Abstract
Worldwide, an increasing number of women use oral or injectable hormonal contraceptives. However, inadequate information is available to aid women and health care professionals in weighing the potential risks of hormonal contraceptive use in individuals living with HIV-1 or at high risk of infection. Numerous epidemiological studies and challenge studies in a rhesus macaque model suggest that progesterone-based contraceptives increase the risk of HIV-1 infection in humans and simian immunodeficiency virus (SIV) infection in macaques, accelerate disease progression, and increase viral shedding in the genital tract. However, because several other studies in humans have not observed any effect of exogenously administered progesterone on HIV-1 acquisition and disease progression, the issue continues to be a topic of intense research and ongoing discussion. In contrast to progesterone, systemic or intravaginal treatment with estrogen efficiently protects female rhesus macaques against the transmission of SIV, likely by enhancing the natural protective properties of the lower genital tract mucosal tissue. Although the molecular and cellular mechanisms underlying the effect of sex steroid hormones on HIV-1 and SIV acquisition and disease progression are not well understood, progesterone and estrogen are known to regulate a number of immune mechanisms that may exert an effect on retroviral infection. This review summarizes current knowledge of the effects of various types of sex steroid hormones on immune processes involved in the biology of HIV-1 infection.
Worldwide, an increasing number of women uses oral or injectable hormonal contraceptives. However, inadequate information is available to aid women and healthcare professionals in weighing the potential risks of hormonal contraceptive use in individuals living with human immunodeficiency virus-1 (HIV-1) or at high risk of infection. Although the molecular and cellular mechanisms underlying the effect of sex steroid hormones on HIV-1 and SIV acquisition and disease progression are not well understood, progesterone and estrogen are known to regulate a number of immune mechanisms that may exert an effect on retroviral infection. This review summarizes current knowledge of the effects of various types of sex steroid hormones on immune processes involved in the biology of HIV-1 infection.
I. Introduction
II. Impact of Progesterone-Based Hormonal Contraception on HIV-1 Acquisition and Disease Progression
III. Nonhuman Primate Studies: Progesterone Increases the Susceptibility Whereas Estrogen Protects against SIV Transmission
IV. Potential Defense Mechanisms Modulated by Sex Steroid Hormones
V. Modulation of the Immune System by Hormonal Cycle
VI. Immunoregulatory Effects of Progesterone
VII. Immunoregulatory Effects of Estrogen
VIII. Possible Mechanisms of the Effect of Progesterone-Based Contraceptives on Disease Progression in HIV-1 Infected Individuals
IX. Conclusions
I. Introduction
Safe and effective methods of contraception represent a critical component of preventive health care reducing maternal and infant mortality, especially in women living in resource-limited settings. Although the number of women using oral or injectable hormonal contraception is rapidly increasing in areas of high HIV seroprevalence such as Sub-Saharan Africa, a clear understanding of the impact of this birth control method on HIV-1 infection is lacking (1,2,3,4). The effect of hormonal contraceptives on the acquisition and immune control of HIV-1 and other coinfecting pathogens represents an important and underinvestigated women’s health-specific issue with potentially large implications for public health policy. The World Health Organization has identified the availability and use of effective contraception by HIV-1-infected women wishing to avoid pregnancy as one of the primary strategies for preventing mother-to-child transmission of HIV-1. Any contraceptive intervention that increases the chance of HIV-1 infection or the shedding of HIV-1 in the female genital tract, or hastens the progression to AIDS has important implications for patients’ health, the spread of the virus in the population, and overall economic and social impacts of the epidemic.
II. Impact of Progesterone-Based Hormonal Contraception on HIV-1 Acquisition and Disease Progression
Whether or not hormonal contraceptives significantly affect the probability of acquiring HIV-1 remains a highly controversial issue and a topic of an intense discussion. To date, more than 10 studies have demonstrated a correlation between the use of hormonal contraception and increased risk of HIV-1 infection (5,6,7,8,9,10,11,12,13,14,15,16) (Table 1). Several studies have focused on injectable contraceptive DMPA (depot medroxyprogesterone acetate; Depo-Provera), a highly effective progesterone-based contraceptive currently used by more than 90 million users worldwide. DMPA, typically administered as a 3-monthly im injection, is gaining increasing popularity in Sub-Saharan Africa. One of the best designed studies was a 10-yr prospective study involving more than 1500 sex workers in Mombasa, Kenya. Women who used DMPA were twice as likely to acquire HIV-1 compared with women with no contraception (6,17,18). A recent report using data from the Demographic and Health Surveys of 4549 young women in four African countries confirmed higher HIV-1 seroprevalence in DMPA users and estimated that 6% of new HIV-1 cases are attributable to DMPA use (16). In contrast, several other studies have failed to observe an overall effect of DMPA or other forms of hormonal contraception on the incidence of HIV-1 infection (19,20,21,22,23,24) or reported increased risk of infection only in subgroups of subjects differing in age and herpes simplex virus (HSV-2) status (25,26,27). It is important to note that the quality of the epidemiological studies addressing the issue varies considerably, and great care should be taken in analyzing their results. Interpretation of these studies is complicated by multiple factors including the type, dose, and method of administration of hormonal contraceptives; method of selection of study subjects; and population sizes. Furthermore, unsafe sharing and reuse of needles and syringes used for delivering DMPA could be a confounding factor for the association between DMPA use and HIV-1 incidence, and future studies should take this into consideration (28). Supporting this point are the results of a study in Tanzania demonstrating a correlation between DMPA use and increased hepatitis C infection rate (29).
Table 1.
Impact of hormonal contraception on HIV-1 infection and disease progression
| Hormonal contraception | Effect | Population size | Refs. |
|---|---|---|---|
| OCa | Increased risk of HIV-1 acquisition | +/++b | 5,8,10,11,13,14,15 |
| DMPA | Increased risk of HIV-1 acquisition | +++ | 7,16 |
| DMPA, OC | Increased risk of HIV-1 acquisition | +++ | 6 |
| DMPA, OC | Increased HIV-1 viral load | +++ | 17,31 |
| OC | Increased risk of HIV-1 acquisition, meta-analysis of 28 studies | +++ | 9 |
| DMPA, OC | No effect on HIV-1 acquisition | ++/+++ | 19,20,21,22,23,24 |
| DMPA, OC | No effect on HIV-1 viral load or disease progression | ++ | 33,34 |
| DMPA, OC | Increased risk of Chlamydia, Gonococcus, and Candida infections in the genital tract | ++ | 39,40,41 |
| DMPA, OC | Accelerated CD4 T cell depletion and disease progression | +++ | 32 |
| DMPA | Increased risk of SIV infection in the rhesus macaque model; higher viral load during acute phase of infection | + | 42,43,44,46 |
OC preparations differ among various studies.
Approximate population size: +, small; ++, medium; +++, large.
Another important issue is whether the use of hormonal contraception during chronic HIV-1 infection affects the rate of viral replication and overall disease progression. A multivariate analysis of the Mombasa study demonstrated a significantly higher HIV-1 viral load at the set-point and acquisition of more diverse viral genotypes in women using hormonal contraception at the time of HIV-1 infection compared with women without contraception (6,17,18). In accordance with the notion that viral load is highly predictive of HIV-1 disease progression (30), Lavreys et al. (31) reported a correlation between higher viral set-point and mortality in the Mombassa cohort. The results of our recent randomized trial suggest that the use of hormonal contraceptives [DMPA or oral contraceptives (OC)] is associated with more rapid disease progression characterized by accelerated loss of CD4+ T cells and increased death rate in HIV-1-infected women (32). In contrast, other studies failed to confirm a relationship between the use of hormonal contraceptives and acceleration of the disease (33,34). Several reports have shown an association between the use of hormonal contraception and increased cervicovaginal shedding of HIV-1 with a significant dose dependency on progesterone levels (35,36,37). Progesterone-based contraceptives also appear to increase the number of inflammatory cells in cervicovaginal fluid (38). Importantly, both of these factors may potentially promote HIV-1 transmission. The effects of hormonal contraception do not seem to be limited to HIV. Application of hormonal contraception, particularly DMPA, was also shown to be associated with an increased acquisition of cervical chlamydial and gonococcal infections and candidiasis, which may increase the susceptibility to HIV-1 or further deteriorate the condition of HIV-1-infected individuals (39,40,41).
In summary, although multiple studies suggest a significant effect of progesterone-based hormonal contraception on the risk of HIV-1 infection, viral load, frequency of opportunistic infections, and the rate of progression to AIDS, the epidemiological data obtained so far are inconclusive, and more comprehensive clinical trials are urgently needed to verify these observations. However, extreme caution must be exercised in the design and analysis of these studies. Research data that would encourage stopping the use of progesterone-based contraceptives in areas with high HIV-1 prevalence could create a multitude of problems including lack of available birth control methods and increased incidence of mother-to-child HIV-1 transmission.
III. Nonhuman Primate Studies: Progesterone Increases the Susceptibility Whereas Estrogen Protects against SIV Transmission
Well-controlled nonhuman primate experiments addressing the effect of exogenous hormones on the acquisition of SIV have yielded more significant results than studies in humans. Rhesus macaque studies showed that administration of DMPA enhances the risk of SIV acquisition by more than 7-fold and significantly increases viral levels in the acute phase of infection (42,43,44). Trunova et al. (43) showed that the genetic complexity of the replicating virus was greater, replication of the CXCR4-using virus was favored, and cellular immune response rate was slower in DMPA-treated animals. Progesterone treatment is routinely used by some laboratories to increase the susceptibility to vaginal SIV infection (44,45). In addition, Christopher Miller and colleagues (46,47) have shown that DMPA abrogates the protective effect of an attenuated lentivirus-induced protection against intravaginal challenge with pathogenic SIVMAC239. Although it is unknown why macaque studies demonstrate a more profound effect of progesterone than the epidemiological studies in humans, multiple factors can play a role in the observed difference, including the kinetic of progesterone concentration in blood and mucosal tissues (declining during the 3-monthly injection period in women), the dose of challenge virus (higher doses are generally used in primate studies), the delivery medium (semen in humans vs. culture medium in macaques), and the responsiveness of genital tissue to progesterone treatment (the effect of progesterone on the thickness of vaginal epithelial layer is more significant in macaques than in humans, as discussed in Section IV).
In sharp contrast to progesterone, estrogen and its derivatives exert a strong protective effect. Smith et al. (48) have demonstrated that estrogen applied systemically in the form of sc implants protects against intravaginal challenge of ovariectomized female rhesus macaques with highly pathogenic SIVMAC251. In this study, six of six control animals but none of the six estrogen-treated animals became infected. The block appears to be at the mucosal tissue level because rechallenging the animals by submucosal injection of SIVMAC251 resulted in a productive infection (48). This was further confirmed in a subsequent study showing that topical treatment with estriol-containing intravaginal cream protected the macaques against intravaginal SIV challenge (49). Only one of 12 treated animals, compared with six of eight untreated controls, became infected. Topical application of estriol resulted in a minimal increase in serum estriol or LH levels. This level of protection against a highly pathogenic virus isolate is unprecedented in any vaccine studies. However, all of the above-mentioned experiments were performed using ovariectomized animals; it remains to be seen whether a similar level of protection would be observed in cycling animals where the effect of estrogen might be counteracted by endogenously produced progesterone.
IV. Potential Defense Mechanisms Modulated by Sex Steroid Hormones
What are the mechanisms responsible for the progesterone-mediated sensitization vs. estrogen-mediated protection against SIV infection in macaques? It is plausible that the main mechanism is the effect of sex steroid hormones on the physiological properties of the vaginal wall. Epithelial and subepithelial layers of the vaginal wall represent a natural barrier protecting the host against pathogenic infections. Indeed, the low probability of women becoming infected with HIV-1 via heterosexual intercourse (1:200–1:2000) is a testimony to the effectiveness of the vaginal and cervical mucosal barrier (50). Although HIV-1 can infect via the single layer of polarized columnar epithelial cells of the endocervical mucosa, most heterosexual transmissions likely occur through the pluristratified epithelial layer of the vaginal and ectocervical mucosa. The combined surfaces of vaginal wall and ectocervix exceed 15 times the surface area of the endocervix. HIV-1 infection occurs in women who lack a uterus at birth and in female macaques after surgical removal of the uterus (50,51,52). In a recent large randomized trial in African women, no significant reduction of HIV-1 infection occurred in women using a diaphragm (53). It has been argued that most transmissions occur through damaged or atrophied vaginal epithelium (50).
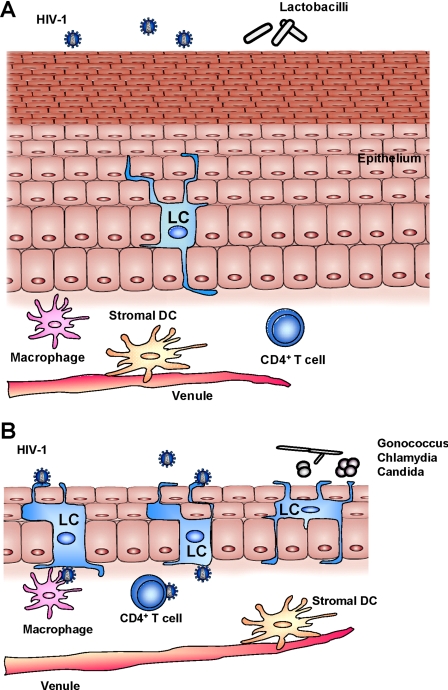
Sex steroid hormones exert a significant effect on the vaginal mucosal tissue. Estrogen induces thickening of the vaginal stratified epithelialium in women and female macaques (48,49,54,55,56,57,58) (Fig. 1). A thick epithelium might block the access of the virus to target Langerhans cells (LCs), CD4+ T cells, and macrophages in the epithelial and subepithelial layers. An inverse correlation between epithelial layer thickness and susceptibility to SIV in estrogen and progesterone-treated macaques was observed (42,48,49). Smith et al. (48) reported that whereas the average thickness of the epithelial layer was about <10 μm in untreated ovariectomized female macaques, it expanded to about 240 μm in the estrogen-treated animals. The estrogen-induced expansion of the epithelial layer is less pronounced in women compared with female macaques (54,55,58). Cornification of epithelial cells appears as soon as 24 h after estrogen treatment, and the response persists for at least 1 wk after the cessation of treatment in macaque vagina and human foreskin (49,59). Macaques intravaginally exposed to SIV appear to be less susceptible to infection when in the follicular ovarian phase (high estrogen) compared with the luteal phase (high progesterone) (60). This susceptibility pattern correlates with the cycling of the thickness of the epithelial layer, which is highest at the peak of the follicular phase around ovulation and lowest during menses (45). Because females are more likely to have intercourse during ovulation, increased thickness of epithelium likely represent a physiological mechanism to prevent traumatic effect of intercourse and infection with pathogens introduced during the intercourse. Estrogen treatment also decreases cervicovaginal pH in women and female macaques, making it hostile to the virus (48,54,61,62). In postmenopausal women, low estrogen is associated with thinning of the vaginal epithelium and atrophy (63). This condition is associated with a dry, friable, thin vaginal epithelium prone to bleeding after minimal trauma. Postmenopausal women are 4- to 8-fold more susceptible to HIV-1 transmission (64,65). Atrophic vaginitis is successfully treated with local or systemic estrogen therapy resulting in thickening of the vaginal wall.
Figure 1.
Potential effects of steroid hormones on the vaginal mucosal tissue. A, Topical or systemic treatment with estrogen results in a thickening of the vaginal epithelial layer in ovariectomized rhesus macaques. Increased thickness of mucosal epithelium may interfere with the access of the virus to target cell populations including LCs, CD4+ T cells, and macrophages in the epithelial and subepithelial layers. Estrogen decreases the frequency of LCs in the vaginal epithelium and lowers cervicovaginal pH, making it hostile to the virus. B, In contrast, thinner epithelial layer in progesterone-treated female macaques allows easier access of the virus to LCs and other target cells. The effect of progesterone treatment on epithelial thickness appears to be less profound in women compared with female macaques. According to some studies, progesterone treatment increases the frequency of LCs in the epithelial layer and induces changes in the vaginal microbiota, namely decreased colonization with H2O2-producing Lactobacillus, resulting in bacterial vaginosis. Use of progesterone-based contraceptives is associated with increased acquisition of cervical candidiasis and chlamydial and gonococcal infections in women. These factors may enhance the risk of HIV-1 acquisition.
In contrast to estrogen, administration of progesterone causes significant thinning of the cervicovaginal epithelium in rhesus macaques, possibly explaining the increased susceptibility to SIV in DMPA-treated animals (42,66). However, the effect of DMPA on the vaginal wall thickness appears to be much less profound in humans, with several studies actually reporting increased epithelial thickness caused by hyperplasia (67,68,69,70). An alternative mechanism of DMPA action has been proposed by Miller et al. (70) who described how DMPA induces changes in the human vaginal microbiota, namely decreased colonization with H2O2-positive Lactobacillus. H2O2-producing Lactobacillus in the vagina may directly kill free virus (71). In addition, the production of H2O2 by Lactobacillus represents an important mechanism by which it maintains dominance over other vaginal microbiota. The acquisition of HIV-1 was found to be significantly higher in women without Lactobacillus or with abnormal vaginal microbiota compared with those with H2O2-producing Lactobacillus (72). Colonization with Lactobacillus might prevent bacterial vaginosis that is associated with increased risk of HIV-1 transmission (7,73,74).
Steroid hormones might also modulate the susceptibility to SIV and HIV-1 by altering the availability of target cells in the vaginal epithelium and stroma. Of particular interest is the population of LCs that is abundant in the epithelial layer of the vaginal and ectocervical mucosa of the female lower reproductive tract. Dendritic processes from LCs extend to the lumen of the vagina to sample antigen. Several reports suggest that LCs represent one of the primary targets of HIV-1 in the female reproductive tract (50,75). They acquire the virus by multiple mechanisms involving CD4, CCR5, and langerin-dependent binding as well as endocytosis (75). Although only a few LCs become productively infected, they might play a key role in viral dissemination by effectively transporting the membrane-associated virus to CD4+ T cells and macrophages in the lamina propria and draining lymph nodes (the Trojan horse theory) (50,75). Importantly, exogenous estrogen decreases (76), whereas progesterone increases (77) the frequency of LCs in the vaginal epithelial and stromal tissue. In contrast, the frequency of LCs appears constant throughout the menstrual cycle in women and female macaques (78,79). Furthermore, peak estrogen levels decrease the recruitment of inflammatory T cells and macrophages through down-regulation of intercellular adhesion molecule-1 (ICAM-1), E-selectins, and vascular cell adhesion molecule-1 (VCAM-1) (80). In contrast, progesterone was reported to increase the expression of CCR5 on human cervical CD4+ T cells, making them possibly more susceptible to infection (81,82). Thus, estrogen might decrease and progesterone might increase the frequency of HIV-1 target cells in the vaginal epithelium and lamina propria. Importantly, steroid hormones might also act by regulating the early response of the innate and adaptive immune systems in the female lower reproductive tract, as discussed in Sections VI and VII.
If the results of macaque studies are reproduced in future studies in humans, vaginally applied estrogen derivatives may represent a new, inexpensive, and safe method of HIV-1 prevention. Ideally, estrogen treatment will enhance the natural protective properties of the genital tract tissue, resulting in a “natural female condom” effectively interfering with HIV-1 transmission. In contrast to microbicides, which have to be applied around the time of exposure, the effect of vaginal estrogen therapy is long lasting (49,59). Estrogen cream has been used by millions of postmenopausal women to treat vaginal atrophy and has not been associated with any negative systemic effects. According to some reports, long-term hormonal replacement therapy has been linked to an increased chance of breast cancer (83). However, estrogen can be replaced by selective estrogen receptor modulators to avoid any potential carcinogenic effect. Vaginal estrogen cream is likely to be affordable because plant-derived phytoestrogens can be produced at a low cost. A potential protective effect of natural products such as soybean, black cohosh, or red clover phytoestrogens that have been used by women worldwide to treat vaginal dryness and atrophy should be investigated in future studies. Topical estrogen therapy is likely to be highly effective in sexually active postmenopausal women, a largely unrecognized subset of population with high susceptibility to HIV-1 infection (64,65). Vaginal estrogen cream can be combined with microbicides and antiretrovirals for additional protection and with contraceptives in women wishing to avoid pregnancy.
V. Modulation of the Immune System by Hormonal Cycle
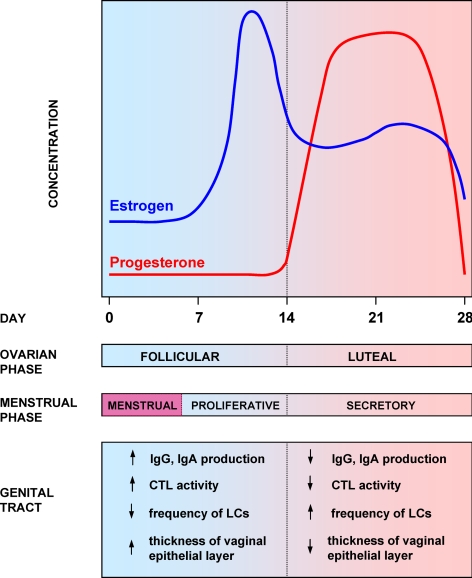
Tightly regulated production of ovarian hormones estrogen and progesterone modulates the menstrual cycle and exerts a dramatic effect on immune processes in the female reproductive tract, other mucosal tissues, as well as the systemic compartment (76,84,85,86) (Fig. 2). Although the effect of hormonal cycle is generally more pronounced in the tissues of the upper reproductive tract (ovaries, fallopian tubes, uterus), a significant effect on immune mechanisms in the lower reproductive tract (endocervix, ectocervix, and vagina) is well documented. We and others have previously demonstrated that the levels of antibodies in genital secretions significantly fluctuate throughout the menstrual cycle (87,88,89,90,91,92). This fluctuation is associated with shifts in the relative frequency of B cell subsets in tissues as well as with the changes in the rate of antibody production and transepithelial transport (78,90). IgA and IgG levels in cervical mucus increase approximately 3 d before ovulation and decrease during the luteal phase of the menstrual cycle, paralleling the increase in the concentration of progesterone (87,88,89,90,91,92,93,94,95). Total and antigen-specific IgG- and IgA-secreting cells in the cervix and vagina of women and female macaques are significantly higher during the periovulatory stage (d 11–15) and decreased before menstruation, coinciding with highest progesterone levels (90,96). Importantly, the cycle-associated effect of sex hormones on antibody-secreting cell frequencies is also observed in nonreproductive tract immune tissues such as the spleen and lymph nodes (90). Gockel et al. (97) have demonstrated that animals immunized orally with tetanus toxoid at the peak of progesterone concentrations during the postestrous stage exhibited 10- to 100-fold lower IgA and IgG responses in fecal extracts and vaginal washes compared with animals immunized at other phases of the menstrual cycle. These data strongly suggest that sex hormones regulate IgA and IgG responses not only in the genital tract but also, importantly, in the gut-associated lymphoid tissue. This may be attributed to the common draining lymphatics, namely the caudal and lumbar lymph nodes, shared by the colon and female genital tract.
Figure 2.
Hormonal cycle and regulation of immune processes in the female genital tract. Sex steroid hormones exert a significant effect on immune processes in the female genital tract mucosa, including the activity of CTLs, B cells, frequency and antigen-presenting activity of LCs and other mechanisms.
Cytotoxic T lymphocytes (CTLs) in the female genital tract exhibit high cytotoxic activity during the follicular phase of hormonal cycle and almost complete loss of this activity during the luteal phase (98). This may be caused by the fact that estrogen inhibits antigen presentation and CTL activity in the female reproductive tract, possibly by inducing TGF-β (77,98,99,100). There is a lack of correlation between genital tract mucosa and peripheral blood CTL activity in HIV-1-infected women, suggesting discrete hormonal regulation (77,101). However, the observed difference may be caused by local inflammation or variations in viral replication in the systemic vs. mucosal compartments. An increased level of estrogen in the luteal phase is associated with increased concentration of TNF-α, IL-4, and sIL-6R in plasma (102,103,104). Natural killer (NK) cell cytotoxicity is higher in the follicular than in the luteal phase, and the number of NK cells per milliliter of blood is highest during the periovulatory phase (105,106).
It remains to be established whether and to what degree the upper female reproductive tract serves as a portal of HIV-1 infection (107,108). Human endometrial epithelial cells express CD4, CCR5, and CXCR4 receptors, and CXCR4 levels gradually increase during the proliferative phase and remain elevated throughout the secretory stage of the menstrual cycle, possibly invoking sex hormones-mediated mechanisms (109,110,111). Endometrial epithelial cells bind and transcytose R5 and X4 HIV-1 viruses to permissive submucosal cells (107,112,113). The endometrial mucosal epithelium expresses innate immune factors, including α- and β-defensins, secretory leukocyte protease inhibitor, and RNA-dependent protein kinase, in a hormonal cycle-dependent fashion (76,108,110,114). Sex hormones regulate the recruitment of lymphocytes into large lymphoid aggregates accumulating in the endometrium of human uterus and consisting of a central core of B lymphocytes surrounded by a large number of CD8+ and a lower number of CD4+ T cells (115). The size of these aggregates peaks at the secretory phase with about 3000 cells/aggregate and decreases at the proliferative stage. Analysis of T cell receptor subtypes within endometrium suggests that these aggregates develop largely by trafficking of cells into the endometrium rather than by local proliferation (116). Progesterone and estradiol decrease the expression of chemokine monocyte chemoattractant protein-1 (MCP-1) by human endometrial stromal cells and decrease the infiltration of leukocytes, neutrophils, and macrophages to the female upper and lower genital tract (117,118,119).
Several studies suggest that plasma HIV-1 load and HIV-1 shedding at the lower reproductive tract are lowest during the follicular ovarian stage and increase during the luteal stage, corresponding to high progesterone levels (120,121,122,123). However, because other studies found no association (124,125), it remains to be established whether endogenously produced sex hormones affect the rate of proliferation of HIV-1 in genital mucosal tissue. Interestingly, women who are pregnant or recently postpartum (conditions characterized by increased progesterone production) have been shown to be more susceptible to HIV-1 infection (126,127). Furthermore, virus excretion in cervicovaginal secretions appears to be elevated in HIV-1-infected pregnant women (128).
VI. Immunoregulatory Effects of Progesterone
Progesterone exerts a strong immunosuppressive effect on the production and transepithelial transport of IgG and IgA (78,87,88,89,90,91,92,93,94,95,96,129,130,131,132,133,134) (Table 2). Treatment of ovariectomized rats with progesterone results in a significant decline in cervicovaginal content of IgA, IgG, and secretory component (SC), which represents an IgA-associated extracellular segment of polymeric Ig receptor (pIgR) expressed by epithelial cells (129,130). Progestin-containing contraceptives have been reported to decrease the levels of IgG and IgA in serum but not in secretions in humans, although the results seem to be dependent on the composition and concentration of particular steroid hormones used (93,131,135). Treatment of mice with DMPA results in decreased specific IgG and IgA responses after intravaginal or intranasal immunization with attenuated HSV-2 and causes a failure to develop protective responses (132,133). The inhibitory effect of progesterone on vaginal humoral responses is routinely used to establish permissive conditions for intravaginal infections of mice with HSV-2 and Chlamydia trachomatis (132,133,134). However, in addition to the effect of progesterone on immune factors, the increased susceptibility to Chlamydia and HSV-2 may be caused by thinning of the vaginal epithelium, allowing easier access of pathogens to basal layer (136). Progesterone derivatives were also reported to alter the pattern of Ig glycosylation, specifically by inducing the synthesis of asymmetric antibodies characterized by the presence of an oligosaccharide group with high mannose content only on one of the two Fab fragments (137,138). This prosthetic group sterically hinders the interaction with antigen, reducing the affinity by about 100-fold. Asymmetric antibodies are increased during pregnancy, mostly associated with the placenta (138).
Table 2.
Reported effects of progesterone and its derivatives on the immune system and immunobiology of HIV-1 infection
| Reported effect of progesterone or its derivatives | Refs. |
|---|---|
| Inhibition of IgG and IgA production and transepithelial transport | 78,87,88,89,90,91,92,93,94,95,96,129,130,131,132,133,134 |
| Decreased frequency of antibody-secreting cells in women and female macaques | 90,96 |
| Decreased specific IgG and IgA responses after mucosal immunization with attenuated HSV-2; induction of permissive conditions for intravaginal infection of mice with HSV-2 and Chlamydia trachomatis | 132,133,134 |
| Inhibition of T cell responses and cytotoxic activity | 139,140,141,142,143,147 |
| Inhibition of perforin expression in T cells | 140,141,142,144,145,146 |
| Decreased proliferation and Th1-type cytokine production by VZV-specific CD4+ T cells in HIV-1 patients | 148 |
| Altered migration and decreased activity of NK cells | 105,106,106,135,159,251,252 |
| PIBF-mediated shift toward Th2 cytokine expression profile | 133,149,150,151,152,153,154 |
| Altered migration and infiltration of lymphocytes, macrophages, and NK cells into the female genital tract tissues | 117,118,157,158,183,191,253 |
| Increased expression of CCR5 on cervical CD4+ lymphocytes | 81,82 |
| Thinning of cervicovaginal epithelium in rhesus macaques | 42,66 |
| Increased frequency of LCs in vaginal epithelium | 76,77 |
| Regulation of HIV replication and LTR activity | 254 |
| Suppression of IL-1, IL-2, and IL-6 release by human lymphocytes | 148,177 |
| Inhibition of TLR-9-induced IFN-α production by human and mouse pDCs | 162 |
| Increased shedding of HIV-1 in the genital tract | 35,36,37 |
| Decreased FcγR expression on monocytes | 159,160 |
| Decreased vaginal colonization with H2O2-producing Lactobacillus | 70 |
VZV, Varicella-zoster virus; TLR, Toll-like receptor; LTR, long terminal repeat; pDC, plasmocytoid dendritic cell.
It is well established that the levels of progesterone reached as a result of administration of injectable contraceptives such as DMPA exert a number of effects on T cell-mediated immune mechanisms including inhibition of cytolytic activity of T cells and blocking perforin expression in T cells, both directly and indirectly (139,140,141,142,143,144,145,146,147). Progesterone and its derivatives also alter the expression of cytokines, generally favoring Th2-type responses (133,148,149,150,151,152,153,154). In a recent study, Enomoto et al. (148) demonstrated that progesterone at concentrations achieved during hormonal therapy decreased the proliferation and Th1-type cytokine production of varicella-zoster virus-specific CD8+ and CD4+ T cells; interestingly, the effect was significantly stronger on cells obtained from HIV-1-infected patients. The effect of progesterone appears to be mediated at least in part by progesterone-induced blocking factor (PIBF), a 34-kDa protein produced by lymphocytes after activation in the presence of progesterone (144,149,150). PIBF binds to IL-4R α-chain and GPI-anchored receptor (155) and blocks CTL-mediated killing by inhibition of perforin expression (156). PIBF induces the production of IL-3, IL-4, and IL-10 and inhibits the production of IL-12 by human peripheral blood mononuclear cells, suggesting its role as a mediator of a progesterone-induced shift toward Th2 cytokine expression profile and dampening Th1-dependent responses (133,149,150,151,152,153,154).
Progestins significantly alter infiltration of female genital tract tissue with NK cells, lymphocytes, and macrophages (115,116,117,118,119,157,158). Progesterone inhibits NK cell activity and FcγR expression on monocytes, thus reducing the two arms of antibody-dependent cell cytotoxicity (106,159,160). Decreased antibody production, CTL, interferon (IFN)-α, and antibody-dependent cell cytotoxicity activity may contribute to the increased shedding of HIV-1 detected in women using contraception (35,36,37,161).
Recently, Hughes et al. (162) demonstrated that medroxyprogesterone, the active compound of DMPA, inhibits Toll-like receptor-9-induced IFN-α production by human and mouse plasmacytoid dendritic cells (DCs), likely through a selective blockade of IFN regulatory factor-7 activation. IFN-α normally induces an antiviral state in nonimmune tissues and primes adaptive antiviral responses by directly activating antigen-presenting cells and T cells. By blocking its production, medroxyprogesterone impairs a critical mechanism of innate antiviral immunity.
Membrane-associated and intracellular progesterone receptors are expressed by a number of cells including B cells, macrophages, NK cells, and γδ-T cells taken from healthy pregnant women (a high progesterone state) (163,164,165). The expression of progesterone receptors in αβ CD4+ and CD8+ T cells remains controversial and appears to be affected by the activation state and other factors (166,167,168,169). Several studies reported the presence of progesterone receptors on T cells isolated from pregnant patients with endometriosis (143,146,168,170,171,172).
Importantly, in contrast to progesterone, medroxyprogesterone does not exhibit its biological effect exclusively via the progesterone receptors, but also by binding to the glucocorticoid receptor (GR) that is widely expressed by various cells of the immune system (173,174,175,176). Medroxyprogesterone displays a higher binding affinity than progesterone for the human GR (Ki 10.8 and 215 nm for medroxyprogesterone and progesterone, respectively) (173). Medroxyprogesterone induces greater phosphorylation at Ser211 and conformational changes in the liganded GR different from those induced by progesterone, and it displays greater glucocorticoid transactivation agonist potency and a higher potency for transrepression than progesterone or norethindrone acetate (an alternative contraceptive) (173). Medroxyprogesterone, but not estrogen or progesterone, suppresses IL-1, IL-2, and IL-6 release by human lymphocytes to a similar extent as classic glucocorticoids dexamethasone and hydrocortisone (148,177). Liganded GR-mediated repression of IL-2 gene expression is thought to be due to the direct interaction of GR with transcriptional enhancers such as activator protein-1 and nuclear factor-κB (178,179,180). Thus, by binding to the GR, medroxyprogesterone, the active component of many contraceptive preparations, may exert significantly stronger immunosuppressive activity than progesterone.
VII. Immunoregulatory Effects of Estrogen
Depending on its concentration, estrogen can exert either a proinflammatory or antiinflammatory effect (80,181) (Table 3). At low levels, estrogen induces TNF-α, IL-6, and IL-1β; inhibits Th2-type cytokines; and increases migration of leukocytes to the site of inflammation (80). In sharp contrast, at high levels, estrogen inhibits cell-mediated immunity and decreases the expression of a number of activation markers (148,182). Estrogen inhibits the production of TNF-α, IL-1β, and IL-6 by T cells, macrophages, and DCs, and induces Th2-type cytokines IL-4, IL-10, and TGF-β, resulting in an antiinflammatory effect (183). Peak estrogen levels reduce the production of Th1-type cytokines such as TNF-α and IFN-γ by T cells, macrophages, and DCs (80,184,185,186).
Table 3.
Effects of estrogen and its derivatives on the immune system
| Reported effect of estrogen and its derivatives | Refs. |
|---|---|
| Protection against SIV vaginal transmission in ovariectomized rhesus macaques | 48,49,60 |
| Increased thickness of vaginal wall | 48,49,54,55,56,57,58 |
| Decreased IgA, IgG, and SC in female genital tract | 129,130,188 |
| Increased expression of pIgR by endocervical and endometrial epithelial cells | 189 |
| Decreased antigen presentation, CTL activity; increased TGF-β in genital tract | 77,98,99,100 |
| Decreased MCP-1 production, decreased macrophage migration into genital tract tissue | 117,191 |
| Decreased T cell migration | 183 |
| Decreased NK cell activity | 252 |
| Decreased frequency of LCs in vaginal epithelium | 76,77 |
| Decreased recruitment of inflammatory T cells and macrophages through down-regulation of ICAM-1, VCAM-1, and E-selectins | 80,183,184,185,186 |
Estrogen enhances antibody production by human peripheral blood mononuclear cells in vitro by enhancing IL-10 production by monocytes (187). However, the effect of estrogen on antibody production in the female genital tract appears to be more complex. Although estradiol elevates the levels of IgG, IgA, and SC in the uterus of ovariectomized rats, it decreases their levels in cervicovaginal secretions (129,130,188). The action of estradiol on cervicovaginal IgA, IgG, and SC appears to be independent of uterine influence, because estrogen treatment of rats with ligations at their uterocervical junction still has decreased cervicovaginal IgA and SC levels. Human vaginal epithelial cells do not express pIgR (87). Instead, pIgR is expressed on the basolateral surfaces of the endocervical and endometrial epithelial cells, and its production is enhanced by estradiol (189). It must be emphasized that uterus, a part of the upper reproductive tract, is the principal source of antibodies in women’s genital tract secretions; after hysterectomy, the levels of IgG are reduced by one half, and levels of IgA by 15-fold (190).
Estrogen and its derivatives also affect the migration and infiltration of lymphocytes, macrophages, NK cells, and other cell types into the female genital tract. Estrogen inhibits MCP-1 expression in endometrial stromal cells, thus possibly controlling endometrial macrophage migration (117,191). Estrogen-mediated inhibition of antigen presentation and CTL activity in the female reproductive tract (98,99,100) is likely caused by its ability to decrease the frequency of LCs in the vaginal tissue (76,77). High estrogen levels decrease the recruitment of inflammatory T cells and macrophages through down-regulation of ICAM-1, E-selectins, and VCAM-1 (80,183,184,185,186). In contrast, estrogen increases recruitment of CD56+ NK cells to human endometrium, likely by up-regulation of CXCL10 and CXCL11 chemokines (192).
VIII. Possible Mechanisms of the Effect of Progesterone-Based Contraceptives on Disease Progression in HIV-1 Infected Individuals
HIV-1 infection is characterized by rapid and extensive infection and depletion of memory CD4+ T cells in mucosal lymphoid tissues, most prominently in the intestinal lamina propria and genital tract-associated tissues early in the acute phase of infection (193,194,195,196,197,198,199,200,201,202). CD4+ T cells play a critical role in the regulation of Ig isotype switching and broadening of antibody diversity by somatic hypermutation in B cells residing in mucosal lymphoid tissues (203,204,205,206). The regulation of class switch recombination, somatic hypermutation, and transepithelial transport of IgA is dependent on stimulatory cytokines, such as IL-4, IFN-γ, IL-5, IL-6, IL-10, and TGF-β, normally produced by CD4+ T cells (204,207,208,209,210). A near-complete elimination of the memory CD4+ T cell population in mucosal lymphoid tissue results in an absence of important regulatory and effector functions these cells normally provide in the control of immune responses to environmental antigens and commensal bacteria on one side and infecting pathogens on the other (211,212). Their depletion, in the long term, compromises mucosal defenses by several mechanisms including reductions in specific IgA responses, decreased barrier function of epithelial cells, and functional alterations of CD8+ cytotoxic T cells (196,203,213,214,215). Extensive evidence suggests that HIV-1 and SIV cause severe damage to gastrointestinal mucosal surface and antimicrobial functions of the mucosal barrier (216,217,218,219,220). Increased mucosal leakiness was observed in SIV-infected macaques (221) and in HIV-1+ patients with low levels of HIV-1-specific IgA antibodies (222,223). Recently obtained data strongly suggest that the impairment of mucosal barrier function associated with increased absorption of environmental microbial antigens to the systemic compartment may be the major cause of the continuous activation of CD4+ and CD8+ T cells in HIV-1 infection (193,216,224,225,226,227,228,229). Importantly, direct evidence of increased microbial translocation was recently obtained by Brenchley et al. (229), who demonstrated that significantly raised levels of plasma bacterial lipopolysaccharide (LPS) in chronically HIV-1-infected individuals and SIV-infected rhesus macaques, evidence for chronic in vivo stimulation of monocytes by LPS, an association between plasma LPS and the level of T cell activation, and an association between the reduction in plasma LPS and CD4+ T cell reconstitution during highly active antiretroviral therapy. However, the underlying causes of increased microbial translocation across the mucosal barrier in HIV-1-infected patients remain unclear.
Recent studies, including those in our laboratory, indicate that HIV-1-specific IgA antibodies are either absent or unexpectedly low in external secretions and sera of HIV-1-infected individuals (230,231,232). In contrast, HIV-1-specific IgG antibodies are easily detectable and quantitatively measurable, even in secretions where IgA is an overwhelmingly dominant isotype (223,230,231,232,233,234,235,236,237,238). These results are in remarkable agreement with studies performed in HIV-1-infected chimpanzees (239) and SIV- infected rhesus macaques (221,240). Thus, HIV-1 infection in humans and SIV infection in primates appear to result in a selective and profound suppression of virus-specific IgA but not IgG responses in the mucosal as well as systemic compartments (204,232,241). These data suggest that the suppression of antigen-specific IgA responses in mucosal tissues is the cause of increased bacterial translocation across the mucosal barrier in the chronic phase of infection. IgA plays a critical role in the regulation of the immune response to microbial community in the gut and in the reduction of inflammation induced by bacterial products and proinflammatory agents (216,224,225). Mucosal IgA antibodies play an essential role in the control of a number of mucosal pathogens, such as Salmonellae spp., Campylobacter jejuni, Shigellae spp., Giardia lamblia, Clostridium difficile, and Cryptosporidium, that cause significant morbidity in HIV-1-infected patients (242,243,244). Impaired mucosal humoral responses to these pathogens are likely to increase the incidence and prolong the prevalence of mucosal infections (245,246,247,248,249,250).
As described above, progesterone and its derivatives exert a strong effect on humoral immune responses in the genital tract and other mucosal tissues including decreasing the frequency of antibody-secreting cells, inhibition of IgG and IgA production, and transepithelial transport, and altered Ig glycosylation pattern. Thus, administration of progesterone-based contraceptives to HIV-1 infected women likely results in further inhibition of specific mucosal IgA and IgG responses either directly via its effect on B cells or indirectly via accelerated depletion of CD4+ T cells or inhibition of receptor-mediated Ig transcytosis. Due to the physiological role of progesterone in the regulation of menstrual cycle and immune responsiveness in the female genital tract, it is likely that progesterone-mediated inhibition of the cytotoxic activity of T and NK cells and altered Ig production will be more pronounced in the genital tract tissue compared with the systemic compartment. Attenuated immune responses against HIV-1 in combination with increased frequency of CD4+CCR5+ T cells are likely to result in preferential HIV-1 proliferation in the genital tissue and increased shedding of HIV-1. This is supported by the observed association between hormonal contraception and increased shedding of HIV-1 in cervicovaginal lavage with a significant dose dependency on progesterone levels (35,36,37).
It has not yet been investigated whether medroxyprogesterone treatment increases the level of HIV-1 proliferation at other mucosal tissues, such as gut-associated lymphoid tissue, the largest lymphoid organ in the body and preferential site of HIV-1 proliferation (193). If this is the case, increased depletion of CD4+ T cells at mucosal lymphoid tissue could represent a key mechanism responsible for accelerated CD4+ T cell decline in progesterone-treated women. We believe this to be a plausible hypothesis due to the documented immunosuppressive effect of progesterone and its derivatives on specific immune responses in mucosal lymphoid tissues. Redistribution of viral proliferation in women using hormonal contraceptives may result in a CD4+ T cell “sink” responsible for the continuous removal of CD4+ T cells from the system.
IX. Conclusions
The data summarized here suggest that hormonal contraception may exert a significant effect on the susceptibility to HIV-1 infection as well as on the progression of the ensuing disease. However, the epidemiological data obtained so far are inconclusive. More detailed and comprehensive studies are needed to provide information as to which type of contraception should be used by HIV-1-infected women and women at high risk of infection. Optimally, these studies should involve large numbers of subjects at high risk of HIV-1 exposure; employ randomized, controlled, and safe administration of defined doses of contraceptives; and control for other confounding factors such as genital infections. Although such studies may be financially demanding, their cost is justified by the importance of the question and the potential impact on the spread of HIV-1 epidemic. Importantly, data obtained in the nonhuman primate model strongly suggest that estrogen enhances the natural protective properties of the female genital tract tissue and decreases its susceptibility to virus transmission. If confirmed by future studies, systemically or topically applied estrogen may represent an inexpensive and safe method of HIV-1 prevention. Further studies are needed to elucidate the basic underlying mechanisms of estrogen-induced mucosal protection against SIV and HIV-1 viruses.
Acknowledgments
We thank Dr. Paul Goepfert and Dr. Phillip Doyle Smith for their helpful comments and stimulating discussions.
Footnotes
This work was supported by National Institutes of Health Grants AI074438 and AI083027.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 10, 2009
Abbreviations: CTL, Cytotoxic T lymphocyte(s); DC, dendritic cell; DMPA, depot medroxyprogesterone acetate; GR, glucocorticoid receptor; HSV-2, herpes simplex virus-2; ICAM-1, intercellular adhesion molecule-1; IFN, interferon; LC, Langerhans cell; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; NK, natural killer; OC, oral contraceptives; PIBF, progesterone-induced blocking factor; pIgR, polymeric Ig receptor; SC, secretory component; SIV, simian immunodeficiency virus; VCAM-1, vascular cell adhesion molecule-1.
References
- Shah I 2005 Contraceptive use patterns in countries with different levels of HIV epidemic. J Acquir Immune Defic Syndr 38(Suppl 1):S5–S6 [DOI] [PubMed] [Google Scholar]
- 2008 World contraceptive use 2007. UN report. New York: United Nations [Google Scholar]
- Cates W 2001 Use of contraception by HIV-infected women. IPPF Med Bull 35:1–3 [Google Scholar]
- Stringer E, Antonsen E 2008 Hormonal contraception and HIV disease progression. Clin Infect Dis 47:945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães MD, Muñoz A, Boschi-Pinto C, Castilho EA 1995 HIV infection among female partners of seropositive men in Brazil. Rio de Janeiro Heterosexual Study Group. Am J Epidemiol 142:538–547 [DOI] [PubMed] [Google Scholar]
- Lavreys L, Baeten JM, Martin Jr HL, Overbaugh J, Mandaliya K, Ndinya-Achola J, Kreiss JK 2004 Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study. AIDS 18:695–697 [DOI] [PubMed] [Google Scholar]
- Martin Jr HL, Nyange PM, Richardson BA, Lavreys L, Mandaliya K, Jackson DJ, Ndinya-Achola JO, Kreiss J 1998 Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis 178:1053–1059 [DOI] [PubMed] [Google Scholar]
- Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T 1996 Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol 12:500–507 [DOI] [PubMed] [Google Scholar]
- Wang CC, Reilly M, Kreiss JK 1999 Risk of HIV infection in oral contraceptive pill users: a meta-analysis. J Acquir Immune Defic Syndr 21:51–58 [DOI] [PubMed] [Google Scholar]
- Sinei SK, Fortney JA, Kigondu CS, Feldblum PJ, Kuyoh M, Allen MY, Glover LH 1996 Contraceptive use and HIV infection in Kenyan family planning clinic attenders. Int J STD AIDS 7:65–70 [DOI] [PubMed] [Google Scholar]
- Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR 1991 Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis 163:233–239 [DOI] [PubMed] [Google Scholar]
- Rehle T, Brinkmann UK, Siraprapasiri T, Coplan P, Aiemsukawat C, Ungchusak K 1992 Risk factors of HIV-1 infection among female prostitutes in Khon Kaen, Northeast Thailand. Infection 20:328–331 [DOI] [PubMed] [Google Scholar]
- Plourde PJ, Plummer FA, Pepin J, Agoki E, Moss G, Ombette J, Ronald AR, Cheang M, D'Costa L, Ndinya-Achola JO 1992 Human immunodeficiency virus type 1 infection in women attending a sexually transmitted diseases clinic in Kenya. J Infect Dis 166:86–92 [DOI] [PubMed] [Google Scholar]
- Chao A, Bulterys M, Musanganire F, Habimana P, Nawrocki P, Taylor E, Dushimimana A, Saah A 1994 Risk factors associated with prevalent HIV-1 infection among pregnant women in Rwanda. National University of Rwanda-Johns Hopkins University AIDS Research Team. Int J Epidemiol 23:371–380 [DOI] [PubMed] [Google Scholar]
- Watson-Jones D, Baisley K, Weiss HA, Tanton C, Changalucha J, Everett D, Chirwa T, Ross D, Clayton T, Hayes R 2009 Risk factors for HIV incidence in women participating in an HSV suppressive treatment trial in Tanzania. AIDS 23:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc PM, Dubois-Colas N, Garenne M 2008 Hormonal contraception and HIV prevalence in four African countries. Contraception 77:371–376 [DOI] [PubMed] [Google Scholar]
- Baeten JM, Lavreys L, Sagar M, Kreiss JK, Richardson BA, Chohan B, Panteleeff D, Mandaliya K, Ndinya-Achola JO, Overbaugh J, Farley T, Mwachari C, Cohen C, Chipato T, Jaisamrarn U, Kiriwat O, Duerr A 2005 Effect of contraceptive methods on natural history of HIV: studies from the Mombasa cohort. J Acquir Immune Defic Syndr 38(Suppl 1):S18–S21 [DOI] [PubMed] [Google Scholar]
- Lavreys L, Baeten JM, Kreiss JK, Richardson BA, Chohan BH, Hassan W, Panteleeff DD, Mandaliya K, Ndinya-Achola JO, Overbaugh J 2004 Injectable contraceptive use and genital ulcer disease during the early phase of HIV-1 infection increase plasma virus load in women. J Infect Dis 189:303–311 [DOI] [PubMed] [Google Scholar]
- Myer L, Denny L, Wright TC, Kuhn L 2007 Prospective study of hormonal contraception and women’s risk of HIV infection in South Africa. Int J Epidemiol 36:166–174 [DOI] [PubMed] [Google Scholar]
- Kiddugavu M, Makumbi F, Wawer MJ, Serwadda D, Sewankambo NK, Wabwire-Mangen F, Lutalo T, Meehan M, Xianbin, Gray RH 2003 Hormonal contraceptive use and HIV-1 infection in a population-based cohort in Rakai, Uganda. AIDS 17:233–240 [DOI] [PubMed] [Google Scholar]
- Kapiga SH, Lyamuya EF, Lwihula GK, Hunter DJ 1998 The incidence of HIV infection among women using family planning methods in Dar es Salaam, Tanzania. AIDS 12:75–84 [DOI] [PubMed] [Google Scholar]
- Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A 1994 Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. AIDS 8:1585–1591 [DOI] [PubMed] [Google Scholar]
- Mati JK, Hunter DJ, Maggwa BN, Tukei PM 1995 Contraceptive use and the risk of HIV infection in Nairobi, Kenya. Int J Gynaecol Obstet 48:61–67 [DOI] [PubMed] [Google Scholar]
- Taneepanichskul S, Phuapradit W, Chaturachinda K 1997 Association of contraceptives and HIV-1 infection in Thai female commercial sex workers. Aust N Z J Obstet Gynaecol 37:86–88 [DOI] [PubMed] [Google Scholar]
- Bulterys M, Smith D, Chao A, Jaffe H 2007 Hormonal contraception and incident HIV-1 infection: new insight and continuing challenges. AIDS 21:97–99 [DOI] [PubMed] [Google Scholar]
- Morrison CS 2007 Commentary: hormonal contraception and HIV acquisition—current evidence and ongoing research needs. Int J Epidemiol 36:175–177 [DOI] [PubMed] [Google Scholar]
- Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, Mugerwa R, Padian N, Rugpao S, Brown JM, Cornelisse P, Salata RA 2007 Hormonal contraception and the risk of HIV acquisition. AIDS 21:85–95 [DOI] [PubMed] [Google Scholar]
- Gisselquist D 2008 Comment on “hormonal contraception and HIV prevalence in four African countries.” Contraception 78:346–347 [DOI] [PubMed] [Google Scholar]
- Stark K, Poggensee G, Höhne M, Bienzle U, Kiwelu I, Schreier E 2000 Seroepidemiology of TT virus, GBC-C/HGV, and hepatitis viruses B, C, and E among women in a rural area of Tanzania. J Med Virol 62:524–530 [DOI] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo Jr CR, Gupta P, White RM, Todd JA, Kingsley LA 1996 Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170 [DOI] [PubMed] [Google Scholar]
- Lavreys L, Baeten JM, Chohan V, McClelland RS, Hassan WM, Richardson BA, Mandaliya K, Ndinya-Achola JO, Overbaugh J 2006 Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis 42:1333–1339 [DOI] [PubMed] [Google Scholar]
- Stringer EM, Kaseba C, Levy J, Sinkala M, Goldenberg RL, Chi BH, Matongo I, Vermund SH, Mwanahamuntu M, Stringer JS 2007 A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am J Obstet Gynecol 197:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejtin HE, Jacobson L, Springer G, Watts DH, Levine A, Greenblatt R, Anastos K, Minkoff HL, Massad LS, Schmidt JB 2003 Effect of hormonal contraceptive use on plasma HIV-1-RNA levels among HIV-infected women. AIDS 17:1702–1704 [DOI] [PubMed] [Google Scholar]
- Richardson BA, Otieno PA, Mbori-Ngacha D, Overbaugh J, Farquhar C, John-Stewart GC 2007 Hormonal contraception and HIV-1 disease progression among postpartum Kenyan women. AIDS 21:749–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostad SB, Overbaugh J, DeVange DM, Welch MJ, Chohan B, Mandaliya K, Nyange P, Martin Jr HL, Ndinya-Achola J, Bwayo JJ, Kreiss JK 1997 Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet 350:922–927 [DOI] [PubMed] [Google Scholar]
- Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, Chohan B, Lavreys L, Ndinya-Achola J, Kreiss JK 2004 The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS 18:205–209 [DOI] [PubMed] [Google Scholar]
- Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, Plummer F, Ndinya-Achola J, Roberts PL, Hillier S 1993 Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA 269:2860–2864 [PubMed] [Google Scholar]
- Ghanem KG, Shah N, Klein RS, Mayer KH, Sobel JD, Warren DL, Jamieson DJ, Duerr AC, Rompalo AM 2005 Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis 191:358–366 [DOI] [PubMed] [Google Scholar]
- Morrison CS, Bright P, Wong EL, Kwok C, Yacobson I, Gaydos CA, Tucker HT, Blumenthal PD 2004 Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis 31:561–567 [DOI] [PubMed] [Google Scholar]
- Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin Jr HL, Mandaliya K, Ndinya-Achola JO, Bwayo JJ, Kreiss JK 2001 Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol 185:380–385 [DOI] [PubMed] [Google Scholar]
- Lavreys L, Chohan V, Overbaugh J, Hassan W, McClelland RS, Kreiss J, Mandaliya K, Ndinya-Achola J, Baeten JM 2004 Hormonal contraception and risk of cervical infections among HIV-1-seropositive Kenyan women. AIDS 18:2179–2184 [DOI] [PubMed] [Google Scholar]
- Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ 1996 Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 2:1084–1089 [DOI] [PubMed] [Google Scholar]
- Trunova N, Tsai L, Tung S, Schneider E, Harouse J, Gettie A, Simon V, Blanchard J, Cheng-Mayer C 2006 Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology 352:169–177 [DOI] [PubMed] [Google Scholar]
- Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP 2003 Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9:343–346 [DOI] [PubMed] [Google Scholar]
- Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS 2006 Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol 190:829–835 [DOI] [PubMed] [Google Scholar]
- Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ 2004 Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis 190:1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genescà M, Li J, Fritts L, Chohan P, Bost K, Rourke T, Blozis SA, McChesney MB, Miller CJ 2007 Depo-Provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239. J Med Primatol 36:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Baskin GB, Marx PA 2000 Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis 182:708–715 [DOI] [PubMed] [Google Scholar]
- Smith SM, Mefford M, Sodora D, Klase Z, Singh M, Alexander N, Hess D, Marx PA 2004 Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS 18:1637–1643 [DOI] [PubMed] [Google Scholar]
- Hladik F, McElrath MJ 2008 Setting the stage: host invasion by HIV. Nat Rev Immunol 8:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell PD, Barton SE, Edmonds DK, Boag FC 1992 HIV infection in a patient with Meyer-Rokitansky-Kuster-Hauser syndrome. J R Soc Med 85:706–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Alexander NJ, Vogel P, Anderson J, Marx PA 1992 Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J Med Primatol 21:64–68 [PubMed] [Google Scholar]
- Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, Blanchard K, Shiboski S, Montgomery ET, Fancher H, Cheng H, Rosenblum M, van der Laan M, Jewell N, McIntyre J 2007 Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 370:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander U, Milsom I, Ekelund P, Mellström D, Eriksson O 1990 Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post-menopause. Maturitas 12:113–120 [DOI] [PubMed] [Google Scholar]
- Gupta S, Kumar N, Singhal N, Manektala U, Jain S, Sodhani P 2006 Cytohormonal and morphological alterations in cervicovaginal smears of postmenopausal women on hormone replacement therapy. Diagn Cytopathol 34:676–681 [DOI] [PubMed] [Google Scholar]
- Sikoski P, Register TC, Lees CJ, Lundeen S, Hutchison J, Brown KH, Cline JM 2007 Effects of two novel selective estrogen receptor modulators, raloxifene, tamoxifen, and ethinyl estradiol on the uterus, vagina and breast in ovariectomized cynomolgus monkeys (Macaca fascicularis). Am J Obstet Gynecol 196:75–77 [DOI] [PubMed] [Google Scholar]
- Cline JM, Register TC, Clarkson TB 2002 Comparative effects of tibolone and conjugated equine estrogens with and without medroxyprogesterone acetate on the reproductive tract of female cynomolgus monkeys. Menopause 9:242–252 [DOI] [PubMed] [Google Scholar]
- Felding C, Mikkelsen AL, Clausen HV, Loft A, Larsen LG 1992 Preoperative treatment with oestradiol in women scheduled for vaginal operation for genital prolapse. A randomised, double-blind trial. Maturitas 15:241–249 [DOI] [PubMed] [Google Scholar]
- Pask AJ, McInnes KJ, Webb DR, Short RV 2008 Topical oestrogen keratinises the human foreskin and may help prevent HIV infection. PLoS ONE 3:e2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora DL, Gettie A, Miller CJ, Marx PA 1998 Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 14(Suppl 1):S119–S123 [PubMed] [Google Scholar]
- Smith P 1993 Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol-releasing vaginal ring. Acta Obstet Gynecol Scand Suppl 157:1–26 [PubMed] [Google Scholar]
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Julia MD 2005 Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas 52(Suppl 1):S46–S52 [DOI] [PubMed] [Google Scholar]
- Nilsson K, Risberg B, Heimer G 1995 The vaginal epithelium in the postmenopause–cytology, histology and pH as methods of assessment. Maturitas 21:51–56 [DOI] [PubMed] [Google Scholar]
- Aaby P, Ariyoshi K, Buckner M, Jensen H, Berry N, Wilkins A, Richard D, Larsen O, Dias F, Melbye M, Whittle H 1996 Age of wife as a major determinant of male-to-female transmission of HIV-2 infection: a community study from rural West Africa. AIDS 10:1585–1590 [DOI] [PubMed] [Google Scholar]
- Comparison of female to male and male to female transmission of HIV in 563 stable couples 1992 European Study Group on Heterosexual Transmission of HIV. BMJ 304:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP 1998 Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res Hum Retroviruses 14(Suppl 1):S125–S130 [PubMed] [Google Scholar]
- Mauck CK, Callahan MM, Baker J, Arbogast K, Veazey R, Stock R, Pan Z, Morrison CS, Chen-Mok M, Archer DF, Gabelnick HL 1999 The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception 60:15–24 [DOI] [PubMed] [Google Scholar]
- Ildgruben AK, Sjöberg IM, Hammarström ML 2003 Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol 102:571–582 [DOI] [PubMed] [Google Scholar]
- Ildgruben A, Sjöberg I, Hammarström ML, Bäckström T 2005 Steroid receptor expression in vaginal epithelium of healthy fertile women and influences of hormonal contraceptive usage. Contraception 72:383–392 [DOI] [PubMed] [Google Scholar]
- Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA 2000 Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol 96:431–439 [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ, Coombs RW 1991 Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med 174:289–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J 1999 Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180:1863–1868 [DOI] [PubMed] [Google Scholar]
- Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG 1998 Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 12:1699–1706 [DOI] [PubMed] [Google Scholar]
- Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS 2008 Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 22:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ 2007 Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira C, Crane-Godreau A, Grant KS 2005 Endocrine regulation of the mucosal immune system in the female reproductive tract. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, eds. Mucosal immunology. New York: Elsevier Academic Press; 1661–1678 [Google Scholar]
- Wieser F, Hosmann J, Tschugguel W, Czerwenka K, Sedivy R, Huber JC 2001 Progesterone increases the number of Langerhans cells in human vaginal epithelium. Fertil Steril 75:1234–1235 [DOI] [PubMed] [Google Scholar]
- Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA 2000 Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol 183:967–973 [DOI] [PubMed] [Google Scholar]
- Ma Z, Lü FX, Torten M, Miller CJ 2001 The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol 100:240–249 [DOI] [PubMed] [Google Scholar]
- Straub RH 2007 The complex role of estrogens in inflammation. Endocr Rev 28:521–574 [DOI] [PubMed] [Google Scholar]
- Prakash M, Kapembwa MS, Gotch F, Patterson S 2002 Oral contraceptive use induces upregulation of the CCR5 chemokine receptor on CD4(+) T cells in the cervical epithelium of healthy women. J Reprod Immunol 54:117–131 [DOI] [PubMed] [Google Scholar]
- Sheffield JS, Wendel Jr GD, McIntire DD, Norgard MV 2009 The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci 16:20–31 [DOI] [PubMed] [Google Scholar]
- Conner P, Lundström E, von Schoultz B 2008 Breast cancer and hormonal therapy. Clin Obstet Gynecol 51:592–606 [DOI] [PubMed] [Google Scholar]
- Paavonen T 1994 Hormonal regulation of immune responses. Ann Med 26:255–258 [DOI] [PubMed] [Google Scholar]
- Beagley KW, Gockel CM 2003 Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol 38:13–22 [DOI] [PubMed] [Google Scholar]
- Grossman CJ 1984 Regulation of the immune system by sex steroids. Endocr Rev 5:435–455 [DOI] [PubMed] [Google Scholar]
- Kutteh WH, Mestecky J, Wira C 2005 Mucosal immunity in the human female reproductive tract. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, eds. Mucosal immunology. New York: Elsevier Academic Press; 1631–1646 [Google Scholar]
- Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J 1996 Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol 104:538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü FX, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller CJ 1999 Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun 67:6321–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ 2002 The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol 128:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sthoeger ZM, Chiorazzi N, Lahita RG 1988 Regulation of the immune response by sex hormones. I. In vitro effects of estradiol and testosterone on pokeweed mitogen-induced human B cell differentiation. J Immunol 141:91–98 [PubMed] [Google Scholar]
- Paavonen T, Andersson LC, Adlercreutz H 1981 Sex hormone regulation of in vitro immune response. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med 154:1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RD, Kutteh WH 1999 Characterization of immunoglobulins and cytokines in human cervical mucus: influence of exogenous and endogenous hormones. J Reprod Immunol 42:93–106 [DOI] [PubMed] [Google Scholar]
- Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P 2003 Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst 95:1128–1137 [DOI] [PubMed] [Google Scholar]
- Shrier LA, Bowman FP, Lin M, Crowley-Nowick PA 2003 Mucosal immunity of the adolescent female genital tract. J Adolesc Health 32:183–186 [DOI] [PubMed] [Google Scholar]
- Lü FX, Ma Z, Moser S, Evans TG, Miller CJ 2003 Effects of ovarian steroids on immunoglobulin-secreting cell function in healthy women. Clin Diagn Lab Immunol 10:944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gockel CM, Bao S, Holland MK, Beagley KW 2003 Influence of the murine oestrous cycle on the induction of mucosal immunity. Am J Reprod Immunol 50:369–379 [DOI] [PubMed] [Google Scholar]
- White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR 1997 CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol 158:3017–3027 [PubMed] [Google Scholar]
- Wira CR, Roche MA, Rossoll RM 2002 Antigen presentation by vaginal cells: role of TGFβ as a mediator of estradiol inhibition of antigen presentation. Endocrinology 143:2872–2879 [DOI] [PubMed] [Google Scholar]
- Wira CR, Rossoll RM, Kaushic C 2000 Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells. Endocrinology 141:2877–2885 [DOI] [PubMed] [Google Scholar]
- White HD, Musey LK, Andrews MM, Yeaman GR, DeMars LR, Manganiello PD, Howell AL, Wira CR, Green WR, McElrath MJ 2001 Human immunodeficiency virus-specific and CD3-redirected cytotoxic T lymphocyte activity in the human female reproductive tract: lack of correlation between mucosa and peripheral blood. J Infect Dis 183:977–983 [DOI] [PubMed] [Google Scholar]
- Willis C, Morris JM, Danis V, Gallery ED 2003 Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod 18:1173–1178 [DOI] [PubMed] [Google Scholar]
- Amory JH, Lawler R, Hitti J 2004 Increased tumor necrosis factor-α in whole blood during the luteal phase of ovulatory cycles. J Reprod Med 49:678–682 [PubMed] [Google Scholar]
- O'Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG 2007 Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation 14:84–90 [DOI] [PubMed] [Google Scholar]
- Souza SS, Castro FA, Mendonça HC, Palma PV, Morais FR, Ferriani RA, Voltarelli JC 2001 Influence of menstrual cycle on NK activity. J Reprod Immunol 50:151–159 [DOI] [PubMed] [Google Scholar]
- Yovel G, Shakhar K, Ben-Eliyahu S 2001 The effects of sex, menstrual cycle, and oral contraceptives on the number and activity of natural killer cells. Gynecol Oncol 81:254–262 [DOI] [PubMed] [Google Scholar]
- Yeaman GR, White HD, Howell A, Prabhala R, Wira CR 1998 The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses 14(Suppl 1):S57–S62 [PubMed] [Google Scholar]
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L 2005 Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206:306–335 [DOI] [PubMed] [Google Scholar]
- Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O'Connell DM, Asin SN, Wira CR, Fanger MW 2003 Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology 109:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA 2005 Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol 53:65–76 [DOI] [PubMed] [Google Scholar]
- Saïdi H, Magri G, Nasreddine N, Réquena M, Bélec L 2007 R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology 358:55–68 [DOI] [PubMed] [Google Scholar]
- Carreno MP, Krieff C, Irinopoulou T, Kazatchkine MD, Belec L 2002 Enhanced transcytosis of R5-tropic human immunodeficiency virus across tight monolayer of polarized human endometrial cells under pro-inflammatory conditions. Cytokine 20:289–294 [DOI] [PubMed] [Google Scholar]
- Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL 2004 Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis 190:236–245 [DOI] [PubMed] [Google Scholar]
- Schaefer TM, Fahey JV, Wright JA, Wira CR 2005 Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J Immunol 174:992–1002 [DOI] [PubMed] [Google Scholar]
- Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, Orndorff KA, Gonzalez J, Stern JE, Wira CR 1997 Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol 61:427–435 [PubMed] [Google Scholar]
- Yeaman GR, Collins JE, Fanger MW, Wira CR, Lydyard PM 2001 CD8+ T cells in human uterine endometrial lymphoid aggregates: evidence for accumulation of cells by trafficking. Immunology 102:434–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G 1999 Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by estrogen and progesterone. Biol Reprod 61:85–90 [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Mukaida N, Wang JB, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K 1998 Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s). J Immunol 160:6159–6165 [PubMed] [Google Scholar]
- Penny LA, Armstrong DG, Baxter G, Hogg C, Kindahl H, Bramley T, Watson ED, Webb R 1998 Expression of monocyte chemoattractant protein-1 in the bovine corpus luteum around the time of natural luteolysis. Biol Reprod 59:1464–1469 [DOI] [PubMed] [Google Scholar]
- Money DM, Arikan YY, Remple V, Sherlock C, Craib K, Birch P, Burdge DR 2003 Genital tract and plasma human immunodeficiency virus viral load throughout the menstrual cycle in women who are infected with ovulatory human immunodeficiency virus. Am J Obstet Gynecol 188:122–128 [DOI] [PubMed] [Google Scholar]
- Hanna L 1999 The menstrual cycle and viral load. BETA 12:18 [PubMed] [Google Scholar]
- Al-Harthi L, Kovacs A, Coombs RW, Reichelderfer PS, Wright DJ, Cohen MH, Cohn J, Cu-Uvin S, Watts H, Lewis S, Beckner S, Landay A 2001 A menstrual cycle pattern for cytokine levels exists in HIV-positive women: implication for HIV vaginal and plasma shedding. AIDS 15:1535–1543 [DOI] [PubMed] [Google Scholar]
- Benki S, Mostad SB, Richardson BA, Mandaliya K, Kreiss JK, Overbaugh J 2004 Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J Infect Dis 189:2192–2201 [DOI] [PubMed] [Google Scholar]
- Mostad SB, Jackson S, Overbaugh J, Reilly M, Chohan B, Mandaliya K, Nyange P, Ndinya-Achola J, Bwayo JJ, Kreiss JK 1998 Cervical and vaginal shedding of human immunodeficiency virus type 1-infected cells throughout the menstrual cycle. J Infect Dis 178:983–991 [DOI] [PubMed] [Google Scholar]
- Villanueva JM, Ellerbrock TV, Lennox JL, Bush TJ, Wright TC, Pratt-Palmore M, Evans-Strickfaden T, Conley LJ, Schnell C, Hart CE 2002 The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis 185:170–177 [DOI] [PubMed] [Google Scholar]
- Quinn TC, Overbaugh J 2005 HIV/AIDS in women: an expanding epidemic. Science 308:1582–1583 [DOI] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, Reynolds SJ, Wawer MJ 2005 Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 366:1182–1188 [DOI] [PubMed] [Google Scholar]
- Hénin Y, Mandelbrot L, Henrion R, Pradinaud R, Coulaud JP, Montagnier L 1993 Virus excretion in the cervicovaginal secretions of pregnant and nonpregnant HIV-infected women. J Acquir Immune Defic Syndr 6:72–75 [PubMed] [Google Scholar]
- Wira CR, Sandoe CP 1980 Hormonal regulation of immunoglobulins: influence of estradiol on immunoglobulins A and G in the rat uterus. Endocrinology 106:1020–1026 [DOI] [PubMed] [Google Scholar]
- Wira CR, Sullivan DA 1985 Estradiol and progesterone regulation of immunoglobulin A and G and secretory component in cervicovaginal secretions of the rat. Biol Reprod 32:90–95 [DOI] [PubMed] [Google Scholar]
- Klinger G, Gräser T, Mellinger U, Moore C, Vogelsang H, Groh A, Latterman C, Klinger G 2000 A comparative study of the effects of two oral contraceptives containing dienogest or desogestrel on the human immune system. Gynecol Endocrinol 14:15–24 [DOI] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL 2003 Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 77:4558– 4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C 2003 Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol 77:9845–9851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushic C, Murdin AD, Underdown BJ, Wira CR 1998 Chlamydia trachomatis infection in the female reproductive tract of the rat: influence of progesterone on infectivity and immune response. Infect Immun 66:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM 2005 Sex hormones and the immune response in humans. Hum Reprod Update 11:411–423 [DOI] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL 1994 A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70:369–380 [PubMed] [Google Scholar]
- Canellada A, Blois S, Gentile T, Margni Idehu RA 2002 In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am J Reprod Immunol 48:334–343 [DOI] [PubMed] [Google Scholar]
- Margni RA, Malan Borel I 1998 Paradoxical behavior of asymmetric IgG antibodies. Immunol Rev 163:77–87 [DOI] [PubMed] [Google Scholar]
- Clemens LE, Siiteri PK, Stites DP 1979 Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol 122:1978–1985 [PubMed] [Google Scholar]
- Mori T, Kobayashi H, Nishimoto H, Suzuki A, Nishimura T, Mori T 1977 Inhibitory effect of progesterone and 20 α-hydroxypregn-4-en-3-one on the phytohemagglutinin-induced transformation of human lymphocytes. Am J Obstet Gynecol 127:151–157 [DOI] [PubMed] [Google Scholar]
- Pavia C, Siiteri PK, Perlman JD, Stites DP 1979 Suppression of murine allogeneic cell interactions by sex hormones. J Reprod Immunol 1:33–38 [DOI] [PubMed] [Google Scholar]
- Wyle FA, Kent JR 1977 Immunosuppression by sex steroid hormones. The effect upon PHA- and PPD-stimulated lymphocytes. Clin Exp Immunol 27:407–415 [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Hadnagy J, Pacsa AS 1985 The suppressive effect of progesterone on lymphocyte cytotoxicity: unique progesterone sensitivity of pregnancy lymphocytes. J Reprod Immunol 7:121–128 [DOI] [PubMed] [Google Scholar]
- Laskarin G, Strbo N, Sotosek V, Rukavina D, Faust Z, Szekeres-Bartho J, Podack ER 1999 Progesterone directly and indirectly affects perforin expression in cytolytic cells. Am J Reprod Immunol 42:312–320 [DOI] [PubMed] [Google Scholar]
- Mannel DN, Falk W, Yron I 1990 Inhibition of murine cytotoxic T cell responses by progesterone. Immunol Lett 26:89–94 [DOI] [PubMed] [Google Scholar]
- Borel IM, Freire SM, Rivera E, Canellada A, Binaghi RA, Margni RA 1999 Modulation of the immune response by progesterone-induced lymphocyte factors. Scand J Immunol 49:244–250 [DOI] [PubMed] [Google Scholar]
- Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL 2008 Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol 181:969–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto LM, Kloberdanz KJ, Mack DG, Elizabeth D, Weinberg A 2007 Ex vivo effect of estrogen and progesterone compared with dexamethasone on cell-mediated immunity of HIV-infected and uninfected subjects. J Acquir Immune Defic Syndr 45:137–143 [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Faust Z, Varga P 1995 The expression of a progesterone-induced immunomodulatory protein in pregnancy lymphocytes. Am J Reprod Immunol 34:342–348 [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L 2001 Progesterone as an immunomodulatory molecule. Int Immunopharmacol 1:1037–1048 [DOI] [PubMed] [Google Scholar]
- Szereday L, Varga P, Szekeres-Bartho J 1997 Cytokine production by lymphocytes in pregnancy. Am J Reprod Immunol 38:418–422 [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Wegmann TG 1996 A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol 31:81–95 [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, Romognani S, Maggi E 1995 Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol 155:128–133 [PubMed] [Google Scholar]
- Piccinni MP, Maggi E, Romagnani S 2000 Role of hormone-controlled T-cell cytokines in the maintenance of pregnancy. Biochem Soc Trans 28:212–215 [DOI] [PubMed] [Google Scholar]
- Kozma N, Halasz M, Polgar B, Poehlmann TG, Markert UR, Palkovics T, Keszei M, Par G, Kiss K, Szeberenyi J, Grama L, Szekeres-Bartho J 2006 Progesterone-induced blocking factor activates STAT6 via binding to a novel IL-4 receptor. J Immunol 176:819–826 [DOI] [PubMed] [Google Scholar]
- Laskarin G, Tokmadziæ VS, Strbo N, Bogoviæ T, Szekeres-Bartho J, Randiæ L, Podack ER, Rukavina D 2002 Progesterone induced blocking factor (PIBF) mediates progesterone induced suppression of decidual lymphocyte cytotoxicity. Am J Reprod Immunol 48:201–209 [DOI] [PubMed] [Google Scholar]
- Inoue T, Kanzaki H, Imai K, Narukawa S, Katsuragawa H, Watanabe H, Hirano T, Mori T 1996 Progesterone stimulates the induction of human endometrial CD56+ lymphocytes in an in vitro culture system. J Clin Endocrinol Metab 81:1502–1507 [DOI] [PubMed] [Google Scholar]
- Whitelaw PF, Croy BA 1996 Granulated lymphocytes of pregnancy. Placenta 17:533–543 [DOI] [PubMed] [Google Scholar]
- Scanlan JM, Werner JJ, Legg RL, Laudenslager ML 1995 Natural killer cell activity is reduced in association with oral contraceptive use. Psychoneuroendocrinology 20: 281–287 [DOI] [PubMed] [Google Scholar]
- Gomez F, Ruiz P, Lopez R, Rivera C 2002 Treatment with megestrol acetate improves human immunodeficiency virus-associated immune thrombocytopenia. Clin Diagn Lab Immunol 9:583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag P, Kim J, Sapiega V, Landay AL, Bremer JW, Mestecky J, Reichelderfer P, Kovacs A, Cohn J, Weiser B, Baum LL 2004 Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis 190:1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GC, Thomas S, Li C, Kaja MK, Clark EA 2008 Cutting edge: progesterone regulates IFN-α production by plasmacytoid dendritic cells. J Immunol 180:2029–2033 [DOI] [PubMed] [Google Scholar]
- Danel L, Vincent C, Rousset F, Klein B, Bataille R, Flacher M, Durie BG, Revillard JP 1988 Estrogen and progesterone receptors in some human myeloma cell lines and murine hybridomas. J Steroid Biochem 30:363–367 [DOI] [PubMed] [Google Scholar]
- Pasanen S, Ylikomi T, Palojoki E, Syvälä H, Pelto-Huikko M, Tuohimaa P 1998 Progesterone receptor in chicken bursa of Fabricius and thymus: evidence for expression in B-lymphocytes. Mol Cell Endocrinol 141:119–128 [DOI] [PubMed] [Google Scholar]
- Polgar B, Barakonyi A, Xynos I, Szekeres-Bartho J 1999 The role of γ/δ T cell receptor positive cells in pregnancy. Am J Reprod Immunol 41:239–244 [DOI] [PubMed] [Google Scholar]
- Savouret JF, Chauchereau A, Misrahi M, Lescop P, Mantel A, Bailly A, Milgrom E 1994 The progesterone receptor. Biological effects of progestins and antiprogestins. Hum Reprod 9(Suppl 1):7–11 [DOI] [PubMed] [Google Scholar]
- Zhao XJ, McKerr G, Dong Z, Higgins CA, Carson J, Yang ZQ, Hannigan BM 2001 Expression of oestrogen and progesterone receptors by mast cells alone, but not lymphocytes, macrophages or other immune cells in human upper airways. Thorax 56:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Szekeres G, Debre P, Autran B, Chaouat G 1990 Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol 125:273–283 [DOI] [PubMed] [Google Scholar]
- Srivastava MD, Anderson DJ 2007 Progesterone receptor expression by human leukocyte cell lines: molecular mechanisms of cytokine suppression. Clin Exp Obstet Gynecol 34:14–24 [PubMed] [Google Scholar]
- Szekeres-Bartho J, Reznikoff-Etievant MF, Varga P, Pichon MF, Varga Z, Chaouat G 1989 Lymphocytic progesterone receptors in normal and pathological human pregnancy. J Reprod Immunol 16:239–247 [DOI] [PubMed] [Google Scholar]
- Roussev RG, Higgins NG, McIntyre JA 1993 Phenotypic characterization of normal human placental mononuclear cells. J Reprod Immunol 25:15–29 [DOI] [PubMed] [Google Scholar]
- Chiu L, Nishimura M, Ishii Y, Nieda M, Maeshima M, Takedani Y, Shibata Y, Tadokoro K, Juji T 1996 Enhancement of the expression of progesterone receptor on progesterone-treated lymphocytes after immunotherapy in unexplained recurrent spontaneous abortion. Am J Reprod Immunol 35:552–557 [DOI] [PubMed] [Google Scholar]
- Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP 2005 Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol 242:23–32 [DOI] [PubMed] [Google Scholar]
- Kontula K, Paavonen T, Luukkainen T, Andersson LC 1983 Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol 32:1511–1518 [DOI] [PubMed] [Google Scholar]
- Palmieri D, Halverson DO, Ouatas T, Horak CE, Salerno M, Johnson J, Figg WD, Hollingshead M, Hursting S, Berrigan D, Steinberg SM, Merino MJ, Steeg PS 2005 Medroxyprogesterone acetate elevation of Nm23-H1 metastasis suppressor expression in hormone receptor-negative breast cancer. J Natl Cancer Inst 97:632–642 [DOI] [PubMed] [Google Scholar]
- Thomas CP, Liu KZ, Vats HS 2006 Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate α-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol 290:F306–F312 [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM 1999 Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab 84:4055–4061 [DOI] [PubMed] [Google Scholar]
- Northrop JP, Crabtree GR, Mattila PS 1992 Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med 175:1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A, Felli MP, Farina AR, Martinotti S, Maroder M, Screpanti I, Meco D, Petrangeli E, Frati L, Gulino A 1992 Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med 175:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS Jr 1995 Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol 15:943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vollenhoven RF, McGuire JL 1994 Estrogen, progesterone, and testosterone: can they be used to treat autoimmune diseases? Cleve Clin J Med 61:276–284 [DOI] [PubMed] [Google Scholar]
- Attanasio R, Gust DA, Wilson ME, Meeker T, Gordon TP 2002 Immunomodulatory effects of estrogen and progesterone replacement in a nonhuman primate model. J Clin Immunol 22:263–269 [DOI] [PubMed] [Google Scholar]
- Zang YC, Halder JB, Hong J, Rivera VM, Zhang JZ 2002 Regulatory effects of estriol on T cell migration and cytokine profile: inhibition of transcription factor NF-κB. J Neuroimmunol 124:106–114 [DOI] [PubMed] [Google Scholar]
- Härkönen PL, Väänänen HK 2006 Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann NY Acad Sci 1089:218–227 [DOI] [PubMed] [Google Scholar]
- Salem ML, Hossain MS, Nomoto K 2000 Mediation of the immunomodulatory effect of β-estradiol on inflammatory responses by inhibition of recruitment and activation of inflammatory cells and their gene expression of TNF-α and IFN-γ. Int Arch Allergy Immunol 121:235–245 [DOI] [PubMed] [Google Scholar]
- Ito A, Buenafe AC, Matejuk A, Zamora A, Silverman M, Dwyer J, Vandenbark AA, Offner H 2002 Estrogen inhibits systemic T cell expression of TNF-α and recruitment of TNF-α(+) T cells and macrophages into the CNS of mice developing experimental encephalomyelitis. Clin Immunol 102:275–282 [DOI] [PubMed] [Google Scholar]
- Kanda N, Tamaki K 1999 Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol 103:282–288 [DOI] [PubMed] [Google Scholar]
- Wira CR, Sandoe CP 1989 Effect of uterine immunization and oestradiol on specific IgA and IgG antibodies in uterine, vaginal and salivary secretions. Immunology 68:24–30 [PMC free article] [PubMed] [Google Scholar]
- Menge AC, Mestecky J 1993 Surface expression of secretory component and HLA class II DR antigen on glandular epithelial cells from human endometrium and two endometrial adenocarcinoma cell lines. J Clin Immunol 13:259–264 [DOI] [PubMed] [Google Scholar]
- Jalanti R, Isliker H 1977 Immunoglobulins in human cervico-vaginal secretions. Int Arch Allergy Appl Immunol 53:402–408 [DOI] [PubMed] [Google Scholar]
- Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR 1998 Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol 39:209–216 [DOI] [PubMed] [Google Scholar]
- Sentman CL, Meadows SK, Wira CR, Eriksson M 2004 Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol 173:6760–6766 [DOI] [PubMed] [Google Scholar]
- Hel Z, McGhee JR, Mestecky J 2006 HIV infection: first battle decides the war. Trends Immunol 27:274–281 [DOI] [PubMed] [Google Scholar]
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M 2005 Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT 2005 Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Kelsall B, Tsai WP, Letvin N, Parks RW, Tryniszewska E, Picker L, Lewis MG, Edghill-Smith Y, Moniuszko M, Pal R, Stevceva L, Altman JD, Allen TM, Watkins D, Torres JV, Berzofsky JA, Belyakov IM, Strober W, Franchini G 2001 Impairment of Gag-specific CD8(+) T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J Virol 75:11483–11495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise C, Miller CJ, Lackner A, Dandekar S 1994 Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis 169:1116–1120 [DOI] [PubMed] [Google Scholar]
- Heise C, Vogel P, Miller CJ, Halsted CH, Dandekar S 1993 Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol 142:1759–1771 [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA 1998 Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- Veazey RS, Lackner AA 2004 Getting to the guts of HIV pathogenesis. J Exp Med 200:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton F, Snow G, Reka S, Kotler DP 1997 Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol 107:288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M 2004 Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T, Kinoshita K, Muramatsu M 2002 Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol 20:165–196 [DOI] [PubMed] [Google Scholar]
- Cerntti A 2008 The regulation of IgA class switching. Nat Rev Immunol 8:421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis JP, Tian M, Alt FW 2002 Mechanism and control of class-switch recombination. Trends Immunol 23:31–39 [DOI] [PubMed] [Google Scholar]
- Van Kooten C, Banchereau J 1996 CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol 61:1–77 [DOI] [PubMed] [Google Scholar]
- Fujihashi K, McGhee JR 2005 Th1/Th2/Th3 cells for regulation of mucosal immunity, tolerance, and inflammation. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, eds. Mucosal immunology. New York: Elsevier/Academic Press; 539–558 [Google Scholar]
- Hörnquist CE, Ekman L, Grdic KD, Schön K, Lycke NY 1995 Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J Immunol 155:2877–2887 [PubMed] [Google Scholar]
- McGhee JR, Mestecky J, Elson CO, Kiyono H 1989 Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol 9:175–199 [DOI] [PubMed] [Google Scholar]
- Strober W, Fagarasan S, Lycke N 2005 IgA B cell development. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, eds. Mucosal immunology. New York: Elsevier/Academic Press; 583–616 [Google Scholar]
- Mestecky J, Moldoveanu Z, Elson CO 2005 Immune response versus mucosal tolerance to mucosally administered antigens. Vaccine 23:1800–1803 [DOI] [PubMed] [Google Scholar]
- Powrie F 2004 Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann NY Acad Sci 1029:132–141 [DOI] [PubMed] [Google Scholar]
- Bevan MJ 2004 Helping the CD8(+) T-cell response. Nat Rev Immunol 4:595–602 [DOI] [PubMed] [Google Scholar]
- Kaetzel CS, Mostov K 2005 Immunoglobulin transport and the polymeric immunoglobulin receptor. In: Mestecky J, Bienenstock J, Lamm ME, Mayer L, McGhee JR, Strober W, eds. Mucosal immunology. New York: Elsevier/Academic Press; 211–250 [Google Scholar]
- Northrop JK, Shen H 2004 CD8+ T-cell memory: only the good ones last. Curr Opin Immunol 16:451–455 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T 2004 Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303:1662–1665 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL 2004 Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 4:478–485 [DOI] [PubMed] [Google Scholar]
- Kotler DP 2005 HIV infection and the gastrointestinal tract. AIDS 19:107–117 [DOI] [PubMed] [Google Scholar]
- Sharpstone D, Neild P, Crane R, Taylor C, Hodgson C, Sherwood R, Gazzard B, Bjarnason I 1999 Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut 45:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MD, Reay E, Sankaran S, Dandekar S 2005 Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol 79:2709–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer F, Kewenig S, Stolte N, Stahl-Hennig C, Stallmach A, Kaup FJ, Zeitz M, Schneider T 2002 Lack of simian immunodeficiency virus (SIV) specific IgA response in the intestine of SIV infected rhesus macaques. Gut 50:608–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, Lindner C, Frieling T, Niederau C, Reinauer H, Häussinger D 1997 Intestinal protein leakage in the acquired immunodeficiency syndrome. J Clin Gastroenterol 25:426–428 [DOI] [PubMed] [Google Scholar]
- Schneider T, Zippel T, Schmidt W, Pauli G, Heise W, Wahnschaffe U, Riecken EO, Zeitz M, Ullrich R 1997 Abnormal predominance of IgG in HIV-specific antibodies produced by short-term cultured duodenal biopsy specimens from HIV-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol 16:333–339 [DOI] [PubMed] [Google Scholar]
- Fernandez MI, Pedron T, Tournebize R, Olivo-Marin JC, Sansonetti PJ, Phalipon A 2003 Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity 18:739–749 [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T 2004 Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann NY Acad Sci 1029:36–43 [DOI] [PubMed] [Google Scholar]
- Mestecky J, Russell MW, Elson CO 1999 Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut 44:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson EC, Gorga JC, Garrett M, Tuncer R, Boyle P, Watkins SC, Alber SM, Parizhskaya M, Trucco M, Rowe MI, Ford HR 1998 Immunoglobulin A supplementation abrogates bacterial translocation and preserves the architecture of the intestinal epithelium. Surgery 124:284–290 [PubMed] [Google Scholar]
- Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P 1999 Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med 190:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC 2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- Bélec L, Dupré T, Prazuck T, Tévi-Bénissan C, Kanga JM, Pathey O, Lu XS, Pillot J 1995 Cervicovaginal overproduction of specific IgG to human immunodeficiency virus (HIV) contrasts with normal or impaired IgA local response in HIV infection. J Infect Dis 172:691–697 [DOI] [PubMed] [Google Scholar]
- Lü FX 2000 Predominate HIV1-specific IgG activity in various mucosal compartments of HIV1-infected individuals. Clin Immunol 97:59–68 [DOI] [PubMed] [Google Scholar]
- Mestecky J, Jackson S, Moldoveanu Z, Nesbit LR, Kulhavy R, Prince SJ, Sabbaj S, Mulligan MJ, Goepfert PA 2004 Paucity of antigen-specific IgA responses in sera and external secretions of HIV-type 1-infected individuals. AIDS Res Hum Retroviruses 20:972–988 [DOI] [PubMed] [Google Scholar]
- Artenstein AW, VanCott TC, Sitz KV, Robb ML, Wagner KF, Veit SC, Rogers AF, Garner RP, Byron JW, Burnett PR, Birx DL 1997 Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: a comparison by site and correlation with clinical information. J Infect Dis 175:265–271 [DOI] [PubMed] [Google Scholar]
- Belec L, Georges AJ, Steenman G, Martin PM 1989 Antibodies to human immunodeficiency virus in vaginal secretions of heterosexual women. J Infect Dis 160:385–391 [DOI] [PubMed] [Google Scholar]
- Belec L, Meillet D, Gaillard O, Prazuck T, Michel E, Ngondi Ekome J, Pillot J 1995 Decreased cervicovaginal production of both IgA1 and IgA2 subclasses in women with AIDS. Clin Exp Immunol 101:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovici F, Mayer KH, Anderson DJ 1997 Quantitation of HIV-1-specific IgG, IgA, and IgM antibodies in human genital tract secretions. J Acquir Immune Defic Syndr Hum Retrovirol 15:185–191 [DOI] [PubMed] [Google Scholar]
- Wolff H, Mayer K, Seage G, Politch J, Horsburgh CR, Anderson D 1992 A comparison of HIV-1 antibody classes, titers, and specificities in paired semen and blood samples from HIV-1 seropositive men. J Acquir Immune Defic Syndr 5:65–69 [PubMed] [Google Scholar]
- Raux M, Finkielsztejn L, Salmon-Céron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, Blondeau C 1999 Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses 15:1365–1376 [DOI] [PubMed] [Google Scholar]
- Israel ZR, Marx PA 1995 Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol 24:53–60 [DOI] [PubMed] [Google Scholar]
- Jackson S, Moldoveanu Z, Mestecky J, Pitts AM, Eldridge JH, McGhee JR, Miller CJ, Marx PA 1995 Decreased IgA-producing cells in the gut of SIV-infected rhesus monkeys. Adv Exp Med Biol 371B:1035–1038 [PubMed] [Google Scholar]
- Mestecky J 2007 Humoral immune responses to the human immunodeficiency virus type-1 (HIV-1) in the genital tract compared to other mucosal sites. J Reprod Immunol 73:86–97 [DOI] [PubMed] [Google Scholar]
- Smith PD, Quinn TC, Strober W, Janoff EN, Masur H 1992 NIH conference. Gastrointestinal infections in AIDS. Ann Intern Med 116:63–77 [DOI] [PubMed] [Google Scholar]
- Jarry A, Cortez A, René E, Muzeau F, Brousse N 1990 Infected cells and immune cells in the gastrointestinal tract of AIDS patients. An immunohistochemical study of 127 cases. Histopathology 16:133–140 [DOI] [PubMed] [Google Scholar]
- Nelson JA, Wiley CA, Reynolds-Kohler C, Reese CE, Margaretten W, Levy JA 1988 Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet 1:259–262 [DOI] [PubMed] [Google Scholar]
- Lewis DJ, Gilks CF, Ojoo S, Castello-Branco LR, Dougan G, Evans MR, McDermott S, Griffin GE 1994 Immune response following oral administration of cholera toxin B subunit to HIV-1-infected UK and Kenyan subjects. AIDS 8:779–785 [DOI] [PubMed] [Google Scholar]
- Eriksson K, Kilander A, Hagberg L, Norkrans G, Holmgren J, Czerkinsky C 1995 Virus-specific antibody production and polyclonal B-cell activation in the intestinal mucosa of HIV-infected individuals. AIDS 9:695–700 [DOI] [PubMed] [Google Scholar]
- Janoff EN, O'Brien J, Thompson P, Ehret J, Meiklejohn G, Duvall G, Douglas JM Jr 1993 Streptococcus pneumoniae colonization, bacteremia, and immune response among persons with human immunodeficiency virus infection. J Infect Dis 167:49–56 [DOI] [PubMed] [Google Scholar]
- Eriksson K, Kilander A, Hagberg L, Norkrans G, Holmgren J, Czerkinsky C 1993 Intestinal antibody responses to oral vaccination in HIV-infected individuals. AIDS 7:1087–1091 [DOI] [PubMed] [Google Scholar]
- Janoff EN, Smith PD, Blaser MJ 1988 Acute antibody responses to Giardia lamblia are depressed in patients with AIDS. J Infect Dis 157:798–804 [DOI] [PubMed] [Google Scholar]
- Perlman DM, Ampel NM, Schifman RB, Cohn DL, Patton CM, Aguirre ML, Wang WL, Blaser MJ 1988 Persistent Campylobacter jejuni infections in patients infected with the human immunodeficiency virus (HIV). Ann Intern Med 108:540–546 [DOI] [PubMed] [Google Scholar]
- Baker DA, Hameed C, Tejani N, Milch P, Thomas J, Monheit AG, Dattwyler RJ 1985 Lymphocyte subsets in women on low dose oral contraceptives. Contraception 32:377–382 [DOI] [PubMed] [Google Scholar]
- Ferguson MM, McDonald FG 1985 Oestrogen as an inhibitor of human NK cell cytolysis. FEBS Lett 191:145–148 [DOI] [PubMed] [Google Scholar]
- De M, Wood GW 1990 Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol 126:417–424 [DOI] [PubMed] [Google Scholar]
- Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL 2008 Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses 24:701–716 [DOI] [PubMed] [Google Scholar]