Abstract
Objective
To determine areas under the curve (AUCs) of oral prednisolone (OP) and intravenous methylprednisolone (IVMP) in patients with juvenile dermatomyositis (DM) and assess the association with nailfold end-row loops (ERLs). Patients with active disease have fewer ERLs that possibly occur in the gastrointestinal tract, impairing absorption of oral medications.
Methods
Six patients with juvenile DM received 50 mg/m2 of OP (day 1) and IVMP (day 2). Blood was drawn at baseline and at 5, 15, 30, 45, 60, and 90 minutes, and hourly (hours 2–8) after each dose. Samples were analyzed by reverse-phase high-performance liquid chromatography for levels of prednisolone and methylprednisolone. AUCs of OP and IVMP were determined by the trapezoid method; pharmacokinetic parameters were obtained using noncompartmental and compartmental analysis. ERLs were determined from freeze-frame video microscopy and nailfold capillaroscopy.
Results
There was a trend toward significance in difference in mean AUC of IVMP (116.72 µg × ml/hour) compared with OP (65.16 µg × ml/hour; P = 0.059). Mean peak concentration was higher for IVMP (34.49 µg/ml) than OP (7.08 µg/ml); mean half-life was shorter for IVMP (1.90 hours) than OP (2.36 hours). There was an inverse association between ΔAUCs (IVMP AUC − OP AUC) and ERLs (R = −0.68, P = 0.044).
Conclusion
Patients with juvenile DM and ERL loss may have decreased bioavailability of OP compared with IVMP. This can provide the rationale for greater efficacy of IVMP in patients with active vasculopathy of juvenile DM. Further studies investigating the pharmacokinetics and pharmacodynamics of high-dose IVMP need to be performed in patients with juvenile DM.
INTRODUCTION
Juvenile dermatomyositis (DM) is the most common pediatric inflammatory myopathy, with an estimated annual incidence of 3.1 per million per year (1). Juvenile DM is a systemic vasculopathy primarily affecting the skeletal muscle and skin, but can involve other organ vasculature. A validated disease activity score (DAS) has been published (2) in which cutaneous criteria and muscle involvement are evaluated. The DAS provides a numerical value with higher scores being associated with more active disease. Nailfold capillaroscopy has been performed in children with juvenile DM, and an association between loss of capillaries of the end-row loops (ERLs) and increased skin manifestations has been demonstrated (3,4). Although nailfold capillaroscopy provides a noninvasive technique to evaluate involvement of cutaneous vessels, there is not an equivalent assessment of vessels of the gastrointestinal tract. If vasculopathy is present in the gastrointestinal tract, the absorption of oral medications may be impaired, resulting in decreased bioavailability.
Optimum therapy for juvenile DM has not been established, but early aggressive treatment with immunosuppressants has demonstrated improved outcomes in these children (5). Corticosteroids are often utilized during the management of juvenile DM, and controversy exists regarding the optimum route of administration. The purpose of the present study was 1) to determine the pharmacokinetics of intravenous methylprednisolone (IVMP) compared with oral prednisolone (OP) in patients with juvenile DM, and 2) to evaluate the association of the difference in bioavailability of IVMP and OP with capillary ERL and DAS.
PATIENTS AND METHODS
Patients
All patients with definite juvenile DM followed at Children’s Memorial Hospital Immunology/Rheumatology Clinic were eligible for this study. The Bohan and Peter criteria (6,7) were utilized to establish a definite diagnosis of juvenile DM. Patients were included if they were receiving corticosteroids and had evidence of active disease, defined as a DAS score >3. Patients were excluded if they had polymyositis or weighed <35 kg (due to the volume of blood required for this study). Our institutional review board approved the protocol; consent and, when age appropriate, assent were obtained from each patient.
Study design
Patients were admitted to the General Clinical Research Center. No patient received corticosteroids or methotrexate within 24 hours of the study. After fasting overnight, all participants received 50 mg/m2 of OP solution mixed in 100 ml of water on day 1 and 50 mg/m2 of IVMP mixed in 20 ml of normal saline and infused over 2 minutes on day 2. Blood samples for prednisolone and methylprednisolone concentration were obtained at 1 minute prior to corticosteroid administration to ensure there was no detectable corticosteroid level and at 5, 15, 30, 45, 60, and 90 minutes, and then hourly from the second through the eighth hours following corticosteroid administration. Samples were centrifuged within 15 minutes of blood draw and stored at −80°C until analyzed.
Nailfold capillaroscopy studies, obtained by freeze-frame video microscopy of each of 8 fingers (thumbs were excluded), were performed within 1 week of the pharmacokinetic study. The images were analyzed for the total number of ERL per mm over the 8 digits with mean ERL (total ERL divided by 8) calculated for each patient.
Laboratory assays
Prednisolone and methylprednisolone serum concentrations were determined by reverse-phase high-performance liquid chromatography (HPLC). Equilin (Sigma, St. Louis, MO) was chosen as a standard because the ring structure is similar for the corticosteroids tested. Equilin was used as the external standard together with the other corticosteroids to construct the standard curves to calibrate the HPLC, and as an internal standard after addition to each sample to correct for extraction efficiency. Stock standards were concentrated at 1 mg/ml in the HPLC mobile phase and dilutions were performed to prepare daily calibration curves targeted at 5, 10, 20, 50, 100, and 500 µg/ml of prednisolone, methylprednisolone, and equilin. The HPLC mobile phase consisted of 68:32 volume/volume mixture of HPLC-grade methanol:10 mM K2HPO4. Twenty microliters of equilin and 1 ml of NaOH (1 mg/ml) were added to each serum sample and passed through C2 Bond Elute Columns (Varian, Palo Alto, CA). Columns were washed with 3 ml of 10:90 v/v mixture of 100% methanol:water and then eluted with 9 ml of 100% methanol. The eluent was evaporated to dryness in a speedvac (Savant, Holbrook, NY), and samples were reconstituted in 0.9 ml of the HPLC mobile phase. Samples were filtered through a 0.22-µm membrane before 0.05 ml was injected through the HPLC system. The HPLC system (Dionex, Sunnyvale, CA) was composed of an AS40 Automated Sampler, GP40 Gradient Pump, and CD20 Conductivity UV Detector, and samples passed through the C18 Monomeric, 100Å Column (Grace Vydac, Hesperia, CA). Chromeleon software, version 6.5 (Dionex, Bannackburn, IL) controlled the system at a flow rate of 0.5 ml/minute and wavelength detection was set to 230 nm.
Pharmacokinetic calculations
Pharmacokinetic parameters were obtained using noncompartmental and compartmental analysis. The bioavailability of IVMP and OP for each patient was defined as the area under the curve (AUC) from the plasma concentration-versus-time curve. The AUC was calculated with the trapezoid method utilizing Microsoft Excel 00 (Microsoft, Redmond, WA). The maximum plasma concentration (Cmax) and time to reach maximum concentration were determined directly from the plasma concentration-versus-time curve. The elimination rate constant (ke) was extrapolated from the terminal slope of a plot of the natural logarithm of the plasma concentration-versus-time curve and the half-life (t1/2) was calculated as 0.693/ke for methylprednisolone and prednisolone. The apparent systemic clearance (Cl) and the volume of distribution (Vd) for methylprednisolone were estimated as dose/AUC and Cl/ke, respectively.
Statistical analysis
The mean blood concentrations for IVMP and OP were compared using the 2-sample t-test. The difference in AUC of IVMP and OP (ΔAUC) was assessed for association with ERL using linear regression. Results were analyzed using Stata statistical software, version 9.0 (StataCorp, College Station, TX) with P values less than 0.05 as the minimum level of significance.
RESULTS
Clinical characteristics of the 6 patients who completed this study are listed in Table 1. There were 4 females and 2 males with a mean age of 16 years (range 12–20 years) and an average disease duration of 36 months (range 1.3–100 months). All patients were receiving oral prednisone (mean daily dosage 16 mg, range 5–30 mg; mean duration 55 months, range 1–119 months) and 4 patients were receiving 1 gm of pulse intravenous methylprednisolone (weekly in 2 patients, every other week in 1, every 3 weeks in 1; mean duration 3 months, range 1–4 months); 4 patients were receiving intravenous methotrexate and 1 patient was receiving subcutaneous methotrexate; 1 patient was taking cyclosporine.
Table 1.
Clinical characteristics*
| Characteristic | Mean ± SD | Range |
|---|---|---|
| Age, years | 16 ± 2.9 | 12–20 |
| Disease duration, months | 36 ± 23.1 | 1.3–100 |
| DAS (0–20)† | 7.7 ± 3.4 | 5–13 |
| Height, cm | 162 ± 12.6 | 149–182 |
| Weight, kg | 63 ± 16.7 | 38–84 |
| M2 | 1.7 ± 0.3 | 1.2–1.9 |
| Corticosteroid dose, mg | 83 ± 13 | 64–97 |
DAS = disease activity score; M2 = meters squared.
A validated measure of cutaneous and muscle involvement, which generates a score between 0 and 20 with higher scores being associated with more active disease.
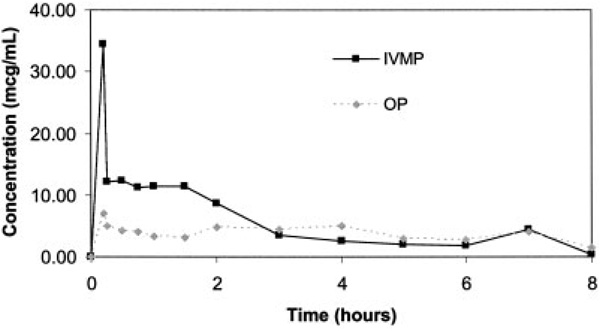
The mean concentration of IVMP and OP over the 8-hour study period is displayed in Figure 1. There were no detectable levels of OP on day 1/day 2 or IVMP on day 2 in the blood obtained 1 minute prior to corticosteroid administration. Overall, there was a trend toward significance in difference in mean AUC of IVMP (116.72 µg × ml/hour) compared with the AUC of OP (65.16 µg × ml/hour; P = 0.059). During the first 3 hours of the study, mean IVMP levels remained elevated compared with OP, whereas during the last 5 hours of the study concentrations were similar in both corticosteroids. Mean pharmacokinetic parameters are listed in Table 2. Mean ± SD peak concentration was higher for IVMP (34.49 ± 9.26 µg/ml) compared with OP (7.08 ± 5.35 µg/ml). The mean ± SD elimination rate constant was decreased for OP compared with IVMP (0.30 ± 0.06 and 0.40 ± 0.14, respectively). This resulted in a longer half-life for OP (mean ± SD 2.36 ± 0.50 hours) compared with IVMP (1.90 ± 0.65 hours).
Figure 1.
Mean concentrations of intravenous methylprednisolone (IVMP) and oral prednisolone (OP). The area under the curves for IVMP and OP were 116.72 µg × ml/hour and 65.16 µg × ml/hour, respectively (P = 0.059).
Table 2.
Pharmacokinetic parameters*
| Parameter | OP | IVMP |
|---|---|---|
| Cmax, µg/ml | 7.08 ± 5.35 | 34.49 ± 9.26 |
| ke, hours−1 | 0.30 ± 0.06 | 0.40 ± 0.14 |
| t1/2, hours | 2.36 ± 0.50 | 1.90 ± 0.65 |
| Vd, liters | – | 10.77 ± 10.11 |
| Cl, liters/hour | – | 3.51 ± 2.62 |
Values are the mean ± SD. OP = oral prednisolone; IVMP = intravenous methylprednisolone; Cmax = maximum plasma concentration; ke = elimination rate constant; t1/2 = half-life; Vd = volume of distribution; Cl = apparent systemic clearance.
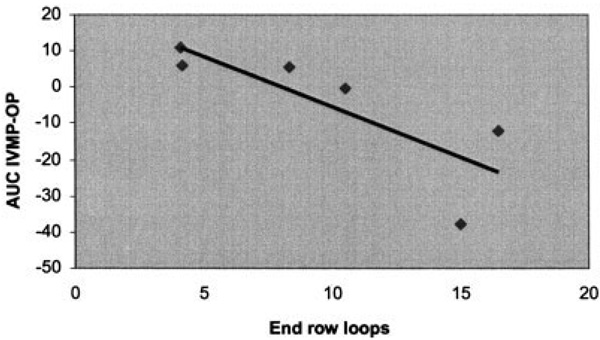
There was a significant inverse association between Δ AUC (AUC IVMP − AUC OP) and ERL (R = −0.68, P = 0.044). Specifically, patients with an increased bioavailability of IVMP compared with OP had fewer ERLs. The linear regression of this relationship is demonstrated in Figure 2. There was no association with ΔAUC and DAS.
Figure 2.
Relationship between Δ area under the curve (ΔAUC; AUC intravenous methylprednisolone [IVMP] − AUC oral prednisolone [OP]) versus nailfold capillary end-row loops. Correlation coefficient: R = −0.68; P = 0.044. The linear regression model was y = −1.57X + 18.18.
DISCUSSION
This study demonstrated that patients with active juvenile DM may have decreased bioavailability of OP compared with IVMP. In addition, the difference in IVMP versus OP bioavailability was inversely associated with nailfold capillary ERLs, suggesting that those patients with capillary obliteration have decreased absorption of OP. These specific corticosteroids were studied due to their relevance in clinical practice, rather than utilizing oral methylprednisolone, which is rarely administered for juvenile DM, or intravenous prednisolone, which is not available. Similar studies comparing the pharmacokinetics of IVMP and OP have been reported in 2 other autoimmune diseases: multiple sclerosis (8) and inflammatory bowel disease (9). The glucocorticoid receptor is utilized by all corticosteroids to initiate their biologic effects; although we compared IVMP and OP at a serum level, the biologic effect of both is to control inflammation. The difference in concentration of IVMP compared with OP may have been underestimated. Studies have indicated that the dose equivalency of prednisolone is 80% of methylprednisolone (10,11). The concentrations of OP that are biologically comparable with methylprednisolone may, in fact, be 20% lower than reported, thus increasing the difference between AUC IVMP and AUC OP. There was interpatient variability in the maximum concentration, clearance, and volume of distribution for both IVMP and OP. A review of studies evaluating corticosteroid pharmacokinetics in adult patients demonstrated less variability (12). However, this included multiple studies involving more than 100 participants, whereas the present study evaluated 6 patients, which is a limitation of the present study. One plausible explanation is that the Vd and Cl have been reported to be less consistent in children compared with adults (13). Because Cmax is related to both Vd and Cl, variability in the latter parameters may result in the interpatient variability of OP Cmax and IVMP Cmax demonstrated in patients with juvenile DM. The ke and t1/2 among patients were less variable and were similar to a study of children with various disorders, including 7 patients with juvenile DM (14). Both investigations demonstrate that the t1/2 of corticosteroids is decreased in the pediatric population. This may be an important factor to consider in a critically ill child.
Another limitation is the age variability of the patients. Although the majority of patients were adolescents, the youngest patient was 12 years old and the oldest patient was 20 years old. This may have altered corticosteroid metabolism (younger patients tend to metabolize corticosteroids more rapidly) and increased variability. Additionally, we only studied 2 male patients; there may be sex differences in the pharmacokinetics of corticosteroids that would not be evident in this study. We attempted to minimize body size effects by administering a dose based on mg/m2, but patients ranged from 1.2 to 1.9 m2; the larger patients may have increased stores of corticosteroids in adipose tissue that impacted results. There was variability in the dose and duration of corticosteroid exposure prior to this study, again potentially influencing adipose stores of corticosteroids. We did not formally analyze intrapatient variability. A final limitation of this study was that the pharmacodynamics of IVMP or OP was not assessed. Examining the biologic effects of corticosteroids in controlling juvenile DM disease activity is pivotal; prior to embarking on this, we wanted to investigate pharmacokinetic parameters that would then guide further pharmacodynamic studies. Because we were not evaluating pharmacodynamics, the order effect of corticosteroids administered (OP on day 1, IVMP on day 2) was not investigated, but should be considered for future studies.
There are few data concerning the pharmacokinetics of corticosteroids in children. To our knowledge, this is the first study evaluating the bioavailability of methylprednisolone and prednisolone in patients with juvenile DM. A similar study was performed in children with inflammatory bowel disease in which there was no difference in corticosteroid pharmacokinetics between active and inactive disease (9). Although there is inflammation in the gastrointestinal mucosa of these patients, the integrity of the blood supply may not be compromised. This is in contrast to patients with juvenile DM, in whom the systemic vasculopathy can potentially result in thrombus formation and vessel obliteration. If vessel occlusion occurs in the gastrointestinal tract, absorption of oral medications may be impaired. Although this study did not visualize vessels in the small intestines where prednisolone is absorbed, this may be a possible mechanism for the decreased absorption of OP observed.
In conclusion, patients with evidence of loss of nailfold capillary ERL by capillaroscopy may have decreased absorption of OP. There was a trend toward significance in the difference in bioavailability of IVMP compared with OP in patients with active juvenile DM. We speculate that these observations can provide a rationale for the greater efficacy of IVMP in the treatment of patients with active vasculopathy of juvenile DM. This study investigated low doses of corticosteroids in an attempt to further understand the pharmacokinetics. Studies evaluating both the pharmacokinetics and the pharmacodynamics of high-dose pulse methylprednisolone need to be performed to establish the role of this particular therapy in treating patients with juvenile DM who have varying degrees of capillary involvement leading to obliteration.
Acknowledgments
Supported by the National Center for Research Resources, NIH (grant M01-RR-0048). Drs. Rouster-Stevens and Pachman’s work was supported by The Cure Juvenile Myositis Foundation.
Footnotes
ClinicalTrials.gov identifier: NCT00004357.
AUTHOR CONTRIBUTIONS
Dr. Rouster-Stevens had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Rouster-Stevens, Ngai, Daru, Pachman.
Acquisition of data. Rouster-Stevens, Gursahaney, Daru, Pachman.
Analysis and interpretation of data. Rouster-Stevens, Ngai, Daru.
Manuscript preparation. Rouster-Stevens, Gursahaney, Pachman.
Statistical analysis. Rouster-Stevens.
REFERENCES
- 1.Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, Dyer A, et al. for the NIAMS Juvenile DM Registry Physician Referral Group. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49:300–305. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- 2.Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49:7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- 3.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–1649. [PubMed] [Google Scholar]
- 4.Christen-Zaech S, Seshadri R, Sunberg J, Abbott K, Paller AS, Pachman LM. Association of nailfold capillaroscopy (NFC) and disease activity score (DAS) over time in previously untreated juvenile dermatomyositis (JDM) [abstract] Arthritis Rheum. 2005;52 Suppl 9:S723. [Google Scholar]
- 5.Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol. 2002;47:505–511. doi: 10.1067/mjd.2002.122196. [DOI] [PubMed] [Google Scholar]
- 6.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 7.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 8.Morrow SA, Stoian CA, Dmitrovic J, Chan SC, Metz LM. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology. 2004;63:1079–1080. doi: 10.1212/01.wnl.0000138572.82125.f5. [DOI] [PubMed] [Google Scholar]
- 9.Faure C, Andre J, Pelatan C, Munck A, Giraud M, Cezard JP, et al. Pharmacokinetics of intravenous methylprednisolone and oral prednisone in paediatric patients with inflammatory bowel disease during the acute phase and in remission. Eur J Clin Pharmacol. 1998;54:555–560. doi: 10.1007/s002280050512. [DOI] [PubMed] [Google Scholar]
- 10.Tse FL, Welling PG. Relative bioavailability of prednisone and methylprednisolone in man. J Pharm Pharmacol. 1979;31:492–493. doi: 10.1111/j.2042-7158.1979.tb13568.x. [DOI] [PubMed] [Google Scholar]
- 11.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43:1216–1227. doi: 10.1177/0091270003258651. [DOI] [PubMed] [Google Scholar]
- 12.Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2006;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TN. Modelling approaches to dose estimates in children. Br J Pharmacol. 2005;59:663–669. doi: 10.1111/j.1365-2125.2005.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green OC, Winter RJ, Kawahara FS, Phillips LS, Lewy PR, Hart RL, et al. Pharmacokinetic studies of prednisolone in children. J Pediatr. 1976;93:299–303. doi: 10.1016/s0022-3476(78)80529-0. [DOI] [PubMed] [Google Scholar]