Abstract
Hydroxy-α-sanshool, the active ingredient in plants of the prickly ash plant family, induces robust tingling paresthesia by activating a subset of somatosensory neurons. However, the subtypes and physiological function of sanshool-sensitive neurons remain unknown. Here we use the ex vivo skin–nerve preparation to examine the pattern and intensity with which the sensory terminals of cutaneous neurons respond to hydroxy-α-sanshool. We found that sanshool excites virtually all D-hair afferents, a distinct subset of ultrasensitive light-touch receptors in the skin and targets novel populations of Aβ and C fiber nerve afferents. Thus, sanshool provides a novel pharmacological tool for discriminating functional subtypes of cutaneous mechanoreceptors. The identification of sanshool-sensitive fibers represents an essential first step in identifying the cellular and molecular mechanisms underlying tingling paresthesia that accompanies peripheral neuropathy and injury.
Introduction
A major goal in somatosensory research is to identify molecular mechanisms of touch, under normal and pathological conditions. Diverse nerve fibers innervate the skin and detect a wide variety of somatosensory stimuli (Brown and Iggo, 1967; Burgess et al., 1968). Myelinated Aβ fibers respond to light touch against the skin and help discriminate texture and object form. A-mechanoreceptors (AM) and C fibers respond to intense or painful pressure. Specialized Aβ and Aδ down hair follicle (D-hair) fibers adapt quickly to touch stimuli, making them exquisitely sensitive to fine textures and movement. Injury and disease affect the function of these neuronal subtypes and lead to altered sensation, ranging from numbing to extreme chronic pain. For instance, tingling paresthesia often accompanies diabetic neuropathy or traumatic nerve injury. This “pins and needles” sensation also accompanies transient limb ischemia. Human microneurography experiments have demonstrated that tingling paresthesia results from the aberrant activity of mechanosensitive neurons (Ochoa and Torebjörk, 1980; Nordin et al., 1984), but little is known about the molecular mechanisms underlying this enigmatic sensation.
A lack of pharmacological tools has hindered our understanding of the physiological mechanisms underlying tingling paresthesia. The utility of natural plant-derived compounds in studying sensation at the molecular level has been illustrated by the use of capsaicin and menthol, to clone the heat-sensitive ion channel TRPV1, and the cold-sensitive ion channel TRPM8, respectively (Caterina et al., 1997; McKemy et al., 2002; Peier et al., 2002). Hydroxy-α-sanshool (sanshool) is a natural plant alkylamide that induces numbing and robust tingling paresthesia in humans (Bryant and Mezine, 1999; Sugai et al., 2005). Thus, understanding the actions of sanshool may provide insight into the cellular and molecular mechanisms underlying tingling paresthesia. Nearly 52% of cultured dorsal root ganglion neurons are excited by sanshool in vitro, including a subset of large-diameter, putative light-touch mechanoreceptors (Bautista et al., 2008). Indeed, lingual nerve recordings (Bryant and Mezine, 1999) and recordings of dorsal horn neurons (Sawyer et al., 2009) confirm that sanshool does activate light-touch receptors, stimulating interest that sanshool may discriminate between subtypes of mechanosensitive fibers. However, no previous study has elucidated the actual types of nerve fibers activated by sanshool or demonstrated the ability of sanshool to differentiate between them.
Using the saphenous skin–nerve preparation to record from primary afferent nerve fibers ex vivo, we demonstrate that sanshool excites specific subtypes of cutaneous mechanoreceptors. Sanshool potently activates all ultrasensitive D-hair fibers and, to a lesser extent, unique populations of pressure-sensitive Aβ fibers and low conduction velocity C fibers. In all fiber types, sanshool evokes action potential bursting, reminiscent of the activity observed in myelinated fibers of human subjects experiencing tingling paresthesia. In addition, sanshool-evoked avoidance behavior is distinct from pain-evoked behaviors and is consistent with the robust activation of low threshold mechanoreceptors. Moreover, we demonstrate that sanshool is an invaluable tool for delineating the function of novel mechanosensitive neuron subtypes.
Materials and Methods
Skin–nerve preparation.
The saphenous skin–nerve preparation was used to record from primary afferent nerve fibers ex vivo (Reeh, 1986; Stucky et al., 1999). Male C57BL/6 mice were killed, and skin from the dorsum of the right hindpaw was dissected free along with the innervating saphenous nerve. This tissue was placed corium side up in a bath superfused with O2 saturated synthetic interstitial fluid containing the following (in mm): 123 NaCl, 3.5 KCl, 0.7 MgSO4, 1.7 NaH2PO4, 2.0 CaCl2, 9.5 sodium gluconate, 5.5 glucose, 7.5 sucrose, and 10 HEPES (to make a 290 mOsm solution). The pH was adjusted to 7.45 ± 0.05, and bath temperature was maintained at 32.0 ± 0.5°C. The saphenous nerve was teased into thin filaments, and single afferent fibers were identified using an electrical search by systematically probing the entire skin with a Teflon-coated steel needle electrode (2 MΩ impedance, un-insulated tip diameter of 10 μm) while square-wave electrical pulses (500 ms, 4.2 mA) were applied. Action potentials with signal/noise ratio >2 were identified, and the surrounding area was probed to find the most electrically sensitive spot. This point reliably coincided with the most mechanically sensitive spot for fibers responding to mechanical stimuli. A fiber was considered mechanically insensitive if it was unresponsive to any force <147 mN or to a glass rod.
Fibers were characterized by conduction velocity and mechanical adaptation. Using the same criteria as previous mouse studies, units conducting slower than 1.2 m/s were classified as unmyelinated C fibers, those conducting between 1.2 and 10 m/s were thinly myelinated Aδ fibers, and those conducting faster than 10 m/s were large myelinated Aβ fibers (Koltzenburg et al., 1997; Stucky et al., 1999). Mechanically sensitive Aβ fibers were further classified as slowly adapting (SA) if they responded throughout a sustained force or as rapidly adapting (RA) if they responded only at the onset and/or offset of the mechanical stimulus. Similarly, mechanically sensitive Aδ fibers were further classified as AM if they responded throughout a sustained force or as D-hairs if they responded only at the onset and/or offset of the mechanical stimulus. The mechanical threshold of all fibers was determined using calibrated von Frey filaments (range, 0.27 to 147 mN). For Aβ fibers, a feedback-controlled mechanical stimulator was placed perpendicular to the most mechanically sensitive part of the receptive field, and square-wave mechanical forces of 5, 10, 20, 40, 100, and 150 mN for 10 s duration were delivered with 1 min intervals between forces. Any elicited action potentials were recorded.
After electrical and mechanical characterization, fibers were exposed to chemical stimuli. A hollow metal cylinder (6 mm in diameter) was used to isolate the cutaneous terminal receptive field of each fiber. A silicon-based lubricant was applied as needed to produce a tight seal. Initially, separate populations of D-hair fibers were tested for concentration–response properties by applying 2, 20, or 200 μm hydroxy-α-sanshool for 2 min each. We subsequently used 200 μm hydroxy-α-sanshool in all skin–nerve experiments because concentration–response analysis revealed that 200 μm was a near-maximal concentration for both number of D-hair afferents activated and action potential firing rate (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
For each unit, 2 min of baseline activity was recorded before any chemical was applied to the receptive field. Next, the cylinder was evacuated and filled with either 200 μm hydroxy-α-sanshool in 26 mm dimethylformamide (DMF) and 100 μm β-cyclodextrin or a control solution containing 26 mm DMF and 100 μm β-cyclodextrin. Solutions were kept in the dark, on ice at 10× concentration. Immediately before use, each solution was diluted 10-fold in warmed synthetic interstitial fluid. The experimenter was blinded as to which mixture was applied first. Action potentials were recorded for 2 min. Then, the cylinder and receptive field were washed for 2 min with synthetic interstitial fluid, and the procedure was repeated using the second mixture. A subset of sanshool-treated fibers (n = 30) were recorded after the removal of sanshool for an additional 5 min wash period. For C fibers, the receptive field was washed for 2 min, exposed to mustard oil (1 mm) for 2 min, washed for 2 min, and then exposed to capsaicin (1 μm). Action potentials were recorded for 30 s before, during, and 2 min after the addition of each chemical. An additional group of C fibers was incubated with a selective TRPA1 antagonist (HC-030031, 100 μm) and TRPV1 antagonist (A-425612, 3 μm) for 2 min preceding and then throughout the application of sanshool (200 μm) for 2 min, a wash period of 2 min, and the dual application of mustard oil (1 mm) and capsaicin (1 μm) for 2 min.
Electrical-, mechanical-, and chemical-evoked action potentials were viewed on an oscilloscope for comparison of shape and profile. Data were recorded and saved to a computer using a Powerlab 4.0 system and Chart software (AD Instruments). Action potentials were discriminated and counted offline using a spike histogram software extension. Fibers were considered “sensitive” to a chemical once they fired more than three action potentials in excess of their baseline firing rate. Latency to response was measured from the addition of chemical to the third action potential over baseline.
Mouse behavior.
Ten- to 16-week-old male C57BL/6 mice were housed with 12 h light/dark cycle at 21°C. All experiments were approved by the University of California, Berkeley Institutional Animal Care and Use Committee. Drinking assays were performed as described previously (Caterina et al., 2000; Bautista et al., 2008). Water containing 0.125% saccharin plus vehicle (1:800 DMF) was provided to animals for 3 h every 24 h. On day 4 or 5, 1 mm sanshool was added to the water in place of vehicle. The amount of water consumed was measured each day. Drinking behavior was video recorded the day before and the day of sanshool addition, and the videos were subsequently scored manually by an observer blinded to chemical treatment to quantitate the amount of time spent drinking. Episodes of drinking separated by <4 s of interruption were counted as a continuous block.
Sensory neuron culture.
Trigeminal ganglion or dorsal root ganglion neurons from postnatal day 0–42 mice were dissociated and cultured as described previously (Bautista et al., 2008). Sensory neurons were incubated in the presence or absence of neurotrophin-4 (NT4) (10–20 ng/ml; Peprotec) in culture media for 16–24 h. The response of neurons to sanshool (100 μm), GABA (100 μm; Sigma), and capsaicin (0.5 μm) was measured using ratiometric calcium imaging, as described (Bautista et al., 2008). Images were collected using MetaFluor (Molecular Devices) and analyzed in MATLAB (MathWorks) using custom scripts. Neurons were considered “responsive” to a chemical once the average fluorescence ratio exceeded 10% of the baseline level (averaged over four time points at ⅓ frames per second).
Pharmacological reagents.
Chemicals were purchased from Sigma-Aldrich. A stock solution of mustard oil (100 mm) was made daily in ethanol and diluted to 1 mm in synthetic interstitial fluid. Aliquots of capsaicin (1 mm) made in 1-methyl-2-pyrrolidinone were stored at −20°C and diluted to 1 μm in synthetic interstitial fluid daily. Hydroxy-α-sanshool was purified from Szechuan peppercorns as described previously (Bautista et al., 2008). A stock of hydroxy-α-sanshool (100 mm) was dissolved in DMF, protected from light, and stored at −80°C. Each experimental day, the stock was diluted to 2 mm in synthetic interstitial fluid containing 1 mm β-cyclodextrin. An equal volume of DMF was added to synthetic interstitial fluid containing 1 mm β-cyclodextrin to make the vehicle control. To prevent breakdown, aliquots were protected from light and kept on ice for up to 4 h before dilution to 200 μm in warm synthetic interstitial fluid. The TRPV1 antagonist A-425619 was obtained from Abbott Laboratories, and the TRPA1 antagonist HC-030031 was obtained from Hydra Biosciences. A-425619 (3 μm) and HC-030031 (100 μm) were dissolved in DMSO and diluted 1:1000 in physiological saline.
Statistical analysis.
Values are reported as either the mean ± SEM or median with 25th and 75th percentiles. For comparison between two groups, a Student's t test was used for parametric data, and a Mann–Whitney U test was used for nonparametric data. For groups of three of more, a one-way ANOVA followed by a Tukey–Kramer post hoc test was used. For analyzing a variable between two or more groups over multiple measurements, a two-way ANOVA was used. For comparison of different proportions between two groups, a Fisher's exact test was used.
Results
Sanshool discriminates between Aδ D-hair and mechanoreceptors
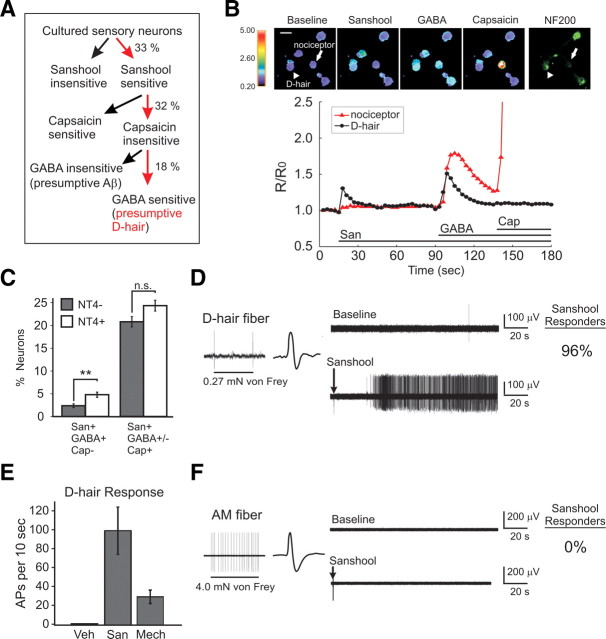
Previous studies have shown that a subpopulation of large-diameter, putative light-touch mechanoreceptors and small-diameter presumptive nociceptors are excited by sanshool (Bautista et al., 2008). We further classified the population of sanshool-sensitive neurons in vitro by examining cell morphology, expression of functional markers, and pharmacological profile. Recently, Aptel et al. (2007) have shown that lightly myelinated Aδ D-hairs can be identified by their ability to depolarize in response to GABA. We therefore asked whether the subset of myelinated [neurofilament 200 (NF200)-immunoreactive] sensory neurons that is excited by sanshool is also sensitive to GABA (Fig. 1A,B). Among the sanshool-sensitive, capsaicin-insensitive population, 18% were excited by GABA. As expected for D-hair neurons, these cells displayed significantly larger cell diameters than sanshool- and capsaicin-sensitive neurons (19.5 ± 0.4 vs 18.9 ± 0.1 μm; p < 0.05) and preferentially stained for NF200 (Fig. 1B). NT4 has been shown to promote the differentiation and survival of D-hairs, and exogenous NT4 increases the number of GABA-sensitive cultured sensory neurons (Stucky et al., 1998, 2002; Aptel et al., 2007). Indeed, the number of neurons sensitive to both sanshool and GABA increased significantly when NT4 was included in the culture media (Fig. 1C). These data suggest that a subset of sanshool-sensitive neurons are putative D-hair receptors.
Figure 1.
Sanshool activates D-hair neurons. A, Classification of cultured sensory neurons based on chemical sensitivity (n = 1187). B, Examples of calcium responses to chemical stimuli and staining with neurofilament 200 antibody in cultured sensory neurons. A presumptive D-hair (arrowhead) and nociceptor (arrow) are indicated. Seventy-four percent of sanshool-sensitive and GABA-sensitive but capsaicin-insensitive neurons stained for NF200 (n = 23) compared with 49% of all neurons tested (n = 669; p < 0.05, Fisher's exact test). C, The proportion of sanshool-sensitive and GABA-sensitive (San+, GABA+) but capsaicin-insensitive (Cap−) sensory neurons in cultures with or without NT4, a growth factor that promotes D-hair development. n = 1187 and 1293, in the absence and presence of NT4, respectively, from three independent cultures. **p < 0.01, two-proportion z test. D, Example of a D-hair fiber response to sustained (suprathreshold) mechanical force and subsequently exposed to 200 μm hydroxy-α-sanshool and percentage of responsive fibers. E, Maximal response of D-hair fibers to sanshool (San) and sustained mechanical stimuli (Mech). AP, Action potentials; Veh, vehicle. F, Example of an AM fiber response to sustained (suprathreshold) mechanical force and subsequently exposed to 200 μm hydroxy-α-sanshool and percentage of responsive fibers.
We next used the ex vivo skin–nerve preparation to identify the physiological function of sanshool-sensitive cutaneous sensory neurons. Subclasses of cutaneous sensory neurons were identified by assessing fiber conduction velocity, action potential waveform, and response properties to mechanical stimuli. We first examined the effects of sanshool on Aδ fibers (conduction velocities between 1.2 and 10 m/s; n = 55). Three subtypes of Aδ fibers were probed: slowly adapting AM fibers (54%) that terminate as free nerve endings in the skin and display high mechanical thresholds, rapidly adapting D-hair fibers (42%) that innervate down hairs and display very low mechanical thresholds, and mechanically insensitive fibers (4%).
D-hair fibers responded robustly to sanshool (200 μm) applied to their receptive field (Fig. 1D). Sanshool evoked an intense and long-lasting barrage of action potentials that displayed an average magnitude approximately fivefold greater than their maximum response to sustained mechanical stimuli (Fig. 1E). D-hair responses to sanshool were concentration dependent in proportion and magnitude (supplemental Fig. 1, available at www.jneurosci.org as supplemental material), and at 200 μm virtually all (96%; 22 of 23) D-hair fibers responded. In contrast to D-hair fibers, AM fibers were completely unresponsive to sanshool (0 of 30) but exhibited robust action potential firing to sustained mechanical stimuli (Fig. 1F). Because some fiber responses to sanshool occurred as the compound was removed from the skin, some AM fibers (n = 8) were expressly recorded throughout a 5 min wash period, during which no responses were observed (data not shown). Like AM fibers, the mechanically insensitive Aδ fibers were also unresponsive to sanshool. These data demonstrate that, among all Aδ fiber types, sanshool selectively and vigorously activates D-hair afferents. Importantly, sanshool sensitivity represents a new functional marker for D-hair afferents.
Sanshool activates fast-conducting, touch-sensitive Aβ fibers
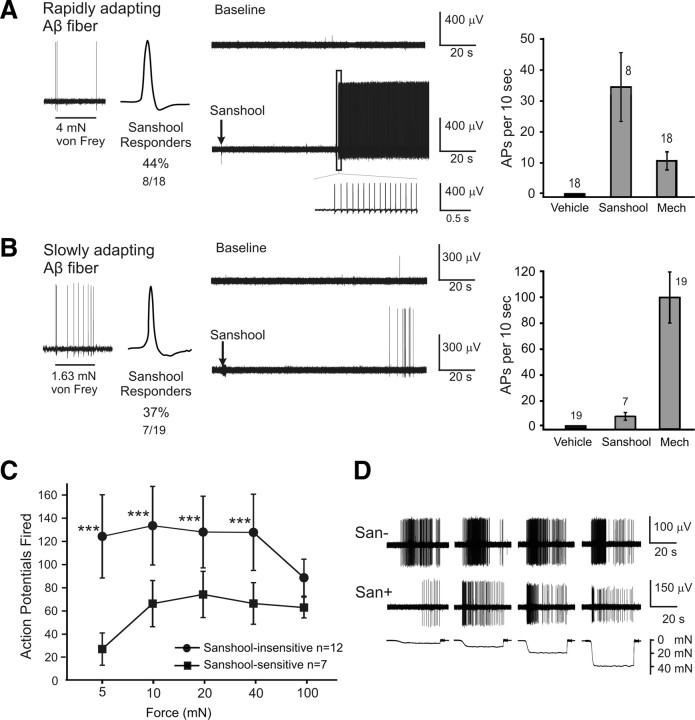
We next probed the effects of sanshool on heavily myelinated Aβ light-touch receptors. Three subtypes of Aβ fibers were examined: SA-Aβ fibers that predominantly terminate on Merkel cells in the basal epidermis, encode low-intensity force, and mediate two-point discrimination (49%), RA-Aβ fibers that enwrap the bulb of large guard hair follicles and respond to hair deflection (46%), and mechanically insensitive fibers (5%). Similar proportions of rapidly adapting (44%) and slowly adapting (37%) Aβ fibers responded to sanshool (Fig. 2A,B), but their response magnitude was quite different. Like rapidly adapting D-hair fibers, sanshool triggered RA-Aβ fibers to fire hundreds of action potentials in a regular pattern at high frequency (Fig. 2A, note inset). In contrast, SA-Aβ fibers fired only a small number of action potentials at frequencies significantly (∼10-fold) lower than those evoked by maximal mechanical stimuli (Fig. 2B, right). Similar to the mechanically insensitive Aδ fibers, two mechanically insensitive Aβ fibers were insensitive to sanshool.
Figure 2.
Sanshool activates subsets of RA-Aβ and SA-Aβ fibers. A, B, Response of rapidly adapting Aβ (A) and slowly adapting Aβ (B) fibers to sanshool. Left, Representative response to a sustained (suprathreshold) mechanical force. Middle, Response to 200 μm hydroxy-α-sanshool. Right, Maximal firing rate in response to sanshool or mechanical stimuli (Mech; 100 mN force; 10 s). AP, Action potentials. C, Number of mechanically evoked action potentials in sanshool-sensitive and sanshool-insensitive slowly adapting Aβ fibers. Sustained force stimuli (5–100 mN; 10 s each) were applied to each receptive field before the receptive field was exposed to sanshool. ***p < 0.001, significant difference between the two cohorts (two-way ANOVA, followed by the Bonferroni post hoc test). D, Representative examples of sanshool-sensitive and sanshool-insensitive slowly adapting Aβ fibers in response to mechanical stimuli.
Close examination of the sanshool-sensitive SA-Aβ fibers revealed a new subset of cutaneous mechanoreceptors. Sanshool-responsive SA-Aβ fibers were significantly less responsive to mechanical force (<100 mN) than the sanshool-insensitive SA-Aβ fibers (Fig. 2C); at low forces (5 mN), they were approximately five times less responsive to sustained force and displayed higher mechanical thresholds (supplemental Table 1, available at www.jneurosci.org as supplemental material). This division of SA-Aβ fibers into two subgroups is based on an intrinsic property of the fibers, because mechanical responsiveness was assessed before sanshool application. No similar trend was found among other Aβ or Aδ fibers (data not shown).
Sanshool excites a subset of slowly conducting C fibers
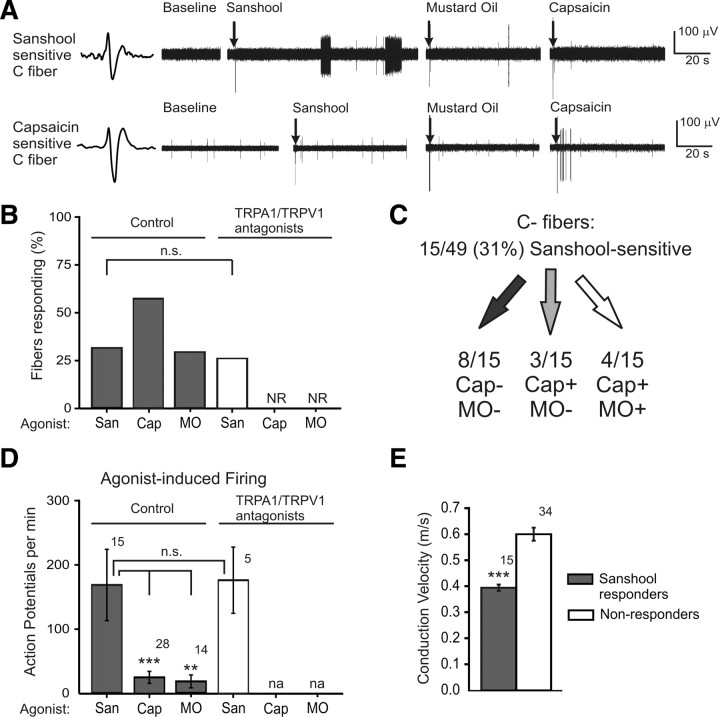
Previous in vitro studies have shown that sanshool activates a subset of putative C-fiber nociceptors. We thus sought to identify the subclass(es) of C-fiber nociceptors activated by sanshool. C fibers were identified using an unbiased electrical search stimulus, classified by their characteristic slow conduction velocity (<1.2 m/s) and subclassified by their sensitivity to TRP channel agonists. Maximal concentrations of sanshool (200 μm), the TRPA1 agonist mustard oil (1 mm), and the TRPV1 agonist capsaicin (1 μm) were applied in succession to the receptive field of isolated C fibers (Fig. 3A). Among all C fibers tested (n = 49), 31% responded to sanshool, 29% responded to mustard oil, 57% responded to capsaicin, and 20% were insensitive to all three chemical stimuli (Fig. 3A,B). No difference in the prevalence of sanshool-evoked responses was observed in the presence of TRPA1 and TRPV1 inhibitors (n = 19) (Fig. 3B); in contrast, these inhibitors completely attenuated responses to capsaicin and mustard oil. The sanshool-evoked action potential firing rate was also not altered by the presence of TRPA1 and TRPV1 inhibitors (p = 0.94) (Fig. 3D). These data suggest that sanshool does not require TRPA1 or TRPV1 to activate sensory nerve fibers. Strikingly, sanshool evoked much stronger responses from C fibers than either capsaicin or mustard oil, which are among the most intense known activators of nociceptors (Fig. 3D).
Figure 3.
Sanshool activates a subset of C fibers. A, Representative C-fiber responses. Top trace, Example of a C fiber that was sensitive to hydroxy-α-sanshool (200 μm) but not to mustard oil (1 mm) or capsaicin (1 μm). Bottom trace, Example of a C fiber insensitive to sanshool and mustard oil but sensitive to capsaicin. B, Proportion of C fibers that respond to sanshool (San), mustard oil (MO), or capsaicin (Cap), in the presence or absence of TRPA1 and TRPV1 inhibitors (HC-030031 at 100 μm and A-425619 at 3 μm, respectively). Fisher's exact test, p = 0.42 for sanshool-sensitive fibers. C, Fraction of C fibers responsive to sanshool and their sensitivity to capsaicin and mustard oil (+ indicates sensitivity to the chemical agonist, whereas − indicates insensitivity; 5 of 32 fibers were insensitive to all chemical agonists). D, Action potentials fired per minute in response to sanshool, capsaicin, or mustard oil, in the presence or absence of TRPA1 and TRPV1 inhibitors. n.s. indicates no significant difference between groups (Student's t test; p = 0.40). **p < 0.01 and ***p < 0.001, significant difference between groups (one-way ANOVA, followed by a Tukey–Kramer post hoc test). E, Conduction velocities of all C fibers that were sanshool sensitive (gray) or insensitive (white). Conduction velocities were measured before sanshool stimulation and are therefore an inherent property of the C fiber. ***p < 0.01, significant difference between sanshool-sensitive and -insensitive fibers (Student's t test).
We observed three types of sanshool-sensitive C fibers. (1) The largest portion of sanshool-sensitive fibers was insensitive to both mustard oil and capsaicin (53%) (Fig. 3A, top, C). This population responded most intensely to sanshool, with firing rates of 242 ± 104 action potentials per minute (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). (2) Some sanshool-sensitive fibers respond to capsaicin but not mustard oil (20%) (Fig. 3A, bottom, C). (3) Another proportion of sanshool-sensitive C fibers responded to both capsaicin and mustard oil (27%) (Fig. 3C). Thus, there was no correlation between sanshool sensitivity and sensitivity to capsaicin or mustard oil. Nerve fibers from all three groups of C fibers exhibited action potential bursting in response to sanshool (11 of 15 fibers, discussed further below). Burst firing was also observed in the presence of TRPA1 and TRPV1 antagonists (four of five fibers) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We identified three mechanically insensitive C fibers that were insensitive to sanshool, similar to the mechanically insensitive A fibers.
We next asked whether any of the sanshool-sensitive C fibers displayed unique biophysical properties. Indeed, the sanshool-sensitive population displayed significantly lower conduction velocities than the sanshool-insensitive population (0.38 ± 0.02 vs 0.59 ± 0.04 m/s) (Fig. 3E). This difference was apparent across all subgroups of C fibers, regardless of TRP channel sensitivity (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Because conduction velocity was always measured before sanshool application, the slower conduction velocity of the sanshool-responsive population is an intrinsic property of these fibers. Interestingly, there was no difference in conduction velocity between the capsaicin-sensitive or -insensitive C fibers or between the mustard oil-sensitive or -insensitive subtypes (supplemental Table 2, available at www.jneurosci.org as supplemental material). Thus, neither TRPV1 nor TRPA1 agonists mark a distinct population of C fibers based on conduction velocity. Together, these data indicate that sanshool activates a unique population of slow-conducting C fibers.
Sanshool-evoked responses are distinct from those evoked by capsaicin
Our data demonstrate that the majority of fibers activated by sanshool are Aβ and D-hair neurons that mediate the detection of light-touch rather than noxious stimuli. However, there is a subset of C fibers that do respond robustly to sanshool. All previously characterized compounds that activate C fibers evoke nocifensive behaviors when applied to the hindpaw. Thus, we asked whether application of sanshool to the top of the hindpaw elicits nocifensive behaviors. Topical application of sanshool (100 mm) failed to elicit any flinching or guarding behaviors typically associated with C-fiber agonists, such as capsaicin or mustard oil (n = 5; data not shown).
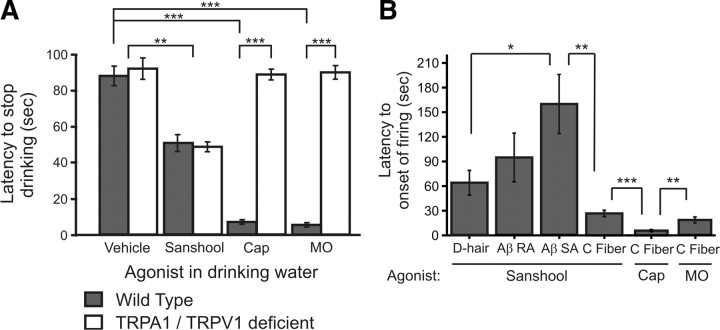
Previous studies have shown that sanshool is aversive when included in drinking water or food (Epple et al., 2001). Thus, we directly compared the acute effects of sanshool versus capsaicin, using the drinking aversion assay. When provided a control solution, water-deprived wild-type mice initially drank for ∼87 s before stopping (87.6 ± 5.4 s) (Fig. 4A). The duration of drinking was significantly curtailed when sanshool was added to the solution (50 ± 4.9 s) (Fig. 4A). However, these latencies were significantly shorter when capsaicin was added to the solution (6.3 ± 1.2 s).
Figure 4.
Slow onset of behavioral and sensory fiber responses to sanshool compared with noxious chemicals. A, Latency to stop drinking after water-deprived mice were given water containing the indicated compounds. Sanshool (1 mm), capsaicin (1 μm; Cap), and mustard oil (100 μm; mo) were added to a 0.125% saccharine solution. **p < 0.01 and ***p < 0.001, significant differences between drinking latencies (n = 8–11 mice per agonist; one-way ANOVA, followed by Tukey–Kramer post hoc test). B, Latency to the first action potential in different fiber types evoked by sanshool (200 μm), capsaicin (1 μm), or mustard-oil (1 mm). Latencies to sanshool were compared across fiber type, and the C-fiber latencies to different agonists were compared. *p < 0.05, **p < 0.01, and ***p < 0.001, significant difference between groups (one-way ANOVA, followed by Tukey–Kramer post hoc test).
We observed a striking similarity between the behavioral latencies and the kinetics of the onset of sanshool- or capsaicin-evoked firing (Fig. 4B). Unmyelinated C fibers responded to sanshool with an average latency of 27 versus 5 s in response to capsaicin. D-hair fibers responded with an average latency of 65 s, and RA-Aβ and SA-Aβ fibers exhibited the longest latency to firing onset (98 ± 33 and 160 ± 37 s, respectively). This finding suggests that capsaicin and sanshool promote aversion through distinct mechanisms. Consistent with this hypothesis, TRPV1−/−/TRPA1−/− mice displayed drinking latencies comparable with wild-type mice when given control or sanshool solution (control, 92 ± 5.9 s; sanshool, 48.7 ± 2.6 s) (Fig. 4A). However, when provided with capsaicin- and mustard oil-laced water, double knock-out animals displayed similar drinking latencies in the presence or absence of these irritants. Thus, neither TRPV1 nor TRPA1 is an essential mediator of the behavioral response to sanshool. These data suggest that activation of the low conduction velocity, sanshool-sensitive C fibers is not sufficient to induce robust nocifensive behaviors.
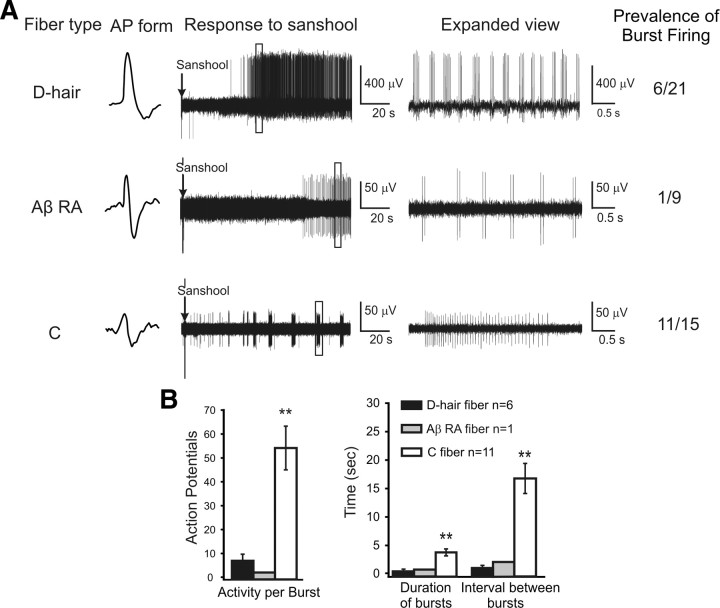
Sanshool evokes periodic burst firing in sensory fibers
In a subset of fibers, sanshool evoked a burst pattern of action potential firing (Fig. 5A). Bursting was most prevalent among sanshool-sensitive C fibers and D-hair fibers, occurring in 73 and 26% of sanshool-sensitive fibers, respectively. Large myelinated Aβ fibers were the least likely to show bursting because only one RA-Aβ fiber exhibited bursting and none of the SA-Aβ fibers displayed bursting (Fig. 5A). Interestingly, the rapidly adapting D-hair fibers and slowly adapting C fibers displayed distinct patterns of burst firing. Rapidly adapting D-hair fibers (and the RA-Aβ fiber) issued quick bursts of action potentials with short intervals, whereas the slowly adapting C fibers issued significantly longer-duration bursts of action potentials at less frequent intervals (Fig. 5B, right). Consequently, the average number of action potentials per burst was considerably higher in C fibers than in D-hair or RA-Aβ fibers (Fig. 5B, left). These data suggest differences in the membrane dynamics of rapidly and slowly adapting fibers.
Figure 5.
Sanshool induces bursting activity in a subset of sensory fibers. A, Representative traces of D-hair, RA-Aβ, and C fibers that exhibited burst pattern action potential (AP) firing and the number of such fibers found in each class. The box in each example at the left indicates a region of interest that is expanded to the right of each trace. Note that the timescale is identical for each fiber, thereby reflecting differences in their pattern of burst firing. No SA-Aβ fibers displayed burst firing. B, Comparison of burst firing properties across fiber types. Left, Average number of action potentials fired per burst. Right, Average duration of individual burst and length of interval between bursts for each fiber type. **p < 0.01, significant difference among groups (one-way ANOVA).
Discussion
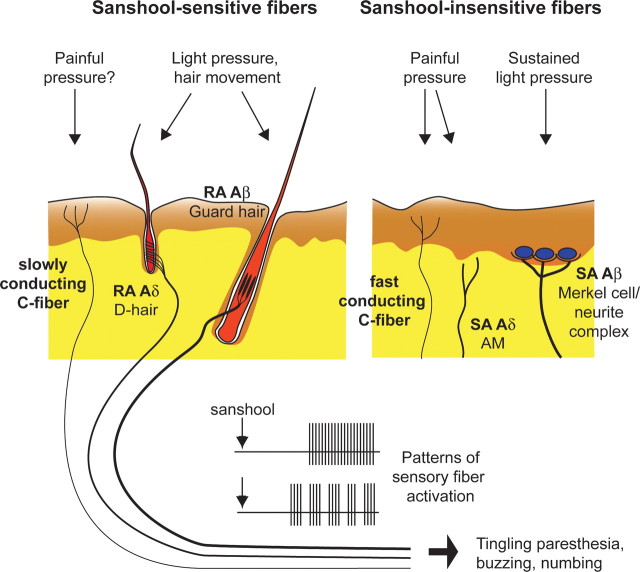
Here, we sought to identify the subtypes of sensory neurons that underlie the tingling paresthesia elicited by hydroxy-α-sanshool. Our findings show that sanshool is the first pharmacological agent identified that can discriminate between distinct subsets of mechanosensory neurons (Fig. 6). Among Aδ fibers, virtually all D-hair afferents were vigorously excited by sanshool, whereas AM nociceptors were completely unresponsive. D-hair afferents are the most sensitive of all mechanoreceptors, with mechanical thresholds below the measurable limit (Brown and Iggo, 1967; Burgess et al., 1968; Koltzenburg et al., 1997). They are a key fiber type required for normal tactile acuity and movement detection (Brown and Iggo, 1967; Wetzel et al., 2007). In addition, D-hairs have also been implicated in diabetic peripheral neuropathy (Shin et al., 2003; Jagodic et al., 2007), which often leads to tingling paresthesia in patients.
Figure 6.
Schematic of cutaneous mechanosensory afferents underlying sanshool-induced tingling paresthesia. The putative cutaneous innervation patterns of sanshool-sensitive (left) and sanshool-insensitive (right) mechanosensory afferents were examined in this study (Brown and Iggo, 1967; Burgess et al., 1968; Luo et al., 2009). The dermal and epidermal layers of the skin are represented in yellow and brown, respectively. Thickness of sensory fibers represents degree of myelination. Application of sanshool to the skin induces tonic or bursting activation of fibers, leading to sensation of tingling paresthesia (bottom).
Sanshool also activated rapidly adapting Aβ mechanoreceptors that encode the movement of guard hair follicles. Similar to rapidly adapting Aδ D-hair receptors, sanshool was more potent in activating Aβ fibers with rapidly adapting properties to mechanical force than those with slowly adapting properties. Thus, among all myelinated fibers, sanshool activates rapidly adapting fibers far more extensively than slowly adapting fibers. Spontaneous activity in rapidly adapting myelinated fibers has been implicated in both injury- and disease-evoked paresthesia, as well as in postischemic paresthesia; however, the exact neuronal subtypes that mediate tingling paresthesia have not been characterized (Ochoa and Torebjörk, 1980; Nordin et al., 1984).
A subset of slowly adapting Aβ fibers also responded to sanshool, albeit with lower firing intensities. Most slowly adapting Aβ fibers are thought to be “SA-I” that innervate Merkel cells and encode sustained pressure to skin, but some slowly adapting Aβ fibers are “SA-II” and sense skin stretch (Srinivasan et al., 1990; LaMotte et al., 1998). Interestingly, in rats 35% of slowly adapting Aβ fibers in the sural nerve and in mice 52% of slowly adapting Aβ fibers in the saphenous nerve are reported to be SA-II stretch sensors (Leem et al., 1993; Maricich et al., 2009). Furthermore, a distinguishing feature of SA-II receptors is that they are approximately six times less sensitive to skin indentation than SA-I receptors (Johansson and Vallbo, 1979, 1980). Thus, two findings support the idea that the sanshool-sensitive slowly adapting Aβ fibers are SA-II type skin stretch sensors. First, the proportion of sanshool-sensitive slowly adapting Aβ fibers (36%) is consistent with the proportion of SA-II type skin stretch sensors. Second, the sanshool-sensitive SA-Aβ fibers were approximately fivefold less sensitive to sustained force than the sanshool-insensitive population.
Sanshool activated a unique subset of C fibers that has an intrinsically slower conduction velocity than other C fibers. Conduction velocity is primarily dependent on fiber diameter and myelination, which influence the internal resistance and membrane capacitance of nerve axons. Thus, we may observe this difference because sanshool-sensitive channels are expressed on the smallest-diameter C fibers. However, conduction velocity also correlates with the length constant of a nerve fiber, which is directly proportional to membrane resistance (Hodgkin and Rushton, 1946; Koester and Siegelbaum, 2000). An intriguing possibility is that sanshool-sensitive channels, potentially KCNK18 channels, decrease membrane resistance and thereby directly slow the conduction velocity. Previous studies of tingling paresthesia in humans have failed to report aberrant activity of Aδ or C fibers (Ochoa and Torebjörk, 1980; Nordin et al., 1984). However, this may be attributable to technical difficulties in recording from patients experiencing tingling paresthesia. Our data implicate both D-hair afferents and the unique population of slowly conducting C fibers in tingling paresthesia.
Our data lend support to the hypothesis that sanshool elicits tingling paresthesia through selective activation of mechanosensitive somatosensory neurons (Fig. 6). Human psychophysical testing shows that sanshool exhibits its sensory effects ∼60 s after application (Bryant and Mezine, 1999). Our behavioral data show that mice respond to the effects of sanshool with a characteristic latency of 50 s, which is strikingly similar to that observed in humans. The sanshool response latency is significantly slower than latencies to capsaicin or mustard oil. In addition, sanshool consumption fails to elicit the nocifensive responses of nose rubbing and wiping that are commonly observed after consumption of capsaicin or mustard oil (our unpublished observations). Moreover, no differences were observed in the sanshool response latency between wild-type and TRPA1−/−/TRPV1−/− animals. Thus, sanshool-evoked behaviors more likely result from tingling paresthesia rather than painful irritation. This is consistent with the activation pattern of Aδ and Aβ fibers by sanshool, as well as with results from human psychophysical studies demonstrating that sanshool does not elicit pain sensations (Bryant and Mezine, 1999; Sugai et al., 2005). Although sanshool also activates a subset of C fibers, it is unclear whether these C fibers actually transmit pain signals. Several studies have demonstrated the existence of C fibers that transmit information other than pain. For example, a recent study demonstrated the existence of unmyelinated C fibers that code for pleasant touch sensations in humans (Löken et al., 2009). In addition, C fibers that transmit sensations of brushing and itch have also been reported (Zotterman, 1939). Finally, specific labeling of neurons that express a Mas-related G-protein-coupled receptor, MrgprB4, revealed a unique subpopulation of C fibers that specifically innervate the skin but not the viscera; these fibers are hypothesized to function as touch receptors rather than nociceptors (Liu et al., 2007). Additional analysis at the molecular and behavioral levels is required to elucidate the exact role of this new class of sanshool-sensitive C fibers.
Common among all subtypes of sanshool-sensitive fibers is the presence of action potential bursting, which we observed in 29% of fibers. Bursting is exhibited by many neurons within the CNS, as well as some peripheral neurons. A short burst of action potentials may temporally summate to provide high-fidelity neuronal transmission (Williams and Stuart, 1999) or foster long-term potentiation to strengthen neuronal synapses (Liu et al., 2008). In the peripheral nervous system, bursting has been described in trigeminal afferents in the brainstem that are thought to play a key role in the central pattern generator circuit regulating mastication in rodents (Brocard et al., 2006; Hsiao et al., 2009). Bursting is also associated with tingling paresthesia. Microelectrode recordings show robust bursting of sensory afferents in normal human subjects experiencing tingling paresthesia (Ochoa and Torebjörk, 1980). In addition, neuronal recordings from patients suffering from activity-dependent tingling paresthesia showed robust bursting of myelinated, rapidly adapting mechanoreceptors that increased with the degree of paresthesia. Finally, in rat models of diabetic neuropathy, robust bursting of medium-diameter fibers increased in diabetic neurons compared with wild-type neurons (Jagodic et al., 2007). Indeed, tingling paresthesia is a common complaint of diabetic patients with neuropathy. We speculate that the bursting pattern may underlie the tingling sensation commonly associated with chewing Szechuan peppers.
Activation of TRPA1 and TRPV1 and inhibition of the two-pore potassium channels KCNK3 (TASK-1), KCNK9 (TASK-3), and KCNK18 (TRESK) have been proposed as mechanisms by which sanshool activates neurons (Koo et al., 2007; Bautista et al., 2008; Menozzi-Smarrito et al., 2009; Riera et al., 2009). However, we demonstrate that sanshool-evoked fiber responses are of similar prevalence and amplitude in the presence or absence of TRPA1 and TRPV1 selective antagonists. Likewise, sanshool-evoked behaviors were similar between wild-type and TRPA1−/−/TRPV1−/− animals. These data suggest that neither TRPA1 nor TRPV1 mediate the excitatory effects of sanshool. In somatosensory neurons, expression and electrophysiological studies show the presence of KCNK18 channels (Dobler et al., 2007; Kang et al., 2008), but expression of KCNK3 and KCNK9 have not been demonstrated; however, KCNK3 and KCNK9 are expressed by keratinocytes in the skin (Kang and Kim, 2006). Thus, sanshool may act directly on KCNK channels in sensory neurons as well as in keratinocytes, which are known to modulate sensory neuron function (Koizumi et al., 2004; Lumpkin and Caterina, 2007) to induce tingling paresthesia. The bursting behavior observed in response to sanshool application is also consistent with a model of potassium channel blockade. Bursting in trigeminal neurons has been linked to the activity of Kv1 channels (Hsiao et al., 2009), and tetraethylammonium-insensitive potassium channel(s) may contribute to burst firing (Brocard et al., 2006). However, analysis of KCNK-deficient mice is required to test this hypothesis. Recently, two other members of the KCNK channel family, KCNK2 (TREK-1) and KCNK4 (TRAAK), have been shown to regulate responses to thermal and mechanical stimuli in nociceptors (Maingret et al., 1999; Noël et al., 2009). Thus, the KCNK family of channels may play key roles in a variety of mechanosensitive sensory fibers. Again, the analysis of mice lacking KCNK channels will be required to test this hypothesis.
Our finding that sanshool robustly activates a distinct subset of D-hair, ultrasensitive light-touch receptors in the skin and targets novel, uncharacterized populations of Aβ- and C-fiber nerve afferents shows that sanshool is an innovative tool for physiological and molecular studies. In addition, characterization of sanshool-sensitive mechanoreceptors represents an essential first step in identifying the cellular and molecular mechanisms underlying tingling paresthesia that accompanies peripheral neuropathy and injury.
Footnotes
This study was supported by National Institutes of Health Grant NS40538 (C.L.S.) and a Burroughs Wellcome Career Award in Biosciences and Alfred P. Sloan Fellowship (D.M.B.). We thank Dr. Philip Kym at Abbott Laboratories for the TRPV1 antagonist A-425619 and Dr. Magdalene Moran at Hydra Biosciences for the TRPA1 antagonist HC-030031.
References
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, Nicoll RA, Julius D. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008;11:772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard F, Verdier D, Arsenault I, Lund JP, Kolta A. Emergence of intrinsic bursting in trigeminal sensory neurons parallels the acquisition of mastication in weanling rats. J Neurophysiol. 2006;96:2410–2424. doi: 10.1152/jn.00352.2006. [DOI] [PubMed] [Google Scholar]
- Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant BP, Mezine I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999;842:452–460. doi: 10.1016/s0006-8993(99)01878-8. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968;31:833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Döring F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in muring dorsal root ganglion neurons. J Physiol. 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple G, Bryant BP, Mezine I, Lewis S. Zanthoxylum piperitum, and Asian spice, inhibits food intake in rats. J Chem Ecol. 2001;27:1627–1640. doi: 10.1023/a:1010462309244. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Rushton WAH. The electrical components of a crustacean nerve fiber. Proc R Soc Lond B Biol Sci. 1946;133:444–479. doi: 10.1098/rspb.1946.0024. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Kaur G, Vong A, Bawa H, Chandler SH. Participation of Kv1 channels in control of membrane excitability and burst generation in mesencephalic V neurons. J Neurophysiol. 2009;101:1407–1418. doi: 10.1152/jn.91053.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagodic MM, Pathirathna S, Nelson MT, Mancuso S, Joksovic PM, Rosenberg ER, Bayliss DA, Jevtovic-Todorovic V, Todorovic SM. Cell-specific alterations of T-type calcium current in painful diabetic neuropathy enhance excitability of sensory neurons. J Neurosci. 2007;27:3305–3316. doi: 10.1523/JNEUROSCI.4866-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Res. 1980;184:353–366. doi: 10.1016/0006-8993(80)90804-5. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim GT, Kim EJ, La JH, Lee JS, Lee ES, Park JY, Hong SG, Han J. Lamotrigine inhibits TRESK regulated by G-protein coupled receptor agonists. Biochem Biophys Res Commun. 2008;367:609–615. doi: 10.1016/j.bbrc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Koester J, Siegelbaum SA. Local signaling: passive electrical properties of the neuron. In: Kandel ER, Schwartz JL, Jessell TM, editors. Principles of neural science. Ed 4. New York: McGraw-Hill; 2000. [Google Scholar]
- Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M, Inoue K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Koo JY, Jang Y, Cho H, Lee CH, Jang KH, Chang YH, Shin J, Oh U. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci. 2007;26:1139–1147. doi: 10.1111/j.1460-9568.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Friedman RM, Lu C, Khalsa PS, Srinivasan MA. Raised object on a planar surface stroked across the fingerpad: responses of cutaneous mechanoreceptors to shape and orientation. J Neurophysiol. 1998;80:2446–2466. doi: 10.1152/jn.1998.80.5.2446. [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu MC, Wang KK. Calpain in the CNS: from synaptic function to neurotoxicity. Sci Signal. 2008;1:re1. doi: 10.1126/stke.114re1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10:946–948. doi: 10.1038/nn1937. [DOI] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, Ginty DD. Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on Ret signaling. Neuron. 2009;64:841–856. doi: 10.1016/j.neuron.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Menozzi-Smarrito C, Riera CE, Munari C, Le Coutre J, Robert F. Synthesis and evaluation of new alkylamides derived from alpha-hydroxysanshool, the pungent molecule in Szechuan pepper. J Agric Food Chem. 2009;57:1982–1989. doi: 10.1021/jf803067r. [DOI] [PubMed] [Google Scholar]
- Noël J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Nyström B, Wallin U, Hagbarth KE. Ectopic sensory discharges and paresthesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain. 1984;20:231–245. doi: 10.1016/0304-3959(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Ochoa JL, Torebjörk HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–853. doi: 10.1093/brain/103.4.835. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Riera CE, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, Vogel H, Simon SA, le Coutre J. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009;157:1398–1409. doi: 10.1111/j.1476-5381.2009.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer CM, Carstens MI, Simons CT, Slack J, McCluskey TS, Furrer S, Carstens E. Activation of lumbar spinal wide-dynamic range neurons by a sanshool derivative. J Neurophysiol. 2009;101:1742–1748. doi: 10.1152/jn.91311.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci. 2003;6:724–730. doi: 10.1038/nn1076. [DOI] [PubMed] [Google Scholar]
- Srinivasan MA, Whitehouse JM, LaMotte RH. Tactile detection of slip: surface microgeometry and peripheral neural codes. J Neurophysiol. 1990;63:1323–1332. doi: 10.1152/jn.1990.63.6.1323. [DOI] [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci. 1998;18:7040–7046. doi: 10.1523/JNEUROSCI.18-17-07040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci. 1999;19:8509–8516. doi: 10.1523/JNEUROSCI.19-19-08509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Shin JB, Lewin GR. Neurotrophin-4: a survival factor for adult sensory neurons. Curr Biol. 2002;12:1401–1404. doi: 10.1016/s0960-9822(02)01072-2. [DOI] [PubMed] [Google Scholar]
- Sugai E, Morimitsu Y, Kubota K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC) and their pungent characteristics. Biosci Biotechnol Biochem. 2005;69:1958–1962. doi: 10.1271/bbb.69.1958. [DOI] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Moshourab R, Kozlenkov A, Labuz D, Caspani O, Erdmann B, Machelska H, Heppenstall PA, Lewin GR. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol. 1999;521:467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotterman Y. Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. J Physiol. 1939;95:1–28. doi: 10.1113/jphysiol.1939.sp003707. [DOI] [PMC free article] [PubMed] [Google Scholar]