Abstract
Opioid abuse has increased in the last decade, primarily as a result of increased access to prescription opioids. Physicians are also increasingly administering opioid analgesics for noncancer chronic pain. Thus, knowledge of the long-term consequences of opioid use/abuse has important implications for fully evaluating the clinical usefulness of opioid medications. Many studies have examined the effect of opioids on the endocrine system; however, a systematic review of the endocrine actions of opioids in both humans and animals has, to our knowledge, not been published since 1984. Thus, we reviewed the literature on the effect of opioids on the endocrine system. We included both acute and chronic effects of opioids, with the majority of the studies done on the acute effects although chronic effects are more physiologically relevant. In humans and laboratory animals, opioids generally increase GH and prolactin and decrease LH, testosterone, estradiol, and oxytocin. In humans, opioids increase TSH, whereas in rodents, TSH is decreased. In both rodents and humans, the reports of effects of opioids on arginine vasopressin and ACTH are conflicting. Opioids act preferentially at different receptor sites leading to stimulatory or inhibitory effects on hormone release. Increasing opioid abuse primarily leads to hypogonadism but may also affect the secretion of other pituitary hormones. The potential consequences of hypogonadism include decreased libido and erectile dysfunction in men, oligomenorrhea or amenorrhea in women, and bone loss or infertility in both sexes. Opioids may increase or decrease food intake, depending on the type of opioid and the duration of action. Additionally, opioids may act through the sympathetic nervous system to cause hyperglycemia and impaired insulin secretion. In this review, recent information regarding endocrine disorders among opioid abusers is presented.
Many studies have examined the effect of opioids on the endocrine system, however a systematic review of the endocrine actions of opioids in both humans and animals has, to our knowledge, not been published since 1984. We include both acute and chronic effects of opioids, with the majority of the studies done on the acute effects although chronic effects are more physiologically relevant. In humans and laboratory animals, opioids generally increase GH and prolactin, and decrease LH, testosterone, estradiol, and OT (oxytocin). In humans, opioids increase TSH, while in rodents, TSH is decreased. Specific information on the prevalence of endocrine disorders being caused by chronic opioid use has been lacking, and recent information regarding endocrine disorders among opioid abusers is presented.
- I. Introduction/Epidemiology
- A. Clinical importance of opioids on the endocrine system
- II. Pharmacology and Physiology of Opioids and Their Derivatives
- A. Opioids and their derivatives
- B. Classes of opioids
- C. Analgesic effects of opioids
- D. Synthetic and semisynthetic opioids
- E. Opioid antagonists
- F. Opioids and the stress response
- III. The Effects of Opioids on Endocrine Systems in Animals and Humans
- A. GH/IGF-I axis
- B. Prolactin
- C. Thyrotropin
- D. ACTH
- E. LH and FSH
- F. Sex steroid hormones (testosterone and estradiol) and sexual behavior
- G. Arginine vasopressin (AVP)
- H. Oxytocin (OT)
- I. Obesity and diabetes
IV. Areas of Future Research
V. Conclusion
I. Introduction/Epidemiology
The National Institute on Drug Abuse (NIDA) “Research Report Series-Prescription Drugs: Abuse and Addiction” (1) defines prescription drug misuse as “taking a medication in a manner other than that prescribed or for a different condition than that for which the medication is prescribed” and prescription drug abuse as “the intentional misuse of a medication outside of the normally accepted standards of its use.” Because prescription analgesics have been increasingly used to treat chronic pain, opioid use/abuse has been on the rise in recent years. Physicians are increasingly prescribing opioid analgesics, such as vicodin (hydrocodone/acetaminophen), darvocet-N (propoxyphen/acetaminophen), fentanyl and methadone because they are very effective in reducing pain and are inexpensive and long-lasting, despite the potential risk for addiction to these drugs (2). These medications are frequently used for osteoarthritis, back pain, sports injuries, and other muscular-skeletal conditions, rheumatological conditions, and headaches. Currently, little information is available on the long-term consequences of chronic opioid use, making it difficult for physicians and their patients to weigh the risks and benefits of prescription opioids before the initiation of therapy. Thus, the goal of this article is to comprehensively review the endocrine effects of opioids to inform clinicians of the endocrine (side) effects that may occur and identify knowledge gaps that need further research.
In the 1990s, there was an increase in the use of opioids, which is illustrated by the almost 10-fold increase in methadone prescriptions from 1997 to 2005 (3). After the increase in the 1990s, the epidemiological data over the mid-late 2000s shows a steady, high prevalence of prescription opioid misuse in the United States (4,5). International prevalence data show similar trends concerning levels of misuse (6). Many factors probably contribute to the increased prevalence of prescription opioid misuse, including changes in medication formulations, pharmaceutical industry marketing, and the aging population (7). However, the situation that has been most associated with prescription opioid misuse is the increased treatment of chronic noncancer pain (8,9). The following surveys give some epidemiological description of opioid use and misuse.
The 2006 National Survey on Drug Use and Health (NSDUH) (10) ascertained drug use patterns of a nationally representative sample of more than 67,000 noninstitutionalized individuals, age 12 and older. According to the NSDUH, an estimated 2.1% of the individuals of that age group were current nonmedical users of prescription pain relievers in 2006. Thus, the number of Americans estimated to use opioid pain relievers was 5.2 million, compared with 1.2 million using stimulants and 2.1 million using sedatives and tranquilizers. Of particular concern is that more persons age 12 and over initiated misuse of pain relievers (2.2 million) than any other illicit substance. By comparison, 2.1 million started using marijuana (10).
Perhaps the most interesting new fact from the recent NSDUH survey relates to the source of pain relievers that were used. According to the 2006 NSDUH (10), 55.7% of those who misused pain relievers said they received their medication from friends and family, and 80.7% of these individuals reported the friend or relative had obtained the drugs from just one doctor. Only 3.9% reported obtaining the drug from a drug dealer or stranger, and only 0.1% reported buying the drug from the Internet, although this may have increased in the last few years.
Monitoring the Future (5) is a series of large, annual surveys of nationally representative samples of public and private secondary school students throughout the United States. Among the 12th-graders, annual prevalence rates for opioids increased substantially between 1990 and 2002, but rates have leveled off since 2003, currently standing at about 9.0% (5).
The Drug Abuse Warning Network (DAWN) is a public health surveillance system that monitors drug-related hospital emergency department (ED) visits and drug-related deaths in select metropolitan areas from 1995 to 2002. DAWN data show that “drug-related” ED visits involving prescription opioids increased yearly in this period from 42,857 to 108,320, representing a 153% increase (11). In 2002, prescription opioids represented 16% of total drug-related ED visits, with hydrocodone and oxycodone the most frequently mentioned opioids. In 2002 and 2003, the past year use of OxyContin (oxycodone hydrochloride) and Vicodin by 12th-graders was reported at about 4 and 10%, respectively. This raises serious concern about prescription opioid abuse among youth. In general, opioids are the most common cause of drug poisoning, pointing out the potential danger of prescription opioids (12).
In addition, evidence points toward an increase in substance abuse among adults aged 60 yr and older (13). A similar increase in substance abuse has been found among aging baby boomers (14). Alcohol and prescription drug misuse may affect as many as 17% of older adults (13,15). An interesting difference between prescription opioid misuse and illicit drug abuse is the greater nonmedical use of opioids by adolescent females (16). This study also showed that the motive for use of prescription opioids was more consistent with a therapeutic indication (79%) than that for other prescription medication misuse. In an adult population, regular opioid use increased with age, decreased with education level, and was more common in females and in non-Hispanic whites (17). African-Americans had slightly lower rates of opioid use than non-Hispanic whites, with Hispanics having the lowest rate (17).
Opioids, primarily Vicodin and heroin, are present and highly sought after within illicit drug markets. The Internet has also been a portal for sales and access to prescription opioids (18), although as stated above, the percentage as reported in surveys is probably low. An Internet search for Vicodin using the search engine “Google” resulted in more than 15 million hits, and several of these are advertisements for this drug that guarantee next day delivery. Thus, there appears to be relatively easy access to opioid drugs, despite governmental attempts to curb illicit drug sales.
In summary, the greatest factor associated with increasing prevalence of prescription opioid misuse appears to be the increased willingness of physicians to prescribe these drugs (19). This appears to be in large part due to the increased number of prescriptions for treatment of chronic noncancer pain. Importantly, the growing use of opioid drugs occurs in the absence of sufficient understanding of the health implications of long-term opioid use. For example, the effects of long-term opioid use/abuse on the endocrine system have not been well studied because most studies on the effects of opioids on the endocrine system focus on acute effects. It is important to realize that chronic effects of opioids on the endocrine system cannot simply be presumed to be a prolongation of acute effects because acute and chronic treatment regimens may produce entirely different profiles with respect to both the magnitude and the direction of the effects.
A. Clinical importance of opioids on the endocrine system
Most clinical studies have focused on hypogonadism in relation to opioid abuse, particularly in men. Currently, the limited clinical awareness of the endocrine effects of opioids, together with the lack of information on their long-term effects, may result in insufficient discussion with patients when initiation of long-term opioid therapy is being considered.
This review includes both clinical and laboratory studies on the effects of opioids and their analogs on the endocrine system as a way to understand the underlying mechanisms and the complications of long-term opioid use/abuse. We analyze and summarize in detail, for both animals and humans, the effects of opioids on the hormones of the anterior pituitary [GH, prolactin (PRL), TSH, ACTH, LH, and FSH] and the posterior pituitary [arginine vasopressin (AVP) and oxytocin (OT)]. An exploration of the differences in the effects of opioids between humans and animals is included, as well as differences between sexes. An understanding of the intricacies of the opioid interaction with the endocrine system and of the resulting medical consequences of use/abuse will increase awareness of the detrimental effects of opioids and hopefully spur more research on their long-term health effects.
II. Pharmacology and Physiology of Opioids and Their Derivatives
A. Opioids and their derivatives
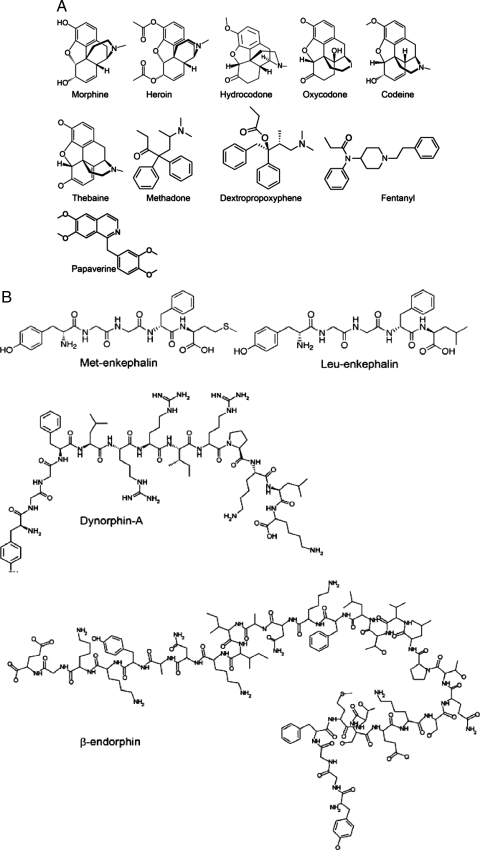
Opiates are derived from opium, which is extracted from the sap of the opium poppy. The term “opiate” is used for the alkaloids of opium, such as morphine, as well as for the synthetic drugs derived from opium alkaloids, such as codeine and heroin. The term opioid includes all opioid analgesics regardless of their source. The endogenous opioid system consists of the endogenous opioid peptides and their corresponding binding sites with which these peptides interact to produce their effects. Figure 1A depicts the structure of common opioids, and Fig. 1B depicts the structure of the main endogenous opioids. Early classical pharmacological studies identified several classes of opioid receptors. At least three classes of opioid receptors, named mu (μ), delta (δ) and kappa (κ), have been cloned (20,21). These classical opioid receptors belong to the family of the guanine regulatory binding (G) protein-coupled receptor and are coupled to the second messenger systems via inhibitory G proteins (Gi/Go). Opioid receptors mediate the actions of endogenous and exogenous opioids and are present in the brain and throughout the body, including the endocrine organs, such as the adrenal cortex and the gonads (22).
Figure 1.
Structure of common exogenous (A) and endogenous (B) opioids.
B. Classes of opioids
Three major classes of endogenous opioid peptides (endorphins, enkephalins, and dynorphins) have been identified (Table 1). These peptides are derived from three distinct precursor proteins: pro-opiomelanocortin (POMC), preproenkephalin A, and preproenkephalin B, respectively (23). The discovery of endomorphin-1 and endomorphin-2 has extended the endogenous opioid peptide family (24). It is generally thought that β-endorphin, enkephalins, and dynorphins are selective agonists for the μ-, δ-, and κ-opioid receptors, respectively (Table 1). Endomorphin-1 and endomorphin-2 have μ-receptor affinity and play a biological role in pain perception, stress responses, and homeostasis (25). However, the endogenous opioid peptides (as well as exogenous opioids) can bind to more than one type of opioid receptor to produce their actions (Table 2). The endogenous opioid peptides exert diverse physiological and pharmacological effects in mammals and humans, including modulation of motor activity (26), seizure threshold (27,28,29), immune responses (30,31), water and food intake (32), and regulation of gastrointestinal (28,33,34), cardiovascular (35,36,37), neuroendocrine (38,39,40,41), and cognitive (28,42) functions. However, their most widely recognized clinical effect is analgesia or pain relief (antinociception in rodents) (43,44). Brain regions containing opioid receptors are given in Table 3.
Table 1.
Overview of endogenous opioids, their precursors, and main receptors
| Endogenous peptide | Precursor | Main opioid receptor |
|---|---|---|
| β-Endorphin | POMC | μ |
| Enkephalins | Preproenkephalin A | δ |
| Dynorphins | Preproenkephalin B | κ |
Table 2.
Endogenous and exogenous opioid ligands and their relative receptor selectivity
| Ligand | Opioid receptors
|
||
|---|---|---|---|
| μ | δ | κ | |
| β-Endorphin | ++++ | ++ | ++ |
| Leu-enkephalin | ++ | +++ | 0 |
| Met-enkephalin | ++ | +++ | 0 |
| Dynorphin A (1-17) | ++ | 0 | +++ |
| Endomorphin-1 | +++++ | 0 | 0 |
| Endomorphin-2 | +++++ | 0 | 0 |
| Morphine | +++ | 0 | + |
| Heroin | +++ | + | 0 |
| Fentanyl | +++ | 0 | 0 |
| Sufentanil | +++ | 0 | 0 |
| Methadone | +++ | 0 | 0 |
| DALA (FK 33-824) | ++ | +++ | 0 |
| DAMME | ++ | ++ | 0 |
| U50,488H | 0 | 0 | ++++ |
| Nalorphine | − | 0 | +++ |
| Naloxone | − | − | − |
| Naltrexone | − | − | − |
| CTOP | − | 0 | 0 |
| Buprenorphine | +/− | − (?) | − |
| Pentazocin | − | 0 | +++ |
Table 3.
Localization of opioid receptors in related brain regions and peripheral tissues
| Brain region | Opioid receptors
|
||
|---|---|---|---|
| μ | δ | κ | |
| Cortex | |||
| Cingulate cortex | +/++++ | +/++++ | +/++ |
| Frontal parietal cortex | ++/+++ | ++/+++ | −/++ |
| Temporal cortex | ++/+++ | +/+++ | −/+ |
| Hippocampus | |||
| Pyramidal cell layers | +++ | ++ | + |
| Dentate gyrus (dorsal) | − | − | − |
| Dentate gyrus (ventral) | +++/++++ | + | + |
| Caudate putamen | ++++ | ++++ | +++ |
| Nucleus accumbens | ++++ | ++++ | +++ |
| Amygdala | |||
| Medial nucleus | +++ | ++ | ++ |
| Central nucleus | − | − | ++ |
| Lateral | ++++ | +++ | +++ |
| Basolateral | ++++ | +++ | +++ |
| Thalamus | |||
| Laterodorsal nucleus | ++ | − | − |
| Central medial nucleus | ++++ | + | ++ |
| Medial geniculate nucleus | ++++ | − | + |
| Zona incerta | − | − | ++ |
| Hypothalamus | |||
| Lateral | + | − | ++ |
| Paraventricular nucleus | − | − | ++ |
| Supraoptic nucleus | − | − | ++ |
| Median eminence | − | − | +++ |
| Arcuate nucleus | − | − | ++ |
| Ventral tegmental area | ++ | − | |
| Raphe nucleus | +++ | − | ++ |
| Pituitary (rat) | +/− | +/− | ++ |
| Pituitary (human) | +++ | +/− | ++ |
C. Analgesic effects of opioids
The analgesic effects of opioids are thought to be mediated at both central and peripheral synapses (43). It is generally accepted that opioids suppress pain transmission at the spinal cord via both pre- and postsynaptic mechanisms involving inhibition of calcium channels and activation of potassium channels, respectively (44). Opioids can also facilitate the function of the descending pain inhibitory systems by inhibiting the activity of γ-amino-butyric acid-ergic interneurons (44,45). In addition, opioids suppress pain perception at the somatosensory cortex and alter the affective component of pain via an action at the level of the limbic structures (46). However, in patients, chronic administration of opioid analgesics, such as morphine, at least under some conditions, produces a state of higher pain sensitivity or hyperalgesia (47). This may lead to higher doses of opioids used with potential complications. Endogenous opioid peptides, particularly enkephalins, are released at numerous central nervous system (CNS) structures that play a critical role in pain modulation (48,49). For example, laminae I, II, and V of the spinal cord are rich in enkephalinergic neurons. Enkephalins are also released in the periaqueductal gray and rostral ventromedial medulla, both of which play an integral role in opioid-induced analgesia (44). Endogenous morphine has been found in low concentrations in the brain (50).
Some natural derivatives of opium such as morphine, thebaine, and papaverine have long been used for their analgesic effects (51). Morphine and other opioids with high abuse potential bind preferentially to the μ-opioid receptors; however, the ability of these drugs to activate opioid receptors and alter signaling varies from partial to full agonists. In addition, morphine binds, albeit with lower affinity, to the δ- and κ-opioid receptors (52). However, the antinociceptive effect of morphine and its rewarding and addictive effects are mediated primarily through the μ-opioid receptors. For example, mice lacking the μ-opioid receptors do not exhibit morphine-induced antinociception, reward, or naloxone-precipitated withdrawal symptoms (53,54).
D. Synthetic and semisynthetic opioids
Synthetic opioids have been used to mimic the effects of endogenous opioids in the body because these drugs and endogenous opioids bind to the same opioid receptors. Heroin, or diacetylmorphine, a semisynthetic opiate derived from morphine, can be used for pain suppression and is used as a recreational street drug. This μ-receptor agonist is highly addictive (55). Other examples of semisynthetic opioids are hydrocodone and oxycodone. Fully synthetic opioids are used as very powerful analgesics; fentanyl is about 75 times more potent than morphine (56). Methadone, another synthetic opioid, is commonly used to prevent relapse in opioid addicts because of its longer-lasting effects that prevent withdrawal syndrome (50). d-Ala2-met-enkephalinamide (DALA), also known as FK 33-824, and d-Ala2,MePhe4,met-(O)enkephalin-ol (DAMME) are synthetic met-enkephalin analogs.
E. Opioid antagonists
Opioid receptor antagonists are commonly used in preclinical studies to determine whether a response is specifically mediated by the endogenous opioid system. These drugs are also used clinically to decrease opioid intake and to reverse the effects of opioid overdose. The most commonly used antagonist is naloxone, which binds to μ-opioid receptors in a competitive manner (57). Naloxone is synthesized from thebaine, a natural derivative of opium. Naltrexone is another opioid receptor antagonist, used similarly to naloxone to block opioids from binding to their receptors. Naltrexone has a slower onset but longer duration of action than naloxone. These antagonists have preference for μ-opioid receptors, but at higher doses they antagonize both δ- and κ-opioid receptors. Accordingly, these drugs are considered nonselective opioid receptor antagonists (Table 2).
F. Opioids and the stress response
The endogenous opioid system is implicated in the response to stress (58,59,60). Opioid-containing neurons have been shown to innervate the median eminence and paraventricular nucleus of the hypothalamus, thereby regulating inputs to ACTH-controlling neurons in the anterior pituitary (61,62). In addition, dynorphin-like peptides have been found to colocalize with CRH and may be cosecreted with CRH in the hypophyseal portal circulation to modulate ACTH release (63). Thus, the hypothalamic-pituitary-adrenal (HPA) axis represents a modulatory target for the action of exogenous and endogenous opioid ligands. Indeed, a growing body of evidence suggests that opioids regulate mechanisms activated during the stress response (58,60). Conversely, the endogenous opioid system is activated by stressful situations (64), raising the possibility that activation of the endogenous opioid system may play a role in stress-mediated events.
Although stress is often linked to unpleasant events, the stress response can be beneficial. For example, exposure to mild stressors has been shown to activate the HPA axis, which is thought to play an important role in mediating cognitive adaptive changes that promote survival (65). The HPA axis is essential in mediating the stress response (66). Activation of the HPA axis is initiated by secretion of CRH from the paraventricular nucleus of the hypothalamus to the portal system of the median eminence. Subsequently, CRH binds to its receptor in the anterior pituitary that leads to the synthesis of POMC, a large precursor molecule that is cleaved into several smaller functional peptides, such as ACTH and β-endorphin (67). ACTH is then secreted and circulates to the adrenal gland, where it causes the release of glucocorticoids, cortisol (in humans), or corticosterone (CS) (in animals). Circulating glucocorticoids mediate the stress response and are essential for survival. Cortisol/CS also modulates CRH and ACTH release through a negative feedback mechanism. The inhibitory action of cortisol/CS, however, can be overcome by further release of CRH under severe or chronic stress situations (65,68). Thus, severe stressors may lead to a greater stress response (69) and possibly to unpredictable and uncontrollable adaptive changes.
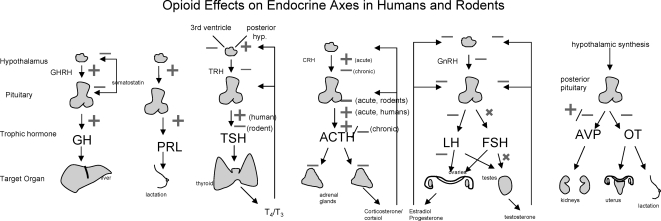
III. The Effects of Opioids on Endocrine Systems in Animals and Humans (Fig. 2 and Table 4)
Figure 2.
Summary of the effects of opioids and opioid analogs on endocrine axes in humans and rodents. X indicates no effect.
Table 4.
Summary of the effects of acute and chronic opioids on the endocrine systems of animals and humans
| Hormone | Acute
|
Chronic
|
||
|---|---|---|---|---|
| Animals | Humans | Animals | Humans | |
| GH | ↑ | ↑ | = | ? |
| PRL | ↑ | ↑ | ↑ | ↑/= |
| TSH | ↓ | ↑ | ? | ?/= |
| ACTH | ↑ | ↓ | ↓/↑ | ↓/= |
| LH | ↓ | ↓↓ | ↓ | ↓↓ |
| FSH | = | = | = | = |
| Estradiol | ↓ | ↓↓ | = | ↓= |
| Testosterone | ↓ | ↓↓ | ↓ | ↓↓ |
| AVP | ↑/↓ | ↑/↓ | ↑/↓ | ↑/↓ |
| OT | ↓ | ↓ | ↓/= | ↓/= |
↑, Stimulation; ↓, inhibition; ↑↓, conflicting; =, no change; ?, not studied.
A. GH/IGF-I axis
1. Animal studies
Opioids and their analogs have been well recognized as stimulants of GH release in animals. Several studies in rats have examined the acute effects of opioids on GH release and the pathways involved. For example, morphine sulfate produced a 3-fold increase in plasma GH concentrations 2 h after intracerebroventricular (i.c.v.) injection and transiently increased the plasma concentration and liver content of IGF-I and IGF binding protein-1 (70). However, morphine-6-glucuronide did not produce any significant alterations in plasma GH and IGF-I levels, suggesting that differential affinities and/or selectivity of morphine and morphine-6-glucuronide to different opioid receptor/subtypes might be responsible for their distinct effects on the GH/IGF-I system (70). β-Endorphin, administered iv or directly into the rat hypothalamus, increased GH levels (71). This increase was attenuated by naloxone (71), suggesting that opioid receptors are involved in this response. α-Adrenergic receptors are also involved in the action of opioids on the release of GH, as phenoxybenzamine, an α-adrenergic blocking agent, inhibited both spontaneous GH release and the response to FK 33-824, a synthetic enkephalin analog (72). In addition, β-endorphin or other endogenous opioid peptides may mediate the GH secretion that is induced by α-2-adrenergic stimulation because β-endorphin antiserum and naloxone reduced the stimulatory effect of the α-2-adrenergic agonist, clonidine, on GH release (73). Induction of stress resulted in a decrease of GH in male rats, whereas sc administration of morphine and methadone increased plasma GH levels (74,75), suggesting that stress and opioids may influence GH release via separate mechanisms. In neonatal rat pups exposed to morphine, naloxone-induced opioid withdrawal suppressed GH levels in a dose-dependent manner (76).
The effect of opioids on GH is influenced by sex hormones. In male but not female rats, morphine treatment for 6–12 h resulted in a 12-fold increase of plasma GH levels that peaked after 3 h (77). The opioid-induced GH increase was significantly blunted during an estradiol/progesterone-induced LH surge (77). In female rats, naloxone treatment reduced GH levels by 64% over a similar time period (78).
Opioid analogs have been studied in animals other than the rat. In wethers, administration of FK 33-824 increased GH concentration for up to 3 h after administration, with a return to basal levels by 2–6 d after the infusion (79). Likewise, a 30-min infusion of DAMME, a μ/δ-opioid agonist, increased plasma GH in Holstein heifer calves via opioid receptors located inside the blood-brain barrier (80). In prepubertal gilts, naloxone attenuated the stress-induced increase in GH secretion (81). The effect of these opioids and their analogs is not specific to mammals because FK 33-824 and a β-adrenergic agonist were seen to affect the amplitude and increase mean plasma concentration of GH secretion in broiler chickens (82,83). Thus, opioids and their analogs have similar stimulatory effects on GH secretion across a number of experimental conditions and animal preparations.
Several investigators have studied the mechanisms by which opioid receptor stimulation affects GH secretion. Acute iv morphine administration results in a GH pulse, suggesting that opioids reset the hypothalamic GH pulse generator (84), an effect that has been shown to be mediated by μ-, κ-, and δ-receptors (85). In neonatal rats, stimulation of μ- and κ-receptors resulted in stimulation and inhibition of GH secretion, respectively, with δ-receptors acting synergistically with the μ-receptors in producing the opioid-induced GH-stimulation (86).
GHRH and somatostatin are also affected by opioids. Treatment of rats with an antiserum against GHRH inhibited the GH stimulatory response to both β-endorphin and morphine (87). The inhibitory effect of somatostatin on GH release was antagonized by endogenous opioids, thus increasing GH pulse frequency and amplitude in hamsters (88). This suggests that opioids stimulate GH secretion through the release of GHRH and the inhibition of somatostatin.
Other studies have focused on the effects of opioids on hormonal gene transcription. Dobado-Berrios et al. (89), using in situ hybridization in rat pituitary, found a 22% decrease in GH mRNA levels after chronic (4-d) morphine administration. Furthermore, a single morphine dose decreased the gene transcripts of both the GH receptor and GH binding protein in male rat hippocampi (90). Both studies reveal that opioids act through mechanisms involving regulation of gene expression and transcription.
Overall, these studies indicate that acute administration of opioids usually results in an increase of GH concentrations, which appears to be mediated primarily via central μ-receptors. Given the acute (several hours) time course, this may well be due to release of already stored rather than newly synthesized GH. The sparse information available suggests that chronic (several days) administration of opioids reduces GH mRNA levels. It is not known whether this reduction is dose-dependent or more profound with longer duration of administration. Furthermore, to our knowledge, the effects of opioid treatments lasting more than 1 wk on GH secretion or expression have not been examined either at baseline or after stress or other stimulatory factors.
2. Human studies
Similar to animals, acute administration of opioids and their analogs in human subjects results in increased GH secretion through mechanisms involving the opioid receptors, feedback levels, and gene transcription. In healthy subjects, the minimum morphine dose required for GH stimulation is approximately 15 mg (91). Naloxone, in a constant infusion for 120 min, attenuated the stimulatory effect of GHRH on the secretion of GH, indicating the existence of an opioid-mediated stimulatory tone on GH secretion (92). In healthy women, naloxone infusion starting 1 h before GHRH administration reduced the GHRH-induced release of GH, whereas in healthy men, naloxone did not change this response (93). This sex-related difference in the effect of naloxone on GH secretion suggests a role of sex steroids in mediating the effects of endogenous opioids on GH secretion.
In contrast to the animal studies, there is one human study on the effect of chronic opioid administration on GH. In a study in patients with severe chronic pain receiving intrathecal opioids for a mean duration of 27 months, Abs et al. (94) found an IGF-I below −2 sd in 12 of 73 patients and a peak GH response to hypoglycemia below 3 mg/liter in about 15% of subjects. This suggests that chronic opioid administration can result in severe GH deficiency in some, but not all subjects. Insulin levels and sensitivity, and perhaps counterregulatory hormones, may affect this response (95). The involvement of opioid receptors in the effect of opioids on GH is supported by a study in human lymphoblastoid IM-9 cells that showed that morphine significantly altered GH receptor gene expression and GH binding in a naloxone-reversible manner (96). It is not clear whether the in vivo effects of opioids are dose-dependent, depend on route (oral or transdermal administration), or to what extent pain and other medications or coexisting conditions may play a role. Furthermore, it is not known whether GH therapy might have any beneficial effects in patients with low GH secondary to opioids.
Other studies used opioid antagonists to examine the effects of opioids on GH. Although chronic administration of naltrexone (50 mg/d for 4 wk) resulted in a 75% decrease in the GHRH-induced GH response in healthy premenopausal women (97), it resulted in a 3-fold increase of the GHRH-induced GH response in obese women (95), even though basal levels of GH, IGF-I, and IGF binding protein-3 were not affected in either group. Opposite effects of naltrexone administration were found in women with polycystic ovary syndrome (PCOS), with an increased GH response after GHRH in lean PCOS women, whereas no effect was seen in obese PCOS women (98). Although these data indicate that opioids alter the response of GH to GHRH and that body composition, sex hormones, and insulin resistance may play a role, the direction and magnitude of the effect of these factors remains poorly understood.
Several human studies involving the effects of opioids on GH levels have been conducted in patients with various diseases. Morphine caused an elevation of GH in both acromegalics and normal subjects. However, higher doses of morphine were required to stimulate GH secretion in normal subjects than in acromegalic subjects, indicating that opioids exert a positive modulating effect on GH secretion in patients with active acromegaly (91). Alternatively, GH secretion in acromegalic patients may be more sensitive to the stimulating effects of opioids. In a retrospective study in patients receiving intrathecal opioids for intractable pain, both IGF-I levels and the GH response to insulin-induced hypoglycemia were significantly decreased compared with controls (94). Fifteen percent of these patients met the criteria for GH deficiency.
3. Summary and overall mechanisms of opioid effects on GH
Overall, whereas acute administration of opioids results in an increase of GH secretion, the effects of chronic opioid administration appear to be much more complex, with GH secretion being inhibited by intrathecal opioids in chronic pain patients and by an opioid antagonist in healthy subjects, whereas the same antagonists increased the GHRH-induced GH response in obese women. Although this response appears to be affected by sex, body composition, and insulin resistance, much research is needed to understand the potential for opioids to induce GH deficiency.
B. Prolactin
1. Animal studies
Generally, opioids and their analogs stimulate PRL release from the anterior pituitary through an effect at the hypothalamus (99). Direct incubation of morphine or morphine analogs on isolated perfused pituitaries had no effect on PRL release (100,101). However, across several species, a single systemic injection of morphine or an opioid agonist, such as DAMME or FK 33-824, has been shown to consistently increase serum PRL concentrations (80,82,102), and this effect was attenuated by naloxone in both heifers and rats (103,104,105). In addition to effects on PRL release, administration of morphine for 4 d increased PRL mRNA by 12%, whereas naloxone decreased PRL mRNA levels by 10% in rats (89), demonstrating that opioids affect morphine gene expression. The mechanism of the effect of opioids on PRL secretion is complex because PRL release is enhanced by serotoninergic but reduced by dopaminergic pathways that interact with the opioid system in rats (106). Additionally, the dopaminergic system develops in neonatal rats, whereas the serotoninergic system is not functional until 10 to 15 d of age and is not able to mediate the effects of opioids on PRL secretion. In contrast, in monkeys, PRL release is enhanced by serotoninergic pathways but is not affected by dopaminergic pathways (107). This study also confirmed a hypothalamic site of regulation of opioids on PRL secretion. Nicotine, morphine, and a serotonin agonist [8-hydroxy-2-(di-n-propylamino) tetralin] were found to utilize a common synaptic pathway for PRL release with serially arranged synapses in the dorsomedial arcuate nucleus of rats (108). Most importantly, this study (108) supported the findings of Bero and Kuhn (106) that inhibition of dopaminergic pathways plays an important role in the induction of PRL release by opioids. However, administration of morphine, leu-enkephalin, and DAMME showed little effect on dopamine-induced inhibition of PRL secretion (100,101), although these experiments using isolated rat pituitaries in culture are not physiological and do not take into account the interactions between the hypothalamus and pituitary. All three opioid receptors are involved in the opioid-induced PRL stimulation (109); however, the μ-opioid receptor appeared to play a primary role in mediating the effect of opioids on PRL secretion (110). These experiments should be confirmed in opioid receptor null mice.
Acute stress resulted in an increase of PRL, an effect that is modulated by endogenous opioids, β-endorphin and dynorphin-A (111). Although one paper in pigs reported that administration of naloxone enhanced the stress-induced increase in PRL secretion (81), this response was mild and was not confirmed statistically. Most articles report that naloxone inhibits PRL release (reviewed in Refs. 112 and 113), which is the expected response.
β-Endorphinergic neurons in the arcuate nucleus participate in the acute response of PRL release after mating in female rats, suggesting that endogenous opioid peptides are involved in the neuronal transmission of genitosensory stimulation inducing PRL secretion (114). Furthermore, medial basal hypothalamus β-endorphin also regulated progesterone-induced PRL secretion in female monkeys (115).
Unlike GH, the effects of opioids on PRL secretion vary considerably and are dependent on the timing of the effects of opioids, particularly in the female cycle. Acute morphine administration increased PRL secretion in diestrous and proestrous rats, but did not produce any effects of PRL levels in lactating rats, possibly as a result of down-regulation of the μ-opioid receptor (116). In contrast, β-endorphin and a leu-enkephalin analog increased PRL during lactation in female rats (116). Additionally, opioids can have an impact on premature reproductive systems, resulting in later deficiencies in adulthood. Morphine administered to juvenile female rats led to reduced suckling-induced PRL secretion in adulthood, but other maternal behaviors were unaffected (117). Thus, opioid use during adolescence has long-lasting effects on PRL secretion.
2. Human studies
Acute morphine administration increased serum PRL concentrations in men (118) as well as euthyroid and hypothyroid volunteers (119). Acute morphine administration also increased PRL levels in postmenopausal women (120). In postmenopausal women, a single dose of iv morphine increased PRL and decreased LH (121), demonstrating that morphine may affect a common receptor or neurotransmitter that controls both PRL and LH secretion from the anterior pituitary. After administration of a submaximal dose of metoclopramide, a dopamine agonist, morphine still increased PRL, whereas after a maximal metoclopramide dose morphine was no longer able to increase PRL. This suggests that in humans, as in animals, the effects of opioids on PRL release are mediated through dopaminergic mechanisms (118).
The effect of chronic opioid administration on PRL is less clear. PRL levels were normal in both male and female chronic pain patients that received opioids either intrathecally (94) or orally (122). In contrast, baseline PRL levels were increased in male opioid addicts on a methadone maintenance program, and decreased after treatment with bromocriptine, a dopamine agonist, for 30 d (123). Eighty-seven percent of opium smokers in Iran had elevated PRL levels (124). The difference between the methadone study and the other studies showing high PRL after opioids may be due to some underlying difference between heroin addicts and patients on pain medicines or specifically due to the effects of methadone on PRL secretion.
The effect of naloxone, the opioid receptor antagonist, on PRL is also ambiguous. In normal men, naloxone did not change basal PRL levels or its release after stimulation with TRH (125). However, in a similar group of men, naloxone was able to reduce the increase of PRL after injection of buprenorphine (126). Two studies which found that naloxone did not affect PRL levels studied both men and women together and used small numbers of subjects (127,128).
In women, sex steroid hormones may also modulate the effects of opioids on PRL. Naloxone did not have any effect on basal PRL secretion in menopausal women, hypogonadal women, or normal women in the early follicular or late luteal phase of their menstrual cycle (120,129,130,131). However, naloxone did induce PRL release when administered for 7 d in the luteal phase of the menstrual cycle of healthy women, as reflected by an increase in LH and PRL pulse frequencies (132). Postmenopausal estrogen-treated women injected with naloxone showed lower plasma PRL concentrations than the untreated reproductive-age control group (131). These articles point to an interaction between LH and PRL that is mediated by opioids and suggest that estrogen levels (as evident by the phase of cycle or by exogenous estrogens) modulate the effects of opioids on PRL levels in women.
3. Summary and overall mechanisms of opioid effects on PRL
In both rodents and humans, acute administration of opioids increased PRL levels, an effect that appears to be mediated by hypothalamic factors rather than via direct action on the pituitary. The opioid-induced PRL release is enhanced by serotoninergic pathways but reduced by dopaminergic pathways, and circulating sex steroids modulate the response. The effects of naloxone on PRL levels and the effect of chronic administration of opioids are both variable. In some studies, naloxone decreased PRL levels, an effect that may be dependent on circulating sex steroids. Chronic administration of opioids occasionally increased PRL levels, an effect that may be due to the type of opioid. More studies need to be performed on the clinical implications of chronic opioids on PRL levels.
C. Thyrotropin
1. Animal studies
In rats, the site of opioid administration determines the effect of opioids on levels of serum TSH. Generally, acute peripheral injection of morphine decreased serum TSH levels without affecting plasma T3 and T4 levels, but this effect was not blocked by naloxone (133). In contrast, Männistö et al. (134) infused a single dose of morphine into different hypothalamic locations and found that the cold-stimulated serum TSH response was inhibited when morphine was infused into the third ventricle and stimulated when the drug was infused in the posterior hypothalamus, with both effects inhibited by naloxone. The effect of morphine on this cold-induced alteration in TSH was also found to be dose-dependent because rat serum TSH levels were stimulated by lower doses of morphine and inhibited by higher amounts of morphine (135).
In the rat hypothalamus, repeated injections (twice daily for 10 d) of morphine, but not a single injection, increased TRH levels in the striatum and hippocampus and increased TRH receptor binding in the striatum, nucleus accumbens, and hippocampus when examined 72 h after the last injection (136). Because most of the changes occurred at 72 h after the last administration of morphine, the findings are more likely related to withdrawal than morphine exposure. Morphine pellet administration for 7 d led to increased TRH biosynthesis as evidenced by increased TRH/5.4 kDa C-terminal proTRH-derived-peptide ratios in the median eminence (137). Although morphine increased TRH levels, opioid peptides exerted a modulatory effect on TRH levels. β-Endorphin decreased TRH secretion in a dose-dependent manner, and leu-enkephalin and met-enkephalin decreased TRH release (138). The effects of these peptides were blocked by naloxone (138). Neither leu-enkephalin nor met-enkephalin affected basal or TRH-stimulated TSH levels in rats (139). Systemic treatment with β-endorphin and met-enkephalin antisera increased both hypothalamic TRH and TSH concentrations (140). These results suggest a hypothalamic mechanism for the regulatory actions of endogenous opioid peptides on the hypothalamic-pituitary-thyroid axis and that exogenous morphine may have different effects than endogenous opioids.
Studies on the effect of antagonists (naloxone or naltrexone) on TSH release show conflicting results. In contrast to earlier reports that naloxone had no effect on basal (141) or cold-stimulated TSH secretion (142), more recent reports have shown an attenuating effect of opioid antagonists. Subcutaneous injection of naltrexone blocked the acute stress-induced decrease in plasma TSH and stopped the decline in TSH after chronic stressors, even slightly elevating TSH levels (143). Naloxone infused into rat pituitary cells increased basal TSH secretion, yet did not augment TRH-stimulated TSH secretion and was not blocked by β-endorphin (144). These experiments were done on isolated pituitary cells, which, as discussed above in the PRL section, lack the hypothalamic factors that may regulate opioid action. Thus, it is possible that a different opioid receptor besides the μ-receptor is involved in the opioid regulation of TSH secretion.
The mechanisms of opioid effects on TSH secretion involve regulation of opioid receptors, feedback mechanisms, and interactions with the thyroid hormones, T3 and T4. In mice, hyperthyroidism increased the binding of opioids to the opioid receptor and thus increased the serum TSH response to morphine and other opioids (145). The increased thyroid hormone levels in hyperthyroidism may facilitate opioid action, although there are no clinical reports suggesting that hyperthyroid patients have an altered pain sensitivity. The locomotor stimulatory action of TRH is mediated by opioid receptors, along with adrenergic and dopaminergic receptors in the ventromedial hypothalamus of rats (146). The κ-agonist MR 2034 acted similarly as morphine, inhibiting the cold-stimulated TSH response when infused into the third ventricle and increasing the TSH response when infused in the posterior hypothalamus (147). Naloxone blocked only the stimulatory, but not the inhibitory effect of MR 2034. DAMME, a μ/δ-receptor agonist, did not affect TSH secretion (147). Thus, κ-receptors appear to be the primary receptors involved in mediating the action of opioids on TSH release.
The hormones secreted by the thyroid gland, T3 and T4, also affect the suppression of TSH by opioids. In rats, the normal TSH decrease after opioid administration was no longer present after thyroidectomy, indicating that circulating thyroid hormones are required for morphine to have its suppressive effects on TSH levels (148). Moreover, replacement with T4 led to a more pronounced suppression of TSH in morphine-treated animals. Thus, morphine may exert its inhibitory effect on TSH secretion by increasing the negative feedback sensitivity to thyroid hormones.
2. Human studies
In contrast to the decrease of TSH secretion by opioids in animals, an increase in TSH is seen in humans. Morphine administration produced a rapid increase in TSH in both normal and hypothyroid subjects, and naloxone attenuated this effect (119). Four other articles report a stimulatory effect of morphine or opioid analogs on TSH levels (149,150,151,152). Opium smokers in Iran had lower TSH levels than cigarette smokers and healthy volunteers (124). One case report found that morphine inhibited TSH secretion during stress (153). This article suggested that the decrease in TSH in rodents that receive opioids is due to the stress on the experimental animals, a condition absent in the human volunteers.
In humans, met-enkephalin has been shown to localize only in TSH immunoreactive cells, suggesting a role in human thyroid function (154). In contrast, nonhumans have little or no met-enkephalin in the anterior lobe of the pituitary (155). The localization of met-enkephalin may provide a possible explanation for the difference in the effects of opioid on TSH secretion between animals and humans.
Particularly in humans, the effects of the opioids and endogenous opioid peptides are more significant during the physiological nocturnal TSH surge. Naloxone decreased TSH levels by suppressing the nocturnal surge in the TSH pulse amplitude, but did not affect daytime TSH levels or pulse frequency (156), supporting the finding that endogenous opioids stimulate TSH levels and affect the circadian rhythm of TSH (157).
The effect of opioids on the hypothalamus-pituitary-thyroid axis can be modulated by thyroid disorders. In hypothyroid patients, an infusion of naloxone did not change serum TSH levels (158), suggesting that endogenous opioids do not modulate TSH in this population. Abs et al. (94) did not find any effect of intrathecal opiate administration on baseline and TRH-stimulated TSH levels and found only slightly higher free T3 levels in patients receiving opiates compared with controls.
3. Summary and overall mechanisms of opioid effects on TSH
Opioids suppress TSH in rodents and stimulate TSH in humans, with the difference possibly due to differences in the localization of endogenous enkephalins in the pituitary of animals compared with humans. The site of opioids on the hypothalamic-pituitary-thyroid axis appears to be at the hypothalamus, with little direct effect at the pituitary. κ-receptors appear to be the primary receptors involved in mediating the action of opioids on TSH.
D. ACTH
1. Animal studies
In rodents, the effect of morphine on ACTH secretion depends on the dose and the time course of its administration. Acute administration of morphine led to a robust increase in ACTH and CS levels (159). In a later study, acute morphine led to an exaggerated response of the HPA axis to stress (160). Another study found that an acute injection of morphine caused a rise in plasma and adenohypophysis ACTH and in hypothalamic CRH content at 5 and 25 min, followed by a fall in these hormones at 90 and 120 min (161). Thus, acute opioid administration results in an increase in ACTH levels.
The effect of chronic opioid administration on ACTH and glucocorticoids is more heterogenous. In rats, chronic ip morphine administration (2 mg/kg daily for 7 d) led to reduced plasma and pituitary ACTH levels and hypothalamic CRH content, whereas no effect was found at a lower dose (0.5 mg/d) (161). In another study also in rats, chronic morphine treatment given sc twice a day for 16 d resulted in a marked elevation of basal CS concentrations (162). It is not clear whether this increase in the HPA axis was the result of chronic morphine administration itself or a response to the stress of repeated injections. Chronic morphine treatment did abolish the ability of either a single morphine injection or stress to increase ACTH and CS levels, suggesting that chronic morphine may desensitize a shared mechanism between acute morphine and stress to stimulate the release of stress hormones (160,163). Interestingly, pretreatment with ACTH enhanced the analgesic response and prevented the development of tolerance to morphine in rats (164). Morphine-dependent rats that had undergone 12-h withdrawal displayed a prolonged CS response to restraint stress (162). In contrast, rats that had undergone 8- and 16-d morphine withdrawal had normal basal pituitary-adrenal activity but displayed significantly reduced and shorter ACTH and CS responses to restraint stress (162). These results suggest that chronic morphine may lead to an attenuated response of the pituitary-adrenal axis to acute stress that may vary depending on duration of the opioid treatment and the length of the withdrawal from the drug.
Further review of the literature supports the notion that the effect of opioids on β-endorphin levels and the HPA axis depends on whether it is given in an acute or chronic manner. In rats, chronic morphine administration (7 d) resulted in a down-regulation of POMC gene (the precursor of β-endorphin and ACTH) mRNA in hypothalamus (165) as well as a decrease of brain and hypophyseal β-endorphin content (166,167). Chronic (8 d) naltrexone administration increased POMC mRNA levels to 140% of control value (168). Gianoulakis et al. (169) found that chronic morphine administration slightly inhibited neurointermediate lobe POMC protein levels as well as the processing of POMC to β-lipotrophin and β-endorphin, an effect that was more pronounced at 3 d than 15 d. Consistent with the later finding, Hollt, et al. (170,171) found a more pronounced decrease in neurointermediate pituitary POMC mRNA, tissue concentrations of β-endorphin and in the in vitro release of β-endorphin from the neurointermediate pituitary; however, there were no alterations in the processing of POMC.
Researchers have long hinted at a second site of opioid action along the HPA axis that is located outside of the hypothalamus-pituitary unit. In hypophysectomized rats, a single dose of morphine potentiated and naloxone inhibited the adrenal steroidogenesis after exogenous ACTH administration (172). The authors suggested that opioids may act via a mechanism that competes with ACTH receptors on the CS-secreting cells of the adrenal cortex (172). More recent studies focused on the specific location and mechanism of these effects. In rat zona glomerulosa and zona fasciculata cells in vitro, Kapas et al. (22) showed that opioid peptides stimulated CS secretion by the inner zona glomerulosa cells, without a specific interaction on the adrenocortical cells with ACTH. The effects were primarily mediated by μ- and, to a lesser extent, κ-receptors (22).
Other endogenous opioid peptides also modulate ACTH levels. For example, met-enkephalin, leu-enkephalin, and the analog FK 33-824 decreased plasma ACTH levels in sheep (173). One enkephalin analog in particular, DALA, has been studied extensively in relation to the adrenocortical cells and response to ACTH. In rats, DALA showed a similar pattern consisting of an increase in ACTH and CS secretion after acute administration of the analog and a decrease after chronic administration (174). DALA also produced a significant decline in the responsiveness of ACTH using dispersed adrenal cells (175). Chronic systemic DALA administration enhanced the trophic action of ACTH in the rat adrenal zona fasciculata (176). These studies suggest that DALA and other opioid peptides modify ACTH secretion and the glucocorticoid response to ACTH at the site of the adrenal cortex, suggesting a peripheral site of action of opioids. However, because DALA did not affect basal ACTH release and stimulation in rat anterior pituitary cell cultures, the authors suggest that DALA acts at the extrahypophyseal level to stimulate ACTH and CS (177). Thus, unlike morphine, the enkephalins do not exert their effects centrally; rather, they are specifically involved in the growth and steroidogenic capacity of the rat adrenal cortex.
Our laboratory has studied the effects of short-term (6 h) and chronic (7 d) morphine administration on levels of the prohormone convertases, PC1/3 and PC2 (137). These enzymes are believed to be responsible for the activation of many neurohormones, including the processing of POMC to β-endorphin and ACTH (178). The expression of these enzymes is dependent on the presence of a cAMP response element in their promoters (179). Specifically, short-term morphine exposure down-regulated, whereas long-term morphine exposure up-regulated phosphorylated cAMP response element binding protein and PC1/3 and PC2 protein levels in the rat hypothalamus (137). The regulation of the prohormone processing system by morphine may lead to alterations in the levels of multiple bioactive hormones, including POMC-derived peptides, and may be a compensatory mechanism whereby the organism tries to restore its homeostatic hormonal milieu. Thus, posttranslational regulation may be another mechanism by which opioids affect hormonal levels and may explain why long-term opioid exposure is necessary for drug addiction to occur (137).
2. Human studies
In normal subjects, in contrast to the effect in rodents, a single dose of a slow-release oral morphine suppressed ACTH, β-endorphin, and cortisol levels both at baseline and after CRH stimulation (180). Acute naloxone administration, on the other hand, increased ACTH and β-endorphin levels (181,182). This was also found for nalmefene, another opioid antagonist (183).
The effect of acute vs. chronic morphine on the HPA response in humans has not been extensively studied. In patients receiving intrathecal opioids, urinary free cortisol excretion was decreased compared with controls (94). Pain patients had elevated basal levels of cortisol and ACTH, which decreased after administration of sustained-release oral morphine for 1 to 12 wk (184).
There is concern regarding the potential development of an insufficient HPA stress response due to chronic opioid administration. In the study on the effects of intrathecal opioid administration, one patient developed adrenal insufficiency, whereas a basal cortisol level below 50 μg/liter (suggesting adrenal insufficiency) was found in 9.2% of opioid-treated patients compared with none of the control patients (94). Several case reports have documented adrenal insufficiency after oral (185) or transdermal (186) opioid administration and during methadone administration (187). Overnight dexamethasone suppression testing resulted in lower cortisol levels in patients receiving methadone-maintenance therapy than in normal volunteers, indicating that methadone affects glucocorticoid feedback and that the interpretation of dexamethasone testing may be affected by treatment with methadone (188). Schluger et al. (189) found that methadone-maintained former heroin addicts displayed a significantly greater increase in plasma ACTH, but not cortisol levels after high-dose but not low-dose human CRH, suggesting that the HPA axis is not completely recovered in methadone maintenance. The authors were unable to determine whether the effects on the HPA axis in methadone-maintained former heroin addicts was a consequence of heroin exposure or may have existed before the addiction. Overall, whereas these studies indicate that intrathecal opioid administration may cause adrenal insufficiency in up to 10% of patients, the risk for and clinical relevance of opioid-induced adrenal insufficiency has not been studied systematically for oral or transdermal administration.
Morphine and opioids affect the circadian rhythm of the HPA axis. Heroin addicts had constant β-endorphin, ACTH, and cortisol plasma levels throughout the day, compared with the normal circadian rhythm with high values in the morning and low values in the evening in healthy controls (190). This lack of differences across the circadian rhythm may explain why the overall long-term cortisol exposure (as measured by hair cortisol levels) was elevated in opioid-treated chronic pain patients compared with control subjects (191).
Studies on the mechanisms of ACTH inhibition by morphine and other endogenous opioid peptides have focused on the release of CRH from the hypothalamus. In healthy subjects, naloxone augmented, whereas morphine inhibited the CRH-induced increase in plasma ACTH and cortisol levels, indicating that opioid peptides inhibit the pituitary ACTH response to CRH (192,193). The primary opioid receptor involved in the regulation of the HPA axis is most likely the κ-receptor (194,195).
Examining the effects of opioids and their analogs in patients with disorders of the HPA axis has also helped to elucidate the effects of opioids on this axis. Patients with Cushing’s disease were tested with FK 33-824, a met- enkephalin analog, and showed no change in ACTH or cortisol levels (196). Moreover, loperamide, a μ-opioid agonist, did not change ACTH and cortisol levels in patients with Cushing’s syndrome, whereas it did suppress these levels in normal volunteers (197). In fact, lack of ACTH and cortisol suppression by loperamide was proposed to be an effective screening test for Cushing’s syndrome (198). In patients with Addison’s disease, FK 33-824 decreased plasma ACTH levels, an effect that was partially reversed by naloxone (196). Additionally, naloxone stimulated ACTH levels in patients with Addison’s disease, but had no effect on ACTH levels in patients with Cushing’s disease (199). This insensitivity to opioid agonists and antagonists in Cushing’s disease suggests that a defect in the inhibitory opioidergic control of ACTH secretion may lead to its hypersecretion (200). On the other hand, Addison’s disease patients treated with loperamide show sensitivity to opioids because the ACTH levels were decreased significantly with loperamide administration (201). Loperamide was seen to modify the effect of CRH on ACTH secretion in a nonadditive manner in Addison’s patients (202), such that ACTH release was inhibited. This evidence shows that opioids primarily act at the pituitary level and also have effects on the peripheral glands, such as the adrenals. This was confirmed by a subsequent study examining the effects of naloxone in patients with primary aldosteronism suggesting that the zona fasciculata of the human adrenal gland may be a secondary site of action for opioids (203), similar to the findings in rodents (10,177).
3. Summary and overall mechanisms of opioid effects on ACTH
Acute opioid administration results in an increase in ACTH and glucocorticoid levels in animals and either no effect or a decrease in humans. Chronic opioid administration may be associated with a decreased glucocorticoid response to acute activation of the HPA axis, despite often chronically elevated glucocorticoid levels, a finding that may be confounded by persistent chronic stress or pain. In occasional patients, opioid administration may cause frank adrenal insufficiency, but specific information on the risk to develop adrenal insufficiency is lacking. The effects of opioids on the HPA axis appear to be primarily mediated via both the hypothalamus and pituitary. In addition, there is evidence for direct stimulatory effects of opioids on adrenal glucocorticoid secretion that are mediated via μ- and κ-receptors.
E. LH and FSH
1. Animal studies
Extensive studies have been performed in laboratory animals to examine the influence of opioids on the hypothalamic-pituitary-gonadal (HPG) axis. These studies focused on the mechanisms of action of opioids, the interaction of opioids with the steroid hormones, and the time course of action of opioids on sexual function. In ovariectomized rats, the effect of a single injection of morphine on LH was dose-dependent, with an increase of LH after a high dose (10 mg/kg), but suppression of LH after a low dose (1 mg/kg) (204). These LH responses were antagonized by naloxone (86,133). Furthermore, naloxone increased LH concentration and pulse frequency when administered alone (105), demonstrating a tonic opioid inhibition of LH secretion.
With respect to endogenous opioid peptides, earlier studies had shown that injections of β-endorphin (at certain doses) stimulated serum LH levels (205); however, recent research points toward β-endorphin as having an inhibitory effect on LH similar to morphine. Injection of β-endorphin iv or into certain specific brain locations (including the ventromedial hypothalamic area, the anterior hypothalamic area, and the preoptic-septal area) decreased LH, suggesting that these brain areas are sites of action for β-endorphin, rather than the pituitary (206). In addition, Petraglia et al. (207) used β-endorphin antiserum to show that β-endorphin participates in the inhibitory action of CRH on LH secretion. Studies on the pituitary as a primary site of action for β-endorphin are conflicting because one study (208) found a direct effect of β-endorphin on LH release, whereas another (206) did not find such a direct effect. These findings suggest that endogenous β-endorphin decreases LH levels by acting on CRH-sensitive neurons and possibly by a direct effect on pituitary LH secretion.
Furthermore, β-endorphin also has modulating effects on the menstrual cycle of animals. A study of rhesus monkeys confirmed the participation of endogenous opioids during the menstrual cycle because the luteal phase of the cycle stimulated β-endorphin levels, which stimulated LH pulse frequency (209). Subsequent reports indicated that β-endorphin likely produced the latter effects via δ-receptors (210).
In contrast to their effects on LH, most opioids and their analogs appear not to affect FSH. Administration of morphine to developing male rats did not change basal FSH levels (211). Furthermore, treatment with opioid antagonists, naloxone and naltrexone, did not alter FSH concentration levels in either sex (212), suggesting that the endogenous opioids are not involved in regulation of FSH secretion. It is interesting to note that FSH levels are unchanged despite the ability of opioid treatments to stimulate hypothalamic GnRH.
The primary mechanism by which opioids affect gonadotropin secretion is through their effects on GnRH. Systemic administration of naloxone stimulated the release of GnRH, thereby stimulating LH release (213). Similarly, in ewes, naloxone treatment created a large amplitude GnRH pulse in the hypothalamus that stimulated a large LH pulse from the pituitary (214). In contrast to these effects of opioid antagonists, exogenous opioids and endogenous opioids inhibited hypothalamic GnRH secretion, leading to suppressed LH levels (215). Chronic morphine administration inhibited GnRH secretion both in vivo and in vitro (216). Li and Pelletier (217) found that opioids down-regulate GnRH mRNA levels as assessed by in situ hybridization, suggesting that morphine may act by decreasing the biosynthesis of GnRH.
In addition to the interaction with the hypothalamic synthesis and release of GnRH, opioids also affect, and conversely are regulated by, the end-product of the axis, the gonadal sex steroid hormones. Opioids also play a role in the feedback inhibition of LH by gonadal steroids. In castrated male and female rats, the decrease of serum LH levels by estradiol or testosterone administration could be reversed by naloxone (218). Administration of estradiol and progesterone decreased the ability of morphine to influence LH secretion (219,220). Thus, because morphine has an effect on steroids through the inhibition of gonadotropins, the gonadal steroids, in turn, reduce the effects of opioids on LH secretion. Bhanot and Wilkinson (221) showed that after gonadectomy, there was an acute reduction in the inhibitory effects of opioids on LH and FSH release, along with a reduced ability of naloxone to stimulate LH and FSH at all stages of development. Studies by Gabriel et al. (222) indicate that chronic morphine has no direct effect on LH, but that it enhances the sensitivity of the hypothalamus to negative feedback by testosterone in male rats. In female rats, chronic opioid treatment increases not only estradiol-mediated negative feedback but also the estradiol surge-induced hypersecretion of LH, indicating that morphine amplifies both negative and positive feedback on gonadotropin secretion (223).
The effect of opioids on the menstrual cycle is correlated with the rise and fall of the gonadal steroids. Exogenous opioids and endogenous opioid peptides caused a significant decrease in LH pulse frequency (224), whereas naloxone promoted an increase in the LH surge compared with saline controls (225). Tonic inhibition of LH levels by central opioid neurons occurred at all stages of the female rat cycle (219), with the luteal phase being the main phase affected. The opioid-induced decrease in the LH surge led to oligomenorrhea and amenorrhea, whereas the naloxone-stimulated increase in LH secretion stimulated the pubertal onset of menses in younger animals relative to controls (226).
There are age- and development-related effects of opioid treatment on gonadal hormones, in particular between prepubertal and postpubertal animals. Specifically, FK 33-824 caused a reduction in the secretion of LH in both male- and female-gonadectomized rats, with prepubertal rats displaying a 4-fold reduced responsiveness to opioids (227). By the same mechanism, naloxone administered to immature female rats advanced the age of onset of puberty (226). However, in very young (10 to 30 d-old) male rats, naloxone failed to increase serum LH (228) and did not induce pubertal changes. These data suggest that endogenous opioids and receptors may shift the timing of sexual maturation. Thus, it is possible that opioid exposure during critical phases of development could produce profound effects on hormonal maturation and sexual development.
2. Human studies
The effect of opioids and their analogs on the HPG axis in humans is similar to that in animals. Intravenous morphine and morphine analogs such as methadone, DAMME, pentazocine, and nalorphine decreased LH levels in healthy men (149,151). In male patients receiving opioids intrathecally (mean duration of 26 months) for nonmalignant chronic pain, LH was less than 2.0 U/liter in 20 of 29 male patients, whereas only two patients had an FSH below this level (94). In adult men, treatment with morphine resulted in a decrease of LH pulse frequency that was returned to normal by coadministration of naloxone (229), and administration of naloxone by itself increased the LH pulse frequency (230). As in animals, none of the opioids significantly altered FSH levels (151). Naloxone and other opioid antagonists increased basal and stimulated LH levels (229,231).
In premenopausal chronic pain patients on long-term intrathecal opioids, LH level was less than 2.0 U/liter in nine of 21 women, with five patients having an FSH below 2.0 U/liter (94). During oral/transdermal opioid administration to 14 premenopausal chronic pain patients, mean LH and FSH levels were 6.9 and 6.3 U/liter, respectively (122). These findings suggest that the suppression of LH may be less profound when opioids are administered orally/transdermally compared with intrathecally. In addition to the route of administration and possibly the dose of opioids, the effect of opioids and their antagonists on the HPG axis in mature females also depends on the levels of circulating sex steroids during the menstrual cycle. A single naloxone injection resulted in a significant rise in LH during the luteal phase but not during the follicular phase (232). In contrast, long-term administration of opioid antagonists did not disrupt LH secretory patterns, temporal organization, or endocrine characteristics of the luteal phase (233). In fact, oral opioid antagonism for 7 d increased LH pulsatile secretions (132). Interestingly, naltrexone is able to induce ovulation in amenorrheic women as seen by stimulated follicular growth and an LH surge (234). A recent article demonstrated that naltrexone induced ovulation and resulted in conception for nine of 27 clomiphene citrate-resistant women with PCOS (235). Hormonal and metabolic profiles also improved with naltrexone. Thus, opioid antagonists have a potential beneficial effect on fertility in women with irregular menses.
In postmenopausal women, the administration of naloxone did not change LH levels (236). Treatment of postmenopausal women with conjugated estrogens and progestin restored the inhibitory effect of dermorphin, an opioid analog, on plasma LH levels compared with normal postmenopausal women (237). In aggregate, these findings indicate that sex steroid hormones are required for opioids to have their full effect on gonadotropin levels.
The effect of opioids on the HPG axis varies with pubertal stage. Most studies used opioid antagonists to determine the development of opioid modulation on this axis. Long-term naloxone administration failed to elevate LH secretion of boys and girls in early puberty (238). In late puberty, the opioid receptor pathway develops such that naltrexone and other opioid antagonists can increase plasma LH levels. This was demonstrated by the opposing effect of naltrexone in sexually developed boys compared with prepubertal boys: in the developed boys in late puberty naltrexone increased, whereas in the sexually immature boys naltrexone decreased LH pulse frequency and concentration (239). In a similar study, naloxone administration in both early and late pubertal boys resulted in stimulation of LH secretion in late pubertal boys, whereas no effect was found in early pubertal boys (240).
In pubertal boys, naloxone administration had no effect on the negative feedback of testosterone on LH and GnRH levels (241). Estradiol suppressed LH secretion through an effect on hypothalamic GnRH secretion in pubertal boys, yet naloxone did not reverse these effects. Estradiol does not require the opioid receptor pathway for its negative feedback on the HPG axis during the early and middle stages of puberty (242). This and other data (240) indicate that the central opioid system affects the LH regulating system only in the presence of sex steroids. Mechanistically, Mauras et al. (243) found that the maturation of opioid antagonist effects during late puberty in boys occurs along the μ-opioid receptor pathway. Overall, these findings are suggestive of changes in opioid regulation along the HPG axis during puberty in both males and females.
3. Summary and overall mechanisms of opioid effects on LH and FSH
In both animals and humans, chronic opioid administration decreases LH, whereas FSH is not or is only minimally affected. The effect on LH occurs primarily by inhibiting hypothalamic GnRH secretion, although opioids also decrease the negative feedback of sex steroids on pituitary LH secretion. In turn, sex steroid hormones are required for and have major modulating effects on the sensitivity of the HPG axis to opioids and their antagonists, explaining why the effects of opioids on the HPG axis vary not only within the menstrual cycle but also with puberty and menopause.
F. Sex steroid hormones (testosterone and estradiol) and sexual behavior
1. Animal studies
As described in the previous section, the interaction of opioids with LH accounts for much of the effect of opioids on the secretion of the gonadal steroid hormones, estradiol and testosterone. As a result of a morphine-induced decrease in LH levels, testosterone and estradiol levels are also decreased (211). The effect of opioids on the steroidal hormones can be observed in the sexual behavior of male and female rats in response to opioids. Morphine, in general, inhibits the sexual receptivity of both male and female rats. In contrast, naloxone facilitated sexual behavior in both male and female rats.
In male rats, treatment with morphine inhibited sexual behavior with dose-dependent reductions in mounting, intromission, and ejaculation frequency (244). Reports from other laboratories suggested that this reduction in sexual behavior was due to failure of sexual arousal, rather than a primary erectile inability (245). Opioid antagonists facilitated sexual behavior in males. In sexually inactive male rats, naloxone was able to induce copulatory behavior (246), and it reduced the number of intromissions before ejaculation and before the time of latency (247,248).
In female rats, morphine decreased sexual behavior in a similar manner. Specifically, morphine administered to female ovariectomized rats pretreated with estradiol benzoate and progesterone inhibited female sexual receptive behaviors (249). Naloxone reversed sexually inhibited behavior (250), induced copulatory behavior in sexually inactive female rats (246), and facilitated sexual receptivity through increased lordosis in females (251). Steroids appear to play a role in the facilitation of sexual behavior because estradiol and progesterone were necessary components for enhanced lordosis.
Mechanistically, the medial preoptic area appears to be involved in the action of opioids on sexual behavior in male rats because injection of morphine or β-endorphin in this area impaired sexual behavior; this effect was attenuated by systemic naloxone administration (252,253). Injection of naloxone into the testes increased Sertoli cell proliferation and secretion (254), which may be linked to facilitation of sexual behavior by naloxone. Thus, peripheral opioid receptors may also play a role in mediating male rat sexual behavior.
In female rats, morphine inhibited sexual behavior via the ventromedial hypothalamus (255). The μ- and δ-receptors play a primary role in modulating sexual behavior; after i.c.v. infusion, high-affinity μ-receptor activation exerted an inhibitory effect on lordosis, whereas low-affinity μ-receptors and/or δ-receptors facilitated lordosis (256).
Morphine and opioid antagonists can also affect sexual development, both prenatally and prepubertally. Prenatal opioid exposure has been shown to have conflicting effects that appear to be sex dependent. Females administered morphine perinatally displayed disrupted ovarian cycles, inhibited lordosis, and reduced plasma estradiol levels (257), significantly reducing sexual behavior and reproductive activity. However, in males, prenatal morphine administration from d 11 to 18 of gestation did not disturb postpubertal testosterone levels, testicular weight, or masculine sexual behavior compared with controls (258,259), but it did shorten the postejactulatory intromission latency (259). Furthermore, prenatal opioid exposure followed by castration and exposure to estrogen of male rats at 10–12 wk resulted in an increased lordosis quotient compared with placebo prenatal (260), which was interpreted as prenatal morphine giving long-lasting feminizing effects. Searching for underlying mechanisms, Vathy et al. (261) found that prenatal morphine exposure did not alter the density of δ-opioid receptors in adult gonadal-intact male and female rats. However, when rats were gonadectomized, the same treatment resulted in decreased levels of cortical δ-opioid receptors in male and increased receptor levels in female rat brain. These morphine-induced changes in δ-opioid receptor density were reversed by male and female hormone replacement (261). Overall, these studies indicate that prenatal exposure to opioids may alter the way by which gonadal hormones affect central opioid levels in adult life, and that this may not be noticeable until significant changes in exposure to testosterone and/or estradiol occur. Regarding long-term effects of morphine exposure, it has also been shown that prepubertal morphine exposure inhibited sexual maturation in the male and displayed reduced testosterone and LH levels, although testicular weights were not altered (211). Thus, exposure of opioids to adolescent male rats also affected postpubertal sexual development.
2. Human studies
In humans, morphine suppresses LH secretion, resulting in lowering of the concentrations of steroid hormones of men and women that manifest with multiple effects. In premenopausal women, endogenous β-endorphin does not seem to have a direct effect on the normal cycling of gonadotropins during menstruation. The plasma levels of β-endorphin vary across the menstrual cycle, increasing during the follicular phase, falling before ovulation, and increasing during the luteal phase (262). This is intriguing in light of the previously mentioned variation of opioid effects during the rat menstrual cycle. One study hypothesized that the decline in β-endorphin levels before ovulation was correlated with symptoms of the premenstrual syndrome (263). Furthermore, naltrexone, an opioid antagonist, alleviated premenstrual syndrome symptoms in women with that syndrome (264). In women with PCOS, endogenous β-endorphin levels were decreased compared with normal controls (265). However, this decrease was less significant in obese women, signifying a relationship with insulin and body mass index (266).
Exogenous opioids have a much more drastic effect on the female menstrual cycle. After long-term intrathecal opioid administration, 14 of 21 premenopausal women developed amenorrhea, and the other seven developed irregularities in menstruation (94). Opioids led to a decline in LH, FSH, estradiol, and progesterone, thus affecting menstruation (94). Amenorrhea and irregular menses as a result of low estrogen levels are signs of hypogonadism in women; long-term opioid abuse is a major cause of this disorder. A more recent study by Daniell (267) presented similar results with oral and transdermal opioids; menstruation ceased shortly after beginning treatment with sustained-action opioids. The author noted a significant decrease in adrenal androgen production suggesting that opioids may also play a role in regulating sexual libido via androgens (267). Heroin users appear to have decreased sexual desire and performance (268), but in general, the effects of chronic opioid treatment on libido and/or fertility in women have not been documented in well-controlled studies.
Opioid antagonists have been found to help patients with amenorrhea, restoring HPG function (234,269,270). However, in women with hypothalamic amenorrhea, this effect is transient and is only effective after acute administration of naloxone or naltrexone (271). In hyperprolactinemic amenorrheic women, naltrexone increased LH concentrations and, accordingly, estradiol concentrations in the first day of treatment, yet these increases were not sustained with continued, chronic treatment, and normal ovulation was not restored (272). Thus, chronic administration of opioid antagonists is not useful in the treatment of central causes of oligomenorrhea/amenorrhea, although as discussed above, opioid antagonists appear to be helpful in treating oligomenorrhea associated with PCOS (235).
In men, the effects of long-term opioid use on gonadal status have been studied more extensively. Heroin addicts show significantly decreased testosterone levels (273,274). Although one study found normal testosterone levels, this study reported abnormal semen analyses in men who used heroin or methadone (275). Using population-based reference ranges for total testosterone, hypogonadism was present in 86% of men receiving intrathecal opioids for chronic pain (94). Oral opioids, including methadone, were associated with hypogonadism in 89% of men and also resulted in decreased levels of estradiol, dihydrotestosterone, LH, and FSH (276). A similar frequency of hypogonadism was found in men receiving oral/transdermal opioids (122). Overall, these studies indicate that opioids, regardless of the administration route, are associated with a high prevalence of male hypogonadism.
SHBG was high in heroin addicts, leading to low free testosterone levels (273), an effect that was not found in men on long-term oral opioids (122). Based on this, we suggest that SHBG and/or free testosterone levels, in addition to total testosterone levels, should be measured in future studies on the prevalence of hypogonadism in men receiving opioids. This would reduce the likelihood that subjects with total testosterone levels at or just above the lower limit of normal would be classified as eugonadal while they in fact are hypogonadal.
Chronic opioid use in males, whether for pain control or through heroin, morphine, or methadone addiction, leads to symptoms of delayed ejaculation, erectile dysfunction, and significant decreases in sexual libido (276,277,278). Administration of opioid antagonists such as naltrexone can improve symptoms of hypogonadism (279). Naltrexone improved erectile function over a period of 7 to 15 d, but in the majority of subjects, it did not sustain the response after cessation of treatment (279). Mechanistically, the antagonist did not increase testosterone or LH levels, suggesting regulation at the central rather than the peripheral level (279). In an open-label study, treatment with exogenous testosterone improved the testosterone levels and sexual function of men exhibiting hypogonadism on prescription opioids (280). The latter finding suggests that testosterone replacement may be helpful for treatment of hypogonadism in male patients using opioids, although a double-blind placebo-controlled study needs to be performed. Alternatively, the type of opioid drug used for chronic pain may also affect the degree of hypogonadism seen. Studies on buprenorphine, a partial μ-opioid agonist used for opioid dependence, found that this drug was associated with significantly higher testosterone levels and lower frequency of sexual dysfunction than methadone (281,282).
In men, hypogonadism may lead to decreased bone mineral density, causing osteopenia or osteoporosis. Chronic heroin users exhibit reduced bone density (283) and lower serum osteocalcin levels (284). Fifty percent of male patients receiving chronic oral/transdermal opioids had osteopenia (122). In epidemiological studies, opioid use is associated with a 1.5- to 6-fold increase of osteoporotic fractures (285). In addition to the effect of hypogonadism, opioids also affect bone directly via opioid receptors on osteoblasts; opioids inhibited osteoblast cell growth in culture, which is also likely to be the case in the whole body (286). Furthermore, hypogonadism in males as a result of opioid use also led to increased depression (280), which by itself or due to antidepressant use, may result in decreased sexual dysfunction. A limitation of these studies is that they do not control for pain or for reduced mobility that may result from pain and depression.
In males, testosterone levels decrease with age, which is also associated with change in body composition, including an increase in fat mass (287). Although the clinical relevance of this age-related decline in testosterone has not been established unequivocally (288), some studies indicate that the decline in testosterone may be associated with a decrease in cognitive performance (289). Although further studies may need to confirm this association and the potential benefit of testosterone therapy (290), it is currently unknown whether opioid-induced hypogonadism is associated with cognitive decline and other signs of hypogonadism.
Opioids are hypothesized to cause a decline in testosterone levels and sexual dysfunction via two mechanisms. de la Rosa and Hennessey (291) suggested that opioids lead to an alteration in normal gonadotropin pulse patterns or affect the response of the anterior pituitary to GnRH, both of which decreased testosterone levels. Morphine down-regulated estrogen receptor-β expression in human vascular endothelial cells, an effect partially blocked by naloxone or a selective μ-opioid receptor antagonist, d-Pen-Cys-Tyr-d-Trp-Orn-Thr-Pen-Thr-NH2, showing the involvement of the μ-opioid receptor in this action of morphine (292). A deeper understanding of the hypogonadal effects of opioids may cause users to reexamine the abuse of opioids.
Male hypogonadism can lead to symptoms of decreased sexual libido, erectile dysfunction, and osteoporosis (293). In chronic pain patients receiving intrathecal morphine, hypogonadism was found in men and in both pre- and postmenopausal women (294). However, the prevalence of hypogonadism as a consequence of oral opioids may not be similar in both genders. Fraser et al. (122) studied chronic noncancer pain patients receiving oral/transdermal opioids and found hypogonadism in 75% of men and 21% of women, although these groups had similar opioid dosage and pain scores. This suggests that opioid use may be associated with a higher prevalence of hypogonadism in men than in women.
3. Summary and overall mechanisms of opioid effects on sex steroids
Chronic opioid administration results in suppression of sex steroids mediated mainly by suppression of LH via effects on GnRH. In addition, there may be direct effects at the gonadal level. Opioids have significant suppressive effects on sexual behavior, which appear to be mediated primarily via activation of μ- and δ-receptors in the medial preoptic area and the ventromedial hypothalamus. Opioid antagonists can facilitate sexual behavior. In humans, the sensitivity to these opioid effects appears to be higher in men than in women.
G. Arginine vasopressin (AVP)
1. Animal studies
Opioids and their analogs not only interact with the hormones of the anterior pituitary but also have an effect on the posterior pituitary (neurohypophysis). AVP, also known as antidiuretic hormone, and OT are both individually and synergistically affected by opioids. Morphine inhibited the electrically evoked release of both OT and AVP after injection into electrically stimulated neurohypophysis cells that hypersecrete both AVP and OT (295). Naloxone reversed the suppression of OT release but was without effect against the opioid block of AVP secretion (295). Morphine injection resulted in a mean decrease in arterial pressure and urine flow in both normal and Brattleboro rats that lack AVP (296), suggesting that the antidiuretic effect of morphine may occur independently of antidiuretic hormone release. However, i.c.v. injection of morphine also caused dose-dependent antidiuresis in rats (297), an effect blocked by naloxone, implying enhanced AVP levels after morphine. In a more detailed study, Michel et al. (298) found that morphine was able to increase or decrease AVP secretion, depending on the hydration state of the rats; morphine increased AVP in water-loaded rats, whereas in dehydrated Brattleboro rats, morphine induced an increased AVP response at higher doses and inhibited the AVP response at lower doses. Thus, the hydration state may regulate the response of the posterior pituitary and kidney to opioid-induced AVP secretion.
Other opioid analogs have also been shown to affect AVP levels, again with contrasting effects. β-Endorphin administration to rat neurointermediate lobe pituitaries in vitro inhibited AVP release in a naloxone-reversible manner (299). However, other endogenous opioid peptides preferentially stimulate secretion of AVP. The secretion, rather than inhibition, of AVP in response to opioid peptides may be the response of the animal to maintain homeostasis during dehydration and in response to an increased plasma osmolality (300,301). Enkephalins, met-enkephalin and leu-enkephalin, did not change plasma AVP concentrations when injected by an i.c.v. or iv route in normal sheep (302,303). However, i.c.v. leu-enkephalin inhibited baseline and angiotensin-stimulated AVP release (304), and another report found that dynorphin (1-17), β-endorphin, and leu-enkephalin inhibited the spontaneous release of AVP from the neurointermediate lobe in vitro. In other studies, i.c.v. and, to a lesser extent, iv leu-enkephalin increased AVP release and contributed to the antidiuretic response in rats (305,306). We are unable to explain the discrepant reports on the effect of leu-enkephalin on AVP. Explanations for the close ties between leu-enkephalin and AVP center around the high immunoreactivity for leu-enkephalin region in the hypothalamus and posterior pituitary of rats (307), an effect that has been demonstrated particularly in AVP terminals (308), suggesting that this opioid peptide may be a significant factor in osmoregulation (308). However, met-enkephalin and leu-enkephalin do not influence spontaneous or stimulated AVP release from isolated neurointermediate lobes (309). Mechanistically, studies suggest that leu-enkephalin, along with met-enkephalin, dynorphin, and CRH are localized in the posterior pituitary in the region innervated by hypothalamic nerve terminals (310,311). Endogenous opioids likely act directly at these neurosecretory nerve endings and not via pituicytes (312).
At the receptor level, the effects of opioids on the secretion of AVP are primarily mediated by the μ- and κ-opioid receptors (313). FK 33-824, an enkephalin analog and μ-receptor agonist, increased plasma AVP levels (302), and another μ-agonist, DAMGO, decreased urine outflow rates, also suggesting increased AVP release after μ-opioid receptor binding (314). The κ-agonist, RU 51599, inhibited AVP secretion, yet also down-regulated AVP mRNA content (315). These contrasting receptor responses to opioid agonists show that activation of μ- and κ-receptors may exert counteracting actions on AVP secretion.
The phenomenon of hormones having effects on endogenous opioids is also seen with AVP and OT. The hyperphagic response to acute morphine was reduced by both AVP and OT (316). AVP also enhanced periaqueductal gray-secreting enkephalin and β-endorphin in the rat, suggesting that central AVP is an important bioactive substance for antinociceptive regulation (317).
2. Human studies
Similar to animal studies, the information on the effect of opioids and their analogs on AVP secretion, although extensively studied, is not conclusive. Some of the difficulties in interpreting human studies of opioids on AVP are that many of these studies used indirect methods for the evaluation of AVP function or failed to consider side effects of opioids, such as hypotension or nausea, which could secondarily stimulate AVP release. The administration of morphine produced antidiuresis that was believed to be the result of increased AVP secretion (318). However, more recently, several studies using a variety of opioids including morphine (319), β-endorphin (304,319), leu-enkephalin analogs (320), and the met-enkephalin analogs, DAMME (321,322,323) and metkephamid acetate (324,325), all indicate that opioids act directly to suppress AVP secretion. However, there are other papers showing that the opioids do raise AVP levels. Intravenous or epidural morphine administered to children undergoing surgery raised AVP levels (326). Infused fentanyl, an opioid stronger than morphine that is used as an anesthetic, increased AVP release in both surgical patients (327) and healthy volunteers (328). Although in mice, κ-receptors are highly involved in AVP regulation, in humans, κ-agonists induced diuresis but did not change plasma AVP levels (329). These data confirm the role of the μ-opioid receptor in modulating AVP.
3. Summary and overall mechanisms of opioid effects on AVP
The effects of opioids on AVP are the most inconclusive of any hormone in both animals and humans. Both animal and human studies find that stimulation and suppression of AVP levels and discrepancies may be due to the fluid status of the subject as well as side effects of the administered opioid. μ- and κ-receptors appear to be involved in the regulation of AVP by opioids. Endogenous opioids likely affect AVP release by acting at neurosecretory nerve endings in the posterior pituitary.
H. Oxytocin (OT)
1. Animal studies
Despite the similar synthesis location of OT and AVP in the magnocellular neurons in the hypothalamus (330), OT is more affected by opioids and their analogs than AVP. In general, acute administration of opioids inhibits OT release (295,301).
Opioids not only inhibit the OT release from the axon terminal in the neural lobe but also suppress the functional activity of OT neurons by opioid action on their perikarya in the hypothalamus (331,332,333,334). Opioids have been found to act directly at the neural lobe of the pituitary (335) to inhibit OT release in a variety of situations. Morphine inhibited OT levels after an isolated rat neural lobe that had been electrically stimulated (295). The hydration state of the animal did not affect the ability of μ- and κ-opioid receptor agonists to suppress OT levels (313). Opioids also inhibited OT levels in dehydrated rats during lactation (336). Hartman et al. (336) proposed that opioid peptides inhibit OT so that the pituitary can store and conserve OT needed for lactation. In normally hydrated rats, morphine caused a dose-dependent reduction in plasma OT levels in virgin but not lactating rats (337), and similar OT levels were found in morphine-treated and untreated lactating rats (338). This finding suggests an interaction between the lactation state and the effects of opioids on OT levels. Interestingly, during parturition, morphine administered sc or by i.c.v. to the mother during birth inhibited the levels of OT and impaired maternal behavior (339).
Chronic morphine treatment in rats affected not only the secretion of OT, but also its synthesis. In the supraoptic nucleus and nucleus accumbens of rats treated with morphine by ip injection for 7 d, decreased OT content and mRNA expression were seen by RIA and in situ hybridization, respectively (340). OT expression after water deprivation was reduced by the μ- or κ-receptor agonists in the neurohypophysis, in a similar manner as AVP expression (313). Stress-induced OT secretion was potentiated by μ- and κ-antagonists, with μ-antagonists potentiating the immobilization response and κ-antagonists potentiating the response to hypertonic saline (341). κ-Receptors are involved in inhibiting OT secretion in times of dehydration (342). On the other hand, μ-receptors are primarily involved in the inhibition of the OT response during pregnancy, with κ-receptors having no effect (343). Thus, the effects of endogenous opioids on OT secretion are mediated by different receptors, depending on the stimulation of OT.
2. Human studies
Women have been studied primarily for the effects of opioids on OT, particularly the effect of analgesics, during pregnancy and labor. Lindow et al. (344) showed that during late pregnancy, morphine and naloxone had no effect on the maternal OT levels. However, during the first stage of labor, morphine significantly inhibited plasma OT levels, whereas naloxone had no effect (345). Furthermore, fetal OT production was not affected by the administration of morphine to the mother (346). Lindow et al. (347) found that after delivery, morphine inhibited the rise in OT secretion in breast-feeding women, whereas naloxone again had no effect. Other opioid analgesics, such as fentanyl or sufentanil, did not suppress OT levels before labor, but suppressed OT levels during the onset of labor (348,349).
Naloxone has been shown to increase the angiotensin II-stimulated rise of OT secretion in male subjects (350). Another study showed that naloxone had no effect on baseline plasma OT levels in males (351). This highlights the need for more studies on the effects of not only naloxone but also other opioids and opioid analogs on OT levels of males and nonpregnant females.
3. Summary and overall mechanisms of opioid effects on OT
Opioids inhibit OT secretion in both animals and humans. The site of action is both directly at the level of the pituitary and at the level of the hypothalamus. Chronic morphine treatment affected both the synthesis and secretion of OT. Both κ- and μ-receptors are involved in mediating the effects of opioids on OT.
I. Obesity and diabetes
1. Animal studies
The effect of opioids on food intake is controversial and includes reports of hyperphagia (352,353,354,355) as well as anorexia (356,357,358,359,360,361,362,363), depending on the dose and dosing regimen (reviewed in Refs. 32,364, and 365). Reviews state that the majority of studies in rodents show that opioid peptides from three families, endorphins, enkephalins, and dynorphins, when administered in the CNS, increase food intake, and opioid antagonists decrease food intake (reviewed in Refs. 32 and 365), although there are probably more studies showing that opioids cause decreased food intake. Opioids bind to multiple opioid receptor types in the CNS, with the dynorphin/α-neo-endorphin κ-opioid receptor likely being the main receptor involved in feeding modulation (365). There is limited evidence to suggest that they affect food intake when administered peripherally (32). Acutely injected heroin increased immunoreactivity of neuropeptide Y in the thalamic paraventricular nucleus and nucleus of stria terminalis (366,367), areas known to stimulate food intake and thus lead to an increased likelihood of obesity. Continuous infusion of morphine increased food intake, and, over time, fat intake decreased whereas carbohydrate intake increased (355). However, in contrast to these reports of hyperphagia, anorexia, characterized by decreased food intake and hyperactivity, is also noted in animals after morphine intake (359,363). The mechanism of morphine’s action on food intake is hypothesized to be a triphasic effect: morphine (15 mg/kg given by ip injection) initially suppressed food intake (1 h), enhanced intake (3 h), then suppressed intake again after 4–24 h (359,368). The main sites of action at which opioid agonists affect feeding include the amygdala, paraventricular nucleus, perifomical area of the lateral hypothalamus, and ventromedial hypothalamus (365,369).
Anghel et al. (370) conducted a study that observed the effects of short- and long-term morphine administration on rodent food intake, body weight, and hypothalamic and pituitary gene expression as determined by microarray technology. They found decreased food intake coupled with marked weight loss in the days after morphine pellet implantation. They also found that long-term morphine treatment increased hypothalamic neuropeptide Y, agouti-related protein, and cocaine and amphetamine regulated transcript expression, whereas short-term morphine decreased leptin receptors and adiponutrin expression. These studies support the anorexic effect of morphine by uncovering changes in gene expression.
Studies of genetically obese mice (ob/ob) showed higher levels of plasma β-endorphin in comparison to control mice. However, plasma β-endorphin levels were similar in obese Zucker rats (fa/fa) compared with controls (371). The increase in β-endorphin levels of obese mice was accompanied by higher δ and κ and lower μ-opioid binding sites, suggesting that altered endogenous opioid levels and receptor affinity may contribute to hyperphagia (372).
Furthermore, obesity can affect the endocrine response to opioids in rats. Obese male rats exhibited a decreased GH response to GHRH and morphine (373). However, TRH and the thyroid hormones, T3 and T4, did not change with obesity in these same rats (373). Studies of obese Zucker female rats concluded that endogenous opioids affect obesity and reproductive dysfunction because the opioid antagonist naltrexone increased sexual receptivity and proceptivity and also decreased food intake (374). These effects were blocked by morphine treatment, suggesting that endogenous opioids promote obesity as well as hypogonadism.
Diabetes and hyperglycemia affect and, in turn, are affected by opioids. Enkephalin agonists for the μ- and δ-receptors increased glucose and insulin levels in both lean and obese mice (375). However, other studies showed that diabetic rats exhibited increased κ binding in the medial preoptic area and decreased μ binding in the lateral habenula (376). Moreover, female mice given oral methadone for 35 d exhibited a rise in serum glucose levels and reduced glycolytic enzymatic activity, producing a metabolic state that is similar to insulin-resistant diabetes (377). Collectively, these studies demonstrate that opioid manipulations can induce diabetic-like states in animals.
The presence of diabetes can affect how opioids affect parameters related to addiction. Shook and Dewey (378) found that diabetic rats were less physically dependent on morphine than nondiabetic controls and suggested that this lower dependence was due to hyperglycemia itself (379). The antinociceptive effects of morphine were reduced in streptozocin-induced diabetic rats, which is explained not by decreasing numbers of opioid receptors or receptor affinities to morphine but by alterations in the pharmacokinetics of morphine (380). However, one study found reduced numbers of functional μ-opioid receptors in the spinal cord dorsal horn of diabetic rats, which was postulated as a mechanism for the reduced analgesic effect of morphine (381). Thus, there is also an interaction between diabetes and the analgesic effect of morphine.
2. Human studies
One of the growing problems in society today is the increasing rate of diabetes and obesity, and thus, research with opioids has come to address these issues. Treatment of obese subjects with the opioid-antagonist naloxone inhibited the insulin and C-peptide responses to glucose administration, suggesting that endogenous β-endorphins increase the responsiveness of the pancreatic β-cells and that opioid administration may contribute to the hyperinsulinemia and the hyperphagia of obesity (382). In contrast, in obese women, naltrexone decreased basal concentration of both insulin and C-peptide, but did not affect the insulin secretory response to an oral glucose tolerance test (95). Interestingly, obese women treated with naloxone showed more significant weight loss than obese men (383), which may be linked to a sex-related difference in opioid effects. Patients with anorexia and bulimia nervosa were found to have elevated levels of endogenous plasma levels of codeine and morphine; it is proposed that endogenous alkaloids are released during an initial dieting period that also reinforces the state of starvation (384). Concordantly, naltrexone administration reduced binge purging tendencies in bulimic and anorexic patients (385).
Opioids also have different effects on the endocrine system in obese persons compared with normal-weight individuals. Obese patients exhibit lowered GH and PRL responses and higher β-endorphin levels in response to raised opioid levels, an effect blocked by naloxone (386). However, Papalia et al. (387) suggested that opioids do not directly regulate GH and PRL levels, but rather they stimulate cortisol and insulin response to insulin-induced hypoglycemia in obese females. In studies of severely obese patients suffering from hypogonadism, treatments with naloxone resulted in more sensitive response to LH in obese men, thus suggesting that endogenous opioids may act to suppress GnRH secretion in obesity (388).
Although naltrexone alone does not have significant weight loss properties by itself, the combination of naltrexone plus buproprion, a drug used for depression and smoking cessation (389) that is a putative stimulator of the melanocortin pathway, leads to weight loss in both an animal model and a small clinical study (390). In the clinical trial, only a small number of subjects were in each group, and there was a high rate of noncompleters. The authors hypothesized that naltrexone would antagonize the normal inhibitory feedback mechanism that limits sustained POMC activation in response to agents such as buproprion and give an additive effect on weight loss. This combination, marketed as Contrave, is in late stage clinical trials (391).
Diabetes in humans and its relationship with endogenous and exogenous opioids has been an interesting area for research. An early finding was that increased sensitivity to endogenous opioids can be associated with type 2 diabetes associated with chlorpropamide alcohol flushing (392). Naloxone administered to type 2 diabetics sharply increased insulin levels in response to glucose and confirmed the endogenous opioid involvement in impairing insulin levels (393,394). Additionally, compared with nonaddicted diabetic patients, opioid-addicted patients with non-insulin-dependent diabetes had higher hemoglobin A1c, potassium, and iron levels, whereas their total serum protein, alanine aminotransferase, and high-density lipoprotein-cholesterol were lower. Overall, serum glucose is increased and so is the risk of metabolic disorders (395). Giugliano (396) hypothesized that opioids and opioid peptides act centrally through the sympathetic nervous system to cause hyperglycemia and impaired insulin secretion. He also suggested that heroin addicts do not respond correctly to insulin signals, implicating a higher prevalence of glucose disorders in this population (396). However, in male heroin addicts, hemoglobin A1c levels were similar to controls (397). Alternatively, opioids may also affect insulin resistance and the risk for metabolic syndrome via the induction of hypogonadism. In males, chronic hypogonadism, either spontaneous or induced by GnRH agonists, is associated with increased insulin resistance and risk for diabetes mellitus, but not frank hyperglycemia (398), and testosterone replacement may improve insulin resistance (399).
Endogenous plasma opioid levels are altered in human subjects with diabetes. For example, patients with diabetes showed a 64% elevation of plasma β-endorphin and a substantial increase in enkephalin-like immunoreactivity (400). A possible function for the increased enkephalins in the plasma is to inhibit insulin secretion because FK 33-824 administration reduced plasma glucose rises in normal and type 2 diabetics (401). In diabetic patients, the analgesic effect of morphine was also lessened in diabetic and hyperglycemic conditions, and higher doses of morphine were needed for effective analgesia during postoperative pain (402). The exact mechanisms in humans have not been extensively studied.
3. Summary and overall mechanisms of opioid effects on obesity and diabetes
Increasing data suggest that opioids play a role in regulating food intake, food choice, and perhaps the reward associated with good-tasting foods. Pharmacological studies suggest that more than one opioid receptor is involved in the regulation of feeding, with the κ-opioid receptor predominantly involved. Opioids appear to exert their effect predominantly within the central nervous system, although peripheral effects on taste and gastrointestinal motility may play a minor role. Chronic administration of opioid antagonists has not shown substantial effects on body weight. In humans, the studies so far suggest a relatively minor role for endogenous opioids in appetite modulation. The opioid antagonist, naltrexone, in combination with other drugs, holds promise for weight loss in obese patients.
In both humans and rodents, opioid administration leads to hyperglycemia and worsening diabetes. The mechanism appears to be decreased insulin secretion. Additionally, hyperglycemia decreased the antinociceptive properties of opioids. More studies are needed to explore the interactions between opioids and insulin secretion/insulin resistance.
IV. Areas of Future Research
The above studies show that opioids affect the endocrine system, but many questions remain. It is not clear whether all effects are dose-dependent or whether there is a threshold opioid dose below which effects do not occur. Many studies are cross-sectional comparisons rather than prospective studies, and results may often be confounded by the presence of other diseases or drugs. The route of administration, e.g., intrathecal vs. oral or transdermal, may change the magnitude and/or direction of the opioid effects on hormonal secretion. The effect of partial agonists, e.g., buprenorphine, on the endocrine system may be different from full opioid agonists and antagonists and remains to be determined. The mechanisms underlying the differential effects of acute vs. chronic opioids are not sufficiently explained. Although chronic opioid use causes hypogonadism, the extent of gender-related differences needs to be confirmed and explained. Furthermore, there is insufficient information on the long-term effects of opioid-induced hypogonadism, including on bone density, sexual function, and fertility.
Because hypogonadism is associated with an increased risk for cardiovascular events (398) and long-term opioid use clearly leads to hypogonadism, studies on cardiovascular events and risk factors of opioid users are warranted, especially because these patients already have a high prevalence of other risk factors, including immobility and nicotine abuse (122). Overall, there is a need for prospective studies on the effect of chronic opioid therapy on the endocrine system. These studies will require adequate controls for severity and perhaps different types of pain because pain itself has multiple effects on the endocrine system (403).
V. Conclusions
The effects of exogenous opioids, opioid analogs, and endogenous opioid peptides on the endocrine system are numerous. In animals and humans, opioids generally increase GH and PRL and decrease LH, testosterone, estradiol, and OT, whereas the AVP reports are conflicting (Table 4 and Fig. 2). In animals, opioids decrease TSH, whereas in humans opioids increase TSH. The results of the effects of opioids on AVP and ACTH are less clear. Specific receptor stimulation and timing of administration play significant roles in the ability of the opioids to affect these endocrine hormones. Moreover, the mechanisms of the opioids are numerous because opioids affect receptors in the endocrine glands yet also affect endogenous opioid peptide gene expression.
In humans, the primary disorder that results from opioid abuse is hypogonadism, particularly in males. Users and abusers must be aware of not only the prevalence of this disorder on their sexual functioning, but also the effects of the opioids on the other hormones in their system, which may lead to harmful long-term effects. Opioids may cause other endocrine disorders, such as hyperprolactinemia and hyperthyroidism. More research must be done to elucidate the prevalence of these and other endocrine disorders associated with opioid use and abuse.
Acknowledgments
We thank Kevin Jones, Ph.D. (Western University and Charles Drew University) for his help in creating the figures of the opioid agonists, and Stacey Teruya, Ed.D. (Charles Drew University) for his helpful comments on the manuscript.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 DA14659 (to T.C.F.); Research Centers at Minority Institutions Program Grant G21 RR03026; NIH Minority Institution Drug Abuse Research Program Grant R24 DA017298; National Institute of Child Health and Human Development, Charles Drew University/University of California, Los Angeles Cooperative Reproductive Science Research Center Grant U54 HD41748; and Center of Clinical Research Excellence Grant U54 RR14616 (to Charles Drew University of Medicine & Science). L.E.O. was supported by NIH Grant R01 DA021274. K.L. was supported by NIH Grant R01 DA16682.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 10, 2009
Abbreviations: AVP, Arginine vasopressin; CNS, central nervous system; CS, corticosterone; DALA, d-ala2-met-enkephalinamide; DAMME, d-Ala2,MePhe4,met-(O)enkephalin-ol; ED, emergency department; HPA, hypothalamic-pituitary-adrenal; HPG, hypothalamic-pituitary-gonadal; i.c.v., intracerebroventricular; OT, oxytocin; PC, prohormone convertase; PCOS, polycystic ovary syndrome; POMC, pro-opiomelanocortin; PRL, prolactin.
References
- National Institute on Drug Abuse 2008 Clinical trials network— research. Retrieved July 22, 2009. http://www.nida.nih.gov/CTN/Research.html [Google Scholar]
- Hoffmann NG, Olofsson O, Salen B, Wickstrom L 1995 Prevalence of abuse and dependency in chronic pain patients. Int J Addict 30:919–927 [DOI] [PubMed] [Google Scholar]
- Manchikanti L 2007 National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician 10:399–424 [PubMed] [Google Scholar]
- 2007 The NSDUH Report: patterns and trends in nonmedical prescription pain reliever use 2002 to 2005. Rockville, MD: Substance Abuse and Mental Health Services Administration [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE 2008 Monitoring the future national results on adolescent drug use: overview of key findings, 2007. Bethesda, MD: National Institute on Drug Abuse [Google Scholar]
- International Narcotics Control Board 2007, March, press release no. 4: Abuse of prescription drugs to surpass illicit drug abuse. Retrieved July 21, 2009. http://www.drugwatch.org/Newsletters/March%202007%20Newsletter.pdf. Drug Watch International 12:14 [Google Scholar]
- Compton WM, Volkow ND 2006 Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend 81:103–107 [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Mao J 2003 Opioid therapy for chronic pain. N Engl J Med 349:1943–1953 [DOI] [PubMed] [Google Scholar]
- Caudill-Slosberg MA, Schwartz LM, Woloshin S 2004 Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain 109:514–519 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration 2007 Results from the 2006 National Survey on Drug Use and Health: national findings, series H-32 (Department of Health and Human Services publication no. SMA 07-4293). Retrieved August 18, 2009. http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.pdf [Google Scholar]
- Substance Abuse and Mental Health Services Administration 2004 Oxycodone, hydrocodone, and polydrug use, 2002. Drug Abuse Warning Network, 2002. The DAWN Report, Office of Applied Studies. Retrieved July 21, 2009. http://www.oas.samhsa.gov/2k4/oxycodone/oxycodone.pdf. [Google Scholar]
- Paulozzi LJ, Ryan GW 2006 Opioid analgesics and rates of fatal drug poisoning in the United States. Am J Prev Med 31:506–511 [DOI] [PubMed] [Google Scholar]
- Helling DK, Lemke JH, Semla TP, Wallace RB, Lipson DP, Cornoni-Huntley J 1987 Medication use characteristics in the elderly: the Iowa 65+ Rural Health Study. J Am Geriatr Soc 35:4–12 [DOI] [PubMed] [Google Scholar]
- Gfroerer J, Penne M, Pemberton M, Folsom R 2003 Substance abuse treatment need among older adults in 2020: the impact of the aging baby-boom cohort. Drug Alcohol Depend 69:127–135 [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Rosomoff HL, Rosomoff RS 1992 Drug abuse, dependence, and addiction in chronic pain patients. Clin J Pain 8:77–85 [DOI] [PubMed] [Google Scholar]
- Boyd CJ, McCabe SE, Cranford JA, Young A 2006 Adolescents’ motivations to abuse prescription medications. Pediatrics 118:2472–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA 2008 Prevalence and characteristics of opioid use in the US adult population. Pain 138:507–513 [DOI] [PubMed] [Google Scholar]
- Forman RF, Woody GE, McLellan T, Lynch KG 2006 The availability of web sites offering to sell opioid medications without prescriptions. Am J Psychiatry 163:1233–1238 [DOI] [PubMed] [Google Scholar]
- Denisco RA, Chandler RK, Compton WM 2008 Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exp Clin Psychopharmacol 16:417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Satoh M 1995 Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res 23:121–145 [DOI] [PubMed] [Google Scholar]
- Satoh M, Minami M 1995 Molecular pharmacology of the opioid receptors. Pharmacol Ther 68:343–364 [DOI] [PubMed] [Google Scholar]
- Kapas S, Purbrick A, Hinson JP 1995 Action of opioid peptides on the rat adrenal cortex: stimulation of steroid secretion through a specific μ opioid receptor. J Endocrinol 144:503–510 [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM 1984 Endogenous opioids: biology and function. Annu Rev Neurosci 7:223–255 [DOI] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ 1997 A potent and selective endogenous agonist for the μ-opiate receptor. Nature 386:499–502 [DOI] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Costentin J, Do Rego JC 2007 The endomorphin system and its evolving neurophysiological role. Pharmacol Rev 59:88–123 [DOI] [PubMed] [Google Scholar]
- Sandyk R 1985 The endogenous opioid system in neurological disorders of the basal ganglia. Life Sci 37:1655–1663 [DOI] [PubMed] [Google Scholar]
- Frenk H 1983 Pro- and anticonvulsant actions of morphine and the endogenous opioids: involvement and interactions of multiple opiate and non-opiate systems. Brain Res 287:197–210 [DOI] [PubMed] [Google Scholar]
- Olson GA, Olson RD, Kastin AJ 1985 Endogenous opiates: 1984. Peptides 6:769–791 [DOI] [PubMed] [Google Scholar]
- Tortella FC, Holaday JW 1986 Dynorphin A (1-13): in vivo opioid antagonist actions and non-opioid anticonvulsant effects in the rat flurothyl test. NIDA Res Monogr 75:539–542 [PubMed] [Google Scholar]
- Plotnikoff NP 1985 The Ying-Yang hypothesis of opioid peptide immunomodulation. Psychopharmacol Bull 21:489 [PubMed] [Google Scholar]
- Wybran J 1985 Enkephalins and endorphins as modifiers of the immune system: present and future. Fed Proc 44:92–94 [PubMed] [Google Scholar]
- Baile CA, McLaughlin CL, Della-Fera MA 1986 Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev 66:172–234 [DOI] [PubMed] [Google Scholar]
- Porreca F, Burks TF 1983 The spinal cord as a site of opioid effects on gastrointestinal transit in the mouse. J Pharmacol Exp Ther 227:22–27 [PubMed] [Google Scholar]
- Schick R, Schusdziarra V 1985 Physiological, pathophysiological and pharmacological aspects of exogenous and endogenous opiates. Clin Physiol Biochem 3:43–60 [PubMed] [Google Scholar]
- Bernton EW, Long JB, Holaday JW 1985 Opioids and neuropeptides: mechanisms in circulatory shock. Fed Proc 44:290–299 [PubMed] [Google Scholar]
- Holaday JW 1983 Cardiovascular effects of endogenous opiate systems. Annu Rev Pharmacol Toxicol 23:541–594 [DOI] [PubMed] [Google Scholar]
- Johnson MW, Mitch WE, Wilcox CS 1985 The cardiovascular actions of morphine and the endogenous opioid peptides. Prog Cardiovasc Dis 27:435–450 [DOI] [PubMed] [Google Scholar]
- Bicknell RJ 1985 Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J Endocrinol 107:437–446 [DOI] [PubMed] [Google Scholar]
- Grossman A, Rees LH 1983 The neuroendocrinology of opioid peptides. Br Med Bull 39:83–88 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Herz A 1985 The endocrinology of the opioids. Int Rev Neurobiol 26:1–83 [DOI] [PubMed] [Google Scholar]
- Yen SS, Quigley ME, Reid RL, Ropert JF, Cetel NS 1985 Neuroendocrinology of opioid peptides and their role in the control of gonadotropin and prolactin secretion. Am J Obstet Gynecol 152:485–493 [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Netto CA 1985 Role of β-endorphin in behavioral regulation. Ann NY Acad Sci 444:162–177 [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Klein GE 2005 Endogenous opiates and behavior: 2004. Peptides 26:2629–2711 [DOI] [PubMed] [Google Scholar]
- Holden JE, Jeong Y, Forrest JM 2005 The endogenous opioid system and clinical pain management. AACN Clin Issues 16:291–301 [DOI] [PubMed] [Google Scholar]
- McFadzean I 1988 The ionic mechanisms underlying opioid actions. Neuropeptides 11:173–180 [DOI] [PubMed] [Google Scholar]
- Pert A, Yaksh T 1975 Localization of the antinociceptive action of morphine in primate brain. Pharmacol Biochem Behav 3:133–138 [DOI] [PubMed] [Google Scholar]
- Chu LF, Angst MS, Clark D 2008 Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 24:479–496 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Abhold R 1987 Enkephalin release into the ventral tegmental area in response to stress: modulation of mesocorticolimbic dopamine. Brain Res 414:339–348 [DOI] [PubMed] [Google Scholar]
- Williams FG, Mullet MA, Beitz AJ 1995 Basal release of met-enkephalin and neurotensin in the ventrolateral periaqueductal gray matter of the rat: a microdialysis study of antinociceptive circuits. Brain Res 690:207–216 [DOI] [PubMed] [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ 2006 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147(Suppl 1):S153–S162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby GW 1967 Biosynthesis of the morphine alkaloids. Science 155:170–173 [DOI] [PubMed] [Google Scholar]
- Herz A 1998 Opioid reward mechanisms: a key role in drug abuse? Can J Physiol Pharmacol 76:252–258 [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL 1996 Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature 383:819–823 [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR 1997 The μ-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol 324:R1–R2 [DOI] [PubMed] [Google Scholar]
- Goldstein A 1991 Heroin addiction: neurobiology, pharmacology, and policy. J Psychoactive Drugs 23:123–133 [DOI] [PubMed] [Google Scholar]
- Friedman Z, Katznelson R, Phillips SR, Zanchetta C, Nistor OI, Eisen LB, Siddiqui N 2008 A randomized double-blind comparison of a morphine-fentanyl combination vs. morphine alone for patient-controlled analgesia following bowel surgery. Pain Pract 8:248–252 [DOI] [PubMed] [Google Scholar]
- Kobylecki RJ, Carling RW, Lord JA, Smith CF, Lane AC 1982 Common anionic receptor site hypothesis: its relevance to the antagonist action of naloxone. J Med Chem 25:116–120 [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF 2001 Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry 25:729–741 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF 1998 Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51:23–47 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC 2001 The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev 53:209–243 [PubMed] [Google Scholar]
- Lightman SL, Young 3rd WS 1987 Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol 394:23–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley JK, Young 3rd WS, Kuhar MJ 1980 Anatomical localization of enkephalin immunoreactive sites in rat forebrain. Adv Biochem Psychopharmacol 22:257–270 [PubMed] [Google Scholar]
- Roth KA, Weber E, Barchas JD, Chang D, Chang JK 1983 Immunoreactive dynorphin-(1-8) and corticotropin-releasing factor in subpopulation of hypothalamic neurons. Science 219:189–191 [DOI] [PubMed] [Google Scholar]
- Lewis JW, Cannon JT, Liebeskind JC 1980 Opioid and nonopioid mechanisms of stress analgesia. Science 208:623–625 [DOI] [PubMed] [Google Scholar]
- Sinha R 2001 How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359 [DOI] [PubMed] [Google Scholar]
- Chrousos GP 1992 Regulation and dysregulation of the hypothalamic-pituitary-adrenal axis. The corticotropin-releasing hormone perspective. Endocrinol Metab Clin North Am 21:833–858 [PubMed] [Google Scholar]
- Mains RE, Eipper BA, Ling N 1977 Common precursor to corticotropins and endorphins. Proc Natl Acad Sci USA 74:3014–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE 2002 Stress and cocaine addiction. J Pharmacol Exp Ther 301:785–789 [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser M 1981 Coping with stress at work. Int J Health Serv 11:491–510 [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Molina PE, Fan J, Lang CH, Abumrad NN 1996 Central opiate modulation of growth hormone and insulin-like growth factor-I. Brain Res Bull 40:99–104 [DOI] [PubMed] [Google Scholar]
- Kato Y, Iwasaki Y, Abe H, Ohgo S, Imura H 1978 Effects of endorphins on prolactin and growth hormone secretion in rats. Proc Soc Exp Biol Med 158:431–436 [DOI] [PubMed] [Google Scholar]
- Katakami H, Kato Y, Matsushita N, Hiroto S, Shimatsu A, Imura H 1981 Involvement of α-adrenergic mechanisms in growth hormone release induced by opioid peptides in conscious rats. Neuroendocrinology 33:129–135 [DOI] [PubMed] [Google Scholar]
- Bruhn TO, Tresco PA, Mueller GP, Jackson IM 1989 β-Endorphin mediates clonidine stimulated growth hormone release. Neuroendocrinology 50:460–463 [DOI] [PubMed] [Google Scholar]
- Briski KP, Quigley K, Meites J 1984 Counteraction by morphine of stress-induced inhibition of growth hormone release in the rat. Proc Soc Exp Biol Med 177:137–142 [DOI] [PubMed] [Google Scholar]
- Bartolome MB, Kuhn CM 1983 Endocrine effects of methadone in rats; acute effects in adults. Eur J Pharmacol 95:231–238 [DOI] [PubMed] [Google Scholar]
- Ceger P, Kuhn CM 2000 Opiate withdrawal in the neonatal rat: relationship to duration of treatment and naloxone dose. Psychopharmacology (Berl) 150:253–259 [DOI] [PubMed] [Google Scholar]
- Singh M, Simpkins JW, Layden MP, Romano TM, Millard WJ 1992 Opiate modulation of growth hormone secretion is compromised during the steroid-induced luteinizing hormone surge. Neuroendocrinology 55:214–220 [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Millard WJ, Berglund LA 1993 Effects of chronic stimulation or antagonism of opiate receptors on GH secretion in male and female rats. Life Sci 52:1443–1450 [DOI] [PubMed] [Google Scholar]
- Armstrong JD, Spears JW 1991 Changes in growth hormone and luteinizing hormone following acute or chronic administration of an opioid agonist, FK33–824, in wethers. J Anim Sci 69:774–781 [DOI] [PubMed] [Google Scholar]
- Johnson DW, Barnes MA, Akers RM, Pearson RE 1993 A synthetic opioid peptide increases plasma growth hormone and prolactin in Holstein calves. J Anim Sci 71:1004–1009 [DOI] [PubMed] [Google Scholar]
- Rushen J, Schwarze N, Ladewig J, Foxcroft G 1993 Opioid modulation of the effects of repeated stress on ACTH, cortisol, prolactin, and growth hormone in pigs. Physiol Behav 53:923–928 [DOI] [PubMed] [Google Scholar]
- Edens FW, Parkhurst CR 1994 Plasma growth hormone and prolactin response to FK 33-824, a synthetic opioid agonist, in broiler chickens. Poult Sci 73:1746–1754 [DOI] [PubMed] [Google Scholar]
- Scanes CG 1989 Influence of β-agonist on plasma concentrations of growth hormone in broiler chickens on a low plane of nutrition. Poult Sci 68:1015–1018 [DOI] [PubMed] [Google Scholar]
- Willoughby JO, Medvedev A 1996 Opioid receptor activation resets the hypothalamic clock generating growth hormone secretory bursts in the rat. J Endocrinol 148:149–155 [DOI] [PubMed] [Google Scholar]
- Janik J, Klosterman S, Parman R, Callahan P 1994 Multiple opiate receptor subtypes are involved in the stimulation of growth hormone release by β-endorphin in female rats. Neuroendocrinology 60:69–75 [DOI] [PubMed] [Google Scholar]
- Eason MG, Francis RS, Kuhn CM 1996 μ-Opioid agonists stimulate growth hormone secretion in immature rats. Neuroendocrinology 63:489–497 [DOI] [PubMed] [Google Scholar]
- Wehrenberg WB, Bloch B, Ling N 1985 Pituitary secretion of growth hormone in response to opioid peptides and opiates is mediated through growth hormone-releasing factor. Neuroendocrinology 41:13–16 [DOI] [PubMed] [Google Scholar]
- Borer KT, Nicoski DR, Owens V 1986 Alteration of pulsatile growth hormone secretion by growth-inducing exercise: involvement of endogenous opiates and somatostatin. Endocrinology 118:844–850 [DOI] [PubMed] [Google Scholar]
- Dobado-Berrios PM, Li S, Garcia de Yebenes E, Pelletier G 1993 Effects of morphine and naloxone on prolactin and growth hormone gene expression in the male rat pituitary gland. J Neuroendocrinol 5:553–556 [DOI] [PubMed] [Google Scholar]
- Thörnwall-Le Grevès M, Zhou Q, Lagerholm S, Huang W, Le Grevès P, Nyberg F 2001 Morphine decreases the levels of the gene transcripts of growth hormone receptor and growth hormone binding protein in the male rat hippocampus and spinal cord. Neurosci Lett 304:69–72 [DOI] [PubMed] [Google Scholar]
- Bhansali A, Velayutham P, Sialy R, Sethi B 2005 Effect of opiates on growth hormone secretion in acromegaly. Horm Metab Res 37:425–427 [DOI] [PubMed] [Google Scholar]
- Tomasi PA, Fanciulli G, Palermo M, Pala A, Demontis MA, Delitala G 1998 Opioid-receptor blockade blunts growth hormone (GH) secretion induced by GH-releasing hormone in the human male. Horm Metab Res 30:34–36 [DOI] [PubMed] [Google Scholar]
- Barbarino A, De Marinis L, Mancini A, D'Amico C, Passeri M, Zuppi P, Sambo P, Tofani A 1987 Sex-related naloxone influence on growth hormone-releasing hormone-induced growth hormone secretion in normal subjects. Metabolism 36:105–109 [DOI] [PubMed] [Google Scholar]
- Abs R, Verhelst J, Maeyaert J, Van Buyten JP, Opsomer F, Adriaensen H, Verlooy J, Van Havenbergh T, Smet M, Van Acker K 2000 Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab 85:2215–2222 [DOI] [PubMed] [Google Scholar]
- De Marinis L, Mancini A, Valle D, Bianchi A, De Luca AM, Fulghesu AM, Villa P, Mancuso S, Lanzone A 1997 Influence of chronic Naltrexone treatment on growth hormone and insulin secretion in obese subjects. Int J Obes Relat Metab Disord 21:1076–1081 [DOI] [PubMed] [Google Scholar]
- Henrohn D, Le Grevés P, Nyberg F 1997 Morphine alters the levels of growth hormone receptor mRNA and [125I]growth hormone binding in human IM-9 lymphoblasts via a naloxone-reversible mechanism. Mol Cell Endocrinol 135:147–152 [DOI] [PubMed] [Google Scholar]
- Villa P, Valle D, De Marinis L, Mancini A, Bianchi A, Fulghesu AM, Caruso A, Mancuso S, Lanzone A 1997 Influence of chronic naltrexone treatment on growth hormone secretion in normal subjects. Eur J Endocrinol 137:631–634 [DOI] [PubMed] [Google Scholar]
- Villa P, Valle D, Mancini A, De Marinis L, Pavone V, Fulghesu AM, Mancuso S, Lanzone A 1999 Effect of opioid blockade on insulin and growth hormone (GH) secretion in patients with polycystic ovary syndrome: the heterogeneity of impaired GH secretion is related to both obesity and hyperinsulinism. Fertil Steril 71:115–121 [DOI] [PubMed] [Google Scholar]
- Dobson PR, Brown BL 1988 Involvement of the hypothalamus in opiate-stimulated prolactin secretion. Regul Pept 20:305–310 [DOI] [PubMed] [Google Scholar]
- Bentley AM, Wallis M 1986 Effects of two enkephalin analogues, morphine sulphate, dopamine and naloxone on prolactin secretion from rat anterior pituitary glands in vitro. J Endocrinol 109:313–320 [DOI] [PubMed] [Google Scholar]
- Login IS, Macleod RM 1979 Failure of opiates to reverse dopamine inhibition of prolactin secretion in vitro. Eur J Pharmacol 60:253–255 [DOI] [PubMed] [Google Scholar]
- Panerai AE, Casanueva F, Martini A, Mantegazza P, Di Giulio AM 1981 Opiates act centrally on GH and PRL release. Endocrinology 108:2400–2402 [DOI] [PubMed] [Google Scholar]
- Brown B, Dettmar PW, Dobson PR, Lynn AG, Metcalf G, Morgan BA 1978 Opiate analgesics: the effect of agonist-antagonist character on prolactin secretion. J Pharm Pharmacol 30:644–645 [DOI] [PubMed] [Google Scholar]
- Leshin LS, Rund LA, Thompson FN, Mahaffey MB, Chang WJ, Byerley DJ, Kiser TE 1990 Serum prolactin and growth hormone responses to naloxone and intracerebral ventricle morphine administration in heifers. J Anim Sci 68:1656–1665 [DOI] [PubMed] [Google Scholar]
- Bedran de Castro JC, Khorram O, Petrovic SL, McCann S 1987 Role of opioid peptides in pulsatile release of gonadotropins and prolactin in the rat. Brain Res Bull 19:539–544 [DOI] [PubMed] [Google Scholar]
- Bero LA, Kuhn CM 1987 Differential ontogeny of opioid, dopaminergic and serotonergic regulation of prolactin secretion. J Pharmacol Exp Ther 240:825–830 [PubMed] [Google Scholar]
- Wehrenberg WB, McNicol D, Wardlaw SL, Frantz AG, Ferin M 1981 Dopaminergic and serotonergic involvement in opiate-induced prolactin release in monkeys. Endocrinology 109:544–547 [DOI] [PubMed] [Google Scholar]
- Flores CM, Hulihan-Giblin BA, Hornby PJ, Lumpkin MD, Kellar KJ 1992 Partial characterization of a neurotransmitter pathway regulating the in vivo release of prolactin. Neuroendocrinology 55:519–528 [DOI] [PubMed] [Google Scholar]
- Kehoe L, Parman R, Janik J, Callahan P 1993 Opiate receptor subtype involvement in the stimulation of prolactin release by β-endorphin in female rats. Neuroendocrinology 57:875–883 [DOI] [PubMed] [Google Scholar]
- Panerai AE, Petraglia F, Sacerdote P, Genazzani AR 1985 Mainly μ-opiate receptors are involved in luteinizing hormone and prolactin secretion. Endocrinology 117:1096–1099 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Vale W, Rivier C 1987 β-Endorphin and dynorphin participate in the stress-induced release of prolactin in the rat. Neuroendocrinology 45:338–342 [DOI] [PubMed] [Google Scholar]
- Barb CR, Kraeling RR, Rampacek GB 1991 Opioid modulation of gonadotropin and prolactin secretion in domestic farm animals. Domest Anim Endocrinol 8:15–27 [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Arbogast LA, Hyde JF 1989 Neuroendocrine [corrected] regulation of prolactin release. Prog Neurobiol 33:399–447 [DOI] [PubMed] [Google Scholar]
- Yang SP, Lee Y, Voogt JL 2000 Involvement of endogenous opioidergic neurons in modulation of prolactin secretion in response to mating in the female rat. Neuroendocrinology 72:20–28 [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Widmann AA, Bethea CL 1996 β-Endorphin, but not oxytocin, substance P or vasoactive-intestinal polypeptide, contributes to progesterone-induced prolactin secretion in monkeys. Neuroendocrinology 63:569–578 [DOI] [PubMed] [Google Scholar]
- Callahan P, Janik J, Grandison L, Rabii J 1988 Morphine does not stimulate prolactin release during lactation. Brain Res 442:214–222 [DOI] [PubMed] [Google Scholar]
- Byrnes EM 2005 Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav 80:445–451 [DOI] [PubMed] [Google Scholar]
- Delitala G, Grossman A, Besser GM 1983 The participation of hypothalamic dopamine in morphine-induced prolactin release in man. Clin Endocrinol (Oxf) 19:437–444 [DOI] [PubMed] [Google Scholar]
- Devilla L, Pende A, Morgano A, Giusti M, Musso NR, Lotti G 1985 Morphine-induced TSH release in normal and hypothyroid subjects. Neuroendocrinology 40:303–308 [DOI] [PubMed] [Google Scholar]
- Lo Dico G, Riggio V, Cossu A, Buscarinu G, Delitala G, Stoppelli I 1983 Role of opiates in the physiological control of prolactin in man. Acta Eur Fertil 14:409–414 [PubMed] [Google Scholar]
- Hemmings R, Fox G, Tolis G 1982 Effect of morphine on the hypothalamic-pituitary axis in postmenopausal women. Fertil Steril 37:389–391 [DOI] [PubMed] [Google Scholar]
- Fraser LA, Morrison D, Morley-Forster P, Paul TL, Tokmakejian S, Larry Nicholson R, Bureau Y, Friedman TC, Van Uum SH 2009 Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp Clin Endocrinol Diabetes 117:38–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi PP, Pezzarossa A, Ceresini G, Rastelli G, Valenti G, Gerra G 1985 Effects of dopamine receptor stimulation on opiate-induced modifications of pituitary-gonadal function. Horm Res 21:155–159 [DOI] [PubMed] [Google Scholar]
- Moshtaghi-Kashanian GR, Esmaeeli F, Dabiri S 2005 Enhanced prolactin levels in opium smokers. Addict Biol 10:345–349 [DOI] [PubMed] [Google Scholar]
- Rampinini A, Iannotta F, Rizzuto G, Colombo F, Giuliani F, Parabiaghi R 1989 Effect of naloxone on TRH-induced PRL and TSH response in normal man. Minerva Endocrinol 14:125–128 [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Teoh SK, Lloyd-Jones JG, Clifford JM 1989 Naloxone suppresses buprenorphine stimulation of plasma prolactin. J Clin Psychopharmacol 9:105–109 [DOI] [PubMed] [Google Scholar]
- Grossman A, Stubbs WA, Gaillard RC, Delitala G, Rees LH, Besser GM 1981 Studies off the opiate control of prolactin, GH and TSH. Clin Endocrinol (Oxf) 14:381–386 [DOI] [PubMed] [Google Scholar]
- Morley JE, Baranetsky NG, Wingert TD, Carlson HE, Hershman JM, Melmed S, Levin SR, Jamison KR, Weitzman R, Chang RJ, Varner AA 1980 Endocrine effects of naloxone-induced opiate receptor blockade. J Clin Endocrinol Metab 50:251–257 [DOI] [PubMed] [Google Scholar]
- Cetel NS, Quigley ME, Yen SS 1985 Naloxone-induced prolactin secretion in women: evidence against a direct prolactin stimulatory effect of endogenous opioids. J Clin Endocrinol Metab 60:191–196 [DOI] [PubMed] [Google Scholar]
- Brzyski RG, Viniegra A, Archer DF 1997 Suppression of luteal phase, but not midcycle, prolactin levels by chronic follicular phase opiate antagonism. Fertil Steril 68:855–859 [DOI] [PubMed] [Google Scholar]
- Steardo L, Monteleone P, Tamminga CA, Canonico PL, Denman D, Scapagnini U, Chase TN 1985 Differential responses in prolactin levels induced by naloxone in humans. Psychoneuroendocrinology 10:203–209 [DOI] [PubMed] [Google Scholar]
- Gindoff PR, Jewelewicz R, Hembree W, Wardlaw S, Ferin M 1988 Sustained effects of opioid antagonism during the normal human luteal phase. J Clin Endocrinol Metab 66:1000–1004 [DOI] [PubMed] [Google Scholar]
- del Valle-Soto ME, Iglesias L, Calzada B, Vega JA, Hernandez LC, Pérez-Casas A 1991 Effects of morphine on the pituitary-thyroid axis: morphological and analytical studies. Funct Dev Morphol 1:3–6 [PubMed] [Google Scholar]
- Männistö PT, Rauhala P, Tuominen R, Mattila J 1984 Dual action of morphine on cold-stimulated thyrotropin secretion in male rats. Life Sci 35:1101–1107 [DOI] [PubMed] [Google Scholar]
- Langer P, Jezová D, Földes O, Gschwendtová K 1987 Dual dose-related action of peripherally administered morphine on cold-stimulated thyrotropin secretion in male rats. Horm Res 27:95–101 [DOI] [PubMed] [Google Scholar]
- Jaworska-Feil L, Budziszewska B, Lasoñ W 1995 The effects of single and repeated morphine administration on the level of thyrotropin-releasing hormone and its receptors in the rat brain. Neuropeptides 29:343–349 [DOI] [PubMed] [Google Scholar]
- Espinosa VP, Liu Y, Ferrini M, Anghel A, Nie Y, Tripathi PV, Porche R, Jansen E, Stuart RC, Nillni EA, Lutfy K, Friedman TC 2008 Differential regulation of prohormone convertase 1/3, prohormone convertase 2 and phosphorylated cyclic-AMP-response element binding protein by short-term and long-term morphine treatment: implications for understanding the “switch” to opiate addiction. Neuroscience 156:788–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D, Veisseire M, Borson-Chazot F, Mornex R 1986 In vitro effects of endogenous opiate peptides on thyrotropin function: inhibition of thyrotropin-releasing hormone release and absence of effect on thyrotropin release. Neurosci Lett 67:289–294 [DOI] [PubMed] [Google Scholar]
- Buydens P, Velkeniers B, Golstein J, Finné E, Vanhaelst L 1988 The effect of β-endorphin on basal and TRH-stimulated TSH release in conscious male rats. Horm Metab Res 20:687–690 [DOI] [PubMed] [Google Scholar]
- Mitsuma T, Hirooka Y, Nogimori T 1993 Effects of immunoneutralization of endogenous opioid peptides on the hypothalamic-pituitary-thyroid axis in rats. Horm Res 39:77–80 [DOI] [PubMed] [Google Scholar]
- Bruni JF, Van Vugt D, Marshall S, Meites J 1977 Effects of naloxone, morphine and methionine enkephalin on serum prolactin, luteinizing hormone, follicle stimulating hormone, thyroid stimulating hormone and growth hormone. Life Sci 21:461–466 [DOI] [PubMed] [Google Scholar]
- Morley JE 1981 The endocrinology of the opiates and opioid peptides. Metabolism 30:195–209 [DOI] [PubMed] [Google Scholar]
- Briski K, Quigley K, Meites J 1984 Counteraction by naltrexone of stress-induced inhibition of TSH release: role of noradrenergic system. Proc Soc Exp Biol Med 177:354–359 [DOI] [PubMed] [Google Scholar]
- Cacicedo L, Sánchez Franco F 1985 Role of naloxone and opioid peptides on thyrotrophin, α subunit and β thyrotrophin by dispersed rat pituitary cells. Acta Endocrinol (Copenh) 110:101–106 [DOI] [PubMed] [Google Scholar]
- Edmondson EA, Bonnet KA, Friedhoff AJ 1990 The effect of hyperthyroidism on opiate receptor binding and pain sensitivity. Life Sci 47:2283–2289 [DOI] [PubMed] [Google Scholar]
- Lin LS, Chiu WT, Shih CJ, Lin MT 1987 Involvement of both opiate and catecholaminergic receptors of ventromedial hypothalamus in the locomotor stimulant action of thyrotropin-releasing hormone. J Neural Transm 68:217–225 [DOI] [PubMed] [Google Scholar]
- Rauhala P, Tuominen RK, Männistö PT 1988 Opiate receptor subtypes in the regulation of thyrotropin and prolactin secretion in the rat. Horm Res 29:218–222 [DOI] [PubMed] [Google Scholar]
- Berglund LA, Millard WJ, Gabriel SM, Simpkins JW 1990 Opiate-thyroid hormone interactions in the regulation of thyrotropin secretion in the rat. Neuroendocrinology 52:303–308 [DOI] [PubMed] [Google Scholar]
- Delitala G, Grossman A, Besser M 1983 Differential effects of opiate peptides and alkaloids on anterior pituitary hormone secretion. Neuroendocrinology 37:275–279 [DOI] [PubMed] [Google Scholar]
- Grossman A 1983 Brain opiates and neuroendocrine function. Clin Endocrinol Metab 12:725–746 [DOI] [PubMed] [Google Scholar]
- Pende A, Musso NR, Montaldi ML, Pastorino G, Arzese M, Devilla L 1986 Evaluation of the effects induced by four opiate drugs, with different affinities to opioid receptor subtypes, on anterior pituitary LH, TSH, PRL and GH secretion and on cortisol secretion in normal men. Biomed Pharmacother 40:178–182 [PubMed] [Google Scholar]
- Roti E, Degli Uberti E, Salvadori S, Bianconi M, Emanuele R, Rotola C, Trasforini G, Robuschi G, Tomatis R, Gnudi A, Pansini R, Braverman LE 1984 Dermorphin, a new opioid peptide, stimulates thyrotropin secretion in normal subjects. J Endocrinol Invest 7:211–214 [DOI] [PubMed] [Google Scholar]
- Ogrin C, Schussler GC 2005 Suppression of thyrotropin by morphine in a severely stressed patient. Endocr J 52:265–269 [DOI] [PubMed] [Google Scholar]
- Roth KA, Lorenz RG, McKeel DW, Leykam J, Barchas JD, Tyler AN 1988 Methionine-enkephalin and thyrotropin-stimulating hormone are intimately related in the human anterior pituitary. J Clin Endocrinol Metab 66:804–810 [DOI] [PubMed] [Google Scholar]
- Pittius CW, Kley N, Loeffler JP, Höllt V 1985 Quantitation of proenkephalin A messenger RNA in bovine brain, pituitary and adrenal medulla: correlation between mRNA and peptide levels. EMBO J 4:1257–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels MH, Kramer P, Wilson D, Sexton G 1994 Effects of naloxone infusions on pulsatile thyrotropin secretion. J Clin Endocrinol Metab 78:1249–1252 [DOI] [PubMed] [Google Scholar]
- Leslie RD, Prescott RW, Kendall-Taylor P, Cook D, Weightman D, Ratcliffe J, Ingram MC 1985 Opiate receptor blockade and diurnal pituitary and adrenal hormone levels. Horm Metab Res 17:86–89 [DOI] [PubMed] [Google Scholar]
- Grossman A, West S, Williams J, Evans J, Rees LH, Besser GM 1982 The role of opiate peptides in the control of prolactin in the puerperium, and TSH in primary hypothyroidism. Clin Endocrinol (Oxf) 16:317–320 [DOI] [PubMed] [Google Scholar]
- Jezová D, Vigas M, Jurcovicová J 1982 ACTH and corticosterone response to naloxone and morphine in normal, hypophysectomized and dexamethasone-treated rats. Life Sci 31:307–314 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA 1984 Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology 38:411–417 [DOI] [PubMed] [Google Scholar]
- el Daly ES 1996 Influence of acute and chronic morphine or stadol on the secretion of adrenocorticotrophin and its hypothalamic releasing hormone in the rat. Life Sci 59:1881–1890 [DOI] [PubMed] [Google Scholar]
- Houshyar H, Cooper ZD, Woods JH 2001 Paradoxical effects of chronic morphine treatment on the temperature and pituitary-adrenal responses to acute restraint stress: a chronic stress paradigm. J Neuroendocrinol 13:862–874 [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM 1995 Ontogenetic studies of tolerance development: effects of chronic morphine on the hypothalamic-pituitary-adrenal axis. Psychopharmacology (Berl) 122:78–84 [DOI] [PubMed] [Google Scholar]
- Hendrie CA 1988 ACTH: a single pretreatment enhances the analgesic efficacy of and prevents the development of tolerance to morphine. Physiol Behav 42:41–45 [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Gutstein HB, Akil H 1993 Effects of chronic morphine treatment on β-endorphin-related peptides in the caudal medulla and spinal cord. J Neurochem 60:2304–2307 [DOI] [PubMed] [Google Scholar]
- Gudehithlu KP, Tejwani GA, Bhargava HN 1991 β-Endorphin and methionine-encephalin levels in discrete brain regions, spinal cord, pituitary gland and plasma of morphine tolerant-dependent and abstinent rats. Brain Res 553: 284–290 [DOI] [PubMed] [Google Scholar]
- Przewłocki R, Höllt V, Duka T, Kleber G, Gramsch C, Haarmann I, Herz A 1979 Long-term morphine treatment decreases endorphin levels in rat brain and pituitary. Brain Res 174:357–361 [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Day NC, Gutstein HB, Trujillo KA, Akil H 1993 Pre- and posttranslational regulation of β-endorphin biosynthesis in the CNS: effects of chronic naltrexone treatment. J Neurochem 60:40–49 [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Drouin JN, Seidah NG, Kalant H, Chrétien M 1981 Effect of chronic morphine treatment on β-endorphin biosynthesis by the rat neurointermediate lobe. Eur J Pharmacol 72:313–321 [DOI] [PubMed] [Google Scholar]
- Hollt V, Haarmann I, Przewlocki R, Jerlicz M 1980 Long-term treatment of rats with morphine decreases in vitro biosynthesis in and release of β-endorphin from intermediate/posterior lobes of pituitary. In: Costa E, Trabucchi M, eds. Neural peptides and neuronal communication. New York: Raven Press; 399–407 [Google Scholar]
- Höllt V, Haarmann I, Herz A 1981 Long-term treatment of rats with morphine reduces the activity of messenger ribonucleic acid coding for the β-endorphin/ACTH precursor in the intermediate pituitary. J Neurochem 37:619–626 [DOI] [PubMed] [Google Scholar]
- Heybach JP, Vernikos J 1981 Naloxone inhibits and morphine potentiates the adrenal steroidogenic response to ACTH. Eur J Pharmacol 75:1–6 [DOI] [PubMed] [Google Scholar]
- Wang XM, Tresham JJ, Scoggins BA, Coghlan JP 1988 Met-enkephalin and the enkephalin analogue FK-33824 centrally inhibit adrenocorticotrophic hormone secretion in sheep. Clin Exp Pharmacol Physiol 15:865–873 [DOI] [PubMed] [Google Scholar]
- De Souza EB, Van Loon GR 1982 D-Ala2-Met-enkephalinamide, a potent opioid peptide, alters pituitary-adrenocortical secretion in rats. Endocrinology 111:1483– 1490 [DOI] [PubMed] [Google Scholar]
- Guaza C, Borrell J 1984 The Met-enkephalin analog D-Ala2-Met-enkephalinamide decreases the adrenocortical response to ACTH in dispersed rat adrenal cells. Peptides 5:895–897 [DOI] [PubMed] [Google Scholar]
- Mazzocchi G, Robba C, Rebuffat P, Gottardo G, Nussdorfer GG 1986 Investigations on the effects of long-term administration of a methionine-enkephalin analogue on the adrenal zona fasciculata of rats treated with dexamethasone or dexamethasone and ACTH. J Steroid Biochem 25:535–540 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Suemaru S, Hattori T, Takao T, Inoue H, Sugawara M, Kageyama J, Ota Z 1986 Effects of (D-ala2, met5)-enkephalinamide and naloxone on ACTH and corticosterone secretion. Endocrinol Jpn 33:813–820 [DOI] [PubMed] [Google Scholar]
- Steiner DF 1998 The proprotein convertases. Curr Opin Chem Biol 2:31–39 [DOI] [PubMed] [Google Scholar]
- Jansen E, Ayoubi TA, Meulemans SM, Van de Ven WJ 1995 Neuroendocrine-specific expression of the human prohormone convertase 1 gene. Hormonal regulation of transcription through distinct cAMP response elements. J Biol Chem 270:15391–15397 [DOI] [PubMed] [Google Scholar]
- Allolio B, Schulte HM, Deuss U, Kallabis D, Hamel E, Winkelman W 1987 Effect of oral morphine and naloxone on pituitary-adrenal response in man induced by human corticotropin-releasing hormone. Acta Endocrinol (Copenh) 114:509–514 [DOI] [PubMed] [Google Scholar]
- Baranowska B, Dorobek W, Misiorowski W, Jeske W, Abdel-Fattah MH 1985 The effect of naloxone on ATCH and β-endorphin in patients with Cushing’s disease. Acta Endocrinol (Copenh) 110:170–175 [DOI] [PubMed] [Google Scholar]
- Naber D, Pickar D, Davis GC, Cohen RM, Jimerson DC, Elchisak MA, Defraites EG, Kalin NH, Risch SC, Buchsbaum MS 1981 Naloxone effects on β-endorphin, cortisol, prolactin, growth hormone, HVA and MHPG in plasma of normal volunteers. Psychopharmacology (Berl) 74:125–128 [DOI] [PubMed] [Google Scholar]
- Geer EB, Landman RE, Wardlaw SL, Conwell IM, Freda PU 2005 Stimulation of the hypothalamic-pituitary-adrenal axis with the opioid antagonist nalmefene. Pituitary 8:115–122 [DOI] [PubMed] [Google Scholar]
- Palm S, Moenig H, Maier C 1997 Effects of oral treatment with sustained release morphine tablets on hypothalamic-pituitary-adrenal axis. Methods Find Exp Clin Pharmacol 19:269–273 [PubMed] [Google Scholar]
- Müssig K, Knaus-Dittmann D, Schmidt H, Mörike K, Häring HU 2007 Secondary adrenal failure and secondary amenorrhoea following hydromorphone treatment. Clin Endocrinol (Oxf) 66:604–605 [DOI] [PubMed] [Google Scholar]
- Oltmanns KM, Fehm HL, Peters A 2005 Chronic fentanyl application induces adrenocortical insufficiency. J Intern Med 257:478–480 [DOI] [PubMed] [Google Scholar]
- Pullan PT, Watson FE, Seow SS, Rappeport W 1983 Methadone-induced hypoadrenalism. Lancet 1:714 [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Ho A, Schluger JH, Perret G, Borg L, Le Moal M, Piazza PV, Kreek MJ 2006 Glucocorticoid negative feedback in methadone-maintained former heroin addicts with ongoing cocaine dependence: dose-response to dexamethasone suppression. Addict Biol 11:84–96 [DOI] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ 2003 Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology 28:985–994 [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Grasso A, Petraglia F, Parrini D, Volpe A, Genazzani AR 1984 Impaired circadian rhythmicity of β-lipotrophin, β-endorphin and ACTH in heroin addicts. Acta Endocrinol (Copenh) 105:149–155 [DOI] [PubMed] [Google Scholar]
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G 2008 Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress 11:483–488 [DOI] [PubMed] [Google Scholar]
- Conaglen JV, Donald RA, Espiner EA, Livesey JH, Nicholls MG 1985 Effect of naloxone on the hormone response to CRF in normal man. Endocr Res 11:39–44 [DOI] [PubMed] [Google Scholar]
- Rittmaster RS, Cutler Jr GB, Sobel DO, Goldstein DS, Koppelman MC, Loriaux DL, Chrousos GP 1985 Morphine inhibits the pituitary-adrenal response to ovine corticotropin-releasing hormone in normal subjects. J Clin Endocrinol Metab 60:891–895 [DOI] [PubMed] [Google Scholar]
- Grossman A, Moult PJ, Cunnah D, Besser M 1986 Different opioid mechanisms are involved in the modulation of ACTH and gonadotrophin release in man. Neuroendocrinology 42:357–360 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Knepel W, Braun S, Meyer HD, Lohmann H, Brantl V 1986 Effects of a κ-opioid agonist on adrenocorticotropic and diuretic function in man. Horm Metab Res 18:842–848 [DOI] [PubMed] [Google Scholar]
- Allolio B, Winkelmann W, Hipp FX, Kaulen D, Mies R 1982 Effects of a met-enkephalin analog on adrenocorticotropin (ACTH), growth hormone, and prolactin in patients with ACTH hypersecretion. J Clin Endocrinol Metab 55:1–7 [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Stalla GK, Lange M, Pfeiffer A, Müller OA 1992 Effects of loperamide on the human hypothalamo-pituitary-adrenal axis in vivo and in vitro. J Clin Endocrinol Metab 75:552–557 [DOI] [PubMed] [Google Scholar]
- Ambrosi B, Bochicchio D, Colombo P, Fadin C, Faglia G 1993 Loperamide to diagnose Cushing’s syndrome. JAMA 270:2301–2302 [PubMed] [Google Scholar]
- Deuss U, Allolio B, Kaulen D, Fischer H, Winkelmann W 1985 Effects of high-dose and low-dose naloxone on plasma ACTH in patients with ACTH hypersecretion. Clin Endocrinol (Oxf) 22:273–279 [DOI] [PubMed] [Google Scholar]
- Allolio B, Deuss U, Kaulen D, Leonhardt U, Kallabis D, Hamel E, Winkelmann W 1986 FK 33-824, a met-enkephalin analog, blocks corticotropin-releasing hormone-induced adrenocorticotropin secretion in normal subjects but not in patients with Cushing’s disease. J Clin Endocrinol Metab 63:1427–1431 [DOI] [PubMed] [Google Scholar]
- Ambrosi B, Bochicchio D, Faglia G 1986 Loperamide, an opiate analogue, inhibits plasma ACTH levels in patients with Addison’s disease. Clin Endocrinol (Oxf) 24:483– 489 [DOI] [PubMed] [Google Scholar]
- Bochicchio D, Ambrosi B, Faglia G 1988 Loperamide, an opiate analog, differently modifies the adrenocorticotropin responses to corticotropin-releasing hormone and lysine vasopressin in patients with Addison’s disease. Neuroendocrinology 48:611–614 [DOI] [PubMed] [Google Scholar]
- Fallo F, Boscaro M, Sonino N, Mantero F 1988 Effects of naloxone on adrenal cortex regulation in patients with primary aldosteronism. J Endocrinol Invest 11:261–265 [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Baby N, Stewart M, Reid RL 1989 The paradoxical stimulatory effect of morphine on LH secretion is dose-dependent and naloxone-reversible. Neuroendocrinology 50:109–116 [DOI] [PubMed] [Google Scholar]
- Takahra J, Kageyama J, Yunoki S, Yakushiji W, Yamauchi J, Kageyama N, Ofuji T 1978 Effects of 2-bromo-α-ergocryptine on β-endorphin-induced growth hormone, prolactin and luteining hormone release in urethane anesthetized rats. Life Sci 22:2205–2207 [DOI] [PubMed] [Google Scholar]
- Wiesner JB, Koenig JI, Krulich L, Moss RL 1984 Site of action for β-endorphin-induced changes in plasma luteinizing hormone and prolactin in the ovariectomized rat. Life Sci 34:1463–1473 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Vale W, Rivier C 1986 β-Endorphin modulates the inhibitory action of corticotropin-releasing factor on luteinizing hormone secretion. NIDA Res Monogr 75: 331–334 [PubMed] [Google Scholar]
- Cacicedo L, Sánchez Franco F 1986 Direct action of opioid peptides and naloxone on gonadotropin secretion by cultured rat anterior pituitary cells. Life Sci 38:617–625 [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Lam NY, Ferin M 1984 Reduced frequency of pulsatile luteinizing hormone secretion in the luteal phase of the rhesus monkey. Involvement of endogenous opiates. Endocrinology 115:1095–1101 [DOI] [PubMed] [Google Scholar]
- Gilbeau PM, Almirez RG, Holaday JW, Smith CG 1985 Opioid effects on plasma concentrations of luteinizing hormone and prolactin in the adult male rhesus monkey. J Clin Endocrinol Metab 60:299–305 [DOI] [PubMed] [Google Scholar]
- Yilmaz B, Konar V, Kutlu S, Sandal S, Canpolat S, Gezen MR, Kelestimur H 1999 Influence of chronic morphine exposure on serum LH, FSH, testosterone levels, and body and testicular weights in the developing male rat. Arch Androl 43:189–196 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Holman SD, Berman M, Neff DA, Goy RW 1984 Effects of opiate antagonists on hormones and behavior of male and female rhesus monkeys. Arch Sex Behav 13:1–25 [DOI] [PubMed] [Google Scholar]
- Blank MS, Roberts DL 1982 Antagonist of gonadotropin-releasing hormone blocks naloxone-induced elevations in serum luteinizing hormone. Neuroendocrinology 35:309–312 [DOI] [PubMed] [Google Scholar]
- Horton RJ, Cummins JT, Clarke IJ 1987 Naloxone evokes large-amplitude GnRH pulses in luteal-phase ewes. J Reprod Fertil 81:277–286 [DOI] [PubMed] [Google Scholar]
- Orstead KM, Spies HG 1987 Inhibition of hypothalamic gonadotropin-releasing hormone release by endogenous opioid peptides in the female rabbit. Neuroendocrinology 46:14–23 [DOI] [PubMed] [Google Scholar]
- Mehmanesh H, Almeida OF, Nikolarakis KE, Herz A 1988 Hypothalamic LH-RH release after acute and chronic treatment with morphine studied in a combined in vivo/in vitro model. Brain Res 451:69–76 [DOI] [PubMed] [Google Scholar]
- Li S, Pelletier G 1993 Opioid regulation of gonadotropin-releasing hormone gene expression in the male rat brain as studied by in situ hybridization. Neuroreport 4:331–333 [DOI] [PubMed] [Google Scholar]
- Van Vugt DA, Sylvester PW, Aylsworth CF, Meites J 1982 Counteraction of gonadal steroid inhibition of luteinizing hormone release by naloxone. Neuroendocrinology 34: 274–278 [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Simpkins JW, Kalra SP 1983 Modulation of endogenous opioid influence on luteinizing hormone secretion by progesterone and estrogen. Endocrinology 113:1806–1811 [DOI] [PubMed] [Google Scholar]
- Berglund LA, Derendorf H, Simpkins JW 1988 Desensitization of brain opiate receptor mechanisms by gonadal steroid treatments that stimulate luteinizing hormone secretion. Endocrinology 122:2718–2726 [DOI] [PubMed] [Google Scholar]
- Bhanot R, Wilkinson M 1984 The inhibitory effect of opiates on gonadotrophin secretion is dependent upon gonadal steroids. J Endocrinol 102:133–141 [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Simpkins JW, Kalra SP, Kalra PS 1985 Chronic morphine treatment induces hypersensitivity to testosterone-negative feedback in castrated male rats. Neuroendocrinology 40:39–44 [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Berglund LA, Simpkins JW 1987 Chronic morphine treatment enhances the negative and positive feedback effects of estradiol on gonadotropin secretion in ovariectomized rats. Endocrinology 120:1799–1805 [DOI] [PubMed] [Google Scholar]
- Ferin M, Vande Wiele R 1984 Endogenous opioid peptides and the control of the menstrual cycle. Eur J Obstet Gynecol Reprod Biol 18:365–373 [DOI] [PubMed] [Google Scholar]
- Orstead KM, Hess DL, Spies HG 1987 Opiatergic inhibition of pulsatile luteinizing hormone release during the menstrual cycle of rhesus macaques. Proc Soc Exp Biol Med 184:312–319 [DOI] [PubMed] [Google Scholar]
- Sirinathsinghji DJ, Motta M, Martini L 1985 Induction of precocious puberty in the female rat after chronic naloxone administration during the neonatal period: the opiate ‘brake’ on prepubertal gonadotrophin secretion. J Endocrinol 104:299–307 [DOI] [PubMed] [Google Scholar]
- Bhanot R, Wilkinson M 1983 Opiatergic control of gonadotropin secretion during puberty in the rat: a neurochemical basis for the hypothalamic ‘gonadostat’? Endocrinology 113:596–603 [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Schmoeker PF, Meyer ER, Miller BT, Bell RD, Cytron SM, Brown CC 1986 Ontogeny of the opioid-mediated control of reproductive endocrinology in the male and female rat. J Pharmacol Exp Ther 236:627–633 [PubMed] [Google Scholar]
- Veldhuis JD, Rogol AD, Samojlik E, Ertel NH 1984 Role of endogenous opiates in the expression of negative feedback actions of androgen and estrogen on pulsatile properties of luteinizing hormone secretion in man. J Clin Invest 74:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves GR, Kennedy TG, Weick RF, Casper RF 1993 The effect of nalmefene on pulsatile secretion of luteinizing hormone and prolactin in men. Hum Reprod 8:1598–1603 [DOI] [PubMed] [Google Scholar]
- Ellingboe J, Veldhuis JD, Mendelson JH, Kuehnle JC, Mello NK 1982 Effect of endogenous opioid blockade on the amplitude and frequency of pulsatile luteinizing hormone secretion in normal men. J Clin Endocrinol Metab 54:854–857 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Porro C, Facchinetti F, Cicoli C, Bertellini E, Volpe A, Barbieri GC, Genazzani AR 1986 Opioid control of LH secretion in humans: menstrual cycle, menopause and aging reduce effect of naloxone but not of morphine. Life Sci 38:2103–2110 [DOI] [PubMed] [Google Scholar]
- Rossmanith WG, Monn M, Benz R 1998 Effects of chronic opioid antagonism on gonadotrophin and ovarian sex steroid secretion during the luteal phase. Clin Endocrinol (Oxf) 49:343–351 [DOI] [PubMed] [Google Scholar]
- Wildt L, Leyendecker G 1987 Induction of ovulation by the chronic administration of naltrexone in hypothalamic amenorrhea. J Clin Endocrinol Metab 64:1334–1335 [DOI] [PubMed] [Google Scholar]
- Ahmed MI, Duleba AJ, El Shahat O, Ibrahim ME, Salem A 2008 Naltrexone treatment in clomiphene resistant women with polycystic ovary syndrome. Hum Reprod 23:2564– 2569 [DOI] [PubMed] [Google Scholar]
- Reid RL, Quigley ME, Yen SS 1983 The disappearance of opioidergic regulation of gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab 57:1107–1110 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Degli Uberti EC, Trasforini G, Facchinetti F, Margutti A, Volpe A, Salvadori S, Tomatis R, Genazzani AR 1985 Dermorphin decreases plasma LH levels in human: evidence for a modulatory role of gonadal steroids. Peptides 6:869–872 [DOI] [PubMed] [Google Scholar]
- Fraioli F, Cappa M, Fabbri A, Gnessi L, Moretti C, Borrelli P, Isidori A 1984 Lack of endogenous opioid inhibitory tone on LH secretion in early puberty. Clin Endocrinol (Oxf) 20:299–305 [DOI] [PubMed] [Google Scholar]
- Mauras N, Veldhuis JD, Rogol AD 1986 Role of endogenous opiates in pubertal maturation: opposing actions of naltrexone in prepubertal and late pubertal boys. J Clin Endocrinol Metab 62:1256–1263 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Bernasconi S, Iughetti L, Loche S, Romanini F, Facchinetti F, Marcellini C, Genazzani AR 1986 Naloxone-induced luteinizing hormone secretion in normal, precocious, and delayed puberty. J Clin Endocrinol Metab 63:1112–1116 [DOI] [PubMed] [Google Scholar]
- Kletter GB, Foster CM, Brown MB, Padmanabhan V, Beitins IZ, Marshall JC, Kelch RP 1991 Naloxone does not reverse the suppressive effects of testosterone infusion on luteinizing hormone secretion in pubertal boys. J Clin Endocrinol Metab 73:1241–1247 [DOI] [PubMed] [Google Scholar]
- Kletter GB, Padmanabhan V, Beitins IZ, Marshall JC, Kelch RP, Foster CM 1997 Acute effects of estradiol infusion and naloxone on luteinizing hormone secretion in pubertal boys. J Clin Endocrinol Metab 82:4010–4014 [DOI] [PubMed] [Google Scholar]
- Mauras N, Rogol AD, Veldhuis JD 1987 Appraising the instantaneous secretory rates of luteinizing hormone and testosterone in response to selective μ opiate receptor blockade in late pubertal boys. J Androl 8:203–209 [DOI] [PubMed] [Google Scholar]
- Agmo A, Paredes R 1988 Opioids and sexual behavior in the male rat. Pharmacol Biochem Behav 30:1021–1034 [DOI] [PubMed] [Google Scholar]
- Clark JT, Gabriel SM, Simpkins JW, Kalra SP, Kalra PS 1988 Chronic morphine and testosterone treatment. Effects on sexual behavior and dopamine metabolism in male rats. Neuroendocrinology 48:97–104 [DOI] [PubMed] [Google Scholar]
- Gessa GL, Paglietti E, Quarantotti BP 1979 Induction of copulatory behavior in sexually inactive rats by naloxine. Science 204:203–205 [DOI] [PubMed] [Google Scholar]
- Myers BM, Baum MJ 1979 Facilitation by opiate antagonists of sexual performance in the male rat. Pharmacol Biochem Behav 10:615–618 [DOI] [PubMed] [Google Scholar]
- Pellegrini-Quarantotti B, Paglietti E, Bonanni A, Petta M, Gessa GL 1979 Naloxone shortens ejaculation latency in male rats. Experientia 35:524–525 [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Södersten P 1984 Spinal opiates affect sexual behaviour in rats. Nature 309:257–258 [DOI] [PubMed] [Google Scholar]
- Forsberg G, Eneroth P, Södersten P 1987 Naloxone stimulates sexual behaviour in lactating rats. J Endocrinol 113:423–427 [DOI] [PubMed] [Google Scholar]
- Olster DH 1994 Opiate receptor blockade enhances the display of progesterone-facilitated lordosis in juvenile female guinea pigs. Horm Behav 28:84–95 [DOI] [PubMed] [Google Scholar]
- van Furth WR, van Emst MG, van Ree JM 1995 Opioids and sexual behavior of male rats: involvement of the medial preoptic area. Behav Neurosci 109:123–134 [DOI] [PubMed] [Google Scholar]
- Band LC, Hull EM 1990 Morphine and dynorphin (1-13) microinjected into the medial preoptic area and nucleus accumbens: effects on sexual behavior in male rats. Brain Res 524:77–84 [DOI] [PubMed] [Google Scholar]
- Gerendai I 1991 Modulation of testicular functions by testicular opioid peptides. J Physiol Pharmacol 42:427–437 [PubMed] [Google Scholar]
- Vathy I, van der Plas J, Vincent PA, Etgen AM 1991 Intracranial dialysis and microinfusion studies suggest that morphine may act in the ventromedial hypothalamus to inhibit female rat sexual behavior. Horm Behav 25:354–366 [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Gorzalka BB 1987 Selective activation of opioid receptors differentially affects lordosis behavior in female rats. Peptides 8:309–317 [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Haq S, Shah BH 1997 Perinatal exposure to morphine disrupts brain norepinephrine, ovarian cyclicity, and sexual receptivity in rats. Pharmacol Biochem Behav 58:243–248 [DOI] [PubMed] [Google Scholar]
- Dutriez-Casteloot I, Bernet F, Dedieu JF, Croix D, Laborie C, Montel V, Lesage J, Beauvillain JC, Dupouy JP 1999 Hypothalamic-pituitary-adrenocortical and gonadal axes and sympathoadrenal activity of adult male rats prenatally exposed to morphine. Neurosci Lett 263:1–4 [DOI] [PubMed] [Google Scholar]
- Vathy IU, Etgen AM, Barfield RJ 1985 Effects of prenatal exposure to morphine on the development of sexual behavior in rats. Pharmacol Biochem Behav 22:227–232 [DOI] [PubMed] [Google Scholar]
- Gagin R, Cohen E, Shavit Y 1997 Prenatal exposure to morphine feminizes male sexual behavior in the adult rat. Pharmacol Biochem Behav 58:345–348 [DOI] [PubMed] [Google Scholar]
- Vathy I, Rimanóczy A, Slamberová R 2000 Prenatal exposure to morphine differentially alters gonadal hormone regulation of δ-opioid receptor binding in male and female rats. Brain Res Bull 53:793–800 [DOI] [PubMed] [Google Scholar]
- Ferrer J, Mtnez-Guisasola J, Díaz F, Alonso F, Guerrero M, Marín B 1997 Plasma levels of β-endorphin during the menstrual cycle. Gynecol Endocrinol 11:75–82 [DOI] [PubMed] [Google Scholar]
- Chuong CJ, Hsi BP, Gibbons WE 1994 Periovulatory β-endorphin levels in premenstrual syndrome. Obstet Gynecol 83:755–760 [PubMed] [Google Scholar]
- Chuong CJ, Coulam CB, Bergstralh EJ, O'Fallon WM, Steinmetz GI 1988 Clinical trial of naltrexone in premenstrual syndrome. Obstet Gynecol 72:332–336 [PubMed] [Google Scholar]
- Martinez-Guisasola J, Ferrer J, Guerrero M, Díaz F, Alonso F, Bodega A, Cordero J, Alonso-Briz E 1999 Circulating levels of immunoreactive β-endorphin in polycystic ovary syndrome. Gynecol Endocrinol 13:26–35 [DOI] [PubMed] [Google Scholar]
- Martínez-Guisasola J, Guerrero M, Alonso F, Díaz F, Cordero J, Ferrer J 2001 Plasma β-endorphin levels in obese and non-obese patients with polycystic ovary disease. Gynecol Endocrinol 15:14–22 [PubMed] [Google Scholar]
- Daniell HW 2008 Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain 9:28–36 [DOI] [PubMed] [Google Scholar]
- Smith DE, Moser C, Wesson DR, Apter M, Buxton ME, Davison JV, Orgel M, Buffum J 1982 A clinical guide to the diagnosis and treatment of heroin-related sexual dysfunction. J Psychoactive Drugs 14:91–99 [DOI] [PubMed] [Google Scholar]
- Genazzani AD, Petraglia F, Gastaldi M, Volpogni C, Gamba O, Genazzani AR 1995 Naltrexone treatment restores menstrual cycles in patients with weight loss-related amenorrhea. Fertil Steril 64:951–956 [DOI] [PubMed] [Google Scholar]
- Wildt L, Sir-Petermann T, Leyendecker G, Waibel-Treber S, Rabenbauer B 1993 Opiate antagonist treatment of ovarian failure. Hum Reprod 8(Suppl 2):168–174 [DOI] [PubMed] [Google Scholar]
- Manieri C, Musso MC, Marolda AR, Pastorino R, Ferrarotti M, Fornengo R, Isolato G, Messina M 1993 Naltrexone must not be considered a real therapy in functional hypothalamic amenorrhea. The results of a double blind controlled study. Panminerva Med 35:214–217 [PubMed] [Google Scholar]
- Matera C, Freda PU, Ferin M, Wardlaw SL 1995 Effect of chronic opioid antagonism on the hypothalamic-pituitary-ovarian axis in hyperprolactinemic women. J Clin Endocrinol Metab 80:540–545 [DOI] [PubMed] [Google Scholar]
- Lafisca S, Bolelli G, Franceschetti F, Danieli A, Tagliaro F, Marigo M, Flamigni C 1985 Free and bound testosterone in male heroin addicts. Arch Toxicol Suppl 8:394–397 [DOI] [PubMed] [Google Scholar]
- Rasheed A, Tareen IA 1995 Effects of heroin on thyroid function, cortisol and testosterone level in addicts. Pol J Pharmacol 47:441–444 [PubMed] [Google Scholar]
- Ragni G, De Lauretis L, Bestetti O, Sghedoni D, Gambaro V 1988 Gonadal function in male heroin and methadone addicts. Int J Androl 11:93–100 [DOI] [PubMed] [Google Scholar]
- Daniell HW 2002 Hypogonadism in men consuming sustained-action oral opioids. J Pain 3:377–384 [DOI] [PubMed] [Google Scholar]
- Paice JA, Penn RD, Ryan WG 1994 Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage 9:126–131 [DOI] [PubMed] [Google Scholar]
- Cicero TJ 1984 Opiate and opioid modulation of reproductive endocrinology in the male and female: development and pregestational aspects. NIDA Res Monogr 55:14–23 [PubMed] [Google Scholar]
- Fabbri A, Jannini EA, Gnessi L, Moretti C, Ulisse S, Franzese A, Lazzari R, Fraioli F, Frajese G, Isidori A 1989 Endorphins in male impotence: evidence for naltrexone stimulation of erectile activity in patient therapy. Psychoneuroendocrinology 14:103–111 [DOI] [PubMed] [Google Scholar]
- Daniell HW, Lentz R, Mazer NA 2006 Open-label pilot study of testosterone patch therapy in men with opioid-induced androgen deficiency. J Pain 7:200–210 [DOI] [PubMed] [Google Scholar]
- Bliesener N, Albrecht S, Schwager A, Weckbecker K, Lichtermann D, Klingmüller D 2005 Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J Clin Endocrinol Metab 90:203–206 [DOI] [PubMed] [Google Scholar]
- Hallinan R, Byrne A, Agho K, McMahon C, Tynan P, Attia J 2008 Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med 5:684–692 [DOI] [PubMed] [Google Scholar]
- Kinjo M, Setoguchi S, Schneeweiss S, Solomon DH 2005 Bone mineral density in subjects using central nervous system-active medications. Am J Med 118:1414 [DOI] [PubMed] [Google Scholar]
- Rico H, Costales C, Cabranes JA, Escudero M 1990 Lower serum osteocalcin levels in pregnant drug users and their newborns at the time of delivery. Obstet Gynecol 75:998–1000 [PubMed] [Google Scholar]
- Vestergaard P, Rejnmark L, Mosekilde L 2006 Fracture risk associated with the use of morphine and opiates. J Intern Med 260:76–87 [DOI] [PubMed] [Google Scholar]
- Elhassan AM, Lindgren JU, Hultenby K, Bergstrom J, Adem A 1998 Methionine-enkephalin in bone and joint tissues. J Bone Miner Res 13:88–95 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Goemaere S, Kaufman JM 1999 Testosterone, body composition and aging. J Endocrinol Invest 22:110–116 [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A 2005 The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26:833–876 [DOI] [PubMed] [Google Scholar]
- Yeap BB, Almeida OP, Hyde Z, Chubb SA, Hankey GJ, Jamrozik K, Flicker L 2008 Higher serum free testosterone is associated with better cognitive function in older men, while total testosterone is not. The Health In Men Study. Clin Endocrinol (Oxf) 68:404–412 [DOI] [PubMed] [Google Scholar]
- Cherrier MM 2009 Testosterone effects on cognition in health and disease. Front Horm Res 37:150–162 [DOI] [PubMed] [Google Scholar]
- de la Rosa RE, Hennessey JV 1996 Hypogonadism and methadone: hypothalamic hypogonadism after long-term use of high-dose methadone. Endocr Pract 2:4–7 [DOI] [PubMed] [Google Scholar]
- Cadet P, Mantione K, Bilfinger TV, Stefano GB 2002 Morphine down regulates human vascular tissue estrogen receptor expression determined by real-time RT-PCR. Neuro Endocrinol Lett 23:95–100 [PubMed] [Google Scholar]
- Hijazi RA, Cunningham GR 2005 Andropause: is androgen replacement therapy indicated for the aging male? Annu Rev Med 56:117–137 [DOI] [PubMed] [Google Scholar]
- Finch PM, Roberts LJ, Price L, Hadlow NC, Pullan PT 2000 Hypogonadism in patients treated with intrathecal morphine. Clin J Pain 16:251–254 [DOI] [PubMed] [Google Scholar]
- Clarke G, Patrick G 1983 Differential inhibitory action by morphine on the release of oxytocin and vasopressin from the isolated neural lobe. Neurosci Lett 39:175–180 [DOI] [PubMed] [Google Scholar]
- Wilson N, Ngsee J 1982 Antidiuretic effect of acute morphine administration in the conscious rat. Can J Physiol Pharmacol 60:201–204 [DOI] [PubMed] [Google Scholar]
- Grell S, Christensen JD, Fjalland B 1985 Morphine antidiuresis in conscious rats: contribution of vasopressin and blood pressure. Acta Pharmacol Toxicol (Copenh) 56: 38–43 [DOI] [PubMed] [Google Scholar]
- Michel C, Montastruc JL, Valdiguié P, Montastruc P 1986 Effects of morphine on urine flow in rats. Involvement of vasopressin. J Pharmacol 17:316–322 [PubMed] [Google Scholar]
- Knepel W, Meyer DK 1983 The effect of naloxone on vasopressin release from rat neurohypophysis incubated in vitro. J Physiol 341:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy-Long JY, Miller DS, Rosella-Dampman LM, Hartman RD, Emmert SE 1984 A functional role for opioid peptides in the differential secretion of vasopressin and oxytocin. Brain Res 309:362–366 [PubMed] [Google Scholar]
- Hartman RD, Rosella-Dampman LM, Emmert SE, Summy-Long JY 1986 Ontogeny of opioid inhibition of vasopressin and oxytocin release in response to osmotic stimulation. Endocrinology 119:1–11 [DOI] [PubMed] [Google Scholar]
- Wang XM, Tresham JJ, Congiu M, Scoggins BA, Coghlan JP 1989 Central effect of the enkephalin analogue FK-33824 on vasopressin secretion in conscious sheep. Acta Endocrinol (Copenh) 120:369–373 [DOI] [PubMed] [Google Scholar]
- Christensen JD, Fjalland B 1982 Lack of effect of opiates on release of vasopressin from isolated rat neurohypophysis. Acta Pharmacol Toxicol (Copenh) 50:113–116 [DOI] [PubMed] [Google Scholar]
- Summy-Long JY, Keil LC, Deen K, Severs WB 1981 Opiate regulation of angiotensin-induced drinking and vasopressin release. J Pharmacol Exp Ther 217:630–637 [PubMed] [Google Scholar]
- Bisset GW, Chowdrey HS, Feldberg W 1978 Release of vasopressin by enkephalin. Br J Pharmacol 62:370–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalamessa F, de Caro G, Perfumi M 1980 Water intake inhibition and vasopressin release induced by eledoisin and leu-enkephalin in rats of the Brattleboro and Wistar strain. Pharmacol Res Commun 12:375–378 [DOI] [PubMed] [Google Scholar]
- Geis R, Weber E, Martin R, Voigt KH 1982 Hypothalamo-posterior pituitary system in Brattleboro rats: immunoreactive levels of leucine-enkephalin, dynorphin (1-17), dynorphin (1-8) and α-neo-endorphin. Life Sci 31:1809–1812 [DOI] [PubMed] [Google Scholar]
- Martin R, Voigt KH 1982 Leucine-enkephalin-like immunoreactivity in vasopressin terminals is enhanced by treatment with peptidases. Life Sci 31:1729–1732 [DOI] [PubMed] [Google Scholar]
- Pitzel L, König A 1984 Lack of response in the release of oxytocin and vasopressin from isolated neurohypophyses to dopamine, met-enkephalin and leu-enkephalin. Exp Brain Res 56:221–226 [DOI] [PubMed] [Google Scholar]
- Zamir N 1985 On the origin of leu-enkephalin and met-enkephalin in the rat neurohypophysis. Endocrinology 117:1687–1692 [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Schmidt P, Sheikh SP, Larsen PJ 1992 Non-vasopressinergic, non-oxytocinergic neuropeptides in the rat hypothalamo-neurohypophyseal tract: experimental immunohistochemical studies. Prog Brain Res 91:367–371 [DOI] [PubMed] [Google Scholar]
- Falke N, Martin R 1985 Opioid binding in a rat neurohypophysial fraction enriched in oxytocin and vasopressin nerve endings. Neurosci Lett 61:37–41 [DOI] [PubMed] [Google Scholar]
- Evans RG, Olley JE, Rice GE, Abrahams JM 1989 μ- and K-opiate receptor agonists reduce plasma neurohypophysial hormone concentrations in water-deprived and normally hydrated rats. Clin Exp Pharmacol Physiol 16:191–197 [DOI] [PubMed] [Google Scholar]
- Tsushima H, Mori M, Matsuda T 1997 Central regulation of urine production by a selective μ-opioid agonist, [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin, in rats. Jpn J Pharmacol 74:45–49 [DOI] [PubMed] [Google Scholar]
- Rossi NF, Kim JK, Summers SN, Schrier RW 1997 κ-Opiate agonist RU 51599 inhibits vasopressin gene expression and osmotically-induced vasopressin secretion in vitro. Life Sci 61:2271–2282 [DOI] [PubMed] [Google Scholar]
- Gulati K, Ray A, Sharma KK 1991 Effects of acute and chronic morphine on food intake in rats: modulation by oxytocin and vasopressin. Pharmacol Biochem Behav 40:27–32 [DOI] [PubMed] [Google Scholar]
- Yang J, Yang Y, Xu HT, Chen JM, Liu WY, Lin BC 2006 Arginine vasopressin enhances periaqueductal gray synthesis and secretion of enkephalin and endorphin in the rat. Brain Res Bull 71:193–199 [DOI] [PubMed] [Google Scholar]
- DeBodo RC 1944 The antidiuretic action of morphine, and its mechanism. J Pharmacol Exp Ther 82:74–85 [Google Scholar]
- van Wimersma Greidanus TB, Thody TJ, Verspaget H, de Rotte GA, Goedemans HJ, Croiset G, van Ree JM 1979 Effects of morphine and β-endorphin on basal and elevated plasma levels of α-MSH and vasopressin. Life Sci 24:579–585 [DOI] [PubMed] [Google Scholar]
- Lightman SL, Iversen LL, Forsling ML 1982 Dopamine and [D-ALA2, D-Leu5]enkephalin inhibit the electrically stimulated neurohypophyseal release of vasopressin in vitro: evidence for calcium-dependent opiate action. J Neurosci 2:78–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Besser GM, Milles JJ, Baylis PH 1980 Inhibition of vasopressin release in man by an opiate peptide. Lancet 2:1108–1110 [DOI] [PubMed] [Google Scholar]
- Brownell J, del Pozo E, Donatsch P 1980 Inhibition of vasopressin secretion by a met-enkephalin (FK 33-824) in humans. Acta Endocrinol (Copenh) 94:304–308 [DOI] [PubMed] [Google Scholar]
- Lightman SL, Forsling ML 1980 The effect of the methionine enkephalin analogue DAMME on the vasopression response to tilt in man. Clin Sci (Lond) 59:501–503 [DOI] [PubMed] [Google Scholar]
- Zerbe RL, Henry DP, Robertson GL 1982 A new met-enkephalin analogue suppresses plasma vasopressin in man. Peptides 3:199–201 [DOI] [PubMed] [Google Scholar]
- Lightman SL, Langdon N, Forsling ML 1980 Effects of the opiate antagonist naloxone and the enkephalin analog DAMME on the vasopressin response to a hypertonic stimulus in man. J Clin Endocrinol Metab 51:1447–1449 [DOI] [PubMed] [Google Scholar]
- Bozkurt P, Kaya G, Yeker Y, Altintas F, Bakan M, Hacibekiroglu M, Kavunoglu G 2003 Effects of systemic and epidural morphine on antidiuretic hormone levels in children. Paediatr Anaesth 13:508–514 [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Wilson N, Turnbull KW, Yip RW 1986 Haemodynamic and plasma vasopressin responses during high-dose fentanyl or sufentanil anaesthesia. Can Anaesth Soc J 33:475–483 [DOI] [PubMed] [Google Scholar]
- Weiskopf RB, Reid IA, Fisher DM, Holmes MA, Rosen JI, Keil LC 1987 Effects of fentanyl on vasopressin secretion in human subjects. J Pharmacol Exp Ther 242:970–973 [PubMed] [Google Scholar]
- Rimoy GH, Bhaskar NK, Wright DM, Rubin PC 1991 Mechanism of diuretic action of spiradoline (U-62066E)–a κ-opioid receptor agonist in the human. Br J Clin Pharmacol 32:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesande F, Dierickx K 1975 Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res 164:153–162 [DOI] [PubMed] [Google Scholar]
- Arnauld E, Cirino M, Layton BS, Renaud LP 1983 Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology 36:187–196 [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Noble R, Clarke G 1983 Effects of morphine and D-Ala, D-Leu enkephalin on the electrical activity of supraoptic neurosecretory cells in vitro. Neuroscience 10:73–81 [DOI] [PubMed] [Google Scholar]
- Inenaga K, Imura H, Yanaihara N, Yamashita H 1990 κ-Selective opioid receptor agonists leumorphin and dynorphin inhibit supraoptic neurons in rat hypothalamic slice preparations. J Neuroendocrinol 2:389–395 [DOI] [PubMed] [Google Scholar]
- Pumford KM, Leng G, Russell JA 1991 Morphine actions on supraoptic oxytocin neurones in anaesthetized rats: tolerance after i.c.v. morphine infusion. J Physiol 440:437–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell RJ, Ingram CD, Leng G 1983 Oxytocin release is inhibited by opiates from the neural lobe, not those from the intermediate lobe. Neurosci Lett 43:227–230 [DOI] [PubMed] [Google Scholar]
- Hartman RD, Rosella-Dampman LM, Summy-Long JY 1987 Endogenous opioid peptides inhibit oxytocin release in the lactating rat after dehydration and urethane. Endocrinology 121:536–543 [DOI] [PubMed] [Google Scholar]
- Evans RG, Olley JE 1988 Comparison of the oxytocin response to water-deprivation, hyperosmolarity and administration of morphine or naltrexone in lactating and virgin female rats. Neurosci Lett 94:177–181 [DOI] [PubMed] [Google Scholar]
- Rayner VC, Robinson IC, Russell JA 1988 Chronic intracerebroventricular morphine and lactation in rats: dependence and tolerance in relation to oxytocin neurones. J Physiol 396:319–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Gosden RG, Humphreys EM, Cutting R, Fitzsimons N, Johnston V, Liddle S, Scott S, Stirland JA 1989 Interruption of parturition in rats by morphine: a result of inhibition of oxytocin secretion. J Endocrinol 121:521–536 [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Wang CH, Lu CL 2000 Chronic morphine treatment inhibits oxytocin synthesis in rats. Neuroreport 11:3113–3116 [DOI] [PubMed] [Google Scholar]
- Carter DA, Lightman SL 1987 Opioid control of oxytocin secretion: evidence of distinct regulatory actions of two opiate receptor types. Life Sci 40:2289–2296 [DOI] [PubMed] [Google Scholar]
- Summy-Long JY, Rosella-Dampman LM, McLemore GL, Koehler E 1990 κ Opiate receptors inhibit release of oxytocin from the magnocellular system during dehydration. Neuroendocrinology 51:376–384 [DOI] [PubMed] [Google Scholar]
- Kutlu S, Yilmaz B, Canpolat S, Sandal S, Ozcan M, Kumru S, Kelestimur H 2004 μ Opioid modulation of oxytocin secretion in late pregnant and parturient rats. Involvement of noradrenergic neurotransmission. Neuroendocrinology 79:197–203 [DOI] [PubMed] [Google Scholar]
- Lindow SW, van der Spuy ZM, Hendricks MS, Nugent FA, Dunne TT 1993 The effect of morphine and naloxone administration on maternal oxytocin concentration in late pregnancy. Clin Endocrinol (Oxf) 39:671–675 [DOI] [PubMed] [Google Scholar]
- Lindow SW, van der Spuy ZM, Hendricks MS, Rosselli AP, Lombard C, Leng G 1992 The effect of morphine and naloxone administration on plasma oxytocin concentrations in the first stage of labour. Clin Endocrinol (Oxf) 37:349–353 [DOI] [PubMed] [Google Scholar]
- Lindow SW, Hendricks MS, Thompson JW, van der Spuy ZM 1998 Effects of morphine administration on the fetal production of oxytocin in labour. Clin Sci (Lond) 95: 91–95 [PubMed] [Google Scholar]
- Lindow SW, Hendricks MS, Nugent FA, Dunne TT, van der Spuy ZM 1999 Morphine suppresses the oxytocin response in breast-feeding women. Gynecol Obstet Invest 48:33–37 [DOI] [PubMed] [Google Scholar]
- Shibli KU, Dhillon AR, Goode JA, Gilbert CL, Thompson JW, Russell IF, Lindow SW 2001 Effect of intrathecal fentanyl on oxytocin secretion in pregnant women not in labour. Clin Sci (Lond) 101:415–419 [PubMed] [Google Scholar]
- Stocche RM, Klamt JG, Antunes-Rodrigues J, Garcia LV, Moreira AC 2001 Effects of intrathecal sufentanil on plasma oxytocin and cortisol concentrations in women during the first stage of labor. Reg Anesth Pain Med 26:545–550 [DOI] [PubMed] [Google Scholar]
- Coiro V, Chiodera P 1991 Naloxone increases the angiotensin II stimulated rise of arginine vasopressin and oxytocin secretion in man. Neuroendocrinology 53:209–213 [DOI] [PubMed] [Google Scholar]
- Honer WG, Thompson C, Lightman SL, Williams TD, Checkley SA 1986 No effect of naloxone on plasma oxytocin in normal men. Psychoneuroendocrinology 11:245–248 [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Sanger DJ 1984 Endorphinergic mechanisms in food, salt and water intake: an overview. Appetite 5:1–6 [DOI] [PubMed] [Google Scholar]
- Grandison L, Guidotti A 1977 Stimulation of food intake by muscimol and β endorphin. Neuropharmacology 16:533–536 [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Gosnell BA, Billington CJ 1984 Which opioid receptor mechanism modulates feeding? Appetite 5:61–68 [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD 1993 The effects of continuous morphine infusion on diet selection and body weight. Physiol Behav 54:853–859 [DOI] [PubMed] [Google Scholar]
- Cooper SJ 1981 Behaviourally-specific hyperdipsia in the non-deprived rat following acute morphine treatment. Neuropharmacology 20:469–471 [DOI] [PubMed] [Google Scholar]
- Frenk H, Rogers GH 1979 The suppressant effects of naloxone on food and water intake in the rat. Behav Neural Biol 26:23–40 [DOI] [PubMed] [Google Scholar]
- Kunihara M, Kanbayashi M, Ohshima T 1983 Opposite effects of morphine on feeding and drinking in rats relative to administration time. Jpn J Pharmacol 33:829–835 [DOI] [PubMed] [Google Scholar]
- Leshem M 1981 Morphine-induced anorexia in lateral hypothalamic rats. Psychopharmacology (Berl) 75:48–53 [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Kanarek RB 1980 Morphine selectively influences macronutrient intake in the rat. Pharmacol Biochem Behav 12:427–430 [DOI] [PubMed] [Google Scholar]
- Sanger DJ, McCarthy PS 1980 Differential effects of morphine on food and water intake in food deprived and freely-feeding rats. Psychopharmacology (Berl) 72:103–106 [DOI] [PubMed] [Google Scholar]
- Wolgin DL, Benson HD 1991 Role of associative and nonassociative mechanisms in tolerance to morphine “anorexia.” Pharmacol Biochem Behav 39:279–286 [DOI] [PubMed] [Google Scholar]
- Buck M, Marrazzi MA 1987 Atypical responses to morphine in mice: a possible relationship to anorexia nervosa? Life Sci 41:765–773 [DOI] [PubMed] [Google Scholar]
- Levine AS, Billington CJ 1989 Opioids. Are they regulators of feeding? Ann NY Acad Sci 575:209–219; discussion 219–220 [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE, Gosnell BA, Billington CJ, Bartness TJ 1985 Opioids and consummatory behavior. Brain Res Bull 14:663–672 [DOI] [PubMed] [Google Scholar]
- D'Este L, Casini A, Pontieri FE, Renda TG 2006 Changes in neuropeptide FF and NPY immunohistochemical patterns in rat brain under heroin treatment. Brain Res 1083:151–158 [DOI] [PubMed] [Google Scholar]
- Pedrazzini T 2004 Importance of NPY Y1 receptor-mediated pathways: assessment using NPY Y1 receptor knockouts. Neuropeptides 38:267–275 [DOI] [PubMed] [Google Scholar]
- Leshem M 1988 Morphine induces delayed anorexia in rats. Psychopharmacology (Berl) 94:254–258 [DOI] [PubMed] [Google Scholar]
- McLean S, Hoebel BG 1983 Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides 4:287–292 [DOI] [PubMed] [Google Scholar]
- Anghel A, Jamieson CAM, Ren X, Young J, Ghods D, Liu Y, Lutfy K, Friedman TC, Gene expression profiling following short-term and long-term morphine exposure in mice uncovers genes involved in food intake. Program of the 90th Annual Meeting of The Endocrine Society, San Francisco, CA, 2008 (Abstract P1-171) [Google Scholar]
- Recant L, Voyles N, Wade A, Awoke S, Bhathena SJ 1983 Studies on the role of opiate peptides in two forms of genetic obesity: ob/ob mouse and fa/fa rat. Horm Metab Res 15:589–593 [DOI] [PubMed] [Google Scholar]
- Khawaja XZ, Bailey CJ, Green IC 1989 Central μ, δ, and κ opioid binding sites, and brain and pituitary β-endorphin and met-enkephalin in genetically obese (ob/ob) and lean mice. Life Sci 44:1097–1105 [DOI] [PubMed] [Google Scholar]
- Millard WJ, Romano TM, Layden MP, Russell WE, Martin RJ 1991 Growth hormone secretion in the obese male rat: modulation by the gonadal and thyroid axes. Growth Dev Aging 55:91–103 [PubMed] [Google Scholar]
- Marín-Bivens CL, Olster DH 1999 Opioid receptor blockade promotes weight loss and improves the display of sexual behaviors in obese Zucker female rats. Pharmacol Biochem Behav 63:515–520 [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Flatt PR 1987 Increased responsiveness to glucoregulatory effect of opiates in obese-diabetic ob/ob mice. Diabetologia 30:33–37 [DOI] [PubMed] [Google Scholar]
- Wolinsky TD, Abrahamsen GC, Carr KD 1996 Diabetes alters μ and κ opioid binding in rat brain regions: comparison with effects of food restriction. Brain Res 738:167–171 [DOI] [PubMed] [Google Scholar]
- Sadava D, Alonso D, Hong H, Pettit-Barrett DP 1997 Effect of methadone addiction on glucose metabolism in rats. Gen Pharmacol 28:27–29 [DOI] [PubMed] [Google Scholar]
- Shook JE, Dewey WL 1986 Morphine dependence and diabetes. I. The development of morphine dependence in streptozotocin-diabetic rats and spontaneously diabetic C57BL/KsJ mice. J Pharmacol Exp Ther 237:841–847 [PubMed] [Google Scholar]
- Shook JE, Kachur JF, Brase DA, Dewey WL 1986 Morphine dependence and diabetes. II. Alterations of normorphine potency in the guinea-pig ileum and mouse vas deferens and of ileal morphine dependence by changes in glucose concentration. J Pharmacol Exp Ther 237:848–852 [PubMed] [Google Scholar]
- Courteix C, Bourget P, Caussade F, Bardin M, Coudore F, Fialip J, Eschalier A 1998 Is the reduced efficacy of morphine in diabetic rats caused by alterations of opiate receptors or of morphine pharmacokinetics? J Pharmacol Exp Ther 285:63–70 [PubMed] [Google Scholar]
- Chen SR, Sweigart KL, Lakoski JM, Pan HL 2002 Functional μ opioid receptors are reduced in the spinal cord dorsal horn of diabetic rats. Anesthesiology 97:1602– 1608 [DOI] [PubMed] [Google Scholar]
- Giugliano D, Salvatore T, Cozzolino D, Ceriello A, Torella R, D'Onofrio F 1987 Sensitivity to β-endorphin as a cause of human obesity. Metabolism 36:974–978 [DOI] [PubMed] [Google Scholar]
- Atkinson RL, Berke LK, Drake CR, Bibbs ML, Williams FL, Kaiser DL 1985 Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther 38:419–422 [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Luby ED, Kinzie J, Munjal ID, Spector S 1997 Endogenous codeine and morphine in anorexia and bulimia nervosa. Life Sci 60:1741–1747 [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Bacon JP, Kinzie J, Luby ED 1995 Naltrexone use in the treatment of anorexia nervosa and bulimia nervosa. Int Clin Psychopharmacol 10:163–172 [DOI] [PubMed] [Google Scholar]
- Plewe G, Schneider U, Krause U, Beyer J 1987 Naloxone increases the response of growth hormone and prolactin to stimuli in obese humans. J Endocrinol Invest 10:137–141 [DOI] [PubMed] [Google Scholar]
- Papalia D, Lunetta M, Di Mauro M 1989 Effects of naloxone on prolactin, growth hormone and cortisol response to insulin hypoglycemia in obese subjects. J Endocrinol Invest 12:777–782 [DOI] [PubMed] [Google Scholar]
- Blank DM, Clark RV, Heymsfield SB, Rudman DR, Blank MS 1994 Endogenous opioids and hypogonadism in human obesity. Brain Res Bull 34:571–574 [DOI] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE 2006 Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 6:1249–1265 [DOI] [PubMed] [Google Scholar]
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O'Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA 2009 Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 17:30–39 [DOI] [PubMed] [Google Scholar]
- Lee MW, Fujioka K 2009 Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother 10:1841–1845 [DOI] [PubMed] [Google Scholar]
- Leslie RD, Pyke DA, Stubbs WA 1979 Sensitivity to enkephalin as a cause of non-insulin dependent diabetes. Lancet 1:341–343 [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, di Pinto P, Saccomanno F, Gentile S, Cappiapuoti F 1982 Impaired insulin secretion in human diabetes mellitus. The effect of naloxone-induced opiate receptor blockade. Diabetes 31:367–370 [DOI] [PubMed] [Google Scholar]
- Mason JS, Heber D 1982 Endogenous opiates modulate insulin secretion in flushing noninsulin-dependent diabetics. J Clin Endocrinol Metab 54:693–697 [DOI] [PubMed] [Google Scholar]
- Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M 2004 Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol 9:53–58 [DOI] [PubMed] [Google Scholar]
- Giugliano D 1984 Morphine, opioid peptides, and pancreatic islet function. Diabetes Care 7:92–98 [DOI] [PubMed] [Google Scholar]
- Sood A, Thakur V, Ahuja MM 1989 Effect of chronic opioid administration on glycosylated haemoglobin levels in heroin addicts. Indian J Med Res 90:51–54 [PubMed] [Google Scholar]
- Shahani S, Braga-Basaria M, Basaria S 2008 Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab 93:2042– 2049 [DOI] [PubMed] [Google Scholar]
- Kapoor D, Goodwin E, Channer KS, Jones TH 2006 Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154:899–906 [DOI] [PubMed] [Google Scholar]
- Awoke S, Voyles NR, Bhathena SJ, Tanenberg RJ, Recant L 1984 Alterations of plasma opioid activity in human diabetics. Life Sci 34:1999–2006 [DOI] [PubMed] [Google Scholar]
- Giugliano D, Quatraro A, Consoli G, Ceriello A, Torella R, D'Onofrio F 1987 Inhibitory effect of enkephalin on insulin secretion in healthy subjects and in non insulin-dependent diabetic subjects. Metabolism 36:286–289 [DOI] [PubMed] [Google Scholar]
- Karci A, Tasdogen A, Erkin Y, Akta° G, Elar Z 2004 The analgesic effect of morphine on postoperative pain in diabetic patients. Acta Anaesthesiol Scand 48:619–624 [DOI] [PubMed] [Google Scholar]
- Chapman CR, Tuckett RP, Song CW 2008 Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain 9:122–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R 1995 Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci 15:3328–3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N 1996 Immunohistochemical localization of μ-opioid receptors in the central nervous system of the rat. J Comp Neurol 367:375–402 [DOI] [PubMed] [Google Scholar]
- Kalyuzhny AE, Arvidsson U, Wu W, Wessendorf MW 1996 μ-Opioid and δ-opioid receptors are expressed in brainstem antinociceptive circuits: studies using immunocytochemistry and retrograde tract-tracing. J Neurosci 16:6490–6503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ME, Pert A, Pert CB, Herkenham M 1983 Opiate receptor localization in rat cerebral cortex. J Comp Neurol 216:339–358 [DOI] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ 2003 Buprenorphine-induced antinociception is mediated by μ-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, Lovering AT 2000 μ and δ Opioid receptor regulation of pro-opiomelanocortin peptide secretion from the rat neurointermediate pituitary in vitro. Neuropeptides 34:69–75 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Rice KC, Jacobson AE, Rothman RB 1986 Opiate receptors in rat pituitary are confined to the neural lobe and are exclusively κ. Brain Res 382:365–371 [DOI] [PubMed] [Google Scholar]
- Rotten D, Leblanc P, Kordon C, Weiner RI, Enjalbert A 1986 Interference of endogenous B endorphin with opiate binding in the anterior pituitary. Neuropeptides 8:377–392 [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ 1987 Autoradiographic differentiation of μ, δ, and κ opioid receptors in the rat forebrain and midbrain. J Neurosci 7:2445–2464 [PMC free article] [PubMed] [Google Scholar]