Abstract
A network of prefrontal and parietal regions has been implicated in executive control processes. However, the extent to which individual regions within this network are engaged in component control processes, such as inhibition of task-irrelevant stimulus attributes or shifting (switching) between attentional foci, remains controversial. Participants (N = 17) underwent functional magnetic resonance imaging while performing a global-local task in which the global and local levels could facilitate or interfere with one another. Stimuli were presented in blocks in which participants either constantly shifted between the global and local levels, or consistently responded to one level only. Activations related to inhibition and shifting processes were observed in a large network of bilateral prefrontal, parietal, and basal ganglia regions. Region of interest analyses were used to classify each region within this network as being common to inhibition and shifting, or preferential to one component process. Several regions were classified as being preferential to inhibition, including regions within the dorsolateral and ventrolateral prefrontal cortex, the parietal lobes, and the temporal-parietal junction. A limited set of regions in the parietal lobes and left dorsolateral prefrontal cortex were classified as preferential to shifting. There was a very large set of regions displaying activation common to both inhibition and shifting processes, including regions within the dorsolateral prefrontal cortex, anterior cingulate, and basal ganglia. Several of these common regions were also involved during facilitation, suggesting that they are responsive to the number of task-salient channels of information, rather than purely to demands on control processes.
Several current conceptions of executive control, broadly defined as the organization and manipulation of information in service of goal-oriented behavior, envision multiple component processes, although the parsing of components and their hierarchical organization in the human brain remains controversial (Banich, 2009; Koechlin et al., 2003; Milham et al., 2003; Miyake et al., 2000; Salthouse et al., 2003). Two prominent hypothesized components of executive control are (1) inhibiting currently irrelevant information and (2) shifting (or switching) between multiple rule sets or information sources. A fundamental question is whether inhibition and shifting are truly distinct psychological processes, or alternative conceptualizations of a single underlying process. Inhibition could be seen as requiring a shift from a dominant stimulus-response mapping to a non-dominant one. Shifting could be reconceptualized as inhibition of prior stimulus dimensions, task sets, or stimulus-response mappings (Aron et al., 2004a; Dreher and Berman, 2002). Behavioral studies of individual differences tend to find evidence that inhibition and shifting are separate, although correlated, processes, but interpretation of the behavioral studies hinges upon the interpretation of the magnitudes of correlations across studies (Hedden and Yoon, 2006). The goal of the present study was to examine whether inhibition and shifting are mediated by common or separable neural mechanisms in the human brain. If inhibition and shifting activate diverse neural networks, these processes are more likely distinct. If, however, they activate a highly similar network, they are more likely unitary. This dichotomy of outcomes is a somewhat simplified view, as activation in similar regions could be associated with both inhibition and shifting processes if those regions interact differently while carrying out specific control functions.
Candidate regions for executive control functions are primarily localized to the lateral and medial prefrontal cortex (PFC), the inferior parietal lobule (IPL), and the basal ganglia. The region most consistently associated with inhibition is a region of the right inferior frontal gyrus in ventrolateral PFC (VLPFC; Aron et al., 2004b; Robbins, 2007), although it has also been implicated in at least some forms of task-shifting (Aron et al., 2004a; Pessoa et al., 2009; Robbins, 2007). The homologous region in the left VLPFC is often associated with interference resolution or selection demands (Badre and Wagner, 2007; Bode and Haynes, 2009; D’Esposito et al., 1999; Forstmann et al., 2006; Forstmann et al., 2008; Leber et al., 2008; Nelson et al., 2009; Rossi et al., 2009; Slagter et al., 2006; Thompson-Schill et al., 2002). Many studies of shifting have observed a region in the left IPL (and sometimes the right homologue) that appears to be primarily engaged during trials on which participants are cued to shift from one stimulus-response mapping to another or to direct their attention toward a different stimulus dimension (Badre and Wagner, 2006; Wager, et al., 2005; Yeung et al., 2006). The presupplementary motor area extending into the dorsal anterior cingulate cortex (ACC) and regions of the middle frontal gyrus are often observed during shifting conditions, but are not observed as consistently across studies as is the left IPL region (Badre and Wagner, 2006; Bode and Haynes, 2009; Forstmann et al., 2006; Leber et al., 2008; Rossi et al., 2009; Slagter et al., 2006; Yeung et al., 2006). The basal ganglia, as a major component of the frontal-striatal circuit, displays coordinated activation with prefrontal and parietal regions at rest and during shifting tasks (Cools et al., 2006;. Di Martino et al., 2008). The importance of the basal ganglia for shifting is indicated by disruption of shifting following dopaminergic depletion, striatal lesions, and in Parkinson’s disease (Cools and Robbins, 2004; Cools et al., 2009; Monchi et al., 2004; Monchi et al., 2007; Nagano-Saito et al., 2008).
Direct comparisons between inhibition and shifting depend on designs in which these processes are free from task and difficulty confounds. Several imaging studies have examined both inhibition and shifting, but have used separate task domains to measure different processes or manipulated task demands such that they become fundamentally different across processes (Barber and Carter, 2005; Brass and von Cramon, 2004; Collette et al., 2005; Derrfuss et al., 2005; Derrfuss et al., 2004; Konishi et al., 1999). Thus, process demands (inhibition or shifting) and task demands cannot be dissociated. For example, one study contrasted inhibition and shifting, but measured inhibition via responses to compatible/incompatible stimulus-response mappings and measured shifting via a counting task that did or did not involve shifting between counters to be updated (Sylvester et al., 2003). Furthermore, interpretation was complicated by reaction time differences up to twelve times greater during shifting than inhibition. Given such complications, findings to date are mixed, with some studies supporting the separate localization of inhibition or shifting processes to particular regions (Aron et al., 2004a; Dreher and Berman, 2002; Robbins, 2007; Sylvester et al., 2003; Wager, et al., 2005; Yeung et al., 2006), and others suggesting a unitary prefrontal-parietal network that undergirds inhibition, shifting, and other control functions (Collette and Van der Linden, 2002; Miller and Cohen, 2001; Nee et al., 2007; Wager et al., 2004).
The current study provides a novel comparison of inhibition and shifting, with two primary contributions. First, task demands and difficulty during inhibition and shifting were controlled; any differences observed should be attributable to the processes themselves. Within a common global-local paradigm (Navon, 1977), participants (1) inhibited one irrelevant stimulus dimension, or (2) shifted between two stimulus dimensions across trials, or (3) simultaneously engaged in inhibition and shifting, or (4) engaged in neither inhibition nor shifting. Thus, shifting and inhibition demands were manipulated independently within a common task, allowing for a direct comparison between shifting and inhibition. Using a specific set of criteria, we set out to identify regions that are selective to one process, preferential to one process, or common to both inhibition and shifting. Regions selective to one process are engaged by that process to the exclusion of the alternative process. A less stringent set of criteria can be applied to identify regions that are preferential to one process; such regions, although associated to some extent with both processes, are more engaged by one process over the other.
Second, we investigated activation during a facilitation condition, in which both stimulus dimensions led to one response. Hence, facilitation could be compared to both a neutral baseline condition and also the conflicting (inhibition and shifting) conditions, with the expectation that regions preferential to shifting or inhibition would not display activation above baseline in the facilitation conditions. Neural correlates of facilitation have been rarely studied, but activation during facilitation could impose firm constraints on theoretical accounts of control processes thought to be subserved by specific brain regions (Badre et al., 2005; Carter et al., 1995; Cohen Kadosh et al., 2008; Weissman et al., 2005). In particular, regions thought to be selective to inhibition should not display activation during facilitation. Although less informative with regard to shifting in isolation, to the extent that regions preferential to shifting or involved in both shifting and inhibition also display activation during facilitation, it is difficult to infer that such regions are involved solely in these processes. Rather, regions involved in facilitation as well as in shifting and/or inhibition are more likely to be involved in more general processes such as the selective allocation of attention to the amount of task–relevant information coming from multiple information sources (or channels).
METHODS
Participants
Eighteen college-aged adults (aged 18-28 years, mean age 21.6 years) participated in the study. All participants gave informed consent and the study was approved by the Stanford University Internal Review Board. One participant’s data were lost due to technical difficulties during the scan session.
Task materials and procedure
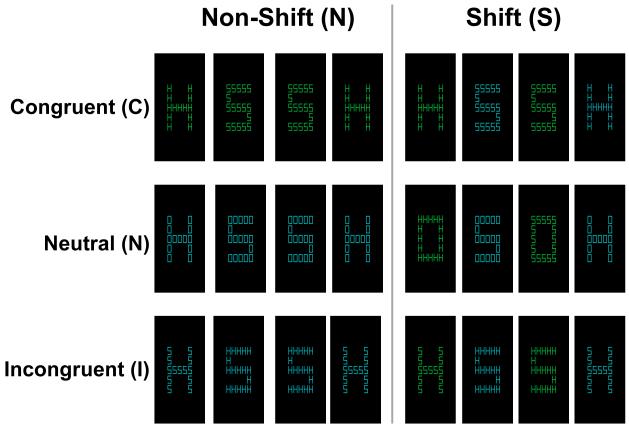
The behavioral task consisted of hierarchical letter stimuli of the type described by Navon (Navon, 1977) presented using the E-prime 1.1 SP2 software (Psychology Software Tools, Pittsburgh, PA). Stimuli consisted of large letters (H, S, and O) made up of smaller letters (H, S, and O) (Figure 1). During scanning, participants viewed the stimuli through a mirror directed at a projection screen. Small letters subtended a visual angle of approximately 0.6° × 0.4°; large letters subtended 3.5° × 2.4°. Stimuli were presented in either green or blue on a black background. Participants were instructed to identify either the global (large letter) or local (small letter) level on each trial, as cued by the color, and to respond with the appropriate response (either H or S). The letter O was never mapped to a response and was used in neutral conditions in which the non-attended level did not have an associated response. Stimuli were combined so as to create 6 experimental conditions in a 3 (congruency) x 2 (shifting) factorial design. Congruent stimuli contained the same letter (either H or S) in both the global and local levels. Neutral stimuli contained an O, which was never associated with a response, at one level, and an H or S at the other level. Incongruent stimuli contained incompatible letters (H and S) at opposing levels. Stimuli were organized into blocks of 12 trials, which could either involve shifting or not. In shifting blocks, participants were cued by the stimulus color to predictably shift between attending to the global and local levels. In non-shifting blocks, participants were cued to constantly attend to either the global or local level. Combining the congruent, neutral, and incongruent stimuli with the non-shifting and shifting blocks resulted in the six possible block types: congruent non-shifting (CN), congruent shifting (CS), neutral non-shifting (NN), neutral shifting (NS), incongruent non-shifting (IN), and incongruent shifting (IS). On each trial, a fixation cross appeared for 100ms, followed by stimulus presentation for 400ms, followed by an ITI of 1350ms during which a fixation cross was displayed. Responses were collected during all phases of the trial using an MRI-compatible button box. Participants received extensive instruction and 96 practice trials before beginning the task in the scanner, which consisted of 8 blocks of trials from each condition.
Figure 1.
Examples of stimuli used in each condition. Stimuli were hierarchical colored letters (H, S, and O) with a larger (global) letter made up of smaller (local) letters. Each stimulus represents a single trial; groups of stimuli represent block types. Each block consisted of 12 stimuli. Each block could either involve shifting between the global and local levels, as cued by the stimulus color, or responding only to one level (non-shifting). Stimuli could be congruent (the global and local levels prompt the same response), neutral (one level has no associated response), or incongruent (the two levels prompt opposing responses). Congruent trials involve facilitation, while incongruent trials involve inhibition of the non-attended level. The six block types were therefore: congruent non-shifting (CN), congruent shifting (CS), neutral non-shifting (NN), neutral shifting (NS), incongruent non-shifting (IN), and incongruent shifting (IS).
Image acquisition
Functional magnetic resonance imaging (fMRI) data were acquired using a 1.5-Tesla General Electric Signa MR scanner (General Electric Medical Systems Signa, Rev. 5.5, Waukesha, Wisconsin) paired with a whole-head coil. Functional data were obtained in 4 runs, each consisting of 150 volumes, using a sequential spiral in-out acquisition sequence for measurement of blood oxygen level-dependent (BOLD) effects (TR = 1850ms, TE = 40ms, flip angle = 70°, 64 × 64 matrix, FOV = 240mm). Twenty-three oblique slices of 5mm thickness were acquired parallel to the plane defined by the anterior and posterior commissures. Anatomical images were obtained using a Spoiled GRASS sequence consisting of 124 sagittal slices of 1.5mm thickness.
Statistical analysis
Behavioral data were analyzed using SPSS software (SPSS Inc., Chicago Illinois). Significant behavioral results are reported using α = .05. Imaging data were analyzed using SPM8 software (Wellcome Department of Imaging Neuroscience, London, UK) and associated scripts. Before analysis, the first 6 volumes of each run were discarded to allow for T1 equilibration effects, leaving 144 volumes per run. Functional volumes were screened for outliers, defined as volumes with a mean global signal greater than 3 SDs from the global mean of the 4 runs combined, which were replaced with an average of the immediately preceding and succeeding volumes. Most participants had no such outliers, and a maximum of 1% of volumes were replaced for any participant. Functional volumes were then corrected for slice time acquisition and motion-corrected by realignment to the first functional volume. The anatomical image was coregistered to the first functional volume and normalized to Montreal Neurological Institute (MNI) space using a standard T1 template image with 2mm3 voxels. The normalization parameters determined from the anatomical image were applied to the functional volumes, which were then smoothed with a 6mm isotropic Gaussian kernel. Condition effects for the 6 stimulus conditions were estimated using box-car regressors convolved with a canonical hemodynamic response function. Unless otherwise specified, all results reported at the whole-brain level are from group-level, random-effects analyses using a False Discovery Rate (FDR) corrected threshold (Genovese, et al., 2002) of p = .05 with a cluster size greater than k = 9. This cluster size was chosen to exclude clusters smaller than 1 voxel in acquired space. FDR correction was chosen in order to maximize the potential for detecting regions potentially related to either shifting or inhibition to be carried forward into region of interest (ROI) analyses, while still controlling the rate of false positive activations. Because the ROI analyses involve comparisons across all conditions (not only those used in defining the ROI), these follow-up analyses additionally reduce the potential for false positives to be interpreted as meaningful. It should be noted that not all of the ROI comparison criteria applied are independent of the contrasts used to identify the ROIs (Kriegskorte, et al., 2009); we have noted such cases when they occur and the contrasts used to identify each ROI are provided in Table 1. ROI analyses were conducted with an alpha level of .05; where we refer to equivalence between two conditions in an ROI, the conditions did not differ at this statistical threshold. Conjunction analyses were performed using the conjunction null hypothesis (Nichols, et al., 2005). Large clusters of activation from the whole-brain analyses were decomposed into regions of interest for further analysis by identifying the intersection of the functional cluster and anatomical subregions (Brodmann areas) within the cluster extent. Anatomical regions were identified using the WFU PickAtlas tool (Maldjian et al., 2004; Maldjian et al., 2003) with a 3-dimensional dilation of 1 voxel. Regions of interest were identified as a 6mm-radius sphere surrounding the voxel of peak activation within each anatomical area. Each ROI encompassed approximately 113 voxels, with some ROIs truncated by boundaries of the brain. Gray matter regions are reported with MNI coordinates and Brodmann areas (BA) for reference.
Table 1.
Activations for inhibition, shifting, and their interaction
| Anatomical Region | L/R | BA | MNI Coordinates |
T-value | Cluster size (k) |
Sel./Pref.? | Paired T-Tests |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Main effects |
|||||||||
| IS > NN | ||||||||||||
| Middle Frontal Gyrus | L | 6 | −32 | 2 | 60 | 12.97 | 44042 | S | In | Ic | cdghjmo | |
| Medial Frontal Gyrus | L | 6 | −10 | 0 | 64 | 9.47 | S | In | Ic | fgno | ||
| Middle Frontal Gyrus | R | 6 | 28 | −4 | 56 | 9.17 | S | In | Ic | cdgjmo | ||
| Inferior Frontal Gyrus | L | 9 | −44 | 10 | 30 | 9.25 | S | In | Ic | cdghjlmo | ||
| Inferior Frontal Gyrus | R | 9 | 52 | 8 | 28 | 9.14 | S | In | Ic | Inh. Pref. | acdghmo | |
| Anterior Cingulate | L | 32 | −12 | 30 | 28 | 6.65 | S | In | Ic | go | ||
| Anterior Cingulate | R | 32 | 10 | 30 | 28 | 6.09 | S | In | Ic | go | ||
| Middle Frontal Gyrus | L | 46 | −42 | 34 | 22 | 12.77 | S | In | Ic | Sh. Pref. | bcdgho | |
| Middle Frontal Gyrus | R | 46 | 48 | 36 | 24 | 11.68 | S | In | cdghjo | |||
| Inferior Frontal Gyrus/Insula | L | 47/13 | −34 | 22 | 4 | 7.69 | S | In | Ic | gmno | ||
| Inferior Frontal Gyrus | R | 47 | 34 | 26 | 2 | 7.44 | S | In | Ic | cdghjmo | ||
| Medial Frontal Gyrus | L | 8/32 | −8 | 18 | 50 | 8.40 | S | In | Ic | cdgjno | ||
| Superior Frontal Gyrus | R | 8/32 | 6 | 12 | 54 | 9.38 | S | In | Ic | cdfgjmno | ||
| Precuneus | L | 7 | −28 | −78 | 38 | 13.93 | S | In | Ic | Sh. Pref. | bcdghjlmno | |
| Precuneus | R | 7 | 30 | −68 | 42 | 14.07 | S | In | Ic | Sh. Pref. | bcdghjlmo | |
| Inferior Parietal Lobule | L | 40 | −46 | −44 | 46 | 14.37 | S | In | Ic | cdghjlmno | ||
| Inferior Parietal Lobule | R | 40 | 40 | −52 | 48 | 9.17 | S | In | Ic | cdgjno | ||
| Caudate Body | L | −20 | −2 | 22 | 6.84 | In | Ic | ghl | ||||
| Caudate Body | R | 22 | −8 | 20 | 9.18 | S | In | Ic | glno | |||
| Cerebellum | L | −30 | −56 | −34 | 14.18 | S | In | Ic | cdfgjmno | |||
| Cerebellum | R | 26 | −62 | −34 | 10.87 | S | In | Ic | cdgjmno | |||
| IN > NS | ||||||||||||
| Precentral Gyrus | L | 44 | −52 | 12 | 8 | 6.37 | 70 | In | Inh. Pref. | aim | ||
| Inferior Frontal Gyrus | R | 47 | 54 | 20 | −6 | 6.14 | 595 | In | Inh. Pref. | aijm | ||
| Precentral Gyrus | R | 44 | 52 | 10 | 12 | 6.08 | In | Ic | Inh. Pref. | am | ||
| Inferior Frontal Gyrus | R | 45 | 58 | 18 | 6 | 5.31 | In | Inh. Pref. | aim | |||
| Middle Frontal Gyrus | R | 6/8 | 32 | 6 | 44 | 5.81 | 40 | S | In | Ic | Inh. Sel. | acgjmo |
| Superior Frontal Gyrus | R | 6 | 6 | 16 | 66 | 5.63 | 369 | In | Inh. Pref. | agijm | ||
| Superior Frontal Gyrus | R | 8 | 6 | 44 | 52 | 5.30 | Inh. Pref. | aim | ||||
| Middle Frontal Gyrus | R | 9 | 42 | 10 | 38 | 4.70 | 52 | In | Ic | Inh. Sel. | acgjm | |
| Inferior Frontal Gyrus | R | 47 | 52 | 44 | −12 | 4.29 | 13 | Inh. Pref. | ak | |||
| Middle Frontal Gyrus | R | 9 | 54 | 24 | 30 | 4.14 | 9 | S | In | Inh. Pref. | acdghjm | |
| Superior Frontal Gyrus | R | 10 | 28 | 66 | 20 | 3.98 | 11 | In | Inh. Sel. | acjm | ||
| Inferior Parietal Lobule | L | 40 | −68 | −34 | 26 | 8.53 | 209 | Inh. Pref. | aik | |||
| Superior Temporal Gyrus | L | 22 | −62 | −38 | 20 | 5.56 | Inh. Pref. | agk | ||||
| Inferior Parietal Lobule | R | 40 | 60 | −34 | 50 | 6.53 | 1234 | In | Ic | Inh. Sel. | acgjmo | |
| Middle Temporal Gyrus | R | 39 | 58 | −60 | 8 | 6.40 | Inh. Pref. | aem | ||||
| Superior Temporal Gyrus | R | 22 | 64 | −50 | 18 | 6.37 | Inh. Pref. | aim | ||||
| Parietal Lobe | L | 40 | −60 | −36 | 52 | 5.43 | 12 | S | In | Ic | Inh. Pref. | afgjmno |
| Postcentral Gyrus | R | 40 | 64 | −24 | 24 | 5.07 | 96 | Inh. Pref. | agi | |||
| Middle Temporal Gyrus | L | 39 | −60 | −60 | 10 | 4.97 | 71 | Inh. Pref. | ag | |||
| Superior Temporal Gyrus | L | 22 | −56 | −2 | 2 | 4.43 | 32 | Inh. Pref. | aefgm | |||
| Middle Temporal Gyrus | R | 21 | 54 | −44 | 4 | 5.74 | Inh. Pref. | akm | ||||
| Caudate Body | R | 10 | 4 | 12 | 6.80 | 178 | In | Ic | Inh. Pref. | ago | ||
| Caudate Body | R | 12 | 16 | 8 | 5.85 | In | Inh. Pref. | a | ||||
| Putamen | L | −16 | 8 | 2 | 5.29 | 76 | In | Ic | Inh. Pref. | amo | ||
| Caudate Head | L | −10 | 16 | 6 | 5.29 | Inh. Pref. | ak | |||||
| Caudate Head | L | −16 | 20 | −4 | 4.30 | 14 | Inh. Pref. | a | ||||
| Cerebellum | L | −22 | −70 | −34 | 5.59 | 96 | S | In | Ic | Inh. Sel. | acgjmo | |
| NS > IN | ||||||||||||
| Inferior Parietal Lobe | L | 7 | −26 | −56 | 40 | 7.68 | 9 | S | In | Ic | Sh. Pref. | bcdghjlo |
Notes. Statistics for peak voxels and surrounding ROIs from each significant cluster of activation. Clusters were identified from the IS > NN, IN > NS, and NS > IN contrasts with an FDR-corrected threshold of p = .05, k = 9. Clusters that also exhibited activation in the main effect factorial analyses for shifting (S), incongruent referenced to neutral (In), and incongruent referenced to congruent (Ic) are noted. Clusters exhibiting multiple main effects are present in the conjunction of those effects. Large clusters of activation were broken into smaller clusters according to anatomical boundaries (Brodmann areas) and were identified as the peak voxel within the anatomical region. ROIs were defined as 6mm spheres around each peak voxel and were interrogated for activation in each of the six experimental conditions. Paired t-tests (α = .05) were conducted to determine which ROIs displayed significantly different activations in each condition. The results of these paired t-tests are given as: a = IN > NS, b = NS > IN, c = IN > NN, d = NS > NN, e = IS > IN, f = IS > NS, g = IS > NN, h = CN > NN, i = CS > NS, j = CS > NN, k = NN > CN, l = NS > CS, m = IN > CN, n = IS > CS, o = IS > CN. Based on these t-tests, regions were labeled as selective or preferential for inhibition or shifting. ROIs identified from the IN > NS and NS > IN contrasts will be classified as preferential (but not selective) to inhibition or shifting by definition. Cortical regions are grouped by cluster and reported in the anterior to posterior direction, followed by subcortical regions. Inhibition selective regions are reported in bold type.
NN = Neutral Non-Shifting, NS = Neutral Shifting, IN = Incongruent Non-Shifting, IS = aIncongruent Shifting, CN = Congruent Non-Shifting, CS = Congruent Shifting, Inh. = Inhibition, Sh. = Shifting, Sel. = Selective, Pref. = Preferential.
RESULTS
Behavioral Results
Accuracy and median reaction times (RTs) for performance in each condition of the global-local task are reported in Figure 2. Accuracy and median RTs for performance in each condition of the global-local task were examined via separate repeated-measures ANOVAs using congruency (3) x shifting (2) as factors. Significant main effects of congruency showed that performance was faster, F(2, 32) = 66.80, MSE = 2941, p < .001, η2 = .81, and more accurate, F(2, 32) = 43.81, MSE = .002, p < .001, η2 = .73, during congruent trials than incongruent trials. Significant main effects of shifting showed that performance was faster, F(1, 16) = 90.48, MSE = 3852, p < .001, η2 = .85, and more accurate, F(1, 16) = 28.26, MSE = .002, p < .001, η2 = .64, for non-shifting trials than for shifting trials. These main effects were modified by Congruency x Shifting interactions, such that trials involving both shifting and incongruent stimuli were the slowest, F(2, 32) = 31.88, MSE = 1434, p < .001, η2 = .67, and least accurate, F(2, 32) = 12.68, MSE = .002, p < .001, η2 = .44.
Figure 2.
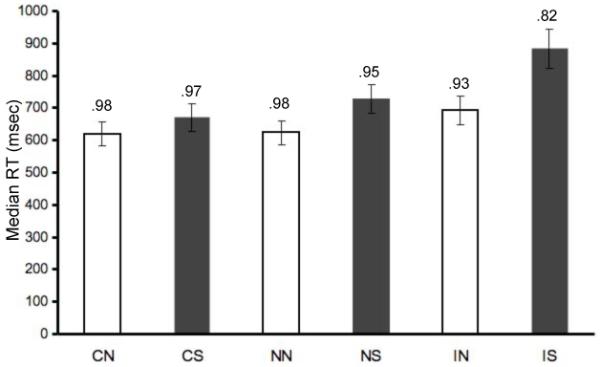
Reaction times for each condition averaged across individuals. Median reaction times were calculated for each individual after trimming all responses greater than 2.5 SD from the individual’s mean. Accuracy scores (mean proportion correct) for each condition are indicated above each bar. Light bars indicate non-shifting conditions, dark bars indicate shifting conditions. Trial type abbreviations are: congruent non-shifting (CN), congruent shifting (CS), neutral non-shifting (NN), neutral shifting (NS), incongruent non-shifting (IN), and incongruent shifting (IS).
Inhibition, shifting, and facilitation effects were calculated by comparing the median RT for the appropriate baseline (either NN or NS) to each condition of interest. The inhibition effect (IN – NN: M = 68, SD = 50) was smaller than the shifting effect during neutral trials (NS – NN: M = 105, SD = 43), t(16) = 3.46, p = .003. Furthermore, the inhibition effect was statistically equivalent when measured relative to the neutral non-shifting baseline (IN – NN: M = 68, SD = 50) and the congruent non-shifting baseline (IN – CN: M = 72, SD = 53), t(16) = 0.42, p = .68. The shifting effect was present during congruent trials (CS – CN: M = 50, SD = 60), t(16) = 3.45, p = .003, but was smaller than during neutral trials (NS – NN: M = 105, SD = 43), t(16) = −4.41, p < .001. As indicated by the above interaction, the combined effect of inhibition and shifting (IS – NN: M = 263, SD = 105) was over-additive. A facilitation effect, in which congruent trials were faster than neutral trials, was observed in the shifting conditions (NS – CS: M = 59, SD = 54), t(16) = 4.53, p < .001, but not in the non-shifting conditions (NN – CN: M = 4, SD = 40), t(16) =0.42, p = .68. Although not a focus of the study, there was a reliable global precedence effect in median reaction times (global: M = 689, SD = 127, local: M = 715, SD = 113), t(16) = 4.11, p = .001, which was about one-third the size of the inhibition effect and one-fourth the size of the shifting effect.
Imaging Results
Regions common to inhibition and shifting
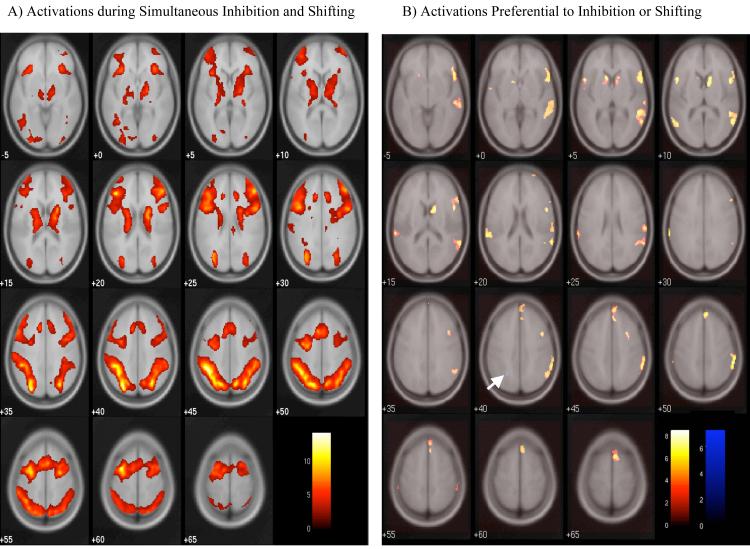
In order to identify regions that may be active in both inhibition and shifting processes, main effects of shifting and inhibition were examined (Table 1). Both analyses resulted in highly similar maps. The conjunction of these main effects can be observed in Table 1 by examining those areas that express main effects of both inhibition and shifting. Main effects of shifting were calculated across all shifting conditions (CS, NS, and IS) versus all non-shifting conditions (CN, NN, and IN). Because the inhibition conditions involved comparisons across three levels, the main effect is reported as two simple effects, representing activations greater in the incongruent conditions (IN and IS) than in the neutral conditions (NN and NS) or the congruent conditions (CN and CS). At the whole-brain level (thresholded at FDR = .05), no voxels exhibited an interaction between inhibition and shifting in the full factorial model, nor were any voxels above-threshold in the direct contrast of simultaneous inhibition and shifting (IS) with the additive effect of inhibition only and shifting only (IN and NS). However, because the main effects of both inhibition and shifting are driven in part by the inclusion of the simultaneous inhibition and shifting (IS) condition, results from this condition compared to the neutral non-shifting condition (IS > NN) were also examined (Table 1; Figure 3A). Regions of activation in this contrast and in the conjunction of the main effects included bilateral activations in the ACC (BA 32), dorsolateral prefrontal cortex (DLPFC, BA 9 and 46), medial regions of the inferior frontal gyrus (BA 45/47) extending into the insula, and the IPL (BA 7 and 40).
Figure 3.
A) Activations during simultaneous inhibition and shifting. Activations are shown from the IS > NN contrast. B) Activations preferential to inhibition or shifting. Inhibition-preferential activations (IN > NS) are displayed in the red-yellow spectrum; shifting-preferential activations (NS > IN) are in blue. The arrow indicates the one above-threshold cluster that was preferential to shifting. In both panels, results are displayed with an FDR-corrected threshold of p = .05, k = 9.
Regions preferential for inhibition or shifting
In order to identify regions that displayed preferential activation during inhibition compared to shifting, and vice versa, the inhibition only (IN) and shifting only (NS) conditions were directly compared to one another (Table 1 and Figure 3B). In the inhibition preferential contrast (IN > NS), several regions of activation were observed, including regions of the right VLPFC (BA 45/47) and bilateral temporal-parietal junction (TPJ, BA 22 and 39). For the shifting preferential contrast (NS > IN), a single region of activation was observed in the left IPL (BA 7). When a less stringent threshold was applied (uncorrected p = .005, k = 9), the spatial extent of this left parietal region increased and activations were observed in the right IPL (BA 7) and in left DLPFC (BA 46).
Region of interest analyses
The above whole-brain analyses revealed regions that were active during inhibition, shifting, or both. However, each contrast or main effect, when viewed independently, may obscure important information about the processes subserved by the active regions. Regions identified in the above comparisons may include regions that are primarily sensitive to task difficulty. Furthermore, the main effects and the inhibition plus shifting (IS > NN) contrast may include regions that are preferential to inhibition or to shifting (because of the inclusion of the simultaneous inhibition and shifting condition in both main effects). The peak voxels identified in all of the above contrasts (listed in Table 1) were therefore used to define regions of interest (ROIs) to be further interrogated. Within each ROI, the beta values associated with each condition were extracted for each participant and the mean beta value per condition was calculated (Figure 4). These mean beta values were subjected to paired t-tests (α = .05), comparing the NN, IN, NS, and IS conditions (Table 1). If the mean beta value was significantly greater for inhibition only (IN) than for shifting only (NS), the ROI was labeled as being preferential for inhibition; if greater for shifting only than for inhibition only, the ROI was labeled as preferential for shifting. Regions that did not differ significantly (at α = .05) for inhibition only and shifting only were labeled as being equivalently involved in inhibition and shifting. A summary of these ROI results is displayed in Figure 5. Because this comparison is not independent of the contrasts used to identify some ROIs (IN > NS and NS > IN, see Table 1), it is important to examine multiple criteria in determining whether a region is preferential for a given process (Kriegeskorte, et al., 2009).
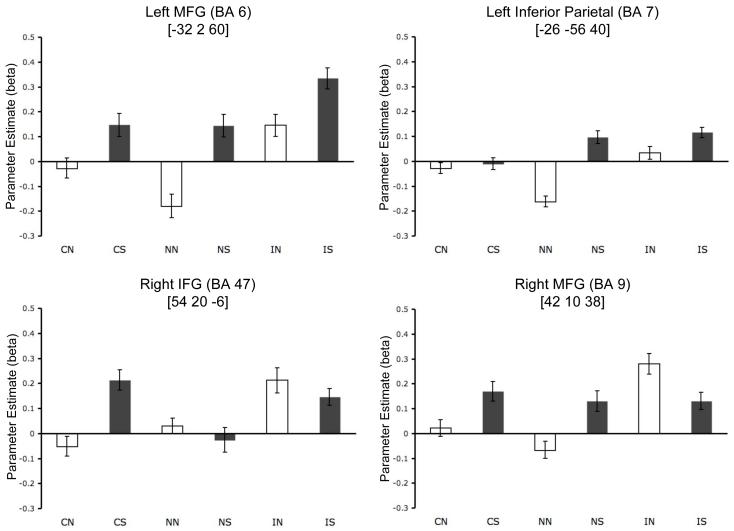
Figure 4.
Mean beta values in each condition for example ROIs. Top left: Left middle frontal gyrus (BA 6, defined from IS > NN contrast) exhibited a pattern common to both inhibition and shifting, with additionally increased activation during simultaneous inhibition and shifting (IS). Top right: Left inferior parietal lobe (BA 7, defined from NS > IN contrast) exhibited a pattern of activation preferential for shifting. Bottom left: Right inferior frontal gyrus (BA 47, defined from IN > NS contrast) exhibited a pattern of activation preferential for inhibition. Activation during the congruent shifting (CS) condition was equivalent to that in the incongruent non-shifting (IN) condition. Bottom right: Right middle frontal gyrus (BA 9, defined from IN > NS contrast) exhibited an activation pattern consistent with selectivity for inhibition. Light bars indicate non-shifting conditions, dark bars indicate shifting conditions. Trial type abbreviations are: congruent non-shifting (CN), congruent shifting (CS), neutral non-shifting (NN), neutral shifting (NS), incongruent non-shifting (IN), and incongruent shifting (IS). Error bars represent average within-subject SE. MNI coordinates (in brackets) indicate the peak voxel of each ROI.
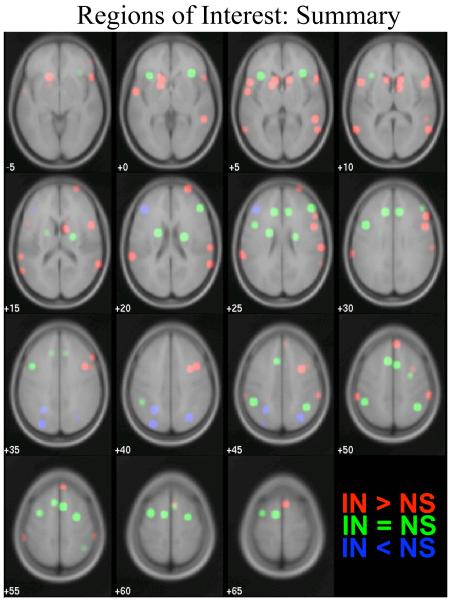
Figure 5.
Summary of region of interest results. ROIs with equivalent activation for inhibition and shifting (IN = NS) are shown in green, ROIs preferential for inhibition (IN > NS) are shown in red, and ROIs preferential for shifting (NS > IN) are shown in blue. Classifications were derived from paired t-tests within each ROI, as described in Table 1.
Two criteria may be applied in determining whether activation within a given region was preferential to inhibition or to shifting. The strong criterion requires that all of the following conditions be met for a region to be considered selective to inhibition: a) activation in the inhibition only condition (IN) must be significantly greater than activation in the shifting only condition (NS), b) activation in the inhibition only (IN) condition must be significantly greater than in the neutral non-shifting baseline (NN), c) activation in the shifting only (NS) condition must not be significantly greater than in the non-shifting baseline (NN), and d) activation in the condition involving simultaneous inhibition and shifting (IS) must not be significantly greater than that for inhibition alone (IN). Comparisons involving no significant difference are limited by the power of our study. For a region to be selective to shifting, the corollaries to the above conditions must all be met. A second, weak, criterion may also be applied, requiring only that the first of the above four conditions be met. However, note that this first criterion is not independent of the method used to identify all ROIs (see Table 1). According to the strong criterion, only five regions were selective to inhibition, namely two distinct regions within the right middle frontal gyrus (BA 6/8 and 9), and regions in the right superior frontal gyrus (BA 10), right inferior parietal lobule (BA 40), and the left cerebellum. However, the regions that met the strong criterion did not include the right inferior frontal gyrus, a region implicated in inhibition by many prior studies (Aron et al., 2004b). Several foci within the right inferior frontal gyrus did meet three out of four conditions, lacking only significantly greater activation in inhibition alone (IN) compared to baseline (NN). Studies of inhibition often compare activation in this region to a congruent condition rather than to a neutral baseline; indeed, when compared to the congruent non-shifting condition (CN), activation during inhibition (IN) was greater in all of the foci within the right inferior frontal gyrus. The neutral baseline potentially invokes perceptual inhibition despite the lack of response inhibition; hence, the congruent non-shifting condition may provide a reference by which both perceptual and response inhibition can be ruled out. As indicated in Table 1 and Figure 5, a large number of other regions within the prefrontal cortex, the parietal lobe, and the temporal-parietal junction displayed preferential activation during inhibition according to the weak criteria. Although a few inhibition-related activations were observed in the left hemisphere, inhibitory processing was biased toward right-lateralized regions. No regions displayed a pattern of activation that met the strong criterion for being selective to shifting. However, four regions, three of which were in the left hemisphere, did meet the weak criteria, and were therefore labeled as being preferential to shifting. All of these regions exhibited greater activation in the simultaneous inhibition and shifting (IS) than in the neutral shifting (NS) conditions, but met the other three criteria for being selective to shifting. These regions were in the left (two separate clusters) and right inferior parietal lobe (BA 7, including the precuneus) and in left DLPFC (BA 46).
Facilitation effects
To investigate the effects of facilitation within the ROIs identified as part of the executive control network, results from the congruent non-shifting versus neutral non-shifting (CN > NN and NN > CN) and congruent shifting versus neutral shifting (CS > NS and NS > CS) contrasts were examined (Table 2). Facilitation effects were observed in both directions, with congruent conditions showing greater activation than neutral baselines in some cases, and less activation than neutral baselines in others. Notably, prefrontal regions, including right VLPFC (BA 47), right DLPFC (BA 46), and MFC (right BA 8 and left BA 6), only displayed the pattern of congruent activation being greater than the neutral baseline. Parietal regions, including the precuneus (BA 7) and inferior parietal cortex (BA 40), displayed both patterns of activation. When restricted to ROIs (p = .001), there were no above-threshold voxels for neutral non-shifting greater than congruent non-shifting (NN > CN). These results were observed only when restricting the analyses to the ROIs identified in the inhibition and shifting analyses; when the main effect of facilitation (congruent conditions versus neutral conditions) was examined at the whole-brain level, no voxels survived the FDR-corrected p = .05 threshold.
Table 2.
Facilitation effects
| Anatomical Region | L/R | BA | MNI Coordinates |
T-value | Cluster size (k) |
||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| CN > NN | |||||||
| Precuneus | R | 7 | 26 | −68 | 42 | 9.03 | 108 |
| Precuneus | L | 7 | −24 | −62 | 42 | 7.37 | 86 |
| Inferior Parietal Lobule | L | −32 | −54 | 42 | 6.07 | ||
| Inferior Parietal Lobule | L | 40 | −42 | −48 | 46 | 5.57 | 58 |
| Inferior Parietal Lobule | L | 40 | −48 | −42 | 40 | 3.87 | |
| Precuneus | L | 19 | −30 | −72 | 36 | 5.51 | 33 |
| Middle Frontal Gyrus | R | 46 | 44 | 40 | 24 | 5.23 | 27 |
| Insula/Inferior Frontal Gyrus | R | 13/47 | 34 | 22 | 0 | 4.76 | 9 |
| Middle Frontal Gyrus | L | 6 | −28 | 2 | 58 | 4.29 | 21 |
| CS > NS | |||||||
| Inferior Frontal Gyrus | R | 47 | 54 | 20 | −6 | 6.39 | 38 |
| Medial Frontal Gyrus | R | 8 | 2 | 42 | 48 | 5.87 | 41 |
| Inferior Parietal Lobule | L | 40 | −68 | −36 | 32 | 4.51 | 10 |
| NS > CS | |||||||
| Precuneus | L | 7 | −28 | −50 | 42 | 6.50 | 73 |
| Precuneus | L | 7 | −20 | −58 | 38 | 5.05 | |
| Putamen | L | −20 | −4 | 16 | 6.16 | 40 | |
| Precuneus | L | 19 | −26 | −78 | 32 | 5.24 | 41 |
| Superior Parietal Lobule | R | 7 | 30 | −70 | 46 | 5.20 | 67 |
| Inferior Parietal Lobule | L | 40 | −40 | −42 | 44 | 5.14 | 36 |
Note. Statistics for peak voxels from each significant cluster of activation. Clusters were restricted to the ROIs identified in Table 1 and Figure 5 and were derived from the CN > NN, CS > NS, and NS > CS contrasts with an uncorrected threshold of p = .001, k = 9. No above-threshold voxels were observed for the NN > CN contrast.
CN = Congruent Non-Shifting, CS = Congruent Shifting, NN = Neutral Non-Shifting, NS = Neutral Shifting.
DISCUSSION
The present study was designed to provide a well-controlled direct comparison between inhibition and shifting component processes of executive control, with the aim of determining the extent to which the neural underpinnings of these control processes are shared or selective. We identified criteria sets by which regions of activation could be labeled as selective (exclusively engaged by either inhibition or shifting), preferential (engaged by both inhibition and shifting but significantly more so by either inhibition or shifting), or common to inhibition and shifting (engaged by both processes and not significantly differentially engaged). We found (1) five regions that were exclusively recruited during inhibition and not at all during shifting; (2) additional regions preferentially recruited during inhibition, but also recruited during shifting; (3) a few regions preferentially recruited during shifting, but also recruited to during inhibition; (4) many regions recruited to a similar extent during both inhibition and shifting; and (5) regions that, despite showing increased activation for inhibition and/or shifting, also showed increased activation during facilitation.
Behavioral results and potential impact of task demands
The reaction time data showed an increasing level of difficulty as inhibitory and shifting demands were required and combined. The behavioral shifting effect was somewhat larger than the inhibition effect, but this does not present a confound for the neural results where inhibition-related processing resulted in greater activation in more regions than did shifting-related processing. Behavioral facilitation effects were present only under circumstances when shifting was required.
Comparison of the neural circuitry of inhibitory and shifting control processes depends upon how precisely task and behavioral factors are controlled. Prior studies making this comparison either (1) used different tasks for the two kinds of control processes, making it difficult to know if the observed brain differences reflected differences in control processes per se or instead reflected multiple other differences in task demands or (2) had large performance differences between the two kinds of control processes, making it difficult to know if brain differences reflected differences in control processes per se or instead differences in task difficulty (Barber and Carter, 2005; Brass and von Cramon, 2004; Collette et al., 2005; Derrfuss et al., 2005; Derrfuss et al., 2004; Konishi et al., 1999; Sylvester et al., 2003). In the current study, we used a task paradigm with common features across conditions. From the participants’ perspective, they were performing a single task with one compound rule: identify the letter presented in the selected stimulus dimension (global or local), with the selected dimension being determined by the color of the stimulus. Although shifting costs were larger than inhibition costs behaviorally (RTs), these differences went against the strongest dissociations we found (i.e., the most compelling dissociations involved regions selective or preferential for inhibition). This apparent dissociation between behavioral and activation data suggests that the involvement or recruitment of additional brain regions may, in some cases, support efficient processing as well as representing the engagement of additional cognitive processes.
Neural systems common to inhibition and shifting
One of the most striking results from this comparison of the control processes invoked by inhibition and shifting is the large amount of overlap between the networks involved in these two component processes (as seen in the conjunction of the main effects of shifting and inhibition). These common regions encompassed an extensive and robust network of prefrontal (including DLPFC, VLPFC, and the ACC), parietal, and basal ganglia regions. In other studies of attention using the global-local paradigm, the role of the ACC (BA 32) in conflict monitoring during incongruent trials has been emphasized (Weissman et al., 2003; Weissman et al., 2005; Weissman et al., 2006). The current results suggest that the ACC is equivalently engaged by conflicts that occur during inhibition and shifting processes. Even for those regions labeled as being preferential to inhibition or shifting, some activation related to the other process was observed in the vast majority of regions. Rather than relying on distinct brain regions, it appears that these control processes are primarily subserved by the same network of regions. This substantial overlap places the current results within a larger context of recent reviews, meta-analyses, and theoretical accounts that have implicated a prefrontal-parietal network in process-general accounts of executive control, despite generalizations across task domains. Furthermore, the lack of any above-threshold voxels in the interaction of inhibition and shifting indicates that although these common regions are involved in both inhibition and shifting, they do not exhibit over-additive activation during simultaneous shifting and inhibition. This stands in contrast to the behavioral results, in which an over-additive effect of reaction time was observed. These results may indicate that although the same regions are involved in both inhibition and shifting, the absolute level of activation is less informative than their interaction with regions that are selective to or preferential for each process. That is, the behavioral interaction may be observed in part because the common regions must share information processing with multiple sets of regions when inhibition and shifting are simultaneously required.
Neural systems for inhibition
Applying a set of strong criteria, the results provide robust evidence for five regions that were selectively recruited during inhibitory processing in right middle frontal gyrus (BA 6/8 and 9; two clusters), right superior frontal gyrus (BA 10), right inferior parietal lobule (BA 40), and the left cerebellum. Notably, all the prefrontal and parietal regions that met the strong criterion were right-lateralized (and the left cerebellum through crossing fibers, is connected to the right cortical hemisphere), suggesting a right-hemisphere bias for inhibitory processing (even for the verbal stimuli used in this study). Other inhibitory-preferential regions identified using less stringent criteria included the right inferior frontal gyrus and the striatum. Although several of these regions have been previously implicated as preferential for inhibition (e.g., Sylvester et al., 2003) the present results are able to distinguish between selective and preferential regions free from task and difficulty confounds and also provide a more comprehensive set of inhibition-related regions. It should be noted, however, that some of the regions meeting only the weak criterion for being preferential to inhibition (or to shifting) were identified on the basis of contrasts that were not independent from the classification criterion (Kriegeskorte, et al., 2009); hence, the role of these regions in the preferential process should be considered in the context of prior results and confirmed in independent datasets.
The inhibitory processing required in the current paradigm involved inhibition of competing stimulus dimensions and their associated responses. There may be several possible types of inhibition, each of which may rely on a slightly different set of neural regions (Friedman and Miyake, 2004; Nee et al., 2007). For example, a study investigating inhibition of prepotent responses (Go/No-Go task), inhibition of competing perceptual properties (Flanker task), and inhibition of stimulus-response mappings (stimulus-response compatibility task), found that although all tasks produced activation in several regions (including ACC, caudate, DLPFC, and parietal cortex), each task also activated unique regions in frontal, striatal, and parietal regions (Nee et al., 2007). Such findings suggest that there is a core system responsible for inhibitory processing, but that some regions may interact differentially depending upon the specific inhibitory requirements. To the extent that inhibition involves multiple sub-processes, it may be more likely that inhibition-selective regions will be identified in comparisons with other types of executive processes.
Neural systems for shifting
No brain region was activated exclusively for shifting relative to inhibition, but applying the less stringent criteria, four brain regions were identified as preferential during shifting relative to inhibition. The strongest evidence for a shifting preferential region implicated the left IPL (BA 7), a region repeatedly implicated in studies of task-shifting (Badre and Wagner, 2006; Bode and Haynes, 2009; Forstmann et al., 2006; Leber et al., 2008; Rossi et al., 2009; Slagter et al., 2006; Wager et al., 2004, 2005; Yeung et al., 2006). However, because all of the shifting-preferential regions, including left IPL, were also engaged during inhibition alone (IN) compared to the neutral baseline (NN), these results suggest that shifting may not be a process entirely separable from inhibition. Inhibition may be a lower-level component process that is required to carry out the deactivation of the no-longer-relevant rule set during the higher-level process of shifting (Dreher and Berman, 2002; Robbins, 2007). This possibility has been supported by results from a comparison of response inhibition during a Go/No-Go task and during set shifting in the Wisconsin Card Sorting Task, in which a similar region of activation in the right VLPFC (BA 44/45) was observed during both task domains (Konishi et al., 1999). It is also possible that because participants were shifting between attentional foci, rather than between rule sets, the shifting involved in this task fundamentally differs from that in studies of task switching (Ravizza and Carter, 2008). However, the similarity of the neural regions observed here to those seen in prior studies and the greater shift cost than inhibitory cost indicate that this attentional shifting manipulation required substantial mental effort of the sort also required in shifting between goals. In a study that compared shifting between task goals and shifting between stimulus features, minor differences were found, indicating that although it may not be possible to fully generalize across the two types of shifting, there is substantial overlap in the neural substrates of shifting processes (Ravizza and Carter, 2008). In another study comparing external to internal shifts of attention and shifts between objects and between attributes of the same object (Wager, et al., 2005), substantial overlap between types of shifting was also observed (particularly in the left parietal lobe), although the different types of shifting were preferentially associated with different areas in the prefrontal cortex. These results suggest that shifting may to some extent be a heterogeneous process, requiring more detailed study to determine how various types of shifting may relate to the neural bases of inhibition.
Facilitation
The results from the facilitation conditions in this study provide constraints on theories regarding the role of various regions during inhibitory processing. That is, if neural activation mirrors behavior, as in studies of repetition priming (Henson, 2003), a region thought to be selectively involved in inhibitory processing should not display increased activation during facilitation conditions, as facilitation may be viewed behaviorally as an inverse of inhibitory processes and would be expected to involve reduced activations relative to baseline in control-related regions. However, findings regarding facilitative stimuli have rarely been reported, with some studies finding decreased activation and others reporting increased activation during facilitation (Carter et al., 1995; Cohen Kadosh et al., 2008; Weissman et al., 2005). In the present study, ROIs in the right VLPFC (BA 44, 45, and 47), a region often implicated in inhibitory control (Aron et al., 2004b), displayed greater activation when processing facilitative stimuli under some conditions (specifically, CS > NS). This result is not compatible with an interpretation that right VLPFC is selective to inhibitory control, and instead suggests that VLPFC may be engaged whenever multiple information channels contain potentially relevant information that must be selected for continued processing. In the present study, each stimulus dimension (global and local) represents a potentially relevant information channel. In the neutral conditions (e.g., a large S made of small Os), no relevant “signal” (in the sense of possessing a response mapping) is carried by one channel; while in the facilitation conditions (e.g., a large H made of small Hs), both channels carry a relevant signal, albeit the same signal is carried over both channels. In keeping with this possibility, a homologous region in left VLPFC (BA 44) that has been implicated in selection processes also displayed activation during both inhibitory and facilitative processing (Badre et al., 2005; Thompson-Schill et al., 2005; Thompson-Schill et al., 1997). A number of other ROIs in the prefrontal-parietal network (BA 6, 8, 40) displayed activation during facilitative processing as well as during inhibition, suggesting that selection processes may be subserved by multiple regions within this network. No ROIs displayed a significant pattern of monotonically increasing activation from congruent to incongruent trials (CN < NN < IN or CS < NS < IS), ruling out explanations that activation within these regions simply tracks behavioral reaction times or task difficulty. One limitation of the current results is that the facilitation effects were observed only within ROIs identified by the inhibition and shifting analyses and were not obtained at the whole-brain level.
Only inclusion of a facilitation condition can reveal whether activation is driven by inhibitory demands (in which case facilitation would lead to reduced activation) or by the number of response cues present in a stimulus independent of whether there are inhibitory demands (in which case both facilitation and inhibition would provoke activation). Thus, activation in some prefrontal and parietal areas often associated with inhibition may be better interpreted as indexing to the number of response-salient cues in a stimulus, whether those cues are inhibitory or facilitating, rather than inhibition per se. Similarly, inclusion of a facilitation condition may be informative with regard to shifting processes, because regions selective for shifting should not exhibit activation during facilitative processing unless shifting processes are simultaneously invoked.
In summary, the results suggest that a largely unified network of prefrontal and parietal regions, along with areas within the basal ganglia, subserves executive control processes of inhibition and shifting and are involved whenever potentially relevant information must be selected from multiple information channels. However, these regions may be preferentially invoked by inhibition or shifting processes, and a few regions may be invoked selectively by inhibitory processing. It remains to be seen how these preferential regions fit within proposed schemes of hierarchical control processes (Banich, 2009; Koechlin et al., 2003) and whether these regions show their component-specific processing in isolation or in conjunction with other regions, in other task domains, or in comparisons with other component processes. Additional research into the activation patterns associated with facilitation may further illuminate the extent to which specific regions are selective to inhibitory processing or are reactive to selection among multiple response cues, thereby providing additional constraints on theories involving component processes of executive control.
Acknowledgements
The authors thank Adam Bernstein for assistance during data collection. Supported by MH061426 from the NIMH; Trey Hedden was supported by AG021847 from the NIA. Trey Hedden is now at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and the Radiology Department, Harvard Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004a;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004b;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci U S A. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directiions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cereb Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Bode S, Haynes JD. Decoding sequential stages of task preparation in the human brain. Neuroimage. 2009;45:606–613. doi: 10.1016/j.neuroimage.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Selection for cognitive control: a functional magnetic resonance imaging study on the selection of task-relevant information. J Neurosci. 2004;24:8847–8852. doi: 10.1523/JNEUROSCI.2513-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Henik A, Linden DE. Processing conflicting information: facilitation, interference, and functional connectivity. Neuropsychologia. 2008;46:2872–2879. doi: 10.1016/j.neuropsychologia.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D’Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Cools R, Rogers R, Barker RA, Robbins TW. Top-Down Attentional Control in Parkinson’s Disease: Salient Considerations. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Jonides J, Smith EE. The neural substrate and temporal dynamics of interference effects in working memory as revealed by event-related functional MRI. Proc Natl Acad Sci U S A. 1999;96:7514–7519. doi: 10.1073/pnas.96.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF. Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci U S A. 2002;99:14595–14600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Brass M, Koch I, von Cramon DY. Voluntary selection of task sets revealed by functional magnetic resonance imaging. J Cogn Neurosci. 2006;18:388–398. doi: 10.1162/089892906775990589. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WP, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. J Cogn Neurosci. 2008;20:1854–1865. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latent-variable analysis. J Exp Psychol Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hedden T, Yoon C. Individual differences in executive processing predict susceptibility to interference in verbal working memory. Neuropsychology. 2006;20:511–528. doi: 10.1037/0894-4105.20.5.511. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double-dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber AB, Turk-Browne NB, Chun MM. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008;105:13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the stroop task. Brain Res Cogn Brain Res. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130:233–244. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon D. Forest before trees: The precedence of global features in visual perception. Cognit Psychol. 1977;9:353–383. [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Persson J, Sylvester CY, Jonides J. Mapping interference resolution across task domains: a shared control process in left inferior frontal gyrus. Brain Res. 2009;1256:92–100. doi: 10.1016/j.brainres.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Rossi A, Japee S, Desimone R, Ungerleider LG. Attentional control during the transient updating of cue information. Brain Res. 2009;1247:149–158. doi: 10.1016/j.brainres.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Carter CS. Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia. 2008;46:2924–2935. doi: 10.1016/j.neuropsychologia.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG. Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn Affect Behav Neurosci. 2006;6:175–189. doi: 10.3758/cabn.6.3.175. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE, Nichols TE. Toward a taxonomy of attention shifting: Individual differences in fMRI during multiple shift types. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:127–143. doi: 10.3758/cabn.5.2.127. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19:1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-task competition and cognitive control in task switching. J Neurosci. 2006;26:1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]