Abstract
Emotional events often lead to particularly strong memory formation. Corticosterone, the final product of HPA-axis activation, has been suggested to play a critical role in this effect. Although a great deal of work has implicated the amygdala as a necessary structure for the effects of corticosterone, other studies have suggested a critical role for the hippocampus in determining the involvement of corticosterone. The current experiments examined this question by disrupting corticosterone synthesis with administration of metyrapone (25 or 100 mg/kg) prior to training in either dorsal hippocampus-independent delay fear conditioning or dorsal hippocampus-dependent trace fear conditioning. Metyrapone administration 2 hrs prior to training significantly attenuated corticosterone secretion during training, but these effects were transient as corticosterone levels were similar to control subjects following the test session. As hypothesized, only trace fear conditioning was impaired. This suggests that only fear conditioning tasks that are dependent on the dorsal hippocampus require HPA-axis activation in order to be learned.
Keywords: fear-potentiated startle, glucocorticoid, memory formation, hippocampus, classical conditioning
The Effects of Metyrapone on Trace and Delay Conditioned Fear-Potentiated Startle in Rats
It has long been understood that emotional and traumatic events lead to the formation of particularly strong memories (e.g., Brown & Kulik, 1977). Although the salient nature of the event certainly has an impact, recent attention has come to focus on the role that stress plays in this facilitation (de Kloet, Oitzl, & Joels, 1998; Gewirtz & Radke, in press). It is now clear that exposure to stressful stimuli induces activation of a host of neurological and endocrine responses including the neuroendocrine pathway termed the hypothalamus-pituitary-adrenal gland (HPA) axis (Wolf, 2003). The output of this pathway includes the release of corticotrophin releasing factor (CRF) in the brain and corticosterone/cortisol (CORT) into the bloodstream, both of which can influence memory. A great deal of work now suggests that the amygdala is a critical site for the interaction between CORT and memory (Roozendaal, 2000). Most of this work has been conducted using inhibitory avoidance tasks, indexed as an increase in latency to make a previously punished response. However, the current paper will demonstrate that in the domain of Pavlovian fear conditioning, corticosterone is required for memory formation only when learning depends on the dorsal portion of the hippocampus, as well as the amygdala.
Several studies have shown that inhibitory (passive) avoidance is impaired by a blockade of CORT and enhanced by administration of an exogenous CORT agonist (Cordero, Kruyt, & Merino, 2002; Roozendaal, Bohus & McGaugh, 1996; Roozendaal, Okuda, Van der Zee, & McGaugh, 2006). Studies using drugs targeting specific receptors have suggested that glucocorticoid receptor type 2 (GR) receptors are primarily responsible for this effect. Moreover, blocking or enhancing GR activation directly in the amygdala mimics the effects of systemic administration of drugs (impairing and enhancing memory respectively) and blocking norepinepherine receptors in the amygdala reverses the memory enhancing effects of GR activation by systemic infusions (e.g., Roozendaal, 2000).
A putative role for amygdalar GRs in mediating the effects of CORT in aversive memory formation is complicated by findings that blocking GR activation does not impair Pavlovian “delay” fear conditioning, in which presentation of an auditory conditioned stimulus (CS) cue overlaps and coterminates with footshock unconditioned stimulus (US) (Pugh, Fleshner, & Rudy 1997), despite evidence that corticosterone agonists can enhance Pavlovian fear conditioning (Hui et al., 2004; Zorawski & Killcross, 2002). As delay fear conditioning is a paradigmatic, amygdala-dependent aversive memory task (Kim & Davis, 1993), this suggests that GR antagonism may not be equally deleterious to all forms of fear conditioning.
In accounting for this apparent disparity, it is worth noting that Pavlovian delay conditioning and inhibitory avoidance learning differ in two important respects. First, although both tasks require the amygdala, delay fear conditioning, as typically conducted, does not require an intact dorsal hippocampus. As GRs are found in high concentrations in both the hippocampus and amygdala, either structure could be a locus of CORT's influence on memory (Reul & de Kloet, 1985). Second, whereas in delay fear conditioning a single, discrete cue becomes associated with the aversive event, inhibitory avoidance learning likely involves a variety of cues associated with the apparatus and the animal's responses. In contrast to delay conditioning, however, Pavlovian contextual fear conditioning by definition involves associations between apparatus cues and the aversive event. Moreover, it is frequently disrupted by lesions of the dorsal hippocampus (Kim & Fanselow, 1992; Phillips & LeDoux, 1992) and by GR antagonism (Pugh et al., 1997). Thus, the nature of the associations formed and/or the neural substrates necessary to form them appear to be critical determinants of the requirement for CORT in the plasticity underlying the acquisition of fearful memories.
The current experiments further investigated this possibility by examining the effects of CORT synthesis disruption on two Pavlovian tasks: trace and delay fear conditioning. Both of these tasks involve the association between an explicit auditory cue and an aversive footshock. They differ only in whether the stimuli overlap (delay conditioning) or are separated in time (trace conditioning). Moreover, trace fear conditioning requires activation of both the dorsal hippocampus and amygdala whereas delay conditioning requires only the amygdala (Burman, Starr & Gewirtz, 2006; Czerniawski, Yoon & Otto, 2009; McEchron, Tseng & Disterhoft, 2000; Quinn, Oommen, Morrison & Fanselow, 2002). Thus if trace, but not delay, conditioning is impaired by corticosterone blockade it would suggest that a surge in corticosterone during training is not a requirement for spatial or contextual tasks alone, but for fear learning that is critically dependent on the dorsal hippocampus more generally.
Methods
Subjects
Eighty-five male albino Charles River Derived Sprague-Dawley rats, weighing between 250 and 400 g at the start of the experiment, were used. Animals were group-housed and maintained on a 12-h light/dark cycle (lights on at 0800), with food and water continuously available. Animals were allowed to acclimate to the experimental housing for at least two weeks after arrival in the colony. Behavioral testing took place at 1200. All experimental procedures conformed to the Guidelines for the Humane Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee at the University of Minnesota.
Apparatus and Stimuli
Animals were trained and tested in four identical 7.5 × 9 × 17-cm stabilimeter devices. Each stabilimeter consisted of a Plexiglas cage, which rested on four compression springs and was located within a ventilated sound-attenuating chamber. Cage movement resulted in displacement of a Type 338B35 accelerometer (PCB Piezotronics, Depew, NY) attached to the top of each cage. The resultant voltage of the accelerometer was proportional to the velocity of the cage displacement. This signal was amplified by a signal-processing unit (# 482820 PCB Piezotronics). An InstruNet 100b board (GW Instruments, Somerville, MA) interfaced to a Macintosh G3 microcomputer digitized the voltage output of the accelerometer on a scale of 0 – 100 units. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 200 ms after onset of the startle stimulus. High frequency speakers (Radio Shack Supertweeters, range 5-40 kHz, #40-1310b) located 5 cm behind each cage delivered the startle stimuli. The startle stimuli were 50-ms (rise-decay: 5 ms) bursts of white noise (low pass, 22 kHz) at various intensities. The ventilation fans of the sound-attenuating chamber elevated background noise to 60-65 dB. The foot shock was a 0.5-s, 0.8-mA constant current scrambled shock, delivered by a shock generator (#SGS-004, by BRS-LVE, Bellsville, ME) through the four bars that made up the bottom of the stabilimeter. Shock intensity was measured with a 1-kΩ resistor across a differential channel of an oscilloscope in series with a 100-kΩ resistor connected between two floor bars in each cage. Current was defined as the RMS voltage across the resistor where mA = 0.707 × 0.5 × peak-to-peak voltage. The CS was a 4-s (trace conditioning) or a 7.5-s (delay conditioning), band pass-filtered noise, raised to a sound pressure level 5dB above background noise (65-70 dB), with high and low cut-offs set at 4 kHz and 24 dB per octave attenuation. The noise was generated by the computer and delivered through a low frequency speaker (Radio Shack woofer, Model # 40-1024A) situated 15 cm from the cage. Previous research demonstrated no unconditioned effects of the CS on the startle response (Burman & Gewirtz, 2004).
Drug Delivery
For five days prior to the beginning of training, including startle habituation sessions (see below) animals were injected with 1 ml of physiological saline per kg body weight. Metyrapone (Sigma-Aldrich or Bio-Mol Research Labs), an 11β-hyrdroxylase synthesis inhibitor was used to disrupt CORT synthesis. The drug was dissolved in propylene glycol and then diluted in physiological saline to 40% propylene glycol at a concentration of 25 mg/ml (for both the trace and delay conditioning experiments; see below) or 100 mg/ml (delay conditioning only). Although metyrapone can have some nonspecific effects on other neuroactive compounds (Rupprecht et al., 1998), this drug was chosen specifically because it reduces, without completely blocking, CORT secretion. Thus, it is likely to reduce primarily stress-related, and not basal, levels of CORT. A 40% propylene glycol-saline solution was used as a vehicle control. All injections were administered 1.5 hrs prior to each conditioning session.
Startle Habituation Sessions
In order to acclimate the rats to the apparatus, procedure and startle stimuli, and to measure levels of baseline startle, the naïve rats underwent two days of startle testing prior to training. Rats were injected with 1 ml/kg physiological saline 1.5 hrs prior to placement in the chamber. Upon placement into the experimental apparatus, rats were given a five-minute acclimation period, after which they were presented with 40 startle stimuli, 20 each at 95 or 105 dB intermixed pseudo-randomly with a 30-s interstimulus interval.
Fear Conditioning Procedure
After baseline startle sessions, acquisition was conducted over three successive days. On each day, rats were presented with 12 tone-shock pairings after a two-minute acclimation period. The interval between shocks was variable with a mean of 2 min (range: 1.5 - 2.5 min). For delay conditioning, the 0.5-s shock overlapped and co-terminated with the 7.5-s CS whereas for trace conditioning, the onset of the shock occurred 3 s after offset of the 4-s CS, procedures that we have previously shown produce robust leaning and differ in their sensitivity to hippocampus manipulations (Burman et al., 2006; Burman & Gewirtz, 2007).
Testing Procedure
The test session occurred 1 day after completion of acquisition. Testing consisted of a two-minute acclimation period, followed by 10 startle stimuli (5 at each of 95 and 105 dB), delivered in an intermixed sequence to habituate the rodents to the stimuli. These startle habituation trials were followed immediately by presentation of the test trials. The startle stimuli were presented either alone (startle-alone trials), 3.5 s after CS onset, or 7 s after CS onset. There were five of each trial type, presented in a pseudorandom order. The interval between successive startle stimuli was 30 s throughout the session.
Blood Sampling
All blood sampling took place in experiments involving delay conditioning. For the study involving repeated blood sampling, blood samples were obtained by tail vein venipuncture. Prior to blood collection, rats were habituated to the sampling procedure. Rats were moved to the room used for blood sampling and restrained for approximately 30 s on each of 3 separate days before the beginning of the blood sampling. Blood samples were collected immediately following each training session during the experiment. Blood collection lasted for no more than one minute per day, and blood samples were collected 24 hrs apart. Because of the potential for stressful effects of the blood-draw procedure to alter learning, behavioral data from these rats were not analyzed.
For the experiment in which blood was sampled at the end of the behavioral study, blood was obtained through rapid decapitation. This procedure occurred immediately following the testing session, and all decapitations occurred within 5 min of the end of the testing session. In all instances, blood samples were combined with 15% w/v EDTA and centrifuged to obtain plasma. Samples were aliquoted and frozen at −80 °C until analysis.
Corticosterone Assay
Concentrations of corticosterone in plasma samples were measured in duplicate using a high-sensitivity corticosterone enzyme-immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ). Briefly, samples were diluted 1:40 in calibrator diluent. Samples and standards were then diluted with a buffer solution, heated to 80 °C for 30 min and cooled to room temperature. Diluted samples and the enzyme conjugated to horseradish peroxidase were added to each well. Following incubation, the plates were washed and substrate solution was added to the wells. Lastly, a stop solution of hydrochloric acid was added, and the absorbance was read at 450 nm using a standard plate reader (Multiskan MCC, Thermo, Shanghei, China). Serially diluted standards were used to generate a curve from which sample values were interpolated. These values were subsequently converted and expressed as ng/ml plasma. Assay sensitivity was 0.17 ng/ml.
Grouping and Statistics
The studies examining the effects of metyrapone on delay and trace conditioning were conducted and analyzed as separate experiments. Startle amplitudes on the second habituation day were averaged across all startle stimuli and were used to match the animals into drug or vehicle groups with similar overall mean startle amplitude (Davis et al., 1989; Falls et al., 1992). Statistics were performed using Statistica 8 (Statsoft, Tulsa OK). Percent fear-potentiated startle was calculated by comparing mean startle obtained on each CS trial type to mean startle obtained on startle-alone trials [i.e., (CS-startle – startle alone)/startle alone × 100%]. These data were analyzed using a two-way mixed design (drug × startle probe time) ANOVAs, followed by Newman-Keuls post-hoc tests where appropriate. Animals were excluded from analysis for failure to demonstrate the startle reflex to the startle stimulus (3 rats) or as statistical outliers if they demonstrated conditioning more than 2.5 standard deviations from the mean of their group (3 rats from the delay conditioning experiment, 1 from each dosing condition).
Results
The Effects of Metyrapone on Trace Fear Conditioning (Figure 1A)
Figure 1.
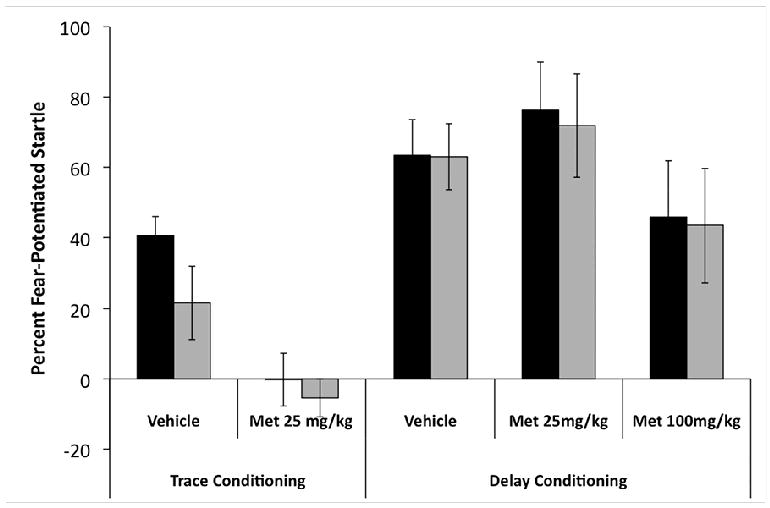
Effects of metyrapone injections made 1.5 hrs prior to each training session of either trace or delay fear conditioning. Data represent the percent increase in the startle response either 3.5 s (black bar) or 7.0 s (grey bar) after CS onset during the drug-free test session 24 hours after the final training session. Note that the 7.0 s probe was after CS offset in the trace conditioning group. A repeated measures ANOVA demonstrated that only trace conditioning was significantly disrupted by metyrapone.
There were 10 metyrapone- and 9 saline-injected rats in this experiment. The data were analyzed with a 2 × 2 repeated measures ANOVA, with drug injection as the between subjects measure and startle probe time as the within subjects measure. Metyrapone administered at a dose of 25 mg/kg blocked fear conditioning when the CS and US were separated by a 3-s trace interval. This was supported by a significant main effect of drug F(1, 17) = 13.55, p < .01. As previously demonstrated (Burman & Gewitz, 2004), there was a significant effect of probe time F(1, 17) = 5.85, p < .05, demonstrating that fear was stronger during the CS than after CS offset. There was no interaction between the factors, p > .10, suggesting that metyrapone administration did not affect the timing of fear expression.
Neuman-Keuls post-hoc tests confirmed that fear-potentiated startle was significantly impaired in the metyrapone-injected animals compared to the saline-injected animals at each probe time ps < .01. Furthermore, in the saline-injected animals, fear after CS offset was significantly lower than during the CS, p < .05. This was not the case for metyrapone-injected animals, in which fear was not significantly different at the two probes ps > .05.
Baseline startle levels did not differ between the metyrapone- and saline-injected animals t(17) = 0.85, p > .10, demonstrating that metyrapone administration during training had no effect on sensory/motor processing at test.
The Effects of Metyrapone on Delay Fear Conditioning (Figure 1B)
There were 16 vehicle, 16 low dose (25 mg/kg) and 11 high dose (100 mg/kg) rats. The data were analyzed with a 3 × 2 repeated measures ANOVA, with drug dose as the between-subjects measure and startle probe time as the within-subjects measure. The data show that neither dose of metyrapone (25 mg/kg or 100 mg/kg) blocked fear conditioning when the CS and US overlap and coterminate. This was confirmed statistically by the lack of a significant main effect of drug F(2, 40) = 1.37, p > .10. As expected, there was no effect of probe time F(1, 40) = 0.18, p > .10. There was no interaction between the factors p > .10.
It is important to note that baseline startle levels did not differ among the groups F(2, 41) = 1.26, p > .10, suggesting that metyrapone injections during training did not impair sensory and motor function on testing day. Furthermore, the intact conditioning in the metyrapone-injected animals confirms that the drug did not impair sensory processing on conditioning days.
Effects of Metryapone Injections Prior to Training on CORT Levels Immediately Following Training (Figure 2A)
Figure 2.
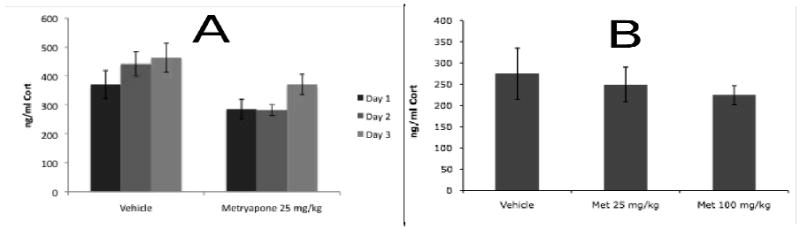
Effects of metyrapone injections made 1.5 hrs prior to each day of training on plasma CORT levels immediately following training (panel A) or drug-free testing (panel B). As expected, ANOVAs demonstrated that plasma CORT levels were significantly reduced by metyrapone in samples taken immediately after each training session (panel A), which was approximately 2.5 hours after drug administration. CORT levels were equivalent in samples taken after the test session (panel B), 24 hours after the last metyrapone administration.
Of the 9 vehicle and 8 metyrapone animals that began this experiment, sufficient blood samples to be included in the final dataset were obtained from only 4 vehicle and 3 metyapone animals due to difficulties in collecting sufficient blood from each animal over multiple days. Nevertheless, a repeated measures ANOVA revealed a significant effect of drug treatment on CORT levels in samples taken immediately after each day of training F(1, 5) = 6.65, p < .05, demonstrating that metyrapone treatment significantly reduced CORT synthesis. There was a trend towards an effect of day F(2, 10) =2.97, p < .10. The interaction between treatment and day was not significant F(2, 10) = 0.61, p > .10.
Effects of Metryapone Injections Prior to Training on CORT Levels after Test (Figure 2B)
Eight vehicle, 8 low-dose and 8 high-dose animals were assessed for plasma CORT levels following the test session. Metyrapone was not administered that day. As expected, there was no effect of previous metyrapone dosing on CORT levels in samples taken immediately after the test session F(2, 21) = 0.42, p > .10. This demonstrates that the effects of metyrapone on conditioning could not be accounted for by reduced expression of conditioning due to lingering effects of the drug during the test session. The behavioral data from these animals are reported above.
Discussion
These data clearly demonstrate that trace conditioning is susceptible to disruption of CORT synthesis, whereas delay conditioning is not disrupted even by injection of a substantially higher dose of the CORT inhibitor metyrapone. Moreover, lasting effects of meytrapone cannot account for these data. Although the drug caused a significant disruption of CORT during training, by the end of the test session, CORT levels were equivalent across groups.
Delay and trace fear conditioning both involve the formation of an association between an auditory conditioned stimulus (CS) and an aversive footshock unconditioned stimulus (US). The only difference between the paradigms is whether the CS terminates prior to the occurrence of the US (as in trace conditioning) or remains present and coterminates with the US (as in delay conditioning). Interestingly, this subtle shift in temporal paramaters alters the neural substrates required in both humans and rodents. Although both forms of conditioning depend upon an intact amygdala, only trace fear conditioning also depends upon the dorsal hippocampus (Burman et al., 2006; Czerniawski et al., 2009; McEchron et al., 2000; Quinn et al., 2002).
Our observation that only hippocampus-dependent trace fear conditioning was impaired by even a modest suppression of circulating corticosterone is consistent with previous findings suggesting that hippocampus-dependent tasks are particularly sensitive to CORT disruption. For example, contextual fear conditioning was impaired by systemic administration of a specific GR-antagonist, whereas specific cue conditioning (using a delay-conditioning protocol) was spared (Pugh et al., 1997). Moreover, previous work has shown that both trace eyeblink conditioning and spatial learning performance is positively correlated with corticosterone levels, at least in male rats (Luine, Martinez, Villegas, Magarinos & McEwen, 1996; Wood, Beylin & Shors, 2001). Further evidence suggesting that hippocampus-dependent learning and memory tasks are sensitive to corticosterone disruption comes from other studies using metyrapone, specific GR antagonists, or adrenalectomy prior to spatial learning and inhibitory avoidance tasks (Oitzl & de Kloet, 1992; Yao, Noble, & Secki, 1999; Roozendaal, 2000).
Despite the clear susceptibility of hippocampus-dependent memory tasks to corticosterone manipulations, CORT may not be acting directly on the hippocampus. First, although direct infusions of a GR antagonist into the basolateral amygdala or the ventral hippocampus blocked contextual fear conditioning, infusions into the dorsal hippocampus had no effect (Donley, Schulkin & Rosen, 2005), suggesting that contextual fear conditioning may have been disrupted via effects in other structures. In addition, although there is some evidence from direct infusions that GRs in the hippocampus can mediate memory formation and storage, the amygdala has also been heavily implicated in the effects of CORT blockade in inhibitory avoidance (Roozendaal, 2000; Roozendaal et al., 2006). Indeed, lesions of the amygdala completely block the memory-enhancing effects of GR agonism on inhibitory avoidance, including agonism of GR receptors via direct intracerebral infusion into the hippocampus. However the current experiments, consistent with previous studies, demonstrate that dorsal hippocampus-independent delay fear conditioning remains intact following CORT disruption. Interestingly, the three fear conditioning tasks shown to depend upon CORT activity: trace fear conditioning (current findings), contextual fear conditioning (Pugh et al., 1997) and inhibitory avoidance (Cordero et al., 2003; Roozendaal, 2000), all depend upon both an intact hippocampus and amygdala. Although there are cortical regions, such as entorhinal and prefrontal cortex, that are involved in these hippocampus-dependent tasks but are not required for delay conditioning, the relative lack of GRs in these regions makes them unlikely targets for CORT's involvement (Reul & de Kloet, 1985). Thus, it appears that a requirement for CORT receptors in fearful memory tasks is limited to tasks that depend on interactions between the hippocampus and amygdala and may primarily occur at the level of amygdalar GRs. This suggests that GR receptor activation may be involved in mediating plasticity in hippocampus-amygdala circuits.
Previous research has demonstrated that GR blockade disrupts the acquisition and consolidation, but not retrieval, of contextual fear conditioning (Cordero & Sandi, 1998; Pugh et al., 1997). Similarly, metyrapone injections prior to training on the water maze disrupt acquisition and both intra-hippocampal and intra-amygdalar GR blockade also disrupt consolidation (de Kloet et al. 1999, Roozendaal, 2000). Our data demonstrate that disrupting CORT synthesis disrupts the acquisition of trace conditioning; however, we did not investigate memory consolidation or retrieval. Future studies comparing the effects of GR receptor antagonism on acquisition, consolidation and retrieval of trace and delay fear conditioning would be necessary to determine whether hippocampus GR receptors are similarly involved in consolidation of trace fear conditioning.
The current studies did not directly control for a possible decrement of memory due to state-dependent learning effects. However, this alternative explanation of the data would require that trace conditioning be particularly susceptible to state-dependent effects compared to delay conditioning, and there is no evidence to support this assumption. In fact, one study examining the effects of endocannabiniod antagonism suggested that delay conditioning was more susceptible to state-dependent learning effects (Reich, Mohammadi, & Alger, 2008). Moreover, the effects of CORT manipulation on contextual fear conditioning and inhibitory avoidance cannot be attributed to state-dependent learning, making it less likely to account for our data.
Overall, the finding that trace, but not delay, fear conditioning is disrupted by metyrapone administration supports the suggestion that hippocampus-dependent memory tasks are particularly sensitive to corticosterone disruption and suggest that this may occur via amygdala-hippocampus interactions.
Acknowledgments
The authors thank Gena Mitchell for technical assistance. Author MB was supported by NIH training grant T32 HD007151 to the Center for Cognitive Science at the University of Minnesota. Author KH was supported by T32 DA 07097. Research funding was provided by a Grant-In-Aid from the University of Minnesota.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Contributor Information
Michael A. Burman, Department of Psychology, University of Minnesota; Center for Excellence in the Neurosciences and Department of Psychology, University of New England
Kathryn L. Hamilton, Department of Psychology, University of Minnesota; Department of Psychology, University of Wisconsin-Stout
Jonathan C. Gewirtz, Department of Psychology, Program in Neuroscience, University of Minnesota
References
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5:73–99. [Google Scholar]
- Burman MA, Gewirtz JC. Timing of fear expression in trace and delay conditioning measured by fear-potentiated startle in rats. Learning & Memory. 2004;11:205–212. doi: 10.1101/lm.66004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–113. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Kruyt ND, Merino JJ, Sandi C. Glucocorticoid involvement in memory formation in a rat model for traumatic memory. Stress. 2002;5:73–79. doi: 10.1080/1025389029000124404. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Research. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- de Kloet E, Oitzl M, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behavioural brain research. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM, et al. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning and Memory. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behavioral Neuroscience. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):0–675. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Restraint stress reversibly enhances spatial memory performance. Physiology & Behavior. 1996;59:27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Fleshner M, Rudy JW. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiology of Learning and Memory. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. Journal of Psychopharmacology. 2008;22:769–777. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Strohle A, Hermann B, di Michele F, Spalletta G, Pasini A, Holsboer F, Romeo E. Neuroactive steroid concentrations following metyrapone administration in depressed patients and healthy volunteers. Biological Psychiatry. 1998;44:912–914. doi: 10.1016/s0006-3223(97)00521-0. [DOI] [PubMed] [Google Scholar]
- Wolf OT. HPA axis and memory. Best Practice & Research. Clinical Endocrinology & Metabolism. 2003;17:287–299. doi: 10.1016/s1521-690x(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. Continuous blockade of brain mineralocorticoid receptors impairs spatial learning in rats. Neuroscience Letters. 1999;277:45–48. doi: 10.1016/s0304-3940(99)00858-7. [DOI] [PubMed] [Google Scholar]
- Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiology of Learning and Memory. 2002;78:458–464. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]


