numbers of cells loaded, and this induction is correlated with increased tenascin-C (TN-C) and phosphorylated FAK (figure 2b, p<0.05) but no change in total FAK (data not shown) TN-C, an extracellular matrix glycoprotein, is known to support SMC proliferation, survival and motility, , as well as fibroblast motility (Jones 2007). Thus SMCs can sense the composition of their substrate and regulate the expression of LPP and palladin, and their expression is correlated with changes of TN-C and phosphorylated FAK.
Figure 2.
Expression of LPP and palladin is up regulated by heat-denatured collagen compared to native collagen. a): R518 cells were cultured on coverslips coated with native and heat denatured collagen or directly on glass for 24 h. Following fixation, LPP, palladin, phosphoFAK, and TN-C were detected by immunofluorescence as detailed in the Methods section. Actin was stained with Alexa fluor 488-conjugated phalloidin. (Column a, c, e ) Scare bar, 20 µm. Cells shown are representative of the population. b): western blot comparing the expression of LPP, palladin, TN-C and pFAK. R518 cells were cultured on native and heat denatured collagen gels. Equal number of cells were loaded for gel. Data is representative of three experiments
Substrate stiffness changes the expression of LPP and paladin
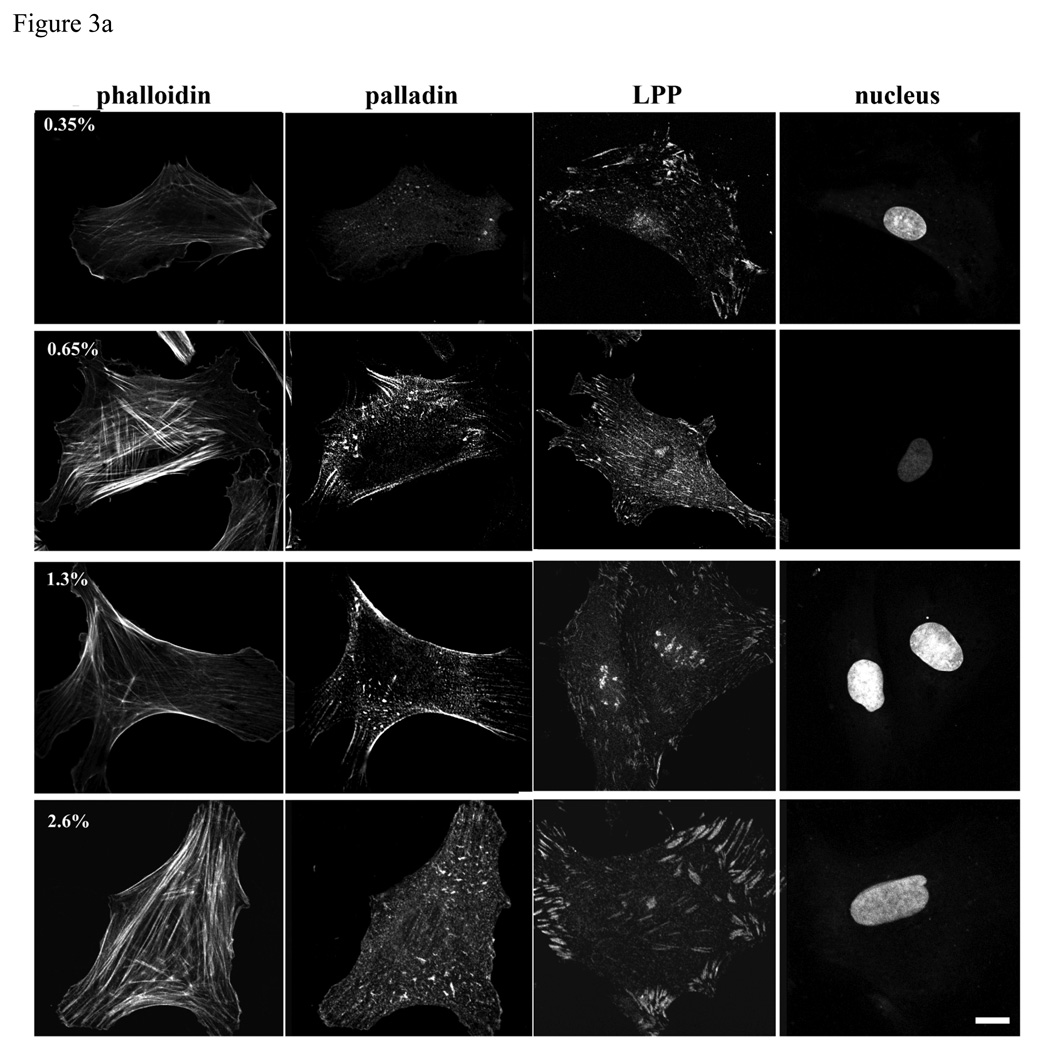
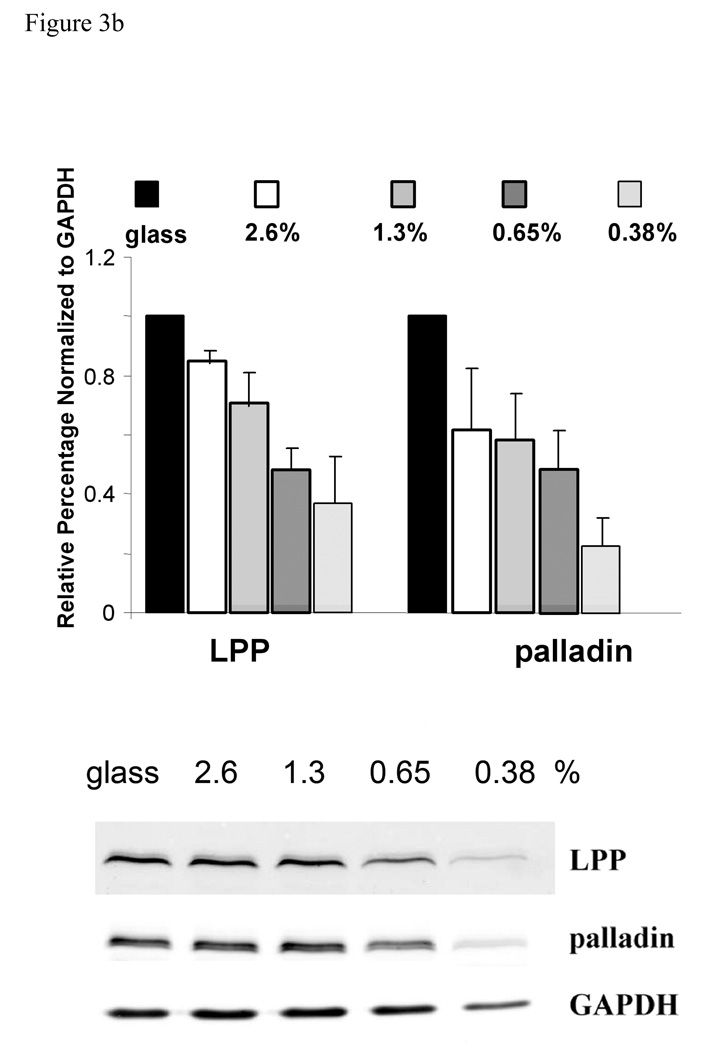
Varying concentration of polyacylamide-based, collagen coated substrate gels display different stiffness measured by Young’s Modulus (Pelham and Wang 1997). R518 SMCs were cultured on collagen-coated polyacrylamide substrates with varying stiffness. On the more flexible compliant substrates, SMCs were less spread and more irregularly shaped than on more rigid substrates (figure 3a), similar to that shown for normal rat kidney epithelial cells on compliant substrates (Pelham and Wang 1997). On the rigid substrate, LPP immunolocalization displayed extensive broad and long focal adhesions at the periphery of cells typical of those seen in cells attached to glass, while on the highly compliant substrate LPP staining was reduced and appeared in punctuate structures and small focal adhesions (figure 3a). Accordingly, western blots showed that the expression of LPP and palladin decreased with increased substrate compliance (figure 3b). Interestingly, a diffuse LPP signal was readily detected in the nucleus by 4h after plating when rat aortic SMCs were cultured on the less rigid substrates. The nucleus LPP signal intensity/ total cell signal intensity was significantly greater in cells grown on 0.35 vs 2.6% polyacrlyamide n=50, p<0.05. LPP also localized to nuclear bodies, being most pronounced at 1.3% and decreasing at 2.6% polyacrylamide (p<0.05). Altogether, the sensitivity of the expression and distribution of LPP and palladin to matrix stiffness and composition suggest that LPP and palladin may function as mechanosensors or are downstream of other mechanosensing molecules responding to environmental cues.
Figure3.
Expression of LPP and palladin is up regulated by increased substrate stiffness. a): R518 cells were cultured on coverslips with varying flexibility generated with polyacrylamide gels coated with type I collagen for 24h, and then fixed and immunolabeled with LPP and palladin antibody. Stress fibers were stained with Alexa fluor 488- conjugated phalloidin. Nuclei were stained with yoyo-1. Cells shown are representative of the population. Scare bar, 50µm. B): summary of changes in the expression of LPP and palladin on substrates of different stiffnesses. Cells were lysed after 24 h incubation. Equal amounts of cell lysate were loaded for western blotting. Bar graph shows quantitative data normalized to GAPDH . p<0.05, n=4
Expression of LPP and palladin is down regulated by oxidative stress
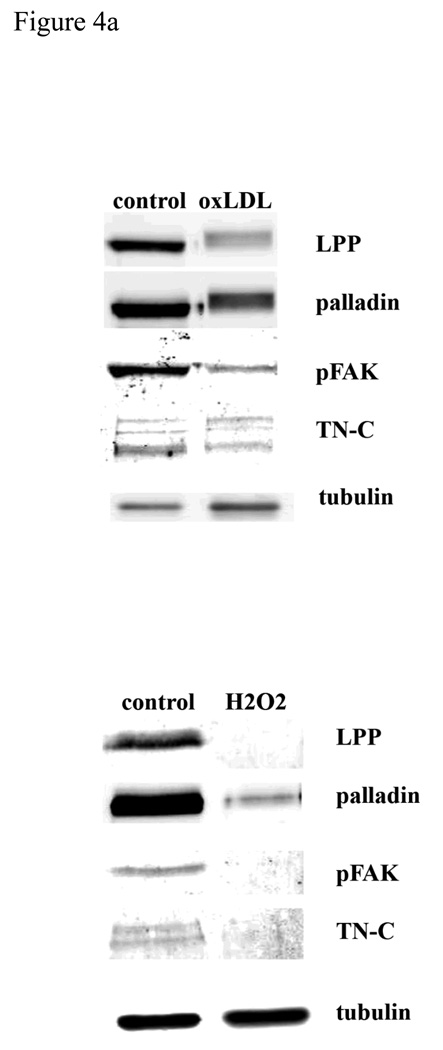
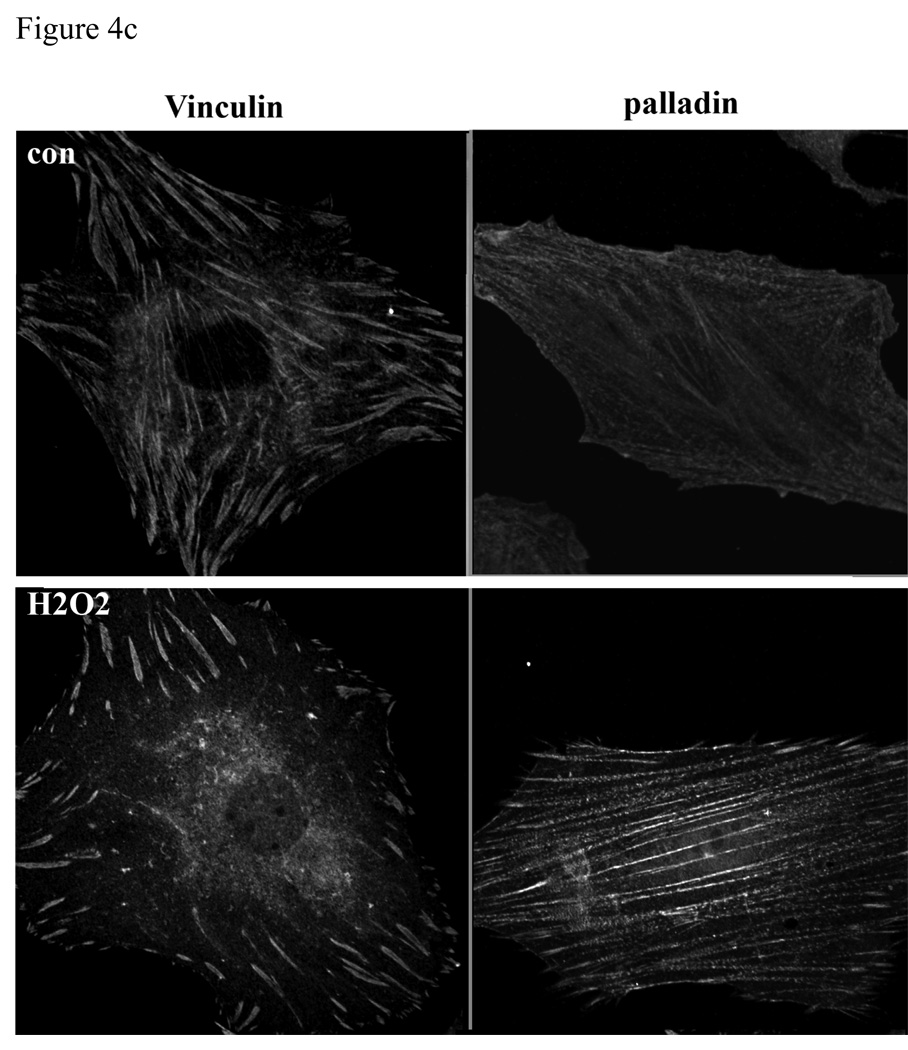
Deposition of low-density lipoproteins (LDLs) in the vessel wall and their oxidative modification seem to initiate, or at least accelerate, the atherosclerotic process by several mechanisms, including promotion of foam cell formation, chemotactic effects on monocytes, and mitogenic effects on SMCs (Ross 1986). Accumulation of SMCs is a hallmark of neointimal formation, and considered as the “soil” of atherosclerosis. Recent reports have shown that oxLDL decreased the level of PINCH-1, another focal adhesion LIM domain protein, in vascular SMCs (Cheng et al. 2007). We have shown that LPP and palladin colocalize at focal adhesions, which are sites connecting integrins to the cytoskeleton (Gorenne et al. 2006; Jin et al. 2007). Therefore, we tested whether oxLDL can modulate the expression and localization of LPP and palladin. When we treated SMCs with 50µg/ml of oxLDL for 6hrs (and 24 hrs, data not shown), we found a significant decrease in the level of LPP and palladin expression (figure 4a, p<0.05), which correlated with the decreased expression of TN-C and pFAK (there is no detectable change of total FAK, data not shown). The cell viability is 90-95% after 6h incubation with oxLDL, while with 24h incubation cell viability is 60–70% as measured by trypan blue staining. As shown in figure 4b, after 1h incubation with oxLDL, LPP is detected in the nucleus, while both pFAK and TN-C signal are decreased (supplement fig1). OxLDL is known to increase reactive oxygen species formation, including a rapid elevation of H2O2 levels (Sukhanov et al. 2006). We further tested whether LPP and palladin are altered by H2O2, which modifies the cellular redox state. As shown in figure 4b, when SMCs were treated with 100 µM of H2O2 for 1hr, a fraction of the LPP signal, both diffuse and punctate was detected in the nuclei with the rest at focal adhesions (nucleus signal intensity/the total signal intensity in untreated vs H2O2 p<0.05 n=50, ). No change for the palladin distribution was detected under these conditions, similar to another focal adhesion protein, vinculin that is known to localize to focal adhesion with H2O2 treatment (figure 4c). Thus, oxLDL and reactive oxygen species, which play a role in the generation of atherosclerotic lesions, lead to translocation of LPP to the nucleus and down regulation of its expression presumably favoring a less migratory state.
Figure 4.
LPP, palladin, phosphoFAK and TN-C expression are down regulated by oxidative stress. A): R518 cells were treated with either 100ug/ml of oxLDL for 6h or 100µM of H2O2 for 24h, and cells were trypsinized and lysed for Western blotting. LPP, palladin, phosphoFAK and TN-C protein expression was significantly decreased from controls, p<0.05, n=3. Equivalent numbers of cells were loaded in each lane. Tubulin was used as loading control. B): LPP translocates to the nucleus, but not vinculin, in response to oxidative stress. Cells were treated with either 100ug/ml of oxLDL or 100µM of H2O2 for 1h, and cells were fixed for immunofluorescence staining with LPP antibody. Phase contrast image shows the cell morphology. Cells shown are representative cells of the population. Scare bar 50µm.
The expression of LPP and palladin is regulated by arterial hemodynamics
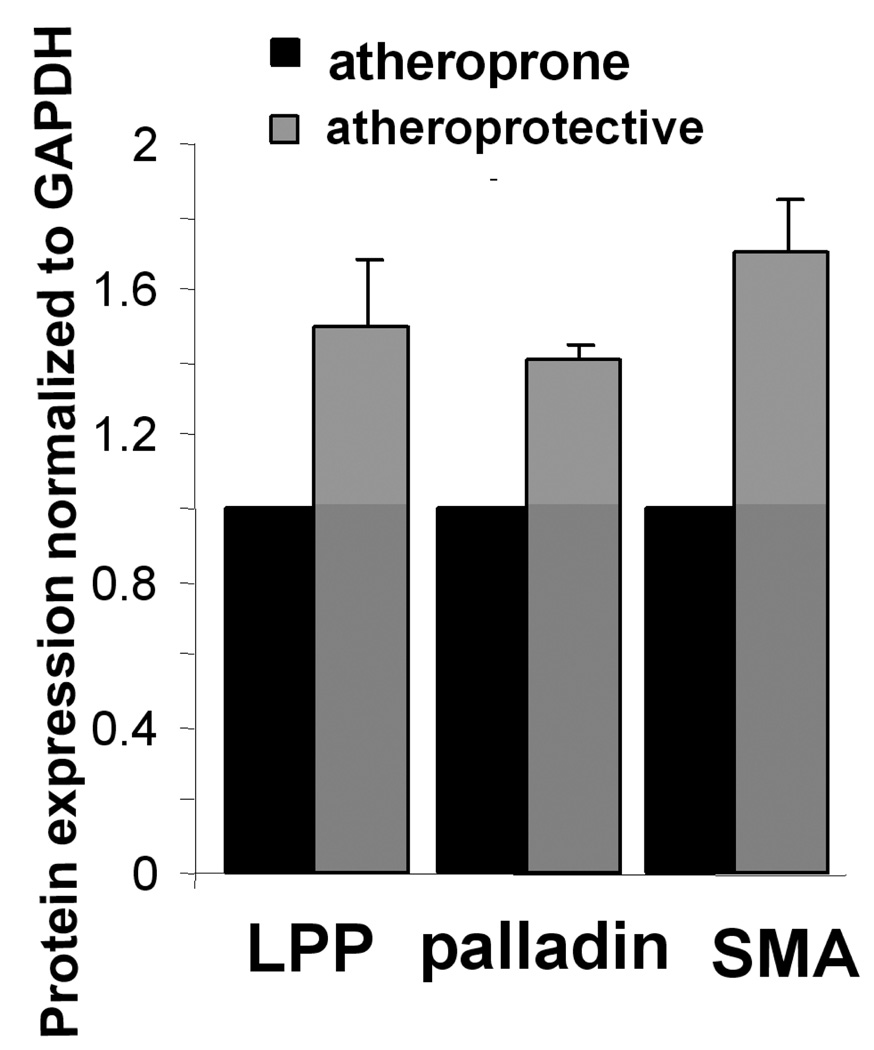
Atherosclerosis is characterized by its focal development at hemodynamically defined regions in large arteries, such as at bifurcations that produce complex flow patterns. Atheroprone regions, susceptible to plaque formation, are subjected to low time-averaged shear stress and "disturbed" oscillatory flow patterns. In contrast, regions which are less susceptible to plaque formation, known as atheroprotective regions, are exposed to relatively higher timeaveraged shear stress and pulsatile laminar flow (Hastings et al. 2007). In an in vitro EC/SMC co-culture model, where atheroprone or atheroprotective flow patterns derived from in vivo MRI measurements, are applied to the EC layer for 24 hrs, the expression of LPP and palladin in the SMCs layer is significantly decreased with atheroprone compared with atheroprotective flow (figure 5, p<0.05). The reduction in LPP and palladin corresponds with previously reported decreases in SMA expression (Hastings et al. 2007).
Figure 5.
the expression of LPP and palladin is down regulated by atheroprone flow. In an in vitro EC/SMC culture system, atheroprone flow was applied to the EC layer for 24h. SMCs were harvested for Western blotting. Bar graph shows the quantification of protein expression, normalized to GAPDH. p<0.05, n=3
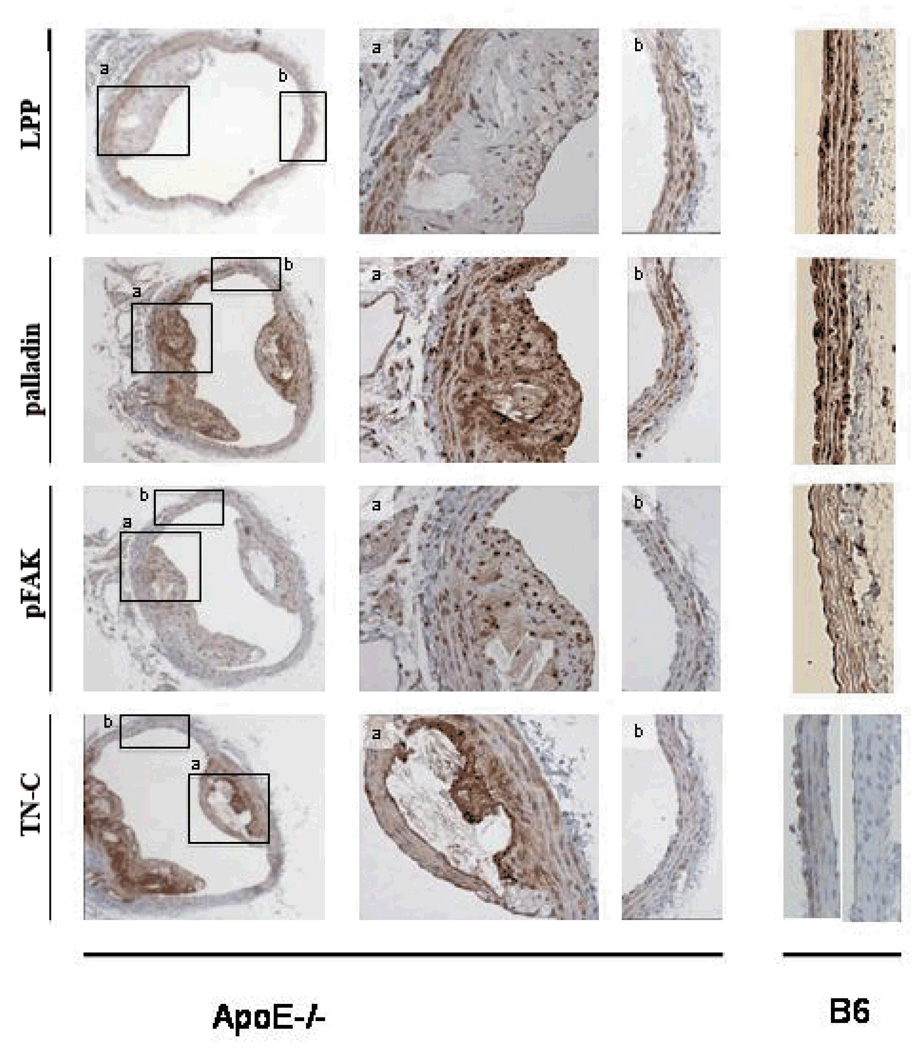
In an in vivo model of atherosclerosis using 32 week old ApoE null mice, LPP and palladin as well as phospho-FAK are decreased compared to WT, in cross sections of the media of the aortic arch (figure 6). However, palladin, unlike LPP is present in the plaque. Phospho-FAK labeling is enhanced in the plaque regions. TN-C expression is also upregulated in the plaque region with a gradient of TN-C expression; plaque > >media underlying the plaque > media without plaque > WT media (lower panel figure 6, B6). Interestingly occasionally discrete otherwise normal areas of the WT aortic arch displayed TN-C staining (right panel, figure 6, B6) perhaps reflecting transient sites of minor injury in regions experiencing atheroprone flow. Prx1 labeling in the media and or regions of lesion did not differ from WT sections of aortic arch (data not shown). Thus, LPP and associated modulators were down regulated in the advanced atherosclerotic lesion containing foam cells, macrophages and lipids, unlike the presence of LPP, palladin, Prx1 and phospho-FAK in SMCs migrating to and proliferating in the neointima following vessel injury from day7 (shown below).
Figure 6.
LPP, palladin, and pFAK are decreased in media of aorta in 32 week apoE null mice (panels labeled b), while TN-C is increased in the atherosclerosis lesion. Columns 2 and 3 are high magnification images of the framed regions in column 1. The right panels show control tissue staining. n=4
The roles of LPP and palladin in the endothelium are currently unstudied. In the EC layer, the expression of LPP and palladin is also decreased with atheroprone flow (data not shown). Although expression of these proteins is lower than SMCs, staining of the endothelium for both LPP and palladin was positive in the ApoE−/− model (Figure 6). Specifically, it was of particular interest that both proteins were strongly induced in endothelium within atherosclerotic lesions, but not in regions absent of plaque formation (ie., atheroprotective regions). This suggests a novel role for LPP and palladin in atherogenesis; however, further investigation is required to understand the exact mechanosensing mechanisms and pathways involved.
Expression of palladin and LPP is regulated by FAK, TN-C and Prx1 in a rat aortic injury model
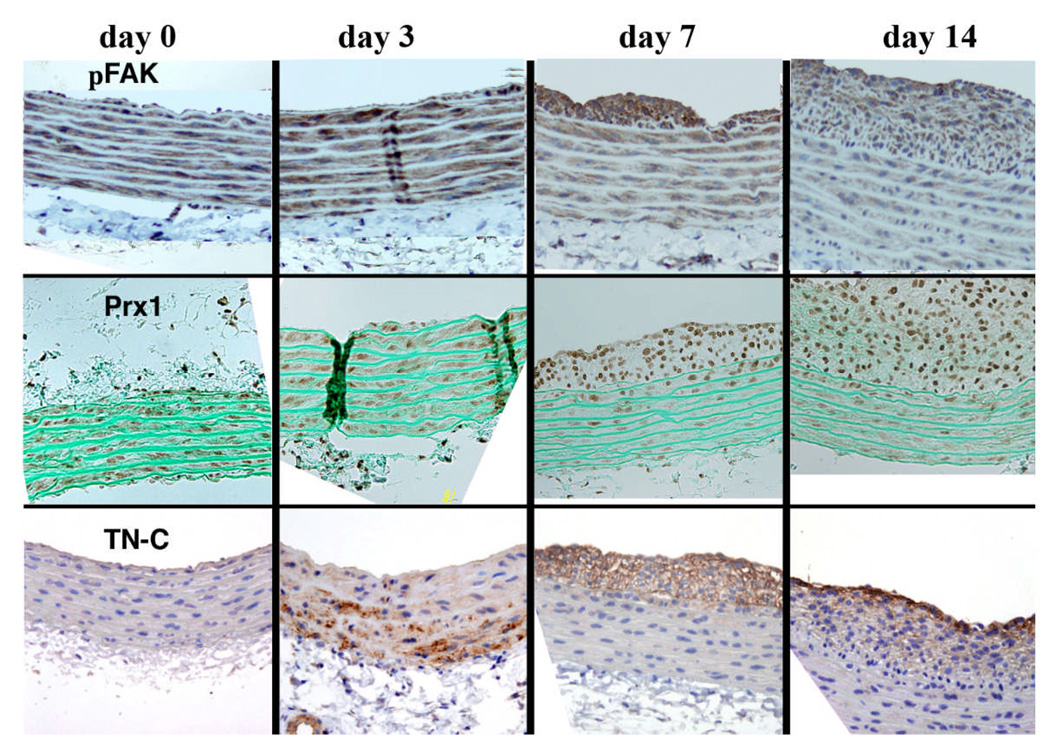
We have shown that both LPP and palladin, like SMA are expressed in medial SMCs but not adventitial fibroblasts and appear in the neointima at 7 days post injury (the first time point measured) (Jin et al. 2007). Based on the high expression of LPP and palladin in SMCs and on their ability to enhance migration in in vitro assays, we suggest a role for these molecules in cytoskeletal remodeling when SMCs transition to migratory cells. In order to test whether the alteration of LPP and palladin in the injured vessel is correlated with their upstream modulators, we further followed over time the expression of phosphoFAK, TN-C and the homeodomain protein, Prx1 in this model. As shown in figure 7, at day 0, Prx1 labeling of media nuclei exhibited a variable intensity suggesting different populations of SMCs. The Prx1 signal shifted to the neointima at day 7, and intense staining of Prx1 occurred in the neointima at day 14. TN-C was first detected in the outer media 3 days post injury and moved over time to the fibrous cap. FAK was detected in the media and enhanced in the neointima (data not shown) as was the expression of phospho-FAK, which was also increased in medial SMCs at day 3 (figure 7 upper). Thus the early expression of Prx1, FAK and phospho-FAK in medial SMCs as well as expression of TN-C at day 3 prior to development of the neointima and at later time points, their enhanced expression in the neointima, is consistent with their functioning upstream of the expression of LPP and palladin in the SMCs which migrate from the media and proliferate in the neointima in response to environmental cues such as vascular injury.
Figure 7.
Immunohistochemistry showing pFAK, TN-C and Prx1 expression in a time and spatial dependent manner in SMCs of the neointima and media of injured rat aortae at day 0 through to day 14. The vessel lumen is oriented to the top of each panel. For each experiment, there were 4 animals and 4 sections were observed. Similar patterns of immunolabeling were observed in each experiment for a given time point.
Discussion
Mechanical signals mediated by the ECM play an essential role in various physiological and pathological processes. Adherent cells seem capable of both responding to applied mechanical forces (Tzima et al. 2005) and probing the mechanical properties of their environment (Discher et al. 2005). For cultured adherent cells, focal adhesions are the structures which mediate mechanosensing through integrin-mediated anchorage to the ECM (Guo et al. 2006), while the mechanism underlying the conversion of physical signals in adhesion sites into chemical ones is still largely unknown. In the current study, we propose that the focal adhesion proteins LPP and palladin play a role in the mechanosensing process in SM based on the following findings: 1) LPP and palladin expression was responsive to substrate composition and rigidity, accompanied by correlative changes in phospho-FAK and TN-C; 2) the expression of LPP and palladin was significantly decreased with atheroprone flow in an in vitro EC/SMC co-culture system and in an in vivo atherosclerosis model; 3) LPP was translocated to the nucleus by oxidative stress and substrate compliance. Furthermore, we propose that mechanotranscriptional regulation of the SMC specific protein LPP and its partner palladin is an early event in the development of atherosclerotic lesions and the formation of the neointima following injury because: 1) the expression of LPP and palladin was down regulated by oxidative stress; 2) LPP and palladin shown to increase SMC migration (Jin et al. 2007), were recruited to podosomes and dorsal ruffles associated with highly motile cells and implicated in the invasion of SMCs in vascular diseases such as atherosclerosis and restenosis (Lener et al. 2006); 3) Prx1, TN-C and phospho- FAK are present in the neointima in a time and spatially dependent manner following injury as we have previously shown for LPP and palladin (Jin et al. 2007). Overall, we propose that the mechanical responsiveness of the SMC specific protein LPP and its partner palladin is an early event in the development of atherosclerotic lesions and that their expression and positive role in SMC migration contribute to the formation of the neointima following injury. Furthermore, these molecules may serve as important markers of these processes.
Within their lifetime, cells are exposed to mechanical forces exerted either by their contactile machinery or by a variety of environment factors. How cells respond to the mechanical properties in their environment is likely critical during development, differentiation and in the etiology of diseases. Mechanical stimulation affects the size and subcellular location of focal adhesions, as well as the activation of specific signaling events (Yoshigi et al. 2005). Moreover, the rigidity of adhesive substrates even in the absence of agonists or growth factors, affects how cells attach, spread, polarize, and migrate (Discher et al. 2005). Sufficient substrate stiffness appears particularly important to anchorage-dependent cells, which often rely on a finite resistance to cell generated forces in order to induce outside-in mechanical signals (Discher et al. 2005). A variety of cell types has been shown to sense substrate stiffness but little is known regarding SMCs. Different cells have different optimal substrate stiffnesses capable of supporting maximal migration speed (Discher et al. 2005). Others have shown in epithelial and fibroblast cells, that vinculin is incorporated into elongated focal adhesions on stiffer gels, while on flexible gels, cells have increased motility, decreased phosphotyrosine and vinculin is localized at punctuate structures which assemble and dissemble quickly (Pelham and Wang 1997). In the current study we show that both LPP and palladin are involved in the signaling cascade that senses the flexibility of the substrates, as the rigidity of the substrates increases, the expression level of these two proteins are increased as is the phosphorylation of FAK (Pelham and Wang 1997). As the compliance of the substrate affects cell spreading and the assembly of actin stress fibers, the tension on the cytoskeleton or the G:F actin may be the critical step for regulating expression of LPP and palladin. We have previously shown that actin dynamics regulated their expression with an increase in F actin increasing LPP and palladin expression and vice versa (Jin et al. 2007). Of particular interest, on compliant substrates, LPP is translocated to the nucleus in SMCs suggesting a transcriptional regulation function that may be through SRF and myocardin pathways (Jin et al. 2007; Petit et al. 2008) and this is currently a focus of investigation. We next explored how might be these changes in LPP and palladin in response to substrate compliance relate to the in vivo vasculature.
Disturbed hemodynamic forces stimulate inflammatory responses in the atherosclerosis-prone arch of aorta that lead to a triggered recruitment of activated monocytes. In an in vitro EC/SMC co-culture system, using human ECs and SMCs, we also showed that atheroprone flow, which represents shear stress profiles in the corresponding atherosclerosis-susceptible carotid artery bifurcation, significantly decreased the expression of LPP and palladin in SMCs. As demonstrated in a previous study, regional hemodynamic flow on the endothelium can cause differential mechanotranscriptional coupling between the EC and SMC layer to influence SMC phenotype in atherosclerosis (Hastings et al. 2007). Specifically, results showed that atheroprone flow reduced classic EC quiescent markers such as eNOS and the SMC contractile genes such as SMA, which coincided with increased inflammatory genes as IL-8. To extend this observation to the in vivo atherosclerosis model, we further show that the expression of LPP and palladin was down regulated in the media of atherosclerotic aorta in ApoE mice on a chow diet. Thus LPP and palladin were down regulated at the onset as well as late stage in the disease. Abnormal hemodynamics also increases the expression of the receptor for oxLDL in endothelial cells, which in turn leads to oxLDL accumulation (Kume et al. 2000). OxLDL is detected in atherosclerotic plaques and in the plasma of atherosclerotic patients, where it contributes to disease evolution. Depending on the extent of its oxidation, oxLDL can induce proliferation, monocyte chemotaxis, apoptosis or necrosis of vascular EC and SMC. OxLDL is believed to be one of the main inducers of oxidative stress. H2O2 is a major oxidative component of oxLDL induced oxidative stress (Sukhanov et al. 2006). We found that oxLDL and H2O2 can significantly decrease the expression of LPP and palladin, and translocate LPP to the nucleus. This may be through the integrin αVβ3- integrin kinase and Rho-ROCK pathways (Cheng et al. 2007). It is also possible that apoptosis is involved in the H2O2 induced downregulation of LPP and palladin (Kim et al.2004) as cell survival was 60–70%. However, our measurements of the effects of oxLDL were made at 6 hr when cell survival was 90–95%, making a contribution from apoptosis to the downregulation of LPP and palladin unlikely. OxLDL and H2O2 treatment resulted in LPP accumulation in discrete areas of the nucleus. LPP, which has a nuclear export signal and in vitro transcriptional activation capacity, has been detected in the nucleus under specific conditions, such as in the presence of a Rho kinase inhibitor or treatment with leptomycin B (Gorenne et al. 2006). However the relevance of this nuclear localization remains to be explored. This suggests that LPP has a potential role in gene transcription and SMC phenotype, and in these conditions such oxLDL and H2O2 as well as with the Rho kinase inhibitor it work via downregulating SM makers.
SMC migration also plays an important role in the development of the atherosclerotic lesion and the formation of the neointima following injury. LPP and palladin contribute to the migratory phenotype. Vascular SMCs are not terminally differentiated and have the ability to switch between a contractile and a synthetic phenotype. Phenotypic modulation and ECM degradation are essential steps for the migration of vascular SMCs from the medial to form the neointima of the artery such as occurs with post angioplasty stenosis (Owens 1995; Owens et al. 2004). Palladin is a key regulator of actin organization and localizes to focal adhesions, cell-cell junctions, dorsal and circular ruffles, and periodically along stress fibers where it colocalizes with a-actinin, and is required for the maintenance of normal stress fibers and focal adhesions in cultured cells (Boukhelifa et al. 2001; Hwang et al. 2001; Boukhelifa et al. 2003; Boukhelifa et al. 2004; Otey et al. 2005; Goicoechea et al. 2006; Rachlin and Otey 2006; Ronty et al. 2006). We have shown that LPP and palladin can enhance cell migration and that they appear in the neointima of injured vessels (Jin et al. 2007). Others (Kim et al. 2004; Li et al. 2008) have shown that ROS can also enhance cell migration. Unexpectively, we found that H2O2 and oxLDL decrease LPP expression which should slow cell migration. A possible explanation of this paradox may lie in spatial distribution and the time course of the ROS signal or in the underlying signaling molecules such as protein tyrosine phosphatases, Src, MAPKs and Rho kinase (Weber et al. 2004). Here, we show that LPP like it partner palladin can be recruited to the motile podosome and dorsal ruffles structures in vascular SMCs supporting their importance in SMC migration. Podosomes are also involved in the invasion of SMCs in vascular diseases (Lener et al. 2006). The detailed mechanisms of how LPP is recruited to these structures and whether LPP can enhance their formation remain to be addressed.
The composition of the extracellular matrix as well as its mechanical properties is altered in vascular disease. For example, the activation of matrix metalloproteinases and generation of extracellular TN-C promoting the breakdown of collagen and cell migration in atherosclerosis and vascular injury would be expected to change the composition and stiffness of the matrix. We found an increase in LPP, palladin, phosphoFAK and TN-C on denatured collagen, with increased substrate stiffness, as well as in the neointima following injury. Thus, this in vitro model mimics some aspects of the injured vessel. For in vitro experiments, heat-denatured collagen has been used in place of MMP-treated collagen, because it is also recognized by vascular SMC via RGD-dependent αvβ3 integrins, and causes the cells to exhibit a phenotype characteristic of the diseased state (Davis 1992). On denatured, in contrast to native collagen gels, the cells assume a highly spread morphology, have well-organized cytoskeletal stress fibers, secrete TN-C, and proliferate (Jones et al. 1997). We have previously shown that FAK can regulate the SMC transcription factor Prx1 and Prx1 has been shown to upregulate expression of TN-C (Jin et al. 2007; Jones 2007), which in turn regulates the expression of LPP and palladin (Jin et al. 2007), which we propose is through destabilization of the extracellular matrix. Expression of Prx1/2 in FAK null cells lead to expression of LPP and palladin (Jin et al. 2007). Likewise in the present study, conditions where LPP and palladin were increased correlated with increased phosphorylated FAK. Thus FAK phosphorylation, an important mediator of integrin-mediated signaling responds to mechanical stimuli, oxidative stress and substrate composition. Our findings are also consistent with our previous results showing the presence of LPP and palladin in the neointima in the rat aortic injury model (Jin et al. 2007). We now show in this model that the activation of the FAK-Prx1-TN-C pathway, has a time course and spatial distribution consistent with their being upstream regulators of LPP and palladin expression.
The up or down regulation of expression of LPP and palladin respectively in the neointima and in the atheroprone region and atherosclerotic lesions highlights the differences and complexities of these two processes. Thus, atheroprone hemodynamics and accompanied oxidative stress may dominate and account for the down regulation of expression of LPP and palladin and their upstream regulators in the models of atherosclerosis. On the other hand, an up regulation of these molecules occurred in the neointima following vascular injury, associated with increased cell migration and proliferation presumably reflecting different changes in substrate composition and stiffness as well as different signaling pathways.
In summary, our findings demonstrate that LPP and palladin, highly expressed in SMCs sense their environment, responding to mechanical cues and to oxidative stress and substrate composition. We propose that these signals underlie their up and down regulation in vessel wall injury and atherosclerotic lesions, respectively as well as their differential distribution across the vessel during lesion development.
Supplementary Material
A) Immuno labeling showing a decrease in phosphorylated FAK and B) TN-C expression by oxidative stress. Cells were treated with either 50ug/ml of oxLDL or 100µM of H2O2 for 1h. Cells were then fixed for immunofluorescence staining as detailed in the Methods section.
Acknowledgements
We gratefully acknowledge Dr. Carol Otey (University of North Carolina at Chapel Hill) for palladin antibody, Dr. Brian Wamhoff for injured rat aorta samples, Mr. John Sanders for immunostaining assistance (University of Virginia)
Sources of Funding:
Supported by NIH grants PO1 HL48807 and PO1 HL 19842 to A.V.S., NIH R01 HL 082836 to B.R.B. N.E.H. is supported by a Basic Cardiovascular Research Training Grant NIH 5-T32-HL 0084.
Footnotes
Disclosures: None.
Reference
- Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA. Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton. 2004;58:17–29. doi: 10.1002/cm.10173. [DOI] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell. 2001;12:2721–2729. doi: 10.1091/mbc.12.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhelifa M, Hwang SJ, Valtschanoff JG, Meeker RB, Rustioni A, Otey CA. A critical role for palladin in astrocyte morphology and response to injury. Mol Cell Neurosci. 2003;23:661–668. doi: 10.1016/s1044-7431(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhang J, Merched A, Zhang L, Zhang P, Truong L, Boriek AM, Du J. Mechanical stretch inhibits oxidized low density lipoprotein-induced apoptosis in vascular smooth muscle cells by up-regulating integrin alphavbeta3 and stablization of PINCH-1. J Biol Chem. 2007;282:34268–34275. doi: 10.1074/jbc.M703115200. [DOI] [PubMed] [Google Scholar]
- Daheron L, Veinstein A, Brizard F, Drabkin H, Lacotte L, Guilhot F, Larsen CJ, Brizard A, Roche J. Human LPP gene is fused to MLL in a secondary acute leukemia with a t(3;11) (q28;q23) Genes Chromosomes Cancer. 2001;31:382–389. doi: 10.1002/gcc.1157. [DOI] [PubMed] [Google Scholar]
- Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Eves R, Webb BA, Zhou S, Mak AS. Caldesmon is an integral component of podosomes in smooth muscle cells. J Cell Sci. 2006;119,24:1386–1392. doi: 10.1242/jcs.02881. [DOI] [PubMed] [Google Scholar]
- Gerthoffer WT, Gunst SJ. Invited review: focal adhesion and small heat shock proteins in the regulation of actin remodeling and contractility in smooth muscle. J Appl Physiol. 2001;91:963–972. doi: 10.1152/jappl.2001.91.2.963. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Arneman D, Disanza A, Garcia-Mata R, Scita G, Otey CA. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–3324. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- Gorenne I, Nakamoto RK, Phelps CP, Beckerle MC, Somlyo AV, Somlyo AP. LPP, a LIM protein highly expressed in smooth muscle. Am J Physiol Cell Physiol. 2003;285:C674–C685. doi: 10.1152/ajpcell.00608.2002. [DOI] [PubMed] [Google Scholar]
- Gorenne I, Jin L, Yoshida T, Sanders JM, Sarembock IJ, Owens GK, Somlyo AP, Somlyo AV. LPP expression during in vitro smooth muscle differentiation and stent-induced vascular injury. Circ Res. 2006;98:378–385. doi: 10.1161/01.RES.0000202802.34727.fd. [DOI] [PubMed] [Google Scholar]
- Guo WH, Frey MT, Burnham NA, Wang YL. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006;90:2213–2220. doi: 10.1529/biophysj.105.070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai CM, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. doi: 10.1006/excr.2002.5592. [DOI] [PubMed] [Google Scholar]
- Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007b;293:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- Hautmann MB, Thompson MM, Swartz EA, Olson EN, Owens GK. Angiotensin II-induced stimulation of smooth muscle alpha-actin expression by serum response factor and the homeodomain transcription factor MHox. Circ Res. 1997;81:600–610. doi: 10.1161/01.res.81.4.600. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Pagliardini S, Boukhelifa M, Parast MM, Otey CA, Rustioni A, Valtschanoff JG. Palladin is expressed preferentially in excitatory terminals in the rat central nervous system. J Comp Neurol. 2001;436:211–224. [PubMed] [Google Scholar]
- Jin L, Kern MJ, Otey CA, Wamhoff BR, Somlyo AV. Angiotensin II, focal adhesion kinase, and PRX1 enhance smooth muscle expression of lipoma preferred partner and its newly identified binding partner palladin to promote cell migration. Circ Res. 2007;100:817–825. doi: 10.1161/01.RES.0000261351.54147.de. [DOI] [PubMed] [Google Scholar]
- Jones PL. Move on!: smooth muscle cell motility paired down. Circ Res. 2007;100:757–760. doi: 10.1161/01.RES.0000263446.33849.ed. [DOI] [PubMed] [Google Scholar]
- Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Min G, Bae YS, Min DS. Phospholipase D is involved in oxidative stress-induced migration of vascular smooth muscle cells via tyrosine phosphorylation and protein kinase C. Exp Mol Med. 2004;36:103–109. doi: 10.1038/emm.2004.15. [DOI] [PubMed] [Google Scholar]
- Kume N, Moriwaki H, Kataoka H, Minami M, Murase T, Sawamura T, Masaki T, Kita T. Inducible expression of LOX-1, a novel receptor for oxidized LDL, in macrophages and vascular smooth muscle cells. Ann N Y Acad Sci. 2000;902:323–327. doi: 10.1111/j.1749-6632.2000.tb06332.x. [DOI] [PubMed] [Google Scholar]
- Lener T, Burgstaller G, Crimaldi L, Lach S, Gimona M. Matrix-degrading podosomes in smooth muscle cells. Eur J Cell Biol. 2006;85:183–189. doi: 10.1016/j.ejcb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Li J, Niu XL, Madamanchi NR. Leukocyte antigen-related protein tyrosine phosphatase negatively regulates hydrogen peroxide-induced vascular smooth muscle cell apoptosis. J Biol Chem. 2008 doi: 10.1074/jbc.M806087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Organizing motility: LIM domains, LPP, and smooth muscle migration. Circ Res. 2006;98:306–308. doi: 10.1161/01.RES.0000208059.16734.35. [DOI] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Nelander S, Mostad P, Lindahl P. Prediction of cell type-specific gene modules: identification and initial characterization of a core set of smooth muscle-specific genes. Genome Res. 2003;13:1838–1854. doi: 10.1101/gr.1197303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MM, Mols R, Schoenmakers EF, Mandahl N, Van de Ven WJ. LPP, the preferred fusion partner gene of HMGIC in lipomas, is a novel member of the LIM protein gene family. Genomics. 1996;36:118–129. doi: 10.1006/geno.1996.0432. [DOI] [PubMed] [Google Scholar]
- Petit MM, Lindskog H, Larsson E, Wasteson P, Athley E, Breuer S, Angstenberger M, Hertfelder D, Mattsson E, Nordheim A, Nelander S, Lindahl P. Smooth muscle expression of lipoma preferred partner is mediated by an alternative intronic promoter that is regulated by serum response factor/myocardin. Circ Res. 2008;103:61–69. doi: 10.1161/CIRCRESAHA.108.177436. [DOI] [PubMed] [Google Scholar]
- Rachlin AS, Otey CA. Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci. 2006;119:995–1004. doi: 10.1242/jcs.02825. [DOI] [PubMed] [Google Scholar]
- Rogalla P, Lemke I, Kazmierczak B, Bullerdiek J. An identical HMGIC-LPP fusion transcript is consistently expressed in pulmonary chondroid hamartomas with t(3;12)(q27-28;q14-15) Genes Chromosomes Cancer. 2000;29:363–366. [PubMed] [Google Scholar]
- Ronty MJ, Leivonen SK, Hinz B, Rachlin A, Otey CA, Kahari VM, Carpen OM. Isoform-Specific Regulation of the Actin-Organizing Protein Palladin during TGF-beta1-Induced Myofibroblast Differentiation. J Invest Dermatol. 2006;126:2387–2396. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- Ross R. Endothelium, monocytes, platelets, and atherosclerosis. Monogr Atheroscler. 1986;14:169–172. [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Itabe H, Ono K, Parthasarathy S, Delafontaine P. Novel effect of oxidized low-density lipoprotein: cellular ATP depletion via downregulation of glyceraldehyde-3-phosphate dehydrogenase. Circ Res. 2006;99:191–200. doi: 10.1161/01.RES.0000232319.02303.8c. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Vervenne HB, Crombez KR, Lambaerts K, Carvalho L, Koeppen M, Heisenberg CP, Van de Ven WJ, Petit MM. Lpp is involved in Wnt/PCP signaling and acts together with Scrib to mediate convergence and extension movements during zebrafish gastrulation. Dev Biol. 2008 doi: 10.1016/j.ydbio.2008.05.529. [DOI] [PubMed] [Google Scholar]
- Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- Wren FE, Schor AM, Schor SL, Grant ME. Modulation of smooth muscle cell behaviour by platelet-derived factors and the extracellular matrix. J Cell Physiol. 1986;127:297–302. doi: 10.1002/jcp.1041270217. [DOI] [PubMed] [Google Scholar]
- Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) Immuno labeling showing a decrease in phosphorylated FAK and B) TN-C expression by oxidative stress. Cells were treated with either 50ug/ml of oxLDL or 100µM of H2O2 for 1h. Cells were then fixed for immunofluorescence staining as detailed in the Methods section.