Abstract
Rationale
Tissue factor pathway inhibitor (TFPI) is a potent regulator of the tissue factor pathway and is found in plasma in association with lipoproteins.
Objective
To determine the role of TFPI in the development of atherosclerosis, we bred mice which overexpress TFPI into the apolipoprotein E−/− (apoE) background.
Methods and Results
On a high fat diet, SM22α-TFPI/apoE−/− mice were shown to have less aortic plaque burden compared to apoE−/−mice. Unexpectedly, SM22α-TFPI/apoE−/− had lower plasma cholesterol levels compared to apoE−/− mice. Furthermore, SM22α-TFPI mice fed a high fat diet had lower cholesterol levels than did wild type mice. Since TFPI is associated with lipoproteins and its carboxy terminus (TFPIct) has been shown to be a ligand for the VLDL receptor, we hypothesized that TFPI overexpression may regulate lipoprotein distribution. We quantified VLDL binding and uptake in vitro in mouse aortic smooth muscle cells (mASMCs) from SM22α-TFPI and wild type mice. mASMCs from SM22α-TFPI mice demonstrated higher VLDL binding and internalization compared to those from wild type mice. Since SM22α-TFPI mice have increased circulating levels of TFPI antigen, we examined whether TFPIct may act to alter lipoprotein distribution. In vitro, TFPIct increased VLDL binding, uptake, and degradation in murine embryonic fibroblasts. Furthermore, this effect was blocked by heparinase treatment. In vivo, systemic administration of TFPIct reduced plasma cholesterol levels in apoE−/− mice.
Conclusions
These studies suggest that overexpression of TFPI lowers plasma cholesterol through the interaction of its carboxy terminus with lipoproteins and heparan sulfate proteoglycans.
Keywords: atherosclerosis, coagulation, murine models, tissue factor, lipoproteins
INTRODUCTION
Tissue factor pathway inhibitor (TFPI), a Kunitz-type serine protease inhibitor, is an endogenous inhibitor of TF-mediated coagulation. TFPI suppresses factor Xa generation by binding via its Kunitz 1 domain to the TF:FVIIa catalytic complex and via its Kunitz 2 domain to factor Xa.1 The formation of a quaternary TF:VIIa:TFPI:Xa complex dampens ongoing FXa generation. Additionally, TFPI also has been shown to regulate coagulation by inducing the internalization and degradation of TF:VIIa complex on cell surfaces.2–4
TFPI is expressed in many cells relevant to atherosclerosis including platelets, endothelial cells, vascular smooth muscle cells, and monocyte/macrophages.5–9 In human carotid plaques, the level of TFPI expression is inversely associated with TF activity suggesting a local regulatory role10. In plasma, TFPI exists in small quantities (<5%) as a free full length protein but is predominantly associated with lipoproteins.11–13 Lipoprotein-associated TFPI has been shown to have less anticoagulant activity than free TFPI13. TFPI is cleared from the plasma by binding the low density lipoprotein receptor related protein (LRP) and heparin sulfate proteoglycans.14, 15 It has been previously shown that the carboxy terminus of TFPI (TFPIct) can bind lipoproteins16–18 and is a ligand for cell surface receptors including the VLDL receptor.19
Vascular overexpression of TFPI has been shown to reduce acute thrombosis and neointimal formation following vascular injury in murine models.20, 21 Similarly, TFPI haploinsufficiency increases the atherosclerotic burden and thrombotic tendency in wild type and apoE deficient backgrounds.22, 23 Taken together, these findings suggest a potentially important role for TFPI in the development and progression of acute and chronic vascular disease.
To further define the role of TFPI in vascular disease, we bred mice in which a murine TFPI transgene was expressed under the control of the SM22α promoter24, 25 into the apoE−/− background. As expected, TFPI overexpression reduced plaque formation in this model. Unexpectedly, this decrease was associated with a reduction in plasma cholesterol suggesting a novel role for TFPI in lipid metabolism.
MTERIAL AND METHODS
Please see Supplemental Methods for additional methods.
Material
125I-hVLDL (human very low density lipoprotein), hVLDL, and LPDS (lipoprotein deficient bovine calf serum) were purchased from Biomedical Technologies Inc. (Stoughton, MA). Culture medium Dulbecco’s modified PBS without Ca2+ or Mg2+ (D-PBS), Hank’s Balanced Salt solution (HBSS), and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). Smooth muscle growth medium (SmGM) was from Lonza (Walkersville, MD). Soybean trypsin inhibitor, bovine serum albumin, heparin, heparinase I and III, collagenase type I, and elastase were purchased from Sigma-Aldrich Corp. (St. Louis, MO). RAP (receptor related protein) was purchased from Innovative Research (Novi, Michigan). Murine TFPI C-terminal 56mer peptide HRFNYTGCGGNNNNFTTRRRCLRSCKTGLIKNKSKGVVKI-QRRKAPFVKVVYESIN (according to NCBI database accession number NP-035706 from amino acid 251 to 306) was synthesized by Mayo Clinic protein core facility. The peptides were purified on a C-4 reversed-phase HPLC column, and the purity was determined by HPLC and confirmed by mass spectral analysis.
Murine models
SM22α-TFPI transgenic+/+ mice with C57BL/6 background were generated as previously described.21 apoE deficient mice on C57BL/6J background were purchased from Jackson Laboratory (Bar Harbor, MI). SM22α-TFPI mice were cross bred into apoE-deficient mice to mice which were heterozygous (SM22α-TFPI ± /apoE ±) for each allele. The double homozygous (SM22α-TFPI+/+/apoE−/−) mice were produced by backcrossing the heterozygous mice, which were identified by polymerase chain reaction (PCR) (for lack of wild type apoE allele) and real-time PCR analysis (for TFPI transgene) of blood DNA samples.21 Mice were maintained on standard chow unless noted. All procedures comply with the standards for care and use of animal subjects as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of sciences, Bethesda, MD) and were approved by Mayo Clinic Institutional Animal Care and Use Committee.
Analysis of atherosclerotic lesions
Mice used in these experiments were 6–8 weeks old and 15–18g in weight. Groups of apoE−/− and SM22α-TFPI/apoE−/− mice were fed a high fat Western diet (TD.88137, Harland, Madison, WI). At the end of 5 weeks and 20 weeks of treatment, the mice were sacrificed; aorta and blood samples were collected and analyzed. Additional methods are available in Supplemental Methods.
Plasma lipid and lipoprotein analysis
Plasma samples
Blood samples from SM22α-TFPI/apoE−/− or apoE−/− mice (pooled groups of 6–8 mice/group) were obtained through retro-orbital bleeding into EDTA-coated vials followed by centrifugation for 20 minutes at 3000rpm at room temperature to obtain a final plasma volume of 1mL. Plasma samples were kept frozen at −20°C until FPLC was performed.
Cholesterol assay
The WAKO Total Cholesterol E kit (Osaka, Japan) was used to determine the cholesterol concentration of each FPLC fraction. See Supplemental Methods
FPLC instrumentation
Fast Protein Liquid Chromatography (FPLC) was performed at 4°C using a Bio-Logic Pathfinder FPLC system interfaced with an automatic fraction collector (Bio-Rad, Hercules, CA).26 See Supplemental Methods.
Cell Culture
To generate murine aortic smooth muscle cells (mASMC), aortas were removed from mice and cleaned of adventitia under a dissecting scope. murine embryonic fibroblasts (MEF), and PEA13 cells were purchased from ATCC and cultured according to provided protocols. See Supplemental Methods.
125I-VLDL binding, uptake, and degradation by mASMC, MEF and PEA13 cells
Studies of binding and uptake of 125I-VLDL in mASMC, MEF and PEA13 cells were performed using modified method of Datta et al.27 See Supplemental Methods.
Measurement of mTFPI in plasma
mTFPI levels in plasma were measured by using mTFPI specific rabbit antibody and Protein Detector ELISA Kit. See Supplemental Methods.
siRNA transfection
MEFs were transfected with siRNA targeted to the VLDL receptor. See Supplemental Methods.
Analysis of mRNA expression of VLDL receptor
Cells were harvested directly for RNA extraction by using RNeasy Plus mini kit (Qiagen, MD). RNA extractions were reverse transcribed using SuperScript III first-Strand synthesis system (Invitrogen, CA). Two microliters of cDNA was used as template to amplify with primer pair (forward 5’-TGACGCAGACTGTTCAGACC-3’, reverse 5’-GCCGTGGATACAGCTACCAT-3’) by polymerase chain reaction (PCR). The products of PCR were analyzed using agarose gel electrophoresis. For normalization of RNA loading, β-actin housekeeping gene was used as control.
VLDL-peptide complex formation and agarose gel electrophoresis
VLDL-peptide complex formation was tested using an agarose gel electrophoresis method.28 Briefly 10ug VLDL and 10ug VLDL plus varying concentrations of TFPIct were incubated at room temperature for 1 hour. The samples of VLDL and VLDL-TFPIct mixtures were electrophoresed on a 0.7% agarose gel at a constant voltage of 100V for 20 minutes. The gel was stained with Coomassie blue and photographed.
In vivo administration of TFPIct
Administration of TFPIct in mice was performed at concentrations previously described for apolipoprotein mimetics.29 ApoE−/− mice (8–12 weeks old) fed high fat or normal chow were anaesthetized, and blood samples were taken from the retro-orbital sinus prior to the injection. In acute studies, 100ul of peptide (1ug/ul in saline) was injected via tail vein. Saline was injected into the control group of mice. Blood samples (100ul) were taken from individual mice at 1 or 3 and 6 hours after injection, and the plasma was separated by centrifugation. Duplicate 10ul aliquots of each plasma sample were used for cholesterol determination. For chronic administration, 6–8 month old apoE−/− mice were used, 100ul of peptide or saline was given intraperitoneally daily. Blood samples were taken at day 3 and 7 for cholesterol determination.
Statistical Analysis
All results are expressed as means ± SEM. In all experiments, differences between control and treated groups were analyzed for statistical significance using a one-way ANOVA, two-way ANOVA or an unpaired Student's t test (two-tailed). When applicable a repeated measures analysis was performed. In the case of ANOVA, a post-test comparison was used to compare all groups.
RESULTS
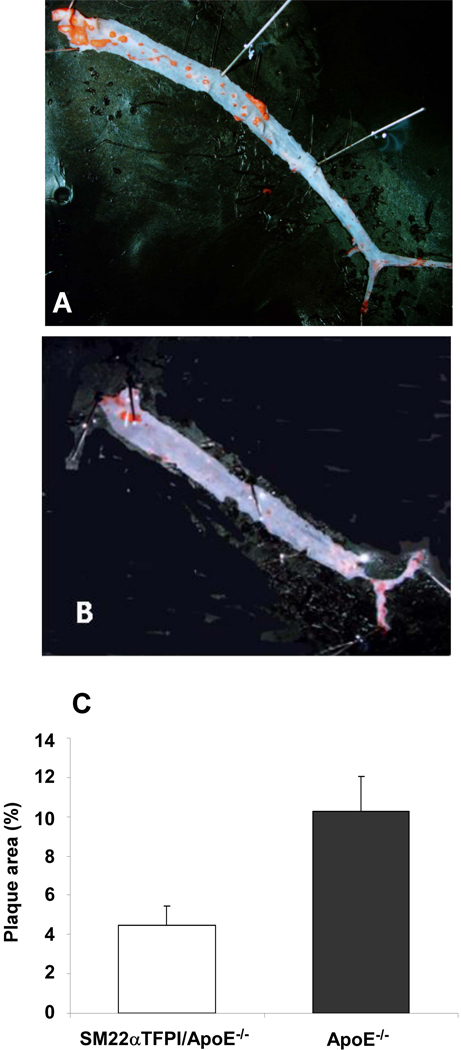
To determine the effect of vascular overexpression of TFPI on the development of atherosclerosis, 6–8 week-old apoE−/− and SM22α-TFPI/apoE−/− mice (n>10 for each group) were fed with a Western diet. After 20 weeks, atherosclerotic lesions were quantified in the aorta (Figure 1A and B). Compared to apoE−/−mice, the extent of plaque surface area was significantly reduced in SM22α-TFPI/apoE−/− mice (Figure 1C). There were no differences in plaque area between sexes in this model (data not shown).
Figure 1.
Vascular directed overexpression of TFPI reduces aortic plaque burden in apoE deficient mice. Atherosclerotic plaques in apoE−/− (A) and SM22αTFPI/apoE−/− (B) mice stained with Sudan IV for en face analysis. C. The comparison of plaque areas in aorta from apoE−/− (n=13) and SM22αTFPI/apoE−/− mice (n=12), * p < 0.02.
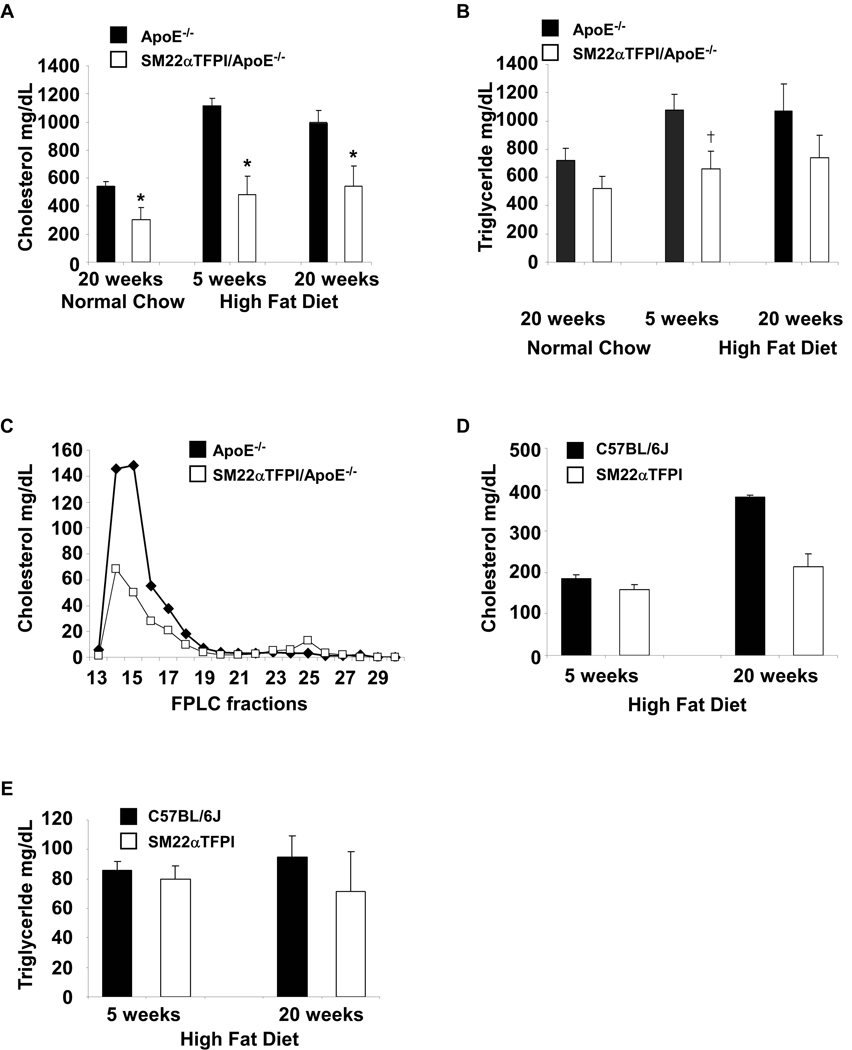
Plasma cholesterol in both groups was measured from mice fed with a high fat diet for 5 weeks and 20 weeks. Surprisingly, the total cholesterol level in SM22α-TFPI/apoE−/− mice was significantly lower at both time points than that in apoE−/− mice (Figure 2A. p < 0.01). Triglyceride levels were significantly lower at 5 weeks in the SM22α-TFPI/apoE−/− mice compared with apoE−/− (165 ± 31mg/dl vs 269 ± 28 mg/dl, p<0.014), but this difference was not significant at 20 weeks (185 ± 40 vs 267 ± 48mg/dl, p=0.21) (Figure 2B). On normal chow, the cholesterol levels were also decreased after 20 weeks (p<.01) (Figure 2A). As with plaque development, no difference was noted between sexes. FPLC analysis on pooled samples confirmed that the differences between the mice were predominantly in the VLDL and LDL fractions (Figure 2C). Baseline plasma cholesterol and triglyceride levels did not differ between SM22α-TFPI and C57BL/6 mice (Figure 2D and E). However when fed a high fat diet for 20 weeks, plasma cholesterol levels in SM22α-TFPI mice were lower than C57BL/6 mice. These data suggest that vascular smooth muscle cell directed overexpression of TFPI attenuates the hyperlipidemia induced by high fat diet or apoE deletion.
Figure 2.
Vascular directed overexpression of TFPI reduces cholesterol levels in wild type and apoE deficient mice. Plasma cholesterol (A) and triglyceride (B) levels from apoE−/− and SM22αTFPI/apoE−/− mice fed a high fat diet for 5 and 20 weeks were analyzed. Plasma from mice fed a high fat diet for 5 weeks were pooled and analyzed by FPLC (C). The fractions 14, 16, and 25 represent VLDL, LDL, and HDL respectively (confirmed by western blot). Plasma cholesterol (D) and triglyceride (E) levels from C57BL/6 and SM22αTFPI mice fed a high fat diet for 5 and 20 weeks. * p < 0.01, †<0.015
The model system studied here allows for several potential mechanisms for the observed effects of TFPI overexpression on plasma cholesterol levels. These mechanisms might include differences in feeding habits or dietary absorption of lipid between mice. To that end, the SM22α-TFPI mice are developmentally normal and have normal body weight and life span which would not be supportive of that mechanism. An alternative mechanism would be that the transgene might be involved in affecting lipoprotein levels.
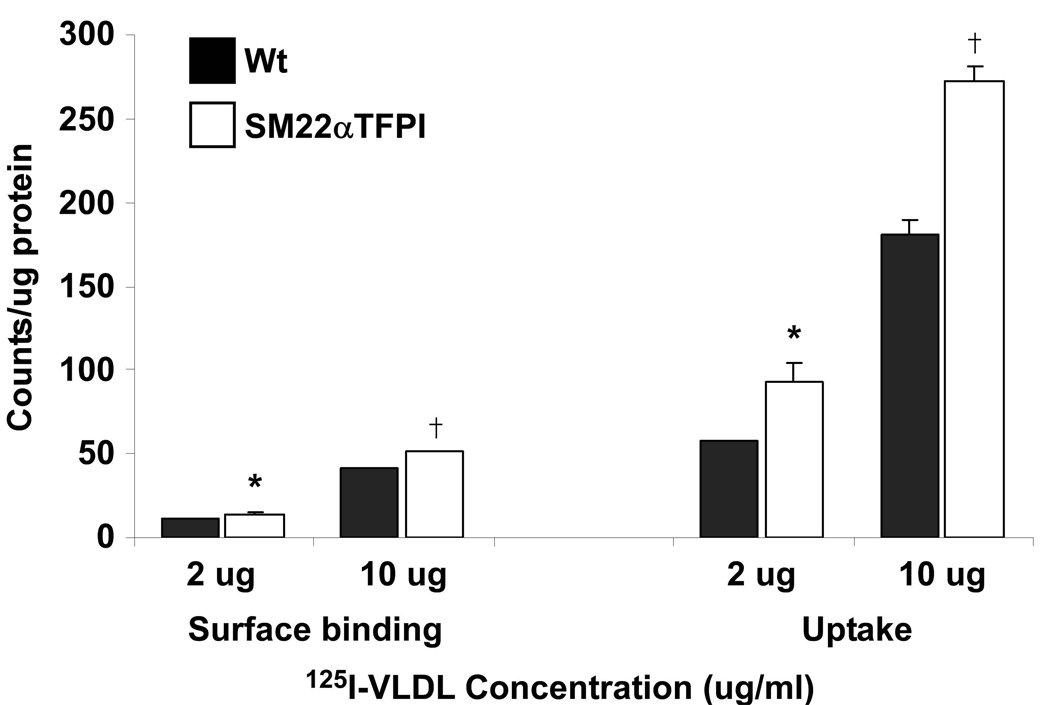
To investigate the possibility that overexpression of TFPI alters local lipoprotein binding and internalization, we isolated mASMC from SM22α-TFPI and wild type mice to study the effect of TFPI overexpression on 125I-VLDL binding and internalization in these cells. At concentrations of 2ug and 10ug/ml of 125I-VLDL, the surface binding of 125I-VLDL on mASMC isolated from SM22α-TFPI mice was increased compared to that of cells isolated from wild type mice (14 ± 1 vs 11 ± 0 for 2ug/ml and 51 ± 0 vs 41 ± 1 for 10ug/ml, Figure 3). Also, the internalization of 125I-VLDL in mASMC isolated from SM22α-TFPI mice was increased by approximately 50% compared with wild type cells (93 ± 11 vs 58+/−0 for 2ug/ml and 272 ± 9 vs 181 ± 8 for 10ug/ml). These data demonstrate that TFPI overexpression in mASMCs can regulate VLDL binding and internalization. However, as shown by Wamhoff,30 the SM22α promoter is not expressed in advanced atherosclerotic lesions in apoE deficient mice; therefore, the effect on lipoprotein handling may be due to overexpresssion of TFPI outside of the plaque itself.
Figure 3.
Binding and internalization of 125I-VLDL in vascular smooth muscle cells. mASMCs from SM22αTFPI transgenic and wild type mice were isolated, grown, and treated with 125I-VLDL at different concentrations. Significant differences between two groups are indicated as * (for 2ug/ml of 125I-VLDL) and † (for 10ug/ml of 125I-VLDL). In binding assay * p < 0.05 and †p < 0.01 vs C57B/6. In the uptake assay * p < 0.05 and †p < 0.001 vs wild type.
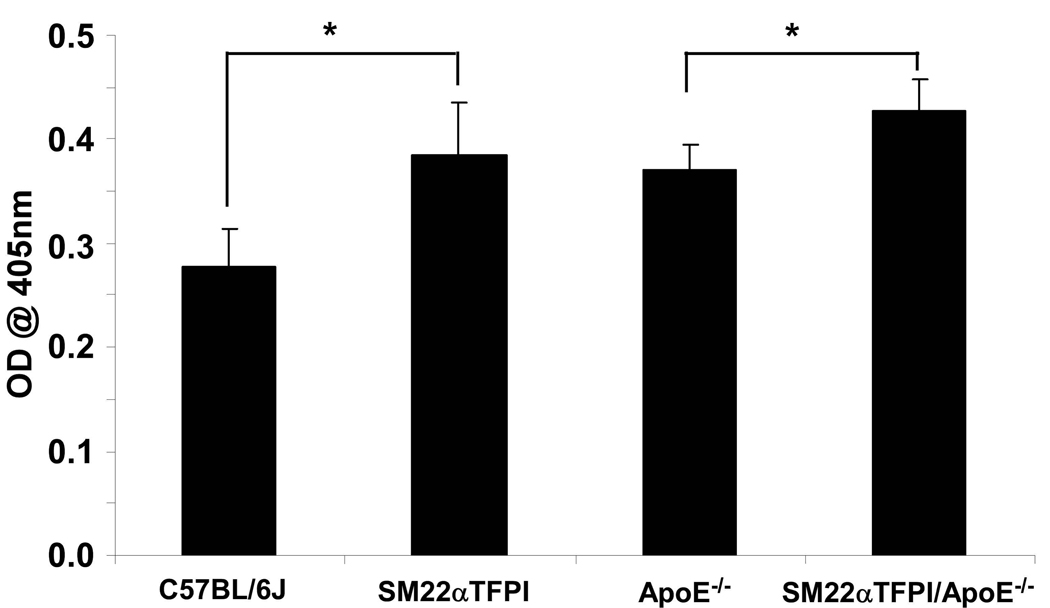
We have previously demonstrated that SM22α-TFPI mice have higher levels of vascular TFPI activity but similar levels of plasma TFPI activity.21 However, using a murine specific ELISA, it was demonstrated that SM22α-TFPI mice had increased levels of plasma TFPI antigen compared to wild type mice (Figure 4). In addition, TFPI antigen was found in VLDL and LDL fractions (data not shown). The increased levels of TFPI in apoE deficient mice parallel findings in humans with atherosclerosis and hyperlipidemia.12 Again similar to humans, we were unable to detect TFPI protein in the livers of transgenic or wild type mice (data not shown). Thus, the SM22α directed overexpression of TFPI resulted in increased circulating TFPI antigen. Therefore, an endocrine/paracrine mechanism for the lipoprotein effect was considered.
Figure 4.
Vascular directed overexpression of TFPI increases circulating levels of TFPI antigen. Mice plasma samples were collected individually and tested by ELISA. (n>10/group) (* p < 0.001)
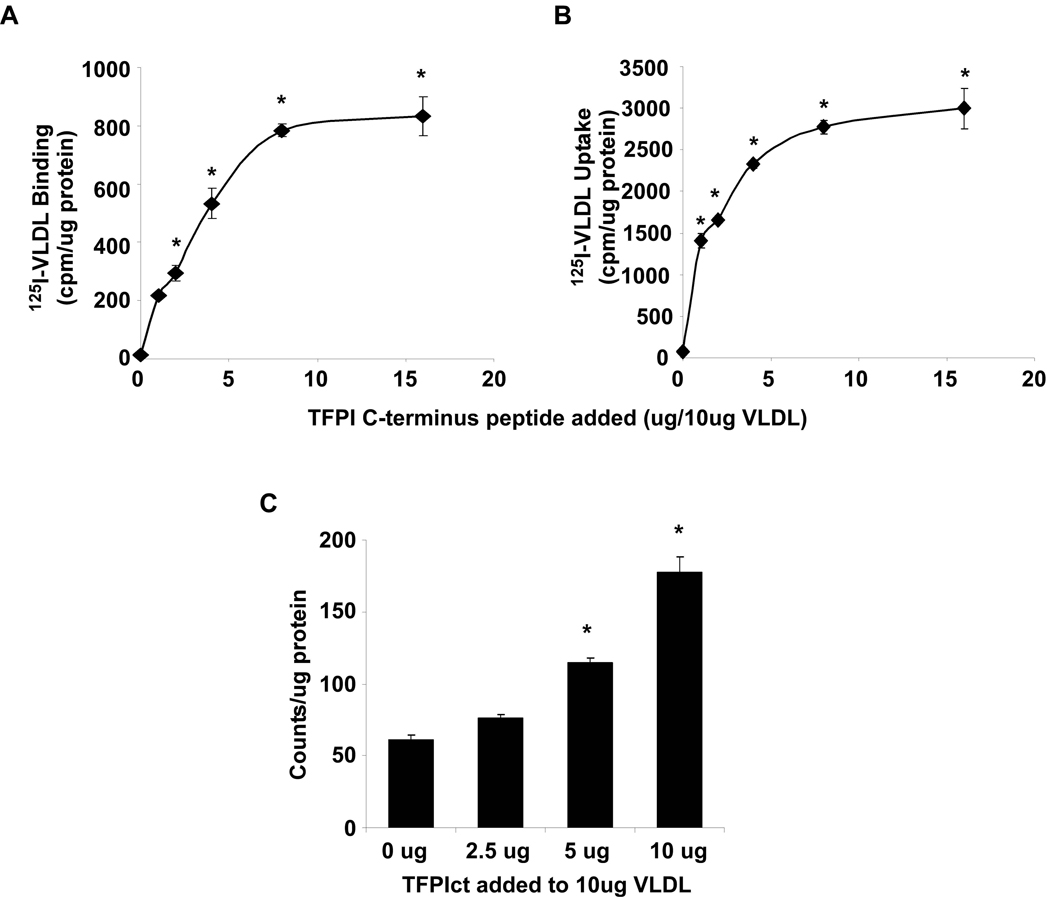
Since TFPI circulates in lipoprotein fractions and the TFPIct is a known ligand of several cell surface receptors, we hypothesized that the TFPI which is overexpressed in our model may associate with VLDL and act to alter lipoprotein binding, uptake and degradation. First, to determine whether TFPIct is capable of altering VLDL binding, uptake, and degradation, an in vitro model system was used. At a constant VLDL concentration, addition of TFPIct enhanced binding and uptake of VLDL in a concentration dependent manner (Figure 5A and B) in MEFs. When 1ug of TFPIct was added to VLDL, it resulted in a 15-fold increase in VLDL binding and 20-fold increase in VLDL internalization. At the highest 16:10 (w/w) ratio of TFPIct:VLDL, a 55-fold increase of VLDL binding and a 40-fold increase in internalization was observed. The effect of TFPIct on degradation of VLDL was also measured after incubation of the cells with or without TFPIct for 6 hours at 37°C. At the highest ratio we tested, 10ug TFPIct mixed with 10ug VLDL, the degradation rate increased by over 100% (Figure 5C). The rate of VLDL degradation accelerated as the TFPIct:VLDL ratio increased.
Figure 5.
Effect of TFPIct peptides on binding, internalization and degradation of 125I-VLDL in MEF. Cells were treated with 125I-VLDL complex containing different concentrations of TFPIct. (A) TFPIct increased 125I-VLDL binding to MEF cell surface, (B) internalization of 125I-VLDL into the cells and (C) degradation of 125I-VLDL in presence of TFPIct in MEF. * p < 0.01.
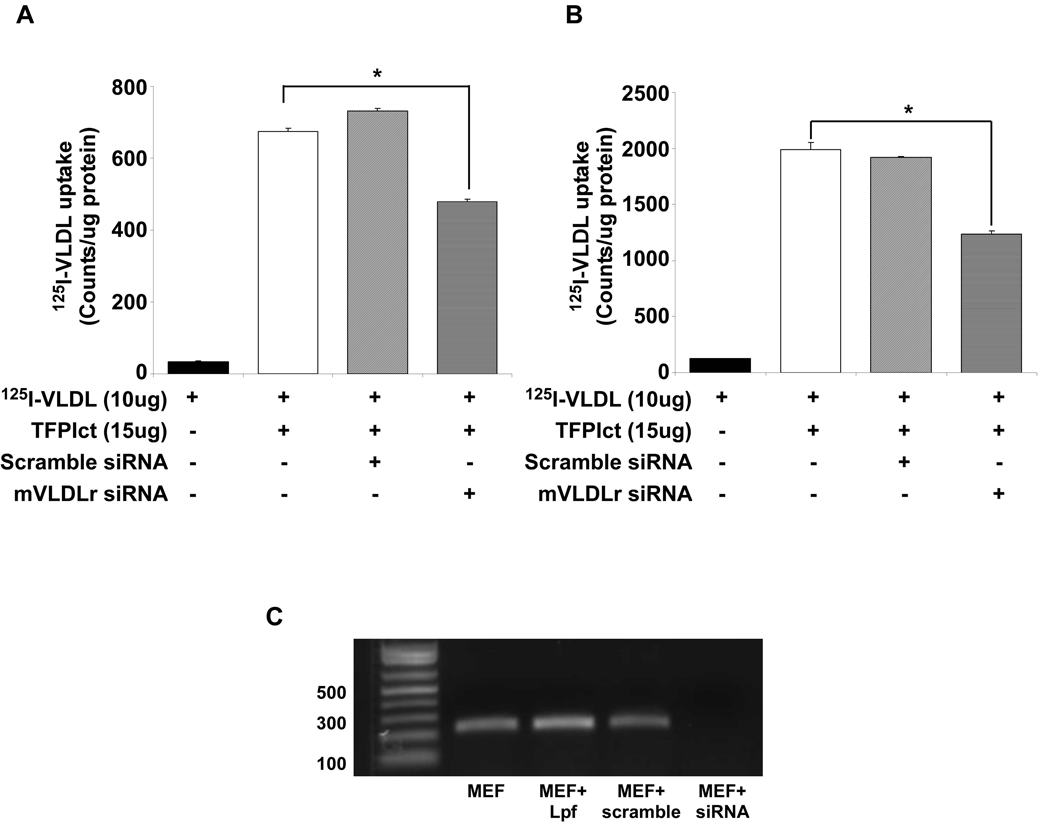
To define the mechanism by which TFPIct alters VLDL internalization, known TFPI receptors were studied. Knockdown of VLDLr expression by siRNA partially attenuated TFPIct mediated binding and uptake of VLDL in these cells (Figure 6). Furthermore, although RAP attenuated VLDL binding and uptake in the absence of TFPIct (Supplemental Figure1A). The same dose of RAP did not affect the increase observed with TFPIct (Supplemental Figure1B). Similarly the increase was only partially attenuated in cells deficient in LRP (PEA13) (Supplemental Figure 2). These results indicate that known receptors for VLDL are only partially responsible for TFPIct-mediated enhancement of VLDL binding and uptake in MEF.
Figure 6.
Knockdown of VLDLr inhibits 125I-VLDL binding to MEF cell surface (A) and internalization (B) of 125I-VLDL into the cells when exposed to TFPIct. (* p<0.01 vs untreated cells). (C) Results of RT-PCR demonstrating specificity of VLDLr knockdown.
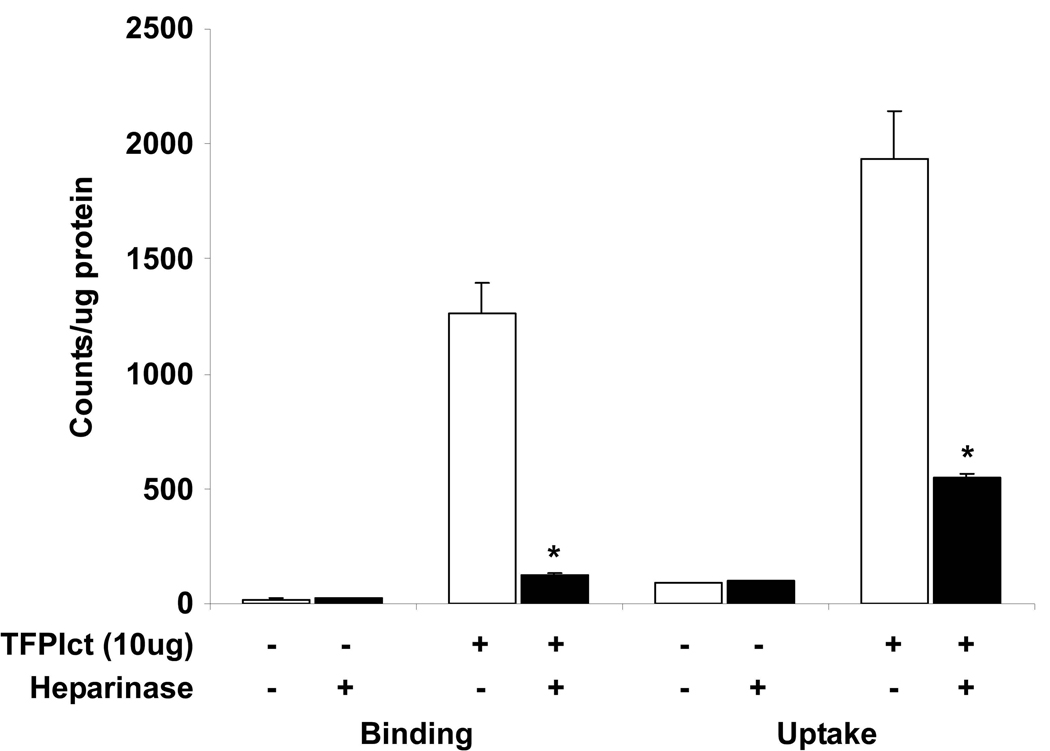
Since TFPI is known to bind heparin sulfate proteoglycans (HSPGs), similar studies were performed following treatment of MEFs with heparinase I and III. Heparinase treatment did not affect baseline VLDL binding and uptake in MEF, but significantly reduced VLDL binding and uptake when TFPIct was added (Figure 7). These results demonstrate that the HSPG pathway is the major route for the TFPI-mediated VLDL binding and subsequent uptake. Taken together, these data suggest that TFPIct enhances the binding, internalization of VLDL via the HSPG pathway in coordination with known receptors for VLDL.
Figure 7.
Effect of heparinases on the binding and uptake of 125I-VLDL or 125I-VLDL:TFPIct complex. MEF cells were pretreated with heparinase I and III (each 3 units/ml) for 2 h at 37 °C and then incubated with 125I-VLDL (10ug) or 125I-VLDL(10ug):TFPIct (10ug) complex. Binding and uptake were attenuated by heparinases only in the presence of TFPIct. * p<0.02
TFPIct has a net positive charge, a feature shared with peptides that have been shown to be apolipoprotein E mimetics.27 To determine whether TFPIct may associate and change the electrophoretic mobility of VLDL, VLDL was incubated with TFPIct at different weight ratios at room temperature for 1 hour, then analyzed by agarose gel electrophoresis (Supplemental Figure 3). VLDL alone migrated toward the anode as a single band. The migration of mixtures of VLDL and TFPIct was impeded, and the degree depended upon the ratio of VLDL to TFPIct. Mobility was impeded at molar ratios as low as 1:15. These results indicated that TFPIct associates with VLDL and alters its electrophoretic properties.
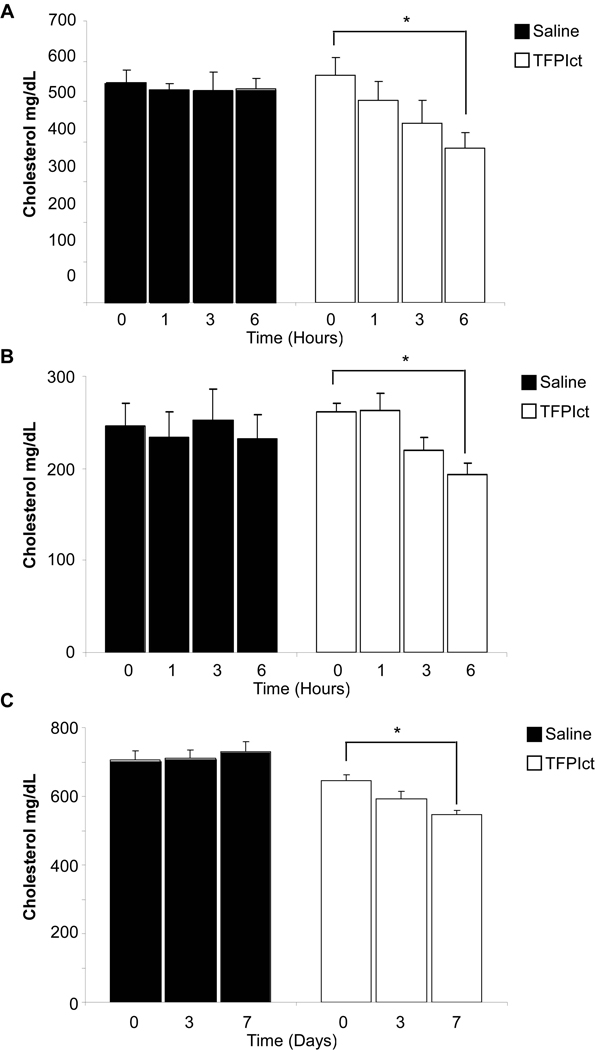
Finally, to determine whether the TFPIct is sufficient to reduce the cholesterol level in vivo, TFPIct was administrated intravenously into apoE deficient mice fed high fat or normal diet utilizing concentrations of peptide previously utilized in studies of apolipoprotein mimetics.29 In apoE deficient mice after intravenous delivery of TFPIct, the cholesterol level was reduced significant after 6 hours compared with baseline in both fed western diet and normal chow mice (Figure 8A and B). Reductions were also observed over seven days time with daily IP injections in these older apoE deficient mice fed normal chow (Figure 8C). Thus, systemic delivery of TFPIct is sufficient to reduce plasma cholesterol levels.
Figure 8.
Reduction of plasma cholesterol level in apoE deficient mice after acute or chronic administration with TFPIct peptide. (A) Cholesterol levels in high fat fed apoE deficient mice after acute peptide injection are shown (* p < 0.05 vs baseline). (B) Cholesterol level in normal chow fed apoE deficient mice after acute peptide injection (* p < 0.01 vs baseline). (C) Cholesterol levels in normal chow fed apoE−/− mice after daily peptide injections (IP) (* p < 0.01 vs baseline).
DISCUSSION
This is the first report that suggests that overexpression of TFPI may regulate atherosclerotic plaque formation. The potential known mechanisms for such effects might include the antithrombotic effects of TFPI in this model 31 and its ability to block signaling via TF32. Unexpectedly we identified that overexpression of TFPI reduced plasma cholesterol. It was this unexpected finding which became the focus of this study. TFPI was originally referred to as lipoprotein associated coagulation inhibitor (LACI) due to its association with lipoproteins.33 As TFPI is cleared from the blood through receptor dependent and independent mechanisms, a potential role in regulating lipoprotein clearance may not have been unexpected. Given the important roles of the tissue factor pathway and lipoprotein metabolism in vascular disease, a biologically important association between the two remains intriguing. We have previously shown that the atherogenic lipoprotein Lp(a) can bind and inhibit TFPI activity through the binding of apo (a) to the TFPIct.16
Overexpression of TFPI resulted in reductions in elevated plasma cholesterol levels in mice from apoE deficient and wild type backgrounds As TFPI has been shown to be a ligand of multiple cell surface receptors which also mediate lipoprotein clearance, we further demonstrated that the carboxy terminus of murine TFPI can associate with and alter the distribution and degradation of VLDL in vitro and lower plasma cholesterol acutely in vivo following systemic injection. Taken together, we suggest that overexpression of TFPI regulates atherosclerotic lesion formation and does so in part through lowering cholesterol through the association of TFPI with atherogenic lipoproteins. These findings provide insights into a novel non-coagulant function for TFPI.
The current data suggest that smooth muscle cell directed overexpression of TFPI can alter cholesterol levels through autocrine or endocrine effects. As a secreted peptide, in vitro and in vivo models of overexpression of TFPI cannot distinguish the two. Analysis of mASMCs which express TFPI from the SM22α promoter demonstrated increased binding, uptake and degradation of VLDL. Our subsequent focus on the TFPIct was based on its known functionality. TFPIct was shown to associate with VLDL and change its electrophoretic properties, and administration of TFPIct increased cell binding, uptake, and degradation of VLDL in vitro. This effect was mediated through enhanced binding of VLDL to HSPGs in a coordinated fashion with members of the LDL receptor family. In addition, acute or daily systemic delivery of TFPIct is sufficient to lower plasma cholesterol levels.
Taken together, these findings define potential mechanisms for the observed effects of TFPI overexpression on lowering cholesterol in the setting of hyperlipidemia due to apoE deletion or hyperlipidemic feeding. TFPI, either on the cell surface or following secretion binds lipoproteins and increases binding and clearance of these lipoproteins through receptor-dependent and HSPG-dependent mechanisms. This HSPG dependent mechanism has been demonstrated to be important in MEFs 34–36 and has been shown to be the mechanism by which apoE mimetics act to lower cholesterol 27.
Previous studies suggest that TFPI plays an important role in pathogenesis of vascular thrombosis and atherosclerosis.10, 37–41 Westrick and colleagues have shown that systemic reduction of TFPI Kunitz 1 expression in mice increased atherosclerotic lesion formation and shortened the occlusion time following ferric chloride exposure in the carotid arteries of TFPI +/− /apoE−/− mice.22 In that study, plasma lipid levels were not reported. It should be noted that the deletion in those mice is specific for the Kunitz 1 domain which leaves the carboxy terminus intact. Indeed, cholesterol levels in heterozygotic mice (K1 deletion) fed normal chow does not differ from wild type mice (data not shown).
Plasma TFPI found in lipoprotein fractions in humans has less anti- FXa and TF-FVIIa activity compared with free TFPI.11 In our animal model, in spite of detecting increased plasma TFPI antigen and increased vascular TFPI activity, we are unable to detect increased circulating TFPI activity.21 Human models of TFPI deficiency, particularly defects in the carboxy terminus, have not been described. In relatively small human studies, there is a positive correlation between circulating TFPI and VLDL levels.12, 42–44 In this study, apoE−/− mice had higher levels of circulating TFPI antigen than did wild type mice suggesting a regulatory role of hyperlipidemia on TFPI expression. These data suggest a potentially important and complex interaction between TFPI and lipoproteins.
Human TFPIct has been shown to be important for its coagulant and noncoagulant properties. It is required for optimal inhibition of FXa.45, 46 It is also required for TFPI binding to cells and cell surface receptors including the VLDL receptor47 and heparin sulfate proteoglycans.48 The carboxy terminus has also been shown to mediate its interaction with lipoproteins.16–18 The broad functionality of the TFPIct is reminiscent of the multifunctional peptides intended to serve as apolipoprotein mimetics.27, 49 These mimetics contain moieties that bind lipoproteins and residues which mimic apoE bind the VLDL receptor. Systemic infusion of these peptides lowers cholesterol acutely in a similar manner to the TFPIct. In addition to the functional similarities, both are positively charged which may play a role in their functionality. In fact, the mechanism for the effect of these peptides has been determined to be through dual pathways; HSPG-mediated and receptor-mediated (similar to our findings).
Although we can not exclude the known anticoagulant functions of TFPI in the attenuation of atherosclerosis in this model, our data support a novel noncoagulant function of TFPI based on the unique multifunctional properties of its carboxy terminus. This functionality adds another potent dimension in which to consider the anti-atherosclerotic effects of TFPI. As such, the opportunities to utilize this functionality to understand its role in pathophysiology and treatment of human disease remain.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by HL65191.
Non-standard Abbreviations and Acronyms
- TFPI
Tissue factor pathway inhibitor
- TFPIct
TFPI carboxy terminus
- apo E
apolipoprotein E
- FPLC
Fast Protein Liquid Chromatography
- mASMCs
murine aortic smooth muscle cells
- LRP
lipoprotein receptor related protein
- hVLDL
human very low density lipoprotein
- LPDS
lipoprotein deficient bovine calf serum
- D-PBS
Dulbecco’s modified PBS
- HBSS
Hank’s Balanced Salt solution
- DMEM
Dulbecco’s Modified Eagle’s Medium
- SmGM
Smooth muscle growth medium
- MEF
murine embryonic fibroblasts
- HSPGs
heparin sulfate proteoglycans
- LACI
lipoprotein associated coagulation inhibitor
Footnotes
Subject Codes: 134, 145, 90, 96, 159
Publisher's Disclaimer: This is an un-copyedited author manuscript accepted for publication in Circulation Research, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circres.ahajournals.org/. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures: None
REFERENCES
- 1.Broze G, Girard T, Novotny W. Regulation of coagulation by multivalent Kunitz-type inhibitor. Biochem. 1990;29(33):7539–7546. doi: 10.1021/bi00485a001. [DOI] [PubMed] [Google Scholar]
- 2.Hamik A, Setiadi H, Bu G, McEver R, Morrissey J. Down-regulation of monocyte tissue factor mediated by tissue factor pathway inhibitor and the low density lipoprotein receptor-related protein. J. Biol. Chem. 1999;274:4962–4969. doi: 10.1074/jbc.274.8.4962. [DOI] [PubMed] [Google Scholar]
- 3.Sevinsky J, Rao L, Ruf W. Ligand-induced protease receptor translocation into caveolae: a mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J Cell Biol. 1996;133:293–304. doi: 10.1083/jcb.133.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott I, Miyagi Y, Miyazaki K, Heeb M, Mueller B, Rao L, Ruf W. Reversible regulation of tissue factor-induced coagulation by glycosyl phosphatidylinositol-anchored tissue factor pathway inhibitor. Arteriolscler. Thromb. Vasc. Biol. 2000;20:874–882. doi: 10.1161/01.atv.20.3.874. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj M, Kuppuswamy M, Saito H, Spitzer S, Bajaj S. Cultured normal human hepatocytes do not synthesize lipoprotein-associated coagulation inhibitor: evidence that endothelium is the principal site of its synthesis. Proc. Natl. Acad. Sci., USA. 1990;87(22):8869–8873. doi: 10.1073/pnas.87.22.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhout T, Blezer R, Schoen P, Nordfang O, Reutelingsperger C, Hemker H. Activation of factor X and its regulation by tissue factor pathway inhibitor in small-diameter capillaries lined with human endothelial cells. Blood. 1992;79(11):2909–2916. [PubMed] [Google Scholar]
- 7.Werling R, Zacharski L, Kisiel W, Bajaj S, Memoli V, Rousseau S. Distribution of tissue factor pathway inhibitor in normal and malignant human tissues. Thromb. Haemost. 1993;69(4):366–369. [PubMed] [Google Scholar]
- 8.van der Logt C, Dirven R, Reitsma P, Bertina R. Expression of tissue factor and tissue factor pathway inhibitor in monocytes in response to bacterial lipopolysaccharide and phorbolester. Blood Coagul. Fibrinolysis. 1994;5:211–220. doi: 10.1097/00001721-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Caplice NM, Mueske CS, Kleppe LS, Peterson TE, Broze GJ, Simari RD. Expression of tissue factor pathway inhibitor in vascular smooth muscle cells and its regulation by growth factors. Circ. Res. 1998;83:1264–1270. doi: 10.1161/01.res.83.12.1264. [DOI] [PubMed] [Google Scholar]
- 10.Caplice NM, Mueske CS, Kleppe LS, Simari RD. Presence of tissue factor pathway inhibitor in human atherosclerotic plaques is associated with reduced tissue factor activity. Circulation. 1998;98(11):1051–1057. doi: 10.1161/01.cir.98.11.1051. [DOI] [PubMed] [Google Scholar]
- 11.Novotny W, Girard T, Miletich J, Broze G., Jr Purification and characterization of the lipoprotein-associated coagulation inhibitor from human plasma. J. Biol. Chem. 1989;264:18832–18837. [PubMed] [Google Scholar]
- 12.Hansen J, Huseby K, Sandset P, Svensson B, Lyngmo V, Norday A. Tissue factor pathway inhibitor and lipoproteins: evidence for association with and regulation by LDL in human plasma. Arterioscler. Thromb. 1994;14:223–229. doi: 10.1161/01.atv.14.2.223. [DOI] [PubMed] [Google Scholar]
- 13.Hansen J, Huseby K, Huseby N, Ezban M, Nordoy A. Tissue factor pathway inhibitor in complex with low density lipoprotein isolated from human plasma does not possess anticoagulant function in tissue factor-induced coagulation in vitro. Thromb. Res. 1997;85:413–425. doi: 10.1016/s0049-3848(97)00029-7. [DOI] [PubMed] [Google Scholar]
- 14.Warshawsky I, GJ Broze J, Schwartz A. The low density lipoprotein receptor-related protein mediates the cellular degradation of tissue factor pathway inhibitor. Proc. Natl. Acad. Sci., USA. 1994;91:6664–6668. doi: 10.1073/pnas.91.14.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narita M, Bu G, Olins G, Higuchi D, Herz J, GJ Broze J, Schwartz A. Two receptor systems are involved in the plasma clearance of tissue factor pathway inhibitor in vivo. J. Biol. Chem. 1995;270(42):24800–24804. doi: 10.1074/jbc.270.42.24800. [DOI] [PubMed] [Google Scholar]
- 16.Caplice N, Peterson T, Kleppe L, Mueske C, Kostner G, Broze G, Jr., Simari R. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: A novel link between lipoproteins and thrombosis. Blood. 2001;98:2980–2987. doi: 10.1182/blood.v98.10.2980. [DOI] [PubMed] [Google Scholar]
- 17.Horie S, Hiraishi S, Hamuro T, Kamikubo Y, Matsuda J. Oxidized low-density lipoprotein associates strongly with carboxy-terminal domain of tissue factor pathway inhibitor and reduces the catalytic activity of the protein. Thromb Haemost. 2002;87(1):80–85. [PubMed] [Google Scholar]
- 18.Ohkura N, Hiraishi S, Itabe H, Hamuro T, Kamikubo Y, Takano T, Matsuda J, Horie S. Oxidized phospholipids in oxidized low-density lipoprotein reduce the activity of tissue factor pathway inhibitor through association with its carboxy-terminal region. Antioxid Redox Signal. 2004;6(4):705–712. doi: 10.1089/1523086041361686. [DOI] [PubMed] [Google Scholar]
- 19.Hembrough TA, Ruiz JF, Swerdlow BM, Swartz GM, Hammers HJ, Zhang L, Plum SM, Williams MS, Strickland DK, Pribluda VS. Identification and characterization of a very low density lipoprotein receptor-binding peptide from tissue factor pathway inhibitor that has antitumor and antiangiogenic activity. Blood. 2004;103(9):3374–3380. doi: 10.1182/blood-2003-07-2234. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Pan S, Mueske CS, Witt T, Kleppe LS, Peterson TE, Slobodova A, Chang J-Y, Caplice NM, Simari RD. Role for tissue factor pathway in murine model of vascular remodeling. Circ. Res. 2001;89:71–76. doi: 10.1161/hh1301.092508. [DOI] [PubMed] [Google Scholar]
- 21.Pan S, Kleppe LS, Witt TA, Mueske CS, Simari RD. The effect of vascular smooth muscle cell-targeted expression of tissue factor pathway inhibitor in a murine model of arterial thrombosis. Thromb Haemost. 2004;92(3):495–502. doi: 10.1160/TH04-01-0006. [DOI] [PubMed] [Google Scholar]
- 22.Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103(25):3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Pan S, Mueske CS, Witt TA, Kleppe LS, Peterson TE, Caplice NM, Simari RD. Tissue factor pathway inhibitor deficiency enhances neointimal proliferation and formation in a murine model of vascular remodeling. Thromb. Haemost. 2003;89:747–751. [PubMed] [Google Scholar]
- 24.Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78(2):188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 25.Solway J, Seltzer J, Samaha FF, Kim S, Alger LE, Niu Q, Morrisey EE, Ip HS, Parmacek MS. Structure and expression of a smooth muscle cell-specific gene, SM22 alpha. J Biol Chem. 1995;270(22):13460–13469. doi: 10.1074/jbc.270.22.13460. [DOI] [PubMed] [Google Scholar]
- 26.Choice E, Ayyobi AF, Pritchard PH, Madden TD. Separation of liposomes from plasma components using fast protein liquid chromatography. Anal Biochem. 1999;270(1):1–8. doi: 10.1006/abio.1999.4049. [DOI] [PubMed] [Google Scholar]
- 27.Datta G, Chaddha M, Garber DW, Chung BH, Tytler EM, Dashti N, Bradley WA, Gianturco SH, Anantharamaiah GM. The receptor binding domain of apolipoprotein E, linked to a model class A amphipathic helix, enhances internalization and degradation of LDL by fibroblasts. Biochem. 2000;39(1):213–220. doi: 10.1021/bi991209w. [DOI] [PubMed] [Google Scholar]
- 28.Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169(3):291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 29.Garber DW, Handattu S, Aslan I, Datta G, Chaddha M, Anantharamaiah GM. Effect of an arginine-rich amphipathic helical peptide on plasma cholesterol in dyslipidemic mice. Atherosclerosis. 2003;168(2):229–237. doi: 10.1016/s0021-9150(03)00101-1. [DOI] [PubMed] [Google Scholar]
- 30.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95(10):981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- 31.Pan S, Kleppe LS, Witt TA, Mueske CS, Simari RD. The effect of vascular smooth muscle cell-targeted expression of tissue factor pathway inhibitor in a murine model of arterial thrombosis. Thromb Haemost. 2004;92(3):495–502. doi: 10.1160/TH04-01-0006. [DOI] [PubMed] [Google Scholar]
- 32.Ahamed J, Belting M, Ruf W. Regulation of tissue factor-induced signaling by endogenous and recombinant tissue factor pathway inhibitor 1. Blood. 2005;105(6):2384–2391. doi: 10.1182/blood-2004-09-3422. [DOI] [PubMed] [Google Scholar]
- 33.Wun T, Kretzmer K, Girard T, Miletich J, Broze G., Jr Cloning and characterizaton of a cDNA coding for the lipoprotein-associated coagulation inhibitor shows that it consists of three tandem Kunitz-type inhibitory domains. J. Biol. Chem. 1988;263:6001–6004. [PubMed] [Google Scholar]
- 34.Al Haideri MGI, Galeano NF, Gleeson A, Vogel T, Gorecki M, Sturley SL, Deckelbaum RJ. Heparan Sulfate Proteglycan-Mediated Uptake of Apolipoprotein E-Triglyceride-Rich Lipoprotein Particles: A Major Pathway at Physiological Particle Concentrations. Biochem. 1997;36:7. doi: 10.1021/bi9631024. [DOI] [PubMed] [Google Scholar]
- 35.Llorente-Cortes VO-VM, Badimon L. Differential Role of Heparan Sulfate Proteoglycans on Aggregated LDL UPtake in Human Vascular Smooth Muscle cells and Mouse Embroyonic Fibroblasts. ATVB. 2002;22:7. doi: 10.1161/01.atv.0000035391.46201.9a. [DOI] [PubMed] [Google Scholar]
- 36.Ji ZSBW, Miranda RD, Hussain MM. Role of Heparan Sulfate Proteglycans in the Binding and Uptake of Apolipoprotien E-Enriched Remnant Lipoproteins by Cultured Cells. The Journal of Biological Chemistry. 1993 May 15;268(No 14):8. [PubMed] [Google Scholar]
- 37.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arteriolscler. Thromb. Vasc. Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 38.Aoki K, Barker C, Danthinne X, Imperiale M, Nabel G. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol Med. 1999;5:224–231. [PMC free article] [PubMed] [Google Scholar]
- 39.Simari R, San H, Rekhter M, Ohno T, Gordon D, Nabel G, Nabel E. Regulation of cellular proliferation and intimal formation following balloon injury in atherosclerotic rabbit arteries. J. Clin. Invest. 1996;98:225–235. doi: 10.1172/JCI118770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toomey J, Kratzer K, Laksy N, Stanton J, Broze G. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 41.Drew A, Davenport P, Apostolopoulos J, Tipping P. Tissue factor pathway inhibitor expression in atherosclerosis. Lab Invest. 1997;77:291–298. [PubMed] [Google Scholar]
- 42.Hansen JB, Grimsgaard S, Huseby N, Sandset PM, Bonaa KH. Serum lipids and regulation of tissue factor-induced coagulation in middle-aged men. Thromb Res. 2001;102(1):3–13. doi: 10.1016/s0049-3848(01)00215-8. [DOI] [PubMed] [Google Scholar]
- 43.Kawaguchi A, Miyao Y, Noguchi T, Nonogi H, Yamagishi M, Miyatake K, Kamikubo Y, Kumeda K, Tsushima M, Yamamoto A, Kato H. Intravascular free tissue factor pathway inhibitor is inversely correlated with HDL cholesterol and postheparin lipoprotein lipase but proportional to apolipoprotein A-II. Arteriolscler. Thromb. Vasc. Biol. 2000;20:251–258. doi: 10.1161/01.atv.20.1.251. [DOI] [PubMed] [Google Scholar]
- 44.Kokawa T, Abumiya T, Kimura T, Harada-Shiba M, Koh H, Tsushima M, Yamamoto A, Kato H. Tissue factor pathway inhibitor activity in human plasma. Measurement of lipoprotein-associated and free forms in hyperlipidemia. Arterioscler Thromb Vasc Biol. 1995;15(4):504–510. doi: 10.1161/01.atv.15.4.504. [DOI] [PubMed] [Google Scholar]
- 45.Nordfang O, Bjorn SE, Valentin S, Nielsen LS, Wildgoose P, Beck TC, Hedner U. The C-terminus of tissue factor pathway inhibitor is essential to its anticoagulant activity. Biochem. 1991;30(43):10371–10376. doi: 10.1021/bi00107a002. [DOI] [PubMed] [Google Scholar]
- 46.Wesselschmidt R, Likert K, Girard T, Wun T, Broze G., Jr. Tissue factor pathway inhibitor: the carboxy-terminus is required for optimal inhibition of factor Xa. Blood. 1992;79:2004–2010. [PubMed] [Google Scholar]
- 47.Hembrough TA, Ruiz JF, Swerdlow BM, Swartz GM, Hammers HJ, Zhang L, Plum SM, Williams MS, Strickland DK, Pribluda VS. Identification and characterization of a very low density lipoprotein receptor-binding peptide from tissue factor pathway inhibitor that has antitumor and antiangiogenic activity. Blood. 2004;103(9):3374–3380. doi: 10.1182/blood-2003-07-2234. [DOI] [PubMed] [Google Scholar]
- 48.Valentin S, Larnkjer A, Ostergaard P, Nielsen JI, Nordfang O. Characterization of the binding between tissue factor pathway inhibitor and glycosaminoglycans. Thromb Res. 1994;75(2):173–183. doi: 10.1016/0049-3848(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 49.Gupta H, White CR, Handattu S, Garber DW, Datta G, Chaddha M, Dai L, Gianturco SH, Bradley WA, Anantharamaiah GM. Apolipoprotein E mimetic Peptide dramatically lowers plasma cholesterol and restores endothelial function in watanabe heritable hyperlipidemic rabbits. Circulation. 2005;111(23):3112–3118. doi: 10.1161/CIRCULATIONAHA.104.497107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.