Abstract
Background
While physically active people have a lower risk of developing colorectal cancer, few studies have examined whether exercise benefits colorectal cancer survivors.
Methods
We studied colorectal cancer-specific and overall mortality in a cohort of 668 men with a history of stage I–III colorectal cancer derived from the Health Professional Follow-up Study according to predefined physical activity categories after diagnosis. To minimize bias by occult recurrences, we excluded men who died within six months of their post-diagnosis physical activity assessment.
Results
In a cohort of men with colorectal cancer and no apparent metastases at diagnosis, 50% exercised at least 18 metabolic equivalent tasks (MET)-hours per week. Increasing amount of physical activity was significantly associated with improved colorectal-cancer specific mortality (p = 0.002) and overall mortality (p = 0.0003). Men who engaged in 27 MET-hours/week of physical activity had an adjusted hazard ratio for colorectal-cancer specific mortality of 0.47 (95% CI, 0.24–0.92), compared to those who did less than 3 MET-hours/week. The apparent benefit of physical activity was seen regardless of age, stage, body mass index, year of diagnosis, location of tumor and pre-diagnosis physical activity (p interaction all non-significant).
Conclusion
In a large cohort of men with history of non-metastatic colorectal cancer, greater physical activity was associated with lower risk of colorectal cancer-specific and overall mortality.
Introduction
Despite appreciable advances in screening and treatment of colorectal cancer, over 148,000 individuals in the United States will be newly diagnosed with colorectal cancer and nearly 50,000 will die of the disease each year.1 A host of modifiable factors have been associated with influencing the likelihood of developing the disease.2 A recent meta-analysis of 52 observational studies found an inverse association between physical activity and colon cancer development, with an overall relative risk of 0.76 (95% confidence interval, 0.72–0.81).3
While there is little debate on the benefit of physical activity in preventing the development of colorectal cancer, the role of physical activity in colorectal cancer survivors is less certain. Recently, several studies suggest that pre-diagnosis physical activity4 or post-diagnosis activity 5, 6 reduces the risk of cancer recurrence and improves mortality in colorectal cancer survivors. However, since randomized data are not available, further studies with prospectively collected data, comprehensive follow-up and post-diagnosis physical activity levels are needed.
We utilized the Health Professional Follow-Up Study, a large prospective cohort of over 50,000 men to test the association between physical activity and colorectal cancer mortality in patients who have been diagnosed with the disease.
Methods
Study population
In 1986, the Health Professional Follow-up Study (HPFS) cohort was established when 51,500 male health professionals answered a mailed questionnaire on risk factors for cancer and cardiovascular disease (details previously reported7) Every two years, participants receive follow-up questionnaires to update information on potential risk factors and report new cancer and disease diagnoses. This study was approved by the human subjects committee at Harvard School of Public Health, Boston, MA.
Ascertainment of colorectal cancer diagnosis
On each biennial follow-up questionnaire, participants were asked whether they had had a diagnosis of colorectal cancer during the prior two years. When a subject (or next of kin for decedents) reported colorectal cancer, we sought permission to obtain records and pathology reports. Study physicians, blinded to exposure data, reviewed all records related to colorectal cancer and recorded stage, histology, and tumor location. For non-responders, we searched the National Death Index to discover deaths and ascertain any diagnosis of colorectal cancer that contributed to death or was a secondary diagnosis. We estimate that we identified 96% of the cases of colorectal cancer through these various methods.8 The subjects in this analysis were HPFS participants with nonmetastatic colorectal cancer diagnosed between 1986 and 2004.
Men were excluded if they presented with metastatic colorectal cancer at time of initial diagnosis. In total, 1,041 men were diagnosed with colorectal cancer in HPFS between 1986 and 2004; however, 182 had metastatic disease and were excluded. Of the 859 remaining men, 680 reported physical activity levels between 6 months and 4 years after diagnosis (see “Exposure assessment” below). To minimize bias by occult recurrence or other undiagnosed major illnesses, we excluded 12 men who died within 6 months of their post-diagnosis physical activity assessment. With these parameters, 668 men were eligible for analysis.
Measurement of mortality
Men were followed until death or January 2006, whichever came first. Ascertainment of deaths included reporting by the family or postal authorities. In addition, the names of persistent nonresponders were searched in the National Death Index.9 The cause of death was assigned by physicians blinded to exposures. In the case of a man who died from colorectal cancer not previously reported, we obtained medical records of the colorectal cancer diagnosis after permission from next of kin. More than 98% of deaths in the HPFS have been identified by these methods.9
Exposure assessment
Leisure-time physical activity was assessed on each biennial questionnaire as previously described.10 Briefly, subjects reported duration of participation (ranging from 0 to 11 or more hours per week) in walking (along with usual pace); jogging; running; bicycling; swimming laps; racket sports; other aerobic exercises; lower intensity exercise (yoga, toning, stretching); or other vigorous activities. The first physical activity assessment collected at least 6 months but no more than 4 years after diagnosis (median 15 months) was used to avoid assessment during the period of active treatment. To avoid bias due to declining activity in a period around recurrence or death, physical activity was not updated (thereby a single post-diagnosis measurement was determined).
Each activity on the questionnaire was assigned a metabolic equivalent task (MET) score.11 One MET is the energy expenditure for sitting quietly. MET scores are defined as the ratio of the metabolic rate associated with specific activities divided by the resting metabolic rate. The values from the individual activities were summed for a total MET-hours per week score. Categories of MET-hours per week were predefined as 3 or less, 3.1 to 9, 9.1 to 18, 18.1 to 27 or greater than 27, to correspond to the equivalent of less than 1, 1 to less than 3, 3 to less than 6, 6 or more hours per week of walking at an average pace, consistent with prior analyses.5,6 Our physical activity questions have been previously validated against activity diaries.12, 13
Covariates
Stage of disease, grade of tumor differentiation, year of diagnosis and location of tumor were extracted from the medical record. The time interval between cancer diagnosis and assessment of activity was also adjusted for in these analyses. Body mass index (BMI) and smoking status (current, past, or never) were also taken from the biennial questionnaire at the time of the physical activity assessment.
Statistical analyses
Cox proportional hazards models were used to calculate hazard ratios of death or death from colorectal cancer, adjusted for other risk factors for cancer survival. In the main analysis, death from colorectal cancer was the primary endpoint and deaths from other causes were censored. In secondary analyses, death from any cause was the endpoint. Participants were followed from the date of return of post-diagnosis physical activity assessment to either death or January 2006, whichever came first. The 2-tailed P value for the linear trend test across categories was calculated by using the median value of each category as a continuous variable. Tests of interactions between physical activity categories and potential effect modifiers were assessed by entering in the model the cross product of the dichotomized division of physical activity (≤ or > 27 MET-hours/week) with the dichotomized covariate. All analyses utilized SAS version 9.1 (SAS Institute Inc, Cary, NC).
Results
Baseline characteristics by physical activity category
Among the 661 eligible participants with stages I, II or III colorectal cancer, there were 258 deaths, of which 88 were classified as colorectal cancer-specific deaths. The median time of follow-up from date of diagnosis of men who are alive was 8.6 years (with 75% followed for 5 or more years). Baseline characteristics of the participants are shown according to categories of physical activity after diagnosis (Table 1). Men who were more active were less likely to have ever smoked cigarettes. There were no appreciable differences in body mass index, change in body mass index before and after diagnosis, site of primary tumor and grade of differentiation across physical activities categories.
Table 1.
Baseline Characteristics by Level of Exercise after Diagnosis
| Post-Diagnosis Activity* |
|||||
|---|---|---|---|---|---|
| ≤ 3 | 3.1 – 9 | 9.1 – 18 | 18.1 – 27 | > 27 | |
| N | 102 | 125 | 101 | 81 | 252 |
| Median MET-hours/week | 0.4 | 6.3 | 13.5 | 23.3 | 49.1 |
| Median age (years) | 72 | 69 | 69 | 68 | 69 |
| Mean body mass index (kg/m2) | 25.9 | 26.2 | 25.6 | 24.7 | 25.6 |
| Change in body mass index | −0.3 | −0.1 | −0.2 | 0 | 0 |
| Stage of disease at diagnosis (%) | |||||
| I/II | 58 | 54 | 62 | 57 | 62 |
| III | 21 | 26 | 24 | 23 | 16 |
| Missing (not metastatic) | 21 | 20 | 14 | 20 | 22 |
| Site of disease (%) | |||||
| Colon | 77 | 79 | 77 | 81 | 76 |
| Rectal | 23 | 21 | 23 | 19 | 24 |
| Grade of differentiation (%) | |||||
| Well/moderate | 61 | 52 | 69 | 64 | 60 |
| Poorly/undifferentiated | 7 | 12 | 10 | 9 | 8 |
| Missing | 32 | 36 | 21 | 27 | 32 |
| Smoking status (%) | |||||
| Never smoker | 37 | 34 | 33 | 43 | 33 |
| Past smoker | 49 | 48 | 58 | 44 | 56 |
| Current smoker | 4 | 7 | 6 | 3 | 3 |
| Missing | 10 | 11 | 3 | 10 | 8 |
| Year of diagnosis (%) | |||||
| Prior to 1990 | 15 | 18 | 20 | 15 | 14 |
| 1990–2000 | 64 | 58 | 64 | 64 | 57 |
| After 2000 | 21 | 24 | 16 | 21 | 29 |
| Median time to physical activity measurement (months) | 15 | 15 | 17 | 17 | 17 |
by total metabolic equivalent tasks (METS) – hours per week
Physical activity after diagnosis
We assessed the influence of physical activity after the diagnosis of non-metastatic colorectal cancer on patient survival (Table 2). Compared to patients who reported less than 3 total MET-hours per week of recreational exercise (15% of the cohort), those reporting greater than 27 MET-hours per week (38% of the cohort) had an adjusted hazard ratio for colorectal cancer-specific mortality of 0.47 (95% confidence interval [CI] 0.24–0.92, p trend = 0.002). Similarly, the adjusted hazard ratio for overall mortality was 0.59 (95% CI, 0.41–0.86; p for trend = 0.0003).
Table 2.
Colorectal Cancer-Specific and Overall Mortality by Level of Post-Diagnosis Physical Activity
| Colorectal Cancer Specific Mortality | Overall Mortality | |||||
|---|---|---|---|---|---|---|
| Events/# of risk | Unadjusted | Adjusted * | Events/# of risk | Unadjusted | Adjusted* | |
| Post-diagnosis Activity (n=661) | ||||||
| ≤ 3 MET-hours/week | 17/102 | Referent | Referent | 52/102 | Referent | Referent |
| 3.1 – 9 MET-hours/week | 21/125 | 0.95 (0.50–1.80) | 1.06 (0.55–2.08) | 57/125 | 0.79 (0.54–1.15) | 1.00 (0.68–1.48) |
| 9.1 – 18 MET-hours/week | 18/101 | 0.98 (0.51–1.91) | 1.30 (0.65–2.59) | 48/101 | 0.79 (0.53–1.17) | 1.12 (0.74–1.70) |
| 18.1 – 27 MET-hours/week | 9/81 | 0.60 (0.27–1.34) | 0.76 (0.33–1.77) | 27/81 | 0.50 (0.31–0.80) | 0.74 (0.46–1.20) |
| > 27 MET-hours/week | 23/252 | 0.51 (0.27–0.96) | 0.47 (0.24–0.92) | 74/252 | 0.51 (0.36–0.73) | 0.59 (0.41–0.86) |
| p for trend | 0.01 | 0.002 | 0.0001 | 0.0003 | ||
MET = metabolic equivalent tasks
adjusted for age at diagnosis (<50, 50–59, 60–69, ≥ 70), stage of disease, grade of tumor differentiation, colon or rectal primary, year of diagnosis, body mass index at diagnosis, time from diagnosis to physical activity measurement, change in body mass index prior and after diagnosis, smoking status (current, past, never)
Since lower levels of physical activity among patients at risk for cancer recurrence could reflect occult cancer recurrence or impending death, we excluded patients who developed cancer recurrence or died within six months of completing the physical activity assessment in our primary analyses (n = 12). When we extended this restriction to 12 months (reducing the sample size to 646), men in the highest category had a hazard ratio of 0.46 (95% CI, 0.23–0.95) for colorectal cancer-specific mortality (p for trend = 0.003) and a hazard ratio of 0.59 (95% CI, 0.40–0.87) for overall mortality (p for trend = 0.0004), compared to the least active men. Further extension of this restriction to 2 years (n = 553) continued to demonstrate similar point estimates for colorectal cancer-specific mortality (HR 0.64 [95% CI, 0.28–1.45], p trend = 0.08) and overall mortality (HR 0.65 [95% CI, 0.43–0.99], p trend = 0.005).
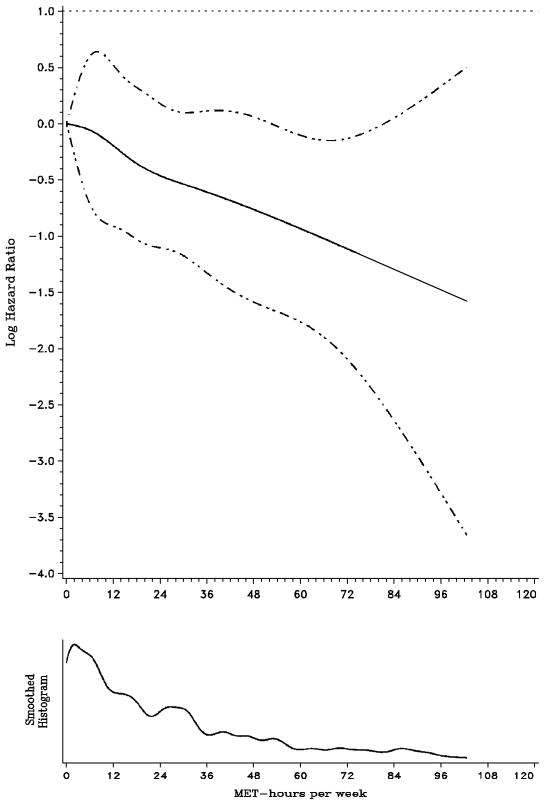
To better characterize the amount of activity necessary to have a benefit, we generated a smoothing spline of log hazard versus the total MET score (Figure 1), a method independent of predetermined MET-hour categorizations. A log hazard less than zero represents a favorable hazard ratio (< 1). The initial inflection of the slope of the spline suggests initial protection between 6 and 12 total MET-hours per week. Furthermore, the leveling of the slope of the curve beyond 35 total MET-hours per week suggests that exercise beyond certain levels do not lead to substantially further improvements in DFS.
Figure 1. Log hazard smoothing spline plot.
Note: MET = metabolic equivalent task. Solid line is the log hazard ratio and dashed lines represent 95% confidence interval
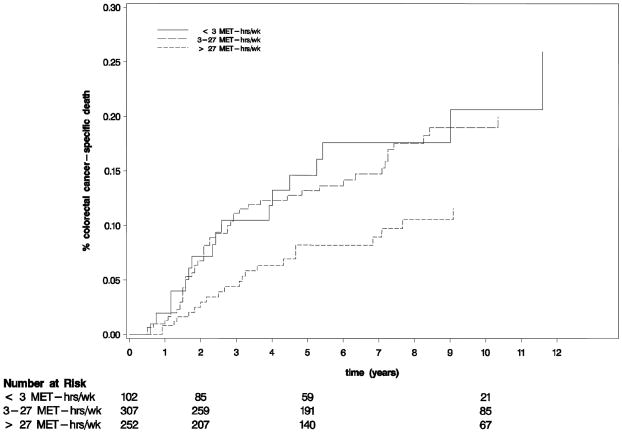
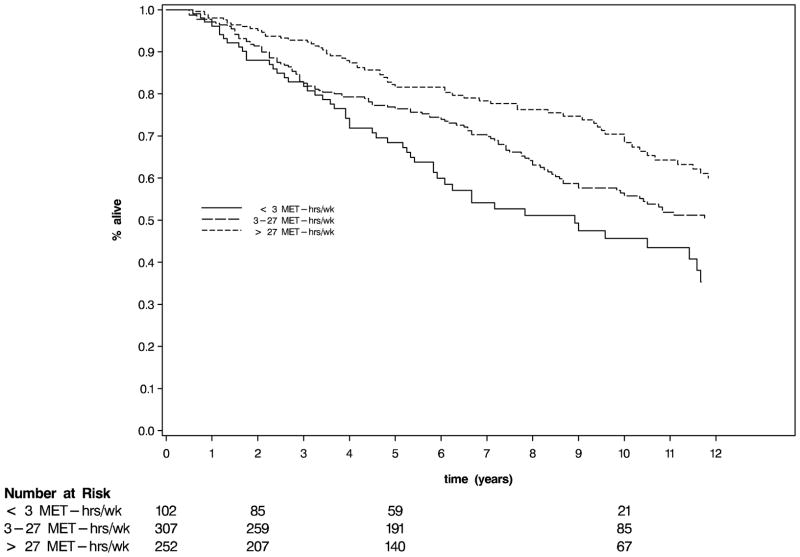
To estimate 5-year survival, activity levels were collapsed into three categories (≤ 3, 3–27, and >27 MET-hours per week). Of note, follow-up in this analysis begins at the time of completion of the questionnaire that assessed physical activity to reduce bias rather than the date of diagnosis of colorectal cancer, as is typically reported in studies of adjuvant chemotherapy. Nonetheless, the proportion of patients free of colorectal cancer-specific deaths at 5-years (Figure 2) was 85% for patients who engaged in no more than 3 MET-hours per week, 87% for 3–27 MET-hours per week, and 92% for more than 27 MET-hours per week (p log rank = 0.05). At 10 years, the proportions of patients free of colorectal cancer-specific deaths were 79%, 81% and 88%, respectively. There was also a statistically significant difference in overall survival across physical activity tertile (p < 0.0007, Figure 3).
Figure 2.
Cumulative incidence of colorectal cancer-specific deaths (log rank p = 0.01)
Figure 3.
Kaplan Meier
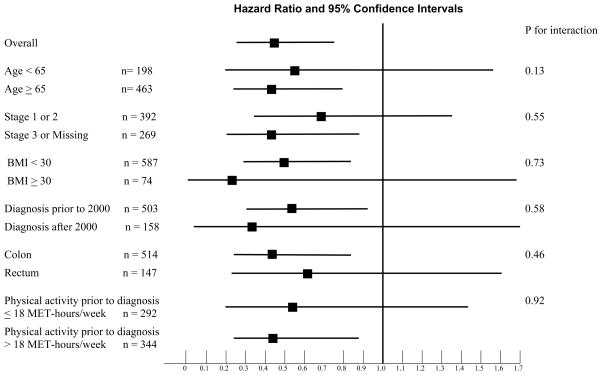
We examined the influence of post-diagnosis physical activity across strata of other predictors of cancer recurrence and mortality (Figure 4). The inverse relation between post-diagnosis physical activity and cancer-specific mortality remained largely unchanged across strata of age, pathological stage, body mass index, site of disease, or year of diagnosis.
Figure 4.
Stratified Analysis of Disease-Free Survival (Comparison of < 27 MET-hours/week to > 27 MET-hours/week of Exercise)
Physical activity prior to diagnosis and mortality
In prior analyses, we demonstrated that level of physical activity prior to diagnosis (most immediate prior questionnaire completed at least 6 months prior to diagnosis) was not significantly associated with mortality in colorectal cancer survivors.5 In this current analysis, we similarly did not detect a statistically significant trend in colorectal cancer-specific mortality (p = 0.65) nor overall mortality (p = 0.26) with increasing exercise. The correlation between pre-diagnosis and post-diagnosis physical activity was relatively modest (correlation coefficient = 0.40, p < 0.001). Pre-diagnosis physical activity did not confound the association between post-diagnosis activity and colorectal cancer-specific mortality. When pre-diagnosis activity was entered in the multivariate model, men who exercised greater than 27 MET-hours/week had an adjusted hazard ratio for colorectal cancer-specific mortality of 0.43 (95% CI, 0.20–0.98), compared to those engaging in no more than 3 MET-hours/week. Similarly, pre-diagnosis activity did not modify the effect of post-activity on colorectal cancer-specific mortality (Figure 4, p for interaction = 0.92)
Comment
Men who were physically active after the diagnosis of non-metastatic colorectal cancer experienced a significantly decreased risk of colorectal cancer-specific death as well as death from any cause. Men who engaged in at least 27 MET-hours per week had > 50% lower risk of colorectal cancer-specific mortality compared to relatively inactive men. This association was consistently detected, regardless of age, stage, body mass index, year of diagnosis, location of tumor and pre-diagnosis physical activity.
These findings are consistent with 2 prior reports of physical activity after diagnosis in prospectively observed colorectal cancer survivors.5, 6 In a cohort of 573 women with colorectal cancer, the most active women had an adjusted hazard ratio for colorectal cancer-specific mortality of 0.39 (95% CI, 0.18 to 0.82), compared to inactive women.5 Similarly, in a cohort of 832 men and women with stage III colon cancer who received adjuvant therapy on a National Cancer Institute-sponsored clinical trial, the adjusted hazard ratio for disease-free survival amongst those was 0.55 (95% CI, 0.33 to 0.91).5 Thus, the adjusted hazard of 0.47 detected in this cohort of men is consistent with those studies. Further, the spline curves in this study and from the study utilizing the clinical trial cohort5 both suggest a measurable risk reduction between 6–12 MET-hours per week, with a potential threshold between 30–35 MET-hours per week. A notable difference in this cohort is that 51% of colorectal cancer survivors in this current cohort engaged in at least 18 MET-hours/week, compared to 26% of survivors in the Nurse’s Health Study and 28% of survivors in the NCI-sponsored trial.5, 6
The mechanism for this consistent association between physical activity and colorectal cancer-specific survival is unknown, however hyperinsulinemia is a potential etiology.14–16 Insulin and the insulin-like growth factor (IGF) family have been associated with enhanced tumor growth and anti-apoptosis 16 and colorectal cancer risk is elevated in individuals with higher circulating levels of insulin or C-peptide (a marker of insulin secretion) and IGF-1.17–22 Patients who develop recurrence of colorectal cancer have micrometastases at diagnosis. An environment that allows such microscopic tumors to proliferate could be detrimental. Physical activity may lower tissue insulin and IGF levels and raise beneficial IGF binding proteins to influence exposure of these growth factors on micrometastases. Other mechanisms proposed in the association between exercise and colon cancer development may also be relevant towards the progression of established cancer, including immune modulation and anti-inflammatory actions.23–25
Advantages of this cohort derived from the Health Professionals Follow-up Study are the prospective collection of exposures, the diligent medical record review of self-reported colorectal cancer and deaths and the sample size. Nonetheless, there are limitations that are worth noting. Beyond cause of mortality, data on cancer recurrences were not available in this cohort. Nonetheless, as median survival for metastatic colorectal cancer was approximately 10 to 12 months during much of the time period of this study,26 colorectal cancer-specific mortality should be a reasonable surrogate for cancer-specific outcomes. Treatment data are not collected in this cohort. However, nearly 60% of patients had stage I or II disease, in which surgery alone would generally be standard of care and no interaction by stage was observed. Further, although there are differences in the likelihood of use of adjuvant chemotherapy based on factors such as socioeconomic class, the fairly homogenous nature of this cohort (professional male health care providers) would likely increase the probability of at least standard therapy.27, 28 Co-morbidities and access to healthcare may also confound these findings. Given the population studied, we would expect the latter to be relatively diminished. Though co-morbidities have been shown to affect mortality in colorectal cancer survivors,29–31 such diseases are less likely to affect disease recurrence and thereby colorectal-cancer specific mortality. Finally, our data are limited to leisure-time exercise; occupational physical activity is not surveyed in our questionnaires.
As in prior analyses5, 6, we considered the possibility that sick patients (with cancer recurrences and limited survival) will exercise less. To minimize the bias by occult cancer recurrence, we excluded recurrences or deaths within six months of the activity assessment in the primary analysis and continued to observe a positive impact of exercise when extending this restriction to 12 months. Finally, we would expect few patients to have undetected recurrences over extended periods of time, given the relatively brief natural history of recurrent colon cancer.
Patients who underwent treatment for colorectal cancer may be considered limited in their ability to exercise. However, Arndt and colleagues reported that one year after surgery of the primary tumor, patients with colorectal cancer reported their physical functioning and global quality of life nearly identical to those of a non-cancer population.32
These results provide further support that physical activity after a colorectal cancer diagnosis may lower the risk of death from that disease. A randomized study for high risk stage II and stage III colon cancer survivors that will compare general education materials to a program with supervised physical activity sessions and behavioral support delivered over 3 years will soon open; the primary endpoint is disease-free survival.33 The findings from this study further support that effort.
Acknowledgments
Acknowledgement of research support: This work was supported by the US National Institute of Health (NIH) grants P01 CA55075 and P50 CA127003.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008 Mar–Apr;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002 Dec;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 3.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009 Feb 24;100(4):611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006 Jan;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006 Aug 1;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006 Aug 1;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991 Aug 24;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E, Rimm EB, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst. 1994 Feb 2;86(3):183–191. doi: 10.1093/jnci/86.3.183. [DOI] [PubMed] [Google Scholar]
- 9.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984 May;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995 Mar 1;122(5):327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993 Jan;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994 Oct;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 13.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996 Jan;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001 Nov;131(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 15.Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60(1):91–106. doi: 10.1079/pns200070. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002 Jul 3;94(13):972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 17.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91(13):1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 18.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000 Oct 4;92(19):1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 19.Palmqvist R, Stattin P, Rinaldi S, et al. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003 Oct 20;107(1):89–93. doi: 10.1002/ijc.11362. [DOI] [PubMed] [Google Scholar]
- 20.Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002 May;50(5):642–646. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999 Apr 7;91(7):620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 22.Manousos O, Souglakos J, Bosetti C, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999 Sep 24;83(1):15–17. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer Epidemiol Biomarkers Prev. 2006 Apr;15(4):690–695. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- 25.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002 Nov;132(11 Suppl):3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005 Feb 3;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 27.VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002 Jul 1;95(1):39–46. doi: 10.1002/cncr.10645. [DOI] [PubMed] [Google Scholar]
- 28.Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL, Begg CB. Influence of hospital procedure volume on outcomes following surgery for colon cancer. Jama. 2000 Dec 20;284(23):3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 29.Payne JE, Meyer HJ. The influence of other diseases upon the outcome of colorectal cancer patients. Aust N Z J Surg. 1995 Jun;65(6):398–402. doi: 10.1111/j.1445-2197.1995.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 30.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998 Jun 1;82(11):2123–2134. [PubMed] [Google Scholar]
- 31.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. Jama. 2004 May 26;291(20):2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 32.Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004 Dec 1;22(23):4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Courneya KS, Booth CM, Gill S, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008 Dec;15(6):271–278. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]