Abstract
Background: Docetaxel is associated with prolonged survival in castration-resistant prostate cancer (CRPC). Platinum compounds have modest but distinct single-agent activity. Carboplatin may have greatest potential for benefit when combined with taxanes. We investigated whether there is a subset of patients with CRPC for whom the efficacy of combination taxane–estramustine–carboplatin (TEC) chemotherapy may be greatest.
Patients and methods: Individual patient data (n = 310) were obtained from seven trials using TEC chemotherapy. Prostate-specific antigen (PSA) response was defined as ≥50% post-therapy decline from baseline. Overall survival was defined from baseline to death from any cause. Logistic and Cox regression were used to investigate heterogeneity in outcome to TEC by patient and disease characteristics. Predicted survival probabilities were calculated from the Halabi Cancer and Leukemia Group B (CALGB) nomogram.
Results: The pooled PSA response proportion was 69% [95% confidence interval (CI) 56% to 80%]. There was no evidence of differential PSA response by disease characteristics. Established prognostic factors were associated with survival. The pooled 12-month survival estimate of 79% (95% CI 71% to 84%) was higher than the median 59% 12-month nomogram-predicted survival.
Conclusions: TEC chemotherapy has significant clinical activity in CRPC. A randomized, controlled trial evaluating the addition of carboplatin to taxane-based chemotherapy is needed to elucidate the value of carboplatin in CRPC.
Keywords: carboplatin, estramustine, prostate cancer, taxanes
introduction
Prostate cancer remains the second leading cause of cancer death in men in the United States [1]. Although initially responsive to androgen deprivation therapy—orchiectomy or luteinizing hormone-releasing hormone agonist therapy—prostate cancer almost always becomes refractory to hormonal manipulation [2]. Two large phase III trials showed that docetaxel combined with either prednisone or estramustine was associated with a survival benefit compared with mitoxantrone and prednisone in the treatment of castration-resistant prostate cancer (CRPC) [3, 4].
Platinum compounds have been studied both as single agents and in combination regimens in the treatment of CRPC [5–18]. Seven phase I and II trials have evaluated the efficacy of taxane, estramustine and carboplatin (TEC) [12–18], observing ≥50% prostate-specific antigen (PSA) declines in 58%–100% of patients and measurable responses in about half of the patients with measurable disease.
Platinum compounds have modest but distinct single-agent activity in CRPC, but when combined with taxanes and estramustine, carboplatin may offer the greatest potential for benefit. It is unknown whether there is a subset of patients with CRPC for whom carboplatin enhances efficacy of the combination chemotherapy. We therefore pooled the data from the seven phase I and phase II trials of TEC and investigated whether there were factors that predicted improved response to TEC and/or survival following treatment with TEC. The ultimate goal was to identify if there were populations most likely to benefit from the carboplatin-containing triplet chemotherapy.
patients and methods
The meta-analysis study was approved by the Dana-Farber/Harvard Cancer Center (DF/HCC) institutional review board. Seven recent phase I or II trials [12–18] using TEC and involving 310 CRPC patients have been published (Table 1). Eligibility criteria and PSA response and progression definitions were comparable across trials and consistent with PSA Working Group definitions for trials in men with CRPC [19]. All trials included CRPC patients with either measurable or nonmeasurable metastatic disease and reported approximately half of the patients having measurable disease. With the exception of the Oh et al. [15] Cancer and Leukemia Group B (CALGB) trial, patients may have received prior chemotherapy, and 16% of patients across all trials were reported to have received prior chemotherapy. In the Kelly et al. [12] and Solit et al. [14] trials, estramustine- and taxane-based regimens were allowed, and in the Urakami et al. [13] and Kikuno et al. [18] trials, most chemotherapy-treated patients received estramustine-based regimens. The majority of patients were enrolled from June 1997 to December 2001, with only the Oh et al. [16] DF/HCC trial completing enrollment in 2002 and the Kikuno et al. trial enrolling from 2001 to 2005.
Table 1.
Seven TEC trials in men with castration-resistant prostate cancer and the PSA response rates and overall survivala
| Publication | Design | Regimen | ≥50% PSA decline | Died | Median survival (months) |
| Kelly et al. [12] | Phase II | 28-day cycle: T (P) 100 mg/m2 i.v., weekly; E 10 mg/kg/day p.o., days −2 to +2 relative to TC; C AUC 6 (30 min) i.v., weekly | 36/56 (64%) | 45 (80%) | 21 |
| Urakami et al. [13] | Phase II | 28-day cycle: T (P) 100 mg/m2 i.v., weekly; E 10 mg/kg/day p.o., daily; C AUC 6 i.v., day 1 | 30/30 (100%) | 23 (77%) | 21 |
| Solit et al. [14] | Phase I/II | 28-day cycle: T (P) 100 mg/m2 i.v., weekly; E 500–1500 mg/m2 i.v., weekly; C AUC 6 (30 min) i.v., day 1 | 19/30 (63%) | 28 (93%) | 16 |
| Oh et al. CALGB [15] | Phase II | 21-day cycle: T (D) 70 mg/m2 i.v., day 2; E 840 mg/day p.o., days 1–5; C AUC 5 i.v., day 2 | 23/40 (58%) | 28 (70%) | 17 |
| Oh et al. DF/HCC [16] | Phase I | 28-day cycle: T (D) 20–43 mg/m2 i.v., days 2, 9 and 16; E 420 mg/day p.o., days 1–5, 8–12 and 15–19; C AUC 5 or 6 i.v., day 2 | 18/30 (60%) | 8 (27%) | 18 |
| Berry et al. [17] | Phase II | 28-day cycle: T (P) 80 mg/m2 i.v., days 2, 9 and 16; E 840 mg/day p.o., days 1–3, 8–10 and 15–17; C AUC 2 i.v., days 2, 9 and 16 | 50/84 (60%) | 52 (62%) | 15 |
| Kikuno et al. [18] | Phase II | 28-day cycle: T (D) 30 mg/m2 i.v., weekly; E 10 mg/kg/day p.o., daily; C AUC 6 i.v., day 1 | 38/40 (95%) | 11 (28%) | 27 |
| All | 214/310 (69%) | 195 (63%) | 18 |
Survival information herein is updated for several trials since the time of publication of the article.
T, taxane; E, estramustine; C, carboplatin; PSA, prostate-specific antigen; P, paclitaxel; p.o., by mouth; AUC, area under the curve (mg·min/ml); CALGB, Cancer and Leukemia Group B; D, docetaxel; DF/HCC, Dana-Farber/Harvard Cancer Center.
Individual patient-level data were requested for the seven trials. The patient and disease data that had been collected in each trial varied, but the requested data included the following information: at diagnosis [year of diagnosis, age, PSA, biopsy Gleason sum, tumor–node–metastasis staging and type of primary definitive therapy]; during the hormone-sensitive period [year of initiation of hormonal therapy (HT), PSA at HT initiation and types of secondary HT that were given]; at trial registration [age, prior HTs, PSA, hemoglobin, lactate dehydrogenase (LDH), alkaline phosphatase (ALP), metastatic sites, Eastern Cooperative Oncology Group performance status (ECOG PS)] and during the trial (year of start and duration of trial treatment, PSA nadir value, maximum PSA decline achieved, duration and number of cycles to achieve nadir, duration from treatment start until PSA progression, survival status and duration from start of treatment). For most trials, longer patient follow-up was available for this analysis than previously published and therefore the number of deaths may be greater and the estimated median survival may differ from that in the published article.
PSA response was defined as whether the patient achieved a post-therapy decline in PSA level from baseline of ≥50% on the trial, as derived for each trial; in two trials (Urakami et al. [13] and Kikuno et al. [18]), PSA response required at least one ≥50% decline and the other five trials required confirmation. Heterogeneity in the response rate was assessed using a mixed-effects meta-analysis approach [20]. Logistic regression, estimated using generalized estimating equations to account for trial, was used to investigate the associations of patient and disease characteristics with PSA response; small-sample-adjusted score statistic P values are reported [21]. Because of unavailable variable values across trials, continuous variables were categorized by quartiles of their distributions and modeling included dummy categories for unavailable values so that all observations entered all models.
Overall survival (OS) was defined as the number of months from start of trial treatment until death from any cause or was censored at the date last known alive. Cox proportional hazards regression modeling, stratified by trial, was used to investigate the association of patient and disease characteristics with survival; Wald chi-square P values were reported. Multivariable modeling implemented forward selection.
Patient data were entered into the Halabi et al. [22] CALGB nomogram to obtain predicted 12- and 18-month OS probabilities. Patients were divided in four groups according to the quartiles of the distribution of predicted probabilities. For each group, the median and interquartile range (IQR) of nomogram-predicted 12- and 18-month OS probabilities were calculated, and the Kaplan–Meier estimates of 12- and 18-month OS probabilities were calculated along with 95% confidence intervals (CIs). Trials were excluded from this analysis if no laboratory data were available (Berry et al. [17] and Kikuno et al. [18]); otherwise, to maximize the number of analyzable patients, the occasional missing values were imputed with the mean value (LDH in the Oh et al. DF/HCC trial and 5.6% of all other values) and a sensitivity analysis repeated the analyses by excluding these patients to ensure consistent conclusions.
results
Across all seven trials, the median patient age at trial initiation was 68 years (IQR 62–74 years), 89% of patients had ECOG PS of zero to one and the median PSA level was 119 ng/ml (IQR 45–326 ng/ml). Among patients for whom data on metastatic disease sites were available, 81% had bone metastases and 54% had extra-skeletal metastases.
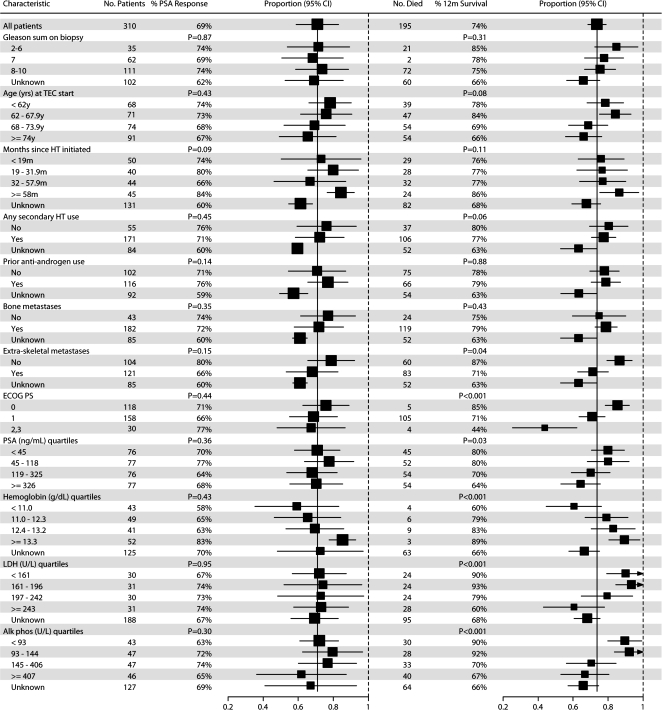
The pooled proportion of patients achieving PSA response of ≥50% PSA decline from baseline on TEC across the seven trials was 69% (214 of 310 patients, 95% CI 56% to 80%; Table 1). There was some evidence of heterogeneity of the response proportions across trials (P = 0.052) contributed primarily by the two Shimane University trials [13, 18]. There was no evidence that PSA response proportions to TEC differed according to patient or disease characteristics (Figure 1).
Figure 1.
Associations of patient and disease characteristics with PSA response and OS. Gleason sum on biopsy, secondary HT and metastatic disease site data not available for the Berry et al. trial. The Berry et al. and Kikuno et al. trials did not have laboratory data other than PSA; Oh et al. Dana-Farber/Harvard Cancer Center trial did not have LDH data. P values were obtained from logistic regression (PSA response) or Cox proportional hazards regression (OS). Extra-skeletal metastases include any metastases other than bone, including visceral, lymph nodes and soft tissue. PSA, prostate-specific antigen; OS, overall survival; HT, hormonal therapy; LDH, lactate dehydrogenase; TEC, taxane, estramustine and carboplatin.
The estimated median survival was 18 months, pooled across trials (Table 1). OS was statistically significantly longer in the most recently conducted trial by Kikuno et al. [18], with estimated median 27-month OS. In univariate analyses stratified by trial, absence of extra-skeletal metastases, lower ECOG PS, higher hemoglobin, lower LDH, lower ALP and lower PSA at trial enrollment were associated with longer survival (each P < 0.05; Figure 1). In multivariable analyses, extra-skeletal metastases, ECOG PS, hemoglobin, LDH and ALP remained statistically significant (each P < 0.05; Table 2).
Table 2.
Multivariable Cox proportional hazards regression model for OS
| Variable | OS, hazards ratio (95% CI) | P value |
| Variables included in the final model | ||
| Extra-skeletal metastases | <0.01 | |
| Absent | 1 (reference) | |
| Present | 2.1 (1.4–3.1) | |
| ECOG performance status | <0.01 | |
| 0 | 1 (reference) | |
| 1 | 1.9 (1.3–2.8) | |
| 2 | 2.6 (1.4–4.8) | |
| 3 | 14.3 (4.7–43.7) | |
| Hemoglobin | <0.01 | |
| First quartile (<11.0 g/dl) | 2.7 (1.5–5.0) | |
| Second quartile (11.0–12.3 g/dl) | 1.7 (1.0–2.9) | |
| Third quartile (12.4–13.2 g/dl) | 2.0 (1.2–3.4) | |
| Fourth quartile (≥13.3 g/dl) | 1 (reference) | |
| Alkaline phosphatase | <0.05 | |
| First quartile (<93 U/l) | 1 (reference) | |
| Second quartile (93–144 U/l) | 1.2 (0.7–2.1) | |
| Third quartile (145–406 U/l) | 1.6 (0.9–2.9) | |
| Fourth quartile (≥407 U/l) | 2.5 (1.2–5.0) | |
| LDH | <0.02 | |
| First quartile (<161 U/l) | 1 (reference) | |
| Second quartile (161–196 U/l) | 1.0 (0.5–2.0) | |
| Third quartile (197–242 U/l) | 0.9 (0.4–1.9) | |
| Fourth quartile (≥243 U/l) | 2.1 (1.1–4.2) | |
| Variables not included in the final model | ||
| Log (PSA) | 1.0 (0.9–1.1) | 0.66 |
| Biopsy Gleason sum | 0.95 | |
| <8 | 1 (reference) | |
| 8–10 | 1.0 (0.7–1.5) |
OS, overall survival; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PSA, prostate-specific antigen.
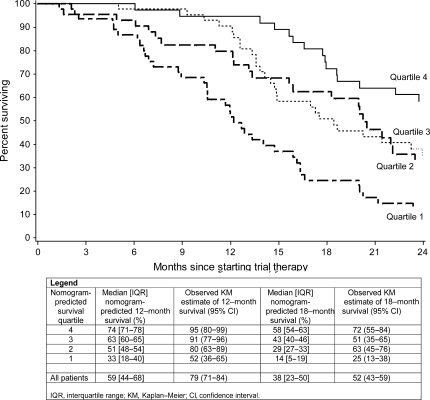
Because of the consistency of these factors with the prognostic nomogram of Halabi et al. [22]—with the exception of biopsy Gleason sum and PSA at treatment initiation (Table 2)—and the absence of a control group comparator, we evaluated patients’ predicted survival based on the Halabi et al. CALGB nomogram. The 185 patients evaluated from five trials were grouped according to quartiles of the distribution of their nomogram-predicted probabilities (Figure 2). The median nomogram predictions of 12-month survival for the four quartile groups were 33%, 51%, 63% and 74%. The corresponding observed 12-month survival estimates were higher among the groups, at 52% (95% CI 36% to 65%), 80% (95% CI 63% to 89%), 91% (95% CI 77% to 96%) and 95% (95% CI 80% to 99%), respectively.
Figure 2.
Kaplan–Meier estimates of overall survival among four groups defined by Halabi et al. Cancer and Leukemia Group B (CALGB) nomogram-predicted survival probabilities.
discussion
This study set out to investigate if there were any factors or specific populations that were most likely to respond to TEC. However, there was no evidence of heterogeneity in the PSA response rate to TEC across patient or disease characteristics.
While the data confirmed the prognostic factors for men with CRPC in the Halabi et al. [22] CALGB nomogram—which identifies Gleason sum, PS, ALP, LDH, hemoglobin, PSA and presence of visceral disease at treatment initiation as statistically significant prognostic factors of OS—the data did not indicate any unique factors or specific populations that would benefit more substantially from this treatment. Consistent with the Halabi et al. nomogram, patients with better PS, higher hemoglobin, lower ALP, lower LDH and presence of extra-skeletal metastases at the time of trial enrollment had better survival. In contrast, PSA was statistically significant only in univariate and not in multivariable analysis, and Gleason sum was not associated with survival among these TEC trial patients.
Patient and disease characteristics between the pooled TEC trials cohort and the CALGB trials cohort used by Halabi et al. [22] to develop the nomogram were comparable for the defined prognostic indicators. However, there were some differences in the characteristics of the two patient populations. The median number of years since diagnosis was 4.5 years in the TEC cohort versus 3 years in the CALGB learning sample. This might indicate that the TEC cohort had slower growing disease type and/or that they had more prior treatments before initiating TEC therapy. There was a substantial difference in the number of patients who received prior secondary HT, 70% in the TEC cohort versus 99% in the CALGB cohort, specifically notable in the use of antiandrogen therapy (54% versus 77%, respectively), though the TEC cohort included 16% of patients who received prior chemotherapy compared with the chemotherapy-naive CALGB cohort. The median patient age in the CALGB cohort was 71 years versus 67 years in the TEC cohort. In terms of metastases, 92% of the CALGB cohort had bone metastases, 32% had lymph node involvement and 13% visceral disease, as compared with 79% with bone and 58% with extra-skeletal metastases in the TEC cohort. The TEC trials reported ∼50% of patients with measurable disease compared with 32% in the CALGB cohort. Thus, the patient and disease characteristics do not clearly indicate that one population had more or less advanced disease than the other at study entry indicating that the longer OS seen in the TEC patients as compared with that predicted by the Halabi CALGB nomogram cannot be completely attributed to patient and disease characteristics.
The combination of a taxane, estramustine and carboplatin is an active regimen in CRPC. The data clearly support a high PSA response rate and indicate a superior OS than that predicted by the Halabi CALGB nomogram. The observed median survival was 18 months in the TEC cohort, whereas the median nomogram-predicted 18-month survival percentage was 38%. It is worth acknowledging that only one of the six CALGB clinical trials used to develop the Halabi nomogram incorporated a taxane as part of the treatment regimen. Since the publication of the seven TEC studies, there has been significant evidence to indicate that taxanes without platinum result in a survival benefit in CRPC. Petrylak et al. [3] compared docetaxel and estramustine with mitoxantrone and prednisone and showed an improved OS of 17.5 versus 15.6 months median survival, respectively. In the TAX327 trial [4], the observed median survival was 18.9 and 17.4 months in the two docetaxel-treated groups and 16.5 months among men treated with mitoxantrone (all patients received prednisone). A nomogram predicting survival of patients with CRPC was recently developed based on the TAX327 trial [23] involving 10 factors, but some of these factors—including pretreatment PSA doubling time and pain—were not collected in the TEC trials and this prevents the TAX327 nomogram from being evaluated in the TEC cohort.
As in the trial of Petrylak et al. [3], these seven phase II trials included estramustine, which may have increased the PSA response rate. However, a recent randomized trial comparing docetaxel, estramustine and prednisone with docetaxel and prednisone [24] has shown that the combination of docetaxel and estramustine has no apparent effect either on PSA response rate (73% versus 69%) or on survival (median 19.3 versus 21 months), with a poorer toxicity profile.
The TEC regimens studied in the seven trials had acceptable toxicity profiles. Hematologic toxic effects, thromboembolism and fatigue were frequently reported, whereas other cardiovascular toxic effects, severe neuropathy and gastrointestinal toxicity were uncommon (Table 3).
Table 3.
Reported grade 3 and higher toxic effects in seven taxane–estramustine–carboplatin trials in men with castration-resistant prostate cancer
| Kelly (n = 56) | Urakami (n = 30) | Solit (n = 30) | Oh CALGB (n = 40) | Oh DFHCC (n = 30) | Berry (n = 82) | Kikuno (n = 40) | |
| Hematologic | |||||||
| Anemia | 1.8 | 59.4 | 3 | 7.5 | 6.7 | 6.1 | 32.5 |
| Leukopenia/neutropenia | 21.4 | 37.5 | 24 | 22.5 | 10.0 | 8.5 | 20.0 |
| Thrombocytopenia | 1.8 | 28.1 | 7 | 12.5 | 10.0 | 4.9 | 17.5 |
| Thrombotic microangiopathy | 5.0 | ||||||
| Cardiovascular | |||||||
| Cardiac dysrhythmia | 3.6 | ||||||
| Cardiac ischemia/infarction | 3.1 | 2.5 | |||||
| Thrombosis/embolism | 25.0 | 20 | 7.5 | 16.7 | 6.1 | ||
| Metabolic | |||||||
| Bilirubin | 3.6 | 3.1 | 3 | ||||
| Transaminase | 5.4 | 6.3 | 23 | 7.5 | 3.3 | 2.5 | |
| Hypophosphatemia | 41.1 | 57 | 7.5 | ||||
| Hyperglycemia | 37.5 | 3.1 | 37 | 10.0 | 3.3 | ||
| Gastrointestinal | |||||||
| Nausea | 7.1 | 5.0 | 20.0 | 9.8 | |||
| Vomiting | 5.4 | 3 | 5.0 | 20.0 | 6.1 | ||
| Anorexia | 3.7 | 2.5 | |||||
| Diarrhea | 1.8 | 10 | 7.5 | 3.3 | 2.4 | ||
| Constipation | 5.4 | 9.4 | 3 | ||||
| Other | |||||||
| Fatigue | 1.8 | 3.1 | 3 | 12.5 | 33.0 | 11.0 | 5 |
| Neurosensory | 12.5 | 3 | 5.0 | ||||
| Dizziness/light-headedness/syncope | 1.8 | 5.0 | 3.3 | 2.4 | |||
| Dyspnea | 6.3 | 6.7 | 2.4 | ||||
| Pleural effusion | 7.5 | ||||||
| Edema | 3.6 | 6.3 | 3.3 |
Values are percent of patients. CALGB, Cancer and Leukemia Group B; DF/HCC, Dana-Farber/Harvard Cancer Center.
Sartor et al. [25] have presented the results of a large phase III trial of the oral platinum analogue, satraplatin. This trial enrolled 950 CRPC patients, evaluating second-line satraplatin plus prednisone versus prednisone alone. Patients treated with satraplatin plus prednisone demonstrated a 42% improvement in progression-free survival (PFS) when compared with prednisone alone, as well as prolonged time to pain progression and a higher PSA response rate [25]. After 6 months, PFS was reported as 30% and 17% for the satraplatin plus prednisone arm and prednisone arm, respectively [26]. At 12 months, satraplatin plus prednisone continued to show an increase in percentage of PFS at 17% versus 7% in prednisone alone. However, no difference in OS was noted and the drug was rejected by the Food and Drug Administration for approval. Many issues have revolved around whether the failure of this drug to improve survival should indicate inactivity of the platinum class of drugs, but this was in fact a second-line study of a single agent, different from the first-line combinations evaluated in this pooled analysis.
It is hard to extrapolate from the existing data whether the favorable OS seen in the TEC studies is gained from the use of taxanes alone or whether combination therapy with taxane and platinum augments the benefit of the taxanes. A randomized, controlled trial of docetaxel versus docetaxel plus carboplatin could elucidate this issue.
funding
Bing Sound Wong Fund for Prostate Cancer Research to W.K.O.; Louis DiGiovanni Fund for Prostate Cancer Research to W.K.O.; Dana-Farber/Harvard Cancer Center Prostate Cancer SPORE (NCI 5P50CA90381); CALGB Statistical Center to S.H. (CA33601); CALGB Participating Center to W.K.O. (CA32291).
Acknowledgments
The research for CALGB 99813 was supported, in part, by grants from the U.S. National Cancer Institute to the CALGB (Richard L. Schilsky, CA31946) and to the CALGB Statistical Center (Stephen George, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Funding for CALGB 99813 was provided, in part, by Sanofi-Aventis, USA. The following CALGB institutions participated in CALGB 99813: Dana-Farber Cancer Institute, Boston, MA (Eric P. Winer, CA32291); Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH (Marc S. Ernstoff, CA04326); Memorial Sloan-Kettering Cancer Center, New York, NY (Clifford A. Hudis, CA77651); The Ohio State University Medical Center, Columbus, OH (Clara D. Bloomfield, CA77658); University of California at San Francisco, San Francisco, CA (Alan P. Venook, CA60138); University of Chicago, Chicago, IL (Gini Fleming, CA41287); University of North Carolina at Chapel Hill, Chapel Hill, NC (Thomas C. Shea, CA47559); Washington University School of Medicine, St Louis, MO (Nancy Bartlett, CA77440).
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.De La Taille A, Vacherot F, Salomon L, et al. Hormone-refractory prostate cancer: a multi-step and multi-event process. Prostate Cancer Prostatic Dis. 2001;4:204–212. doi: 10.1038/sj.pcan.4500534. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared to mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Yagoda A, Watson RC, Natale RB, et al. A critical analysis of response criteria in patients with prostatic cancer treated with cis-diamminedichloride platinum II. Cancer. 1979;44:1553–1562. doi: 10.1002/1097-0142(197911)44:5<1553::aid-cncr2820440502>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Merrin CE. Treatment of genitourinary tumours with cis-dichlorodiammineplatinum(II): experience in 250 patients. Cancer Treat Rep. 1979;63:1579–1584. [PubMed] [Google Scholar]
- 7.Moore MR, Troner MB, DeSimone P, et al. Phase II evaluation of weekly cisplatin in metastatic hormone-resistant prostate cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986;70:541–542. [PubMed] [Google Scholar]
- 8.Wagstaff AJ, Ward A, Benfield P, et al. Carboplatin. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the treatment of cancer. Drugs. 1989;37:162–190. doi: 10.2165/00003495-198937020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Miglietta L, Cannobbio L, Boccardo F. Assessment of response to carboplatin in patients with hormone-refractory prostate cancer: a critical analysis of drug activity. Anticancer Res. 1995;15:2825–2828. [PubMed] [Google Scholar]
- 10.Jungi WF, Bernhard J, Hurny C, et al. Effect of carboplatin on response and palliation in hormone-refractory prostate cancer. Swiss Group for Clinical Cancer Research (SAKK) Support Care Cancer. 1998;6:462–468. doi: 10.1007/s005200050195. [DOI] [PubMed] [Google Scholar]
- 11.Steineck G, Reuter V, Kelly WK, et al. Cytotoxic treatment of aggressive prostate tumors with or without neuroendocrine elements. Acta Oncol. 2002;418:668–674. doi: 10.1080/028418602321028292. [DOI] [PubMed] [Google Scholar]
- 12.Kelly WK, Curley T, Slovin S, et al. Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol. 2001;19:44–53. doi: 10.1200/JCO.2001.19.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Urakami S, Igawa M, Kikuno N, et al. Combination chemotherapy with paclitaxel, estramustine and carboplatin for hormone refractory prostate cancer. J Urol. 2002;168:2444–2450. doi: 10.1016/S0022-5347(05)64164-X. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Morris M, Slovin S, et al. Clinical experience with intravenous estramustine phosphate, paclitaxel, and carboplatin in patients with castrate, metastatic prostate adenocarcinoma. Cancer. 2003;98:1842–1848. doi: 10.1002/cncr.11754. [DOI] [PubMed] [Google Scholar]
- 15.Oh WK, Halabi S, Kelly WK, et al. A phase II study of estramustine, docetaxel, and carboplatin with granulocyte-colony-stimulating factor support in patients with hormone-refractory prostate carcinoma: Cancer and Leukemia Group B 99813. Cancer. 2003;98:2592–2598. doi: 10.1002/cncr.11829. [DOI] [PubMed] [Google Scholar]
- 16.Oh WK, Hagmann E, Manola J, et al. A phase I study of estramustine, weekly docetaxel and carboplatin chemotherapy in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2005;11:284–289. [PubMed] [Google Scholar]
- 17.Berry W, Friedland D, Fleagle J, et al. A phase II study of weekly paclitaxel/estramustine/carboplatin in hormone-refractory prostate cancer. Clin Genitourin Cancer. 2006;5:131–137. doi: 10.3816/CGC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- 18.Kikuno N, Urakami S, Nakamura S, et al. Phase-II study of docetaxel, estramustine phosphate, and carboplatin in patients with hormone-refractory prostate cancer. Eur Urol. 2007;51:1252–1258. doi: 10.1016/j.eururo.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 19.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 20.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Pan W, Connett JE, et al. Small-sample performance of the robust score test and its modifications in generalized estimating equations. Stat Med. 2005;24:3479–3495. doi: 10.1002/sim.2161. [DOI] [PubMed] [Google Scholar]
- 22.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in med with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Garrett-Mayer ES, Yang Y-CO, et al. A contemporary prognostic nomogram for men with hormone-refractory metastatic prostate cancer: a TAX327 study analysis. Clin Cancer Res. 2007;13:6396–6402. doi: 10.1158/1078-0432.CCR-07-1036. [DOI] [PubMed] [Google Scholar]
- 24.Machiels JP, Mazzeo F, Clausse M, et al. Prospective randomized study comparing docetaxel, estramustine, and prednisone with docetaxel and prednisone in metastatic hormone-refractory prostate cancer. J Clin Oncol. 2008;26:5261–5268. doi: 10.1200/JCO.2008.16.9524. [DOI] [PubMed] [Google Scholar]
- 25.Sartor AO, Petrylak DP, Witjes JA, et al. Satraplatin in patients with advanced hormone-refractory prostate cancer: overall survival results from the phase III satraplatin and prednisone against refractory cancer (SPARC) trial. J Clin Oncol. 2008;26(Suppl) (Abstr 5003) [Google Scholar]
- 26.Armstrong A, George D. Satraplatin in the treatment of hormone-refractory metastatic prostate cancer. Ther Clin Risk Manag. 2007;3:877–883. [PMC free article] [PubMed] [Google Scholar]