Abstract
The pros and cons of estrogen therapy for use in postmenopausal women continue to be a major topic of debate in women’s health. Much of this debate focuses on the potential benefits vs. harm of estrogen therapy on the brain and the risks for cognitive impairment associated with aging and Alzheimer’s disease. Many animal and human studies suggest that estrogens can have significant beneficial effects on brain aging and cognition and reduce the risk of Alzheimer’s-related dementia; however, others disagree. Important discoveries have been made, and hypotheses have emerged that may explain some of the inconsistencies. This review focuses on the cholinergic hypothesis, specifically on evidence that beneficial effects of estrogens on brain aging and cognition are related to interactions with cholinergic projections emanating from the basal forebrain. These cholinergic projections play an important role in learning and attentional processes, and their function is known to decline with advanced age and in association with Alzheimer’s disease. Evidence suggests that many of the effects of estrogens on neuronal plasticity and function and cognitive performance are related to or rely upon interactions with these cholinergic projections; however, studies also suggest that the effectiveness of estrogen therapy decreases with age and time after loss of ovarian function. We propose a model in which deficits in basal forebrain cholinergic function contribute to age-related changes in the response to estrogen therapy. Based on this model, we propose that cholinergic-enhancing drugs, used in combination with an appropriate estrogen-containing drug regimen, may be a viable therapeutic strategy for use in older postmenopausal women with early evidence of mild cognitive decline.
This paper reviews evidence from both animal and human literature that estrogens affect cognition, and in particular that beneficial effects of estrogens on cognition are related to interactions with cholinergic projections emanating from the basal forebrain. In addition, a model is proposed in which deficits in basal forebrain cholinergic function contribute to age-related changes in the response to estrogen therapy. Based on this model, it is speculated that cholinergic enhancing drugs, used in combination with an appropriate estrogen-containing drug regimen, may be an effective therapeutic strategy for use in older post-menopausal women, particularly women with early evidence of cognitive decline.
I. Introduction
II. The Neurobiology of Cognition
- III. Estrogens and Cognition
- A. Animal studies
- B. Studies in humans
- IV. The Role of Basal Forebrain Cholinergic Neurons in Cognitive Performance
- A. Alzheimer’s disease
- B. Cholinergic lesion studies
- C. Effects on visuospatial attention and stimulus processing
V. Effects of Estrogen Therapy on Basal Forebrain Cholinergic Function
- VI. Evidence That Effects on Basal Forebrain Cholinergic Projections Are Relevant to Estrogen Effects on Cognitive Performance
- A. Relevance to navigational performance in humans
- VII. Estrogen Therapy for the Prevention and Treatment of Cognitive Decline Associated with Aging and AD
- A. The importance of timing
VIII. The Cholinergic Basis of the Critical Period Hypothesis
IX. Testing the Cholinergic Hypothesis
- X. Cellular Mechanisms Underlying Estrogen Effects on Cholinergic Function and Cognitive Performance
- A. Hippocampus
- B. The role of specific estrogen receptors
- C. Mood/affect and effects on monoamines
XI. Concluding Remarks
I. Introduction
Evidence pertaining to the risks and benefits of hormone therapy on brain aging and cognition in postmenopausal women has created much confusion and debate. On the one hand, animal studies show unequivocally that estrogens can have many beneficial effects on the brain, including reducing neuronal loss after cardiac arrest and stroke (reviewed in Ref. 1), increasing neuronal connectivity (2), improving cognitive performance (3), and preventing or slowing age-related cognitive decline (4,5). Most of these studies used 17ß-estradiol (also referred to as estradiol in this review), which is the primary active estrogen in both animals and humans. As in animals, studies suggest that estrogens also can have beneficial effects on brain aging and cognition in humans. For example, several studies have reported enhanced performance on specific cognitive tasks, particularly in the realm of verbal memory and executive functioning (6). Other studies have reported estrogen-mediated increases in cerebral blood flow (7,8), and reductions in hippocampal and cortical atrophy associated with aging and AD (9,10), and more than a dozen retrospective and observational studies have reported substantial reductions (up to 83%) in the risk of developing Alzheimer’s-related dementia among women who received postmenopausal hormone therapy compared with those who did not (11). In most of the human studies, hormone therapy consisted of daily oral administration of conjugated equine estrogens (CEEs) either alone or in combination with medroxyprogesterone acetate (MPA). CEE is a mixture of conjugated estrogens isolated from the urine of pregnant mares. CEE contains at least 10 active estrogens, the most abundant of which are estrone (∼50%), equilin (∼24%), and 17ß-dihydroequilin (∼15%) in sulfated form (12). 17ß-Estradiol accounts for only 0.7% of the estrogens in CEE. MPA is a synthetic progestin commonly used as a component of oral contraceptives and is included in postmenopausal hormone therapy to prevent uterine hyperplasia.
In contrast to the positive findings noted above, the results of several important randomized clinical trials have been less promising. The largest of these trials is the Women’s Health Initiative Memory Study (WHIMS). This trial included 7479 women drawn from the larger Women’s Health Initiative (13). The women enrolled in WHIMS had a mean age of 69 yr at enrollment. Forty percent (n = 2947) had prior hysterectomy and received CEE alone. The remaining 60% (n = 4532) received CEE + MPA. Results of this study showed an increased (∼2-fold) risk of dementia among women receiving CEE + MPA (14) and a trend toward increased risk of dementia among women receiving CEE alone (15). Estrogen therapy, particularly oral therapy, also is associated with increased risk of thromboembolism and stroke as well as a significant increased risk of breast and endometrial cancer (16,17,18,19). Some of these adverse effects can be avoided by selecting transdermal as opposed to oral therapy (20). There also is evidence that treatment with 17ß-estradiol as opposed to CEE may be more effective (21,22) (discussed in greater detail in Section III.B). These issues have raised important concerns about the risks and benefits of using estrogen-containing therapies in postmenopausal women.
The mechanisms by which estrogens enhance cognitive performance have been the subject of intense study. Several important discoveries have been made that may explain some of the inconsistencies in the animal and human literatures. It is clear that a better understanding of the mechanisms that underlie the effects of estrogens on cognition will lead to the development of more effective and targeted therapies that benefit the aging brain. This review focuses on the cholinergic hypothesis, i.e., on evidence that beneficial effects of estrogens on brain aging and cognition are related to interactions with cholinergic projections emanating from the basal forebrain. A model in which deficits in basal forebrain cholinergic function contribute to age-related changes in the response to estrogen therapy is proposed. Based on this analysis, it is proposed that cholinergic-enhancing drugs, used in combination with an appropriate estrogen-containing drug regimen, may be a viable therapeutic strategy for use in older postmenopausal women with early evidence of mild cognitive decline.
II. The Neurobiology of Cognition
A rudimentary understanding of cognition and the functional organization of cognitive processes is necessary to appreciate how estrogen therapy influences performance within specific cognitive domains. Cognition is a general term that encompasses the totality of information processing which, in turn, can be broken down into different functional domains (e.g., learning, memory, attention, pattern recognition, problem solving, abstract reasoning, etc.). Each dimension consists of component processes that are subserved by specific brain regions and circuits, which have been identified through the study of brain lesions and through the use of modern functional imaging methods.
With respect to memory, information is divided into declarative (e.g., episodic, semantic) and nondeclarative (e.g., procedural, implicit) memories depending on whether the memories are accessible to conscious reflection and can be explicitly stated (e.g., a date, time, place, or event) or are subconscious (e.g., memory for skills, classical conditioning, priming) (23). Studies show that in both animals and humans, the consolidation of declarative memories (the process of converting short-term memories into a stable and lasting form) relies heavily on medial temporal lobe structures, the hippocampus in particular (24). Hence, humans, rodents, and nonhuman primates with hippocampal injuries are unable to form new declarative memories. In contrast, memories that have been fully consolidated before injury are intact (25). The hippocampus also plays an important role in tasks involving spatial rotation and navigation, as well as in temporal sequencing (24). Again, this is true for both animals and humans. This has led to the effective use of both rodent and nonhuman primate models for studying the role of the hippocampus and related structures in memory impairments associated with aging and disease.
Procedural memories differ from declarative memories in that they are not accessible to conscious reflection. Consolidation of procedural memories relies greatly on extrapyramidal structures such as the basal ganglia and cerebellum (26,27). This is particularly evident in humans where damage to striatal and cerebellar circuits impairs the acquisition of procedural tasks. The same is true for animals where striatal and cerebellar circuits are involved in the acquisition of specific forms of classical conditioning (27,28).
Working memory is a form of short-term memory and refers to the process by which trial-unique events are temporarily stored and manipulated in consciousness. Working memory is essential for tasks in which memory for recent events is used in decision making—for example, remembering which arms of a maze have been entered, or the location of a reward cue on a given trial. In rats, the hippocampus plays a principal role in working memory processes—damage to the hippocampus severely disrupts the ability to perform working memory tasks. This is not the case in humans. In humans, working memory is subserved by specific regions of dorsal prefrontal cortex and is modulated to a degree by inputs from the hippocampus (29). This reflects the tremendous neocortical development in humans, which has resulted in both the development and redistribution of higher-order cognitive functions.
An example of higher-order cognitive functions is a collection of processes known as executive functioning. Executive functioning refers to abilities associated with planning, cognitive flexibility, abstract thinking, and the ability to initiate or inhibit specific responses (30). Studies show that in humans, executive functioning is associated with neural activation within specific subregions of the prefrontal cortex; the specific subregion(s) depend on the domain of executive functioning being examined (31,32). For example, distinct processes involved in decision making, such as memory for recent unique events, cost-benefit evaluation, consideration of different strategies and outcomes, and ultimately the decision to act, are each subserved by specific regions of prefrontal cortex. Rodents can display some forms of executive functioning. For example, rodents can undergo reversal learning, adopt strategies, and make decisions based on differentially weighted cues. Nevertheless, the rodent prefrontal cortex is far less developed than in humans and nonhuman primates. Much of the current efforts to understand higher-order cognitive processes is devoted to mapping out the cortical circuits and neural activity that underlie executive cognitive functions. Behavioral endpoints that often are used to study executive cognitive functioning include visuospatial attention, perseveration, problem solving, decision making, and behavioral inhibition. Note that verbal declarative memory, which appears to be particularly sensitive to estrogen therapy (discussed in Section III.B), relies heavily on both temporal and prefrontal lobe structures and the interconnections between them (e.g., temporo-thalamo-frontal, temporo-parieto-frontal) (33,34,35).
Lastly, cognitive functions including memory also are strongly influenced by emotional context. Effects of emotional context are mediated by stress hormones and rely greatly on projections to and from the amygdala, which also are modulated by inputs from the hippocampus (36,37). Gonadal hormones can modulate the effects of stress on memory consolidation (38).
In summary, cognition is an umbrella term that represents a collection of discrete but interacting processes subserved by specific brain regions. Some of these brain regions (e.g., hippocampus) support similar processes in both rodents and humans. Others (e.g., neocortex) are far more developed in humans and contain specialized regions that support working memory and higher-order executive functions. The effects of estrogens on task performance, therefore, logically depend on the cognitive domain(s) being evaluated and the extent to which performance in a specific domain relies on the neural circuits being affected.
III. Estrogens and Cognition
A. Animal studies
Several laboratories have shown that estrogens (most often 17ß-estradiol) administered to young ovariectomized rats and mice will enhance performance on a variety of cognitive tasks and do so in a task-specific way (3,39). Many studies have used spatial tasks in which animals must navigate a maze or arena and, by making appropriate choices, either escape an aversive stimulus or attain a food reward. For example, studies by Dohanich and co-workers (40,41,42) demonstrated significant enhancement of reinforced alternation in a T-maze, as well as significant improvement of working memory performance on an eight-arm radial maze. As mentioned above, working memory refers to a process by which trial-unique events are temporarily stored, used, and manipulated in short-term memory. One way of testing for effects on working memory is to test memory span, e.g., the amount of information that can be maintained in short-term memory at a given time. Using a water escape version of a radial arm maze, Bimonte et al. (43,44) demonstrated that animals treated with estradiol performed better when faced with increased working memory load. Another test of working memory is to test the ability to maintain and manipulate information in short-term memory for longer periods of time. Studies show that rats treated with estradiol are better able to use information to solve a radial maze task when delays are introduced during performance of the task (45,46).
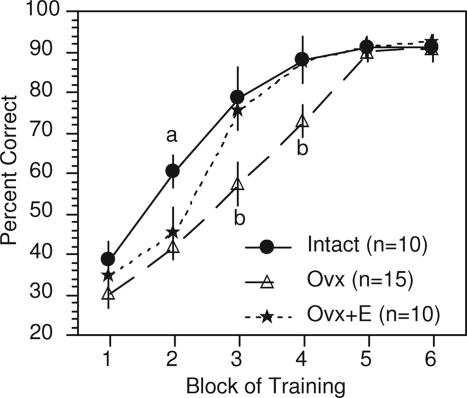
Our own studies have used a delayed matching-to-position (DMP) T-maze task. This is a spatial learning task in which rats typically use extramaze cues to identify correct vs. incorrect arm choices and are rewarded for returning to the maze arm visited on the immediately preceding trial. Our studies show that ovariectomy causes rats to learn the task more slowly and that estradiol (in physiological or supraphysiological range; e.g., ∼30–120 pg/ml) restores the rate of learning to that of gonadally intact controls (Fig. 1) (47,48,49). This effect, however, does not appear to be due to effects on working memory, and it may reflect effects on strategy or on cognitive flexibility (discussed in Section IV.B). This effect also declines with age and time after ovariectomy (50).
Figure 1.
Effects of ovariectomy (Ovx) and estradiol (E) treatment [SILASTIC (Dow Corning Corp., Midland, MI) capsule containing 17ß-estradiol and implanted sc] on acquisition of a delayed matching-to-position T-maze task. Values indicate mean percentage correct ± sem. Each block represents 3 consecutive days of training. a, Intact differs significantly from both Ovx and Ovx+E; P < 0.05. b, Ovx differs significantly from both Intact and Ovx+E; P < 0.05.
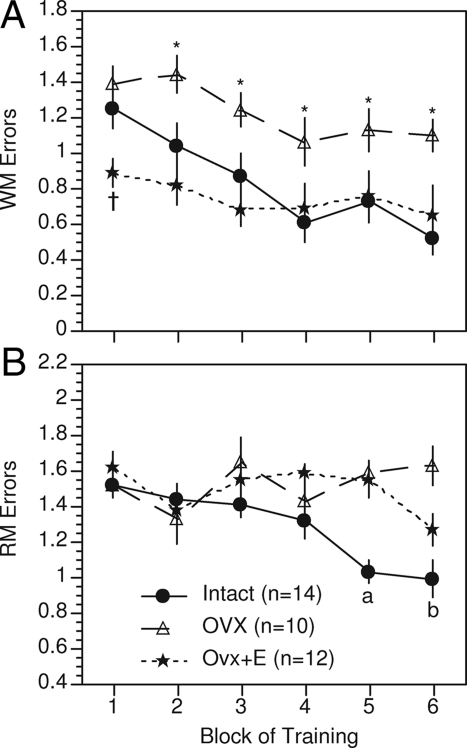
More recently, we demonstrated robust effects of ovariectomy and estradiol treatment on a 12-arm radial maze task (51). The radial arm maze task is a spatial learning task, but it allows for a more rigorous analysis of working vs. reference memory. Reference memory is a term used in animal literature and, in contrast to working memory, refers to memory for events that do not vary from trial to trial. For example, the ability to learn and recall that a particular arm of a maze is never baited is an example of reference memory. On the radial maze task, ovariectomy significantly impaired acquisition of both working and reference memory components of the task, and performance was restored by estradiol treatment (mean serum levels of 28.8 pg/ml) (Fig. 2). Other laboratories likewise have reported effects on working and reference memory (reviewed in Ref. 39), as well as on place recognition tasks (52,53,54), visual object recognition (52,55,56,57), and contextual and cued fear conditioning (58).
Figure 2.
Effects of ovariectomy (Ovx) and estradiol (E) treatment (SILASTIC capsule containing 17ß-estradiol and implanted sc) on working memory (A) and reference memory (B) components of a 12-arm radial maze task. Values indicate the mean number ± sem of working memory and reference memory errors conducted per group for each block of training. Each block represents 7 consecutive days of training. †, Ovx+E differs significantly from both Intact and Ovx; P < 0.05. *, Ovx differs significantly from both Intact and Ovx+E; P < 0.05. a, Intact differs significantly from both Ovx and Ovx+E; P < 0.05. b, Intact differs significantly from Ovx; P < 0.05. [Adapted from R. B. Gibbs and D. A. Johnson: Endocrinology 149:3176–3183, 2008 (51) © The Endocrine Society].
Estradiol does not, however, enhance performance on all tasks. For example, estradiol has been shown to impair performance on certain versions of a Morris water maze task (59), on the reference memory component of a water radial arm maze task (60), on an operant-delayed spatial alternation task, and on a task of differential reinforcement (61). Likewise, estradiol has little effect on acquisition of a configural association negative patterning task (50,62). This task uses the same food reward as the DMP task and requires rats to distinguish sensory cues and to inhibit responses to a configural stimulus. The fact that estradiol has no effect on the configural association task, but enhances acquisition of the DMP task, suggests that the effects on DMP acquisition are not due to effects on motivation or the ability to respond to sensory cues. In short, these data suggest that estradiol enhances performance in a task-specific way by affecting performance within specific cognitive domains.
Task-specific effects also have been observed in nonhuman primates. For example, Voytko (63) showed that estradiol modulates visuospatial attention in young ovariectomized cynomologus monkeys tested on a visuospatial cuing task and, more recently, that chronic treatment with estradiol alone or with progesterone enhances visual recognition by middle-aged rhesus monkeys tested on a delayed matching to sample task (64). Treatments did not, however, enhance visual or spatial memory on a delayed response task. Using aged ovariectomized monkeys, Lacreuse et al. (65,66) reported that estradiol enhanced performance on a delayed recognition span task, a task of spatial working memory, but not on a delayed response task or a modified version of a Wisconsin Card Sorting Task. Note that the monkeys in this study were ovariectomized as young adults and remained estrogen deprived for many years before treatment. In a separate study, Rapp et al. (67) showed that long-term cyclical administration of estradiol to aged ovariectomized monkeys reversed a marked age-related impairment on a delayed response task and produced modest improvement on a delayed non-matching-to-sample task but did not prevent deficits on an object discrimination task. In this case, monkeys were ovariectomized at an advanced age, and hormone treatments began approximately 30 wk after ovariectomy.
Hence, in primates as in rodents, estrogen therapy has task-specific effects on cognitive performance. In addition, we begin to see that effects may vary depending on age, timing, and regimen of treatment. The issues of age and timing are discussed in much greater depth in Section VII. Collectively, both the rodent and primate studies suggest that estradiol affects specific neural circuits that underlie performance in a task-selective way.
B. Studies in humans
Human studies also demonstrate positive effects of hormone treatment on performance within specific cognitive domains, although not all studies agree. The human studies have been discussed extensively in recent reviews (6,68), so they are described here only briefly. Several randomized clinical trials have reported positive effects of estrogen therapy on cognitive performance (69,70,71,72,73,74,75,76,77). One study evaluated women treated with estradiol, an androgen, a combination of estradiol + androgen, or placebo after hysterectomy and oophorectomy for benign disease. Women who received placebo showed postoperative declines on tests of short-term and long-term verbal memory and logical reasoning, whereas performance was maintained in women who received any of the hormone treatments (77). Performance also was maintained in women who had a hysterectomy but whose ovaries were retained, suggesting that removal of the ovaries has direct effects on cognitive performance in women. A replication of this study confirmed that treatment of surgically menopausal women with estradiol (10 mg estradiol valerate injected im every 4 wk) maintained performance on tests of short- and long-term verbal memory relative to placebo-treated controls (69). Notably, benefits were not observed on tests of visual memory or spatial abilities. This is consistent with a well-documented sex difference in performance showing a female advantage on verbal memory tasks and male advantage on visual memory and spatial abilities (70). A much smaller trial used a within-subject crossover design to evaluate the effects of short-term (3 d) transdermal estradiol on tests of prefrontal and hippocampal function in naturally menopausal women (age 51–64 yr). In this case, estradiol treatment was associated with improved scores primarily on tests of prefrontal function, including a digit-ordering task, short-term memory of event sequences in an unfamiliar story, a test of selective attention, as well as a test of immediate recall (71).
Using a different approach, Sherwin et al. (72) tested the effects of estrogen depletion and replacement on cognitive performance in relatively young women treated with leuprolide acetate (a GnRH receptor agonist) for benign uterine myoma. As expected, leuprolide suppressed ovarian function and produced a significant decline in ovarian hormones. After 12 wk of treatment, women were randomly selected to receive add-back estradiol or placebo. Performance on tests of verbal memory declined during the period of ovarian suppression and remained low after treatment with placebo, suggesting that suppression of GnRH release and ovarian function had direct effects on performance. Scores improved significantly in women receiving add-back estradiol. This is consistent with the idea that estrogen therapy can restore performance, at least on certain tasks, after loss of ovarian function.
Not all studies agree. A number of randomized clinical trials failed to find any beneficial effects of estrogen therapy on cognitive performance in postmenopausal women. This includes trials of surgically (78,79) and naturally (80,81,82,83,84) menopausal women and tests of attention, working memory, and visual memory. Notably, tests of short-term verbal memory, which appear to be particularly sensitive to estrogen manipulation, were less often used. Hence, one factor that might have contributed to the negative results is the use of tests that rely on neural circuits that are not particularly sensitive to estrogens.
Another factor could be the specific estrogen therapy that was used. Most of the trials that generated positive results treated women with estradiol, whereas most of the negative trials used CEE, which, as mentioned above, contains at least 10 distinct estrogens. Mean circulating levels of estradiol produced by CEE are well below mean physiological levels in normal cycling women (22), and many of the other constituents of CEE, including estrone, are far less active than estradiol at estrogen receptor (ER) α receptors and less effective at mediating interactions between ERα and specific cofactor proteins (12). This suggests that discrepancies in the human vs. animal literatures could well be due to differences in the pharmacology of estradiol vs. CEE (22).
Although the biological efficacy of estrone and other constituents of CEE on neuronal function and cognitive performance is not well characterized, specific neuroprotective effects have been described. For example, work by Brinton et al. (85) shows that CEE can induce neurite outgrowth from hippocampal neurons in culture and protect hippocampal, cortical, and basal forebrain neurons from ß-amyloid and glutamate-induced toxicity (85,86). An evaluation of the major constituents of CEE demonstrated that many are neuroprotective in preventing neuronal membrane damage induced by glutamate toxicity in vitro, and that two (17β-estradiol and Δ8,9-dehydroestrone) are particularly effective at protecting against ß-amyloid-induced intracellular ATP decline (87). Using specific constituents in combination was even more effective (87).
How these effects translate to neuroprotection in vivo and to effects on cognitive performance still needs to be determined. Walf and Frye (88) recently reported that in middle-aged rats, treatment with CEE improved object recognition memory, decreased anxiety, and increased social interaction similar to estradiol, demonstrating that on these tasks CEE and estradiol can have similar effects. Likewise, Acosta et al. (89) recently showed that CEE decreases overnight forgetting on a Morris water maze task, increases acquisition of a delayed match to sample plus maze task, and dose-dependently protects against scopolamine-induced amnesia on this task. CEE also increased the number of choline acetyltransferase (ChAT)-immunoreactive cells detected in the vertical limb of the diagonal band of Broca (DBB), which demonstrates that CEE has an effect on these cholinergic neurons. These data demonstrate that CEE affects cognitive performance in rats similar to estradiol and suggests that effects may be related to effects on basal forebrain cholinergic neurons. It is possible that results could differ somewhat in humans. A recent functional magnetic resonance imaging study compared the effects of CEE vs. estradiol on figural memory and verbal memory, and on hippocampal activation while performing the figural memory task (90). Participants were women (mean age, 58.5 yr) receiving opposed CEE (n = 10), opposed estradiol (n = 4), or no hormone (n = 9). Women receiving either CEE or estradiol showed greater activation of the right hippocampus during retrieval of previously studied vs. novel line drawings, demonstrating efficacy of both hormone treatments on this measure. Women receiving CEE, however, performed significantly worse on the verbal memory task compared with controls, whereas women receiving estradiol performed comparable to controls. Although the sample sizes are small, this clearly demonstrates that differences in the effects of CEE vs. estradiol on performance can be task specific as well as dissociated from effects on hippocampal activity. Hence, the question of whether discrepancies in the human vs. animal literatures can be explained by differences in the pharmacology of CEE vs. estradiol is complex and is far from settled.
Other important factors are age and time after the loss of ovarian function before the initiation of treatment. Positive effects have more often been observed in younger subjects treated soon after surgical menopause or after chemically-induced ovarian suppression. Verbal memory in particular may benefit from early hormone therapy. In six of six randomized clinical trials involving women younger than age 65, verbal memory was enhanced more with estrogen-containing therapy than with placebo (69,73,74,75,76,77). In contrast, of two randomized clinical trials involving women over age 65, one showed no benefit in verbal memory (81), and another showed a trend toward harm (84). These findings are consistent with animal studies showing task-specific effects of estrogens on performance within specific cognitive domains and suggest that positive effects may decrease with age and time after loss of ovarian function.
IV. The Role of Basal Forebrain Cholinergic Neurons in Cognitive Performance
The idea that estrogens might influence cognitive performance by affecting cholinergic neurons in the basal forebrain was introduced more than 25 yr ago by the influential studies of Luine and colleagues (91,92,93). Basal forebrain cholinergic projection neurons are organized into clusters that begin rostrally in the medial septal nucleus (MS), extend caudally and laterally through the DBB, the magnocellular preoptic nucleus, the substantia innominata (SI), and then further caudally into the ventral pallidum where clusters of large cholinergic neurons are referred to as the nucleus basalis magnocellularis (NBM) (94,95). Neurons in the MS, DBB, and NBM are the primary source of cholinergic inputs to the hippocampus and cerebral cortex (94,95). The hippocampus receives cholinergic inputs primarily from the MS and the vertical limb of the DBB, whereas neocortex, including medial prefrontal cortex, receives cholinergic inputs primarily from the magnocellular preoptic nucleus, SI, and NBM (96,97). Subsets of cholinergic neurons in the DBB and SI also project to the olfactory bulbs, thalamus, and amygdala.
Cholinergic projections to the hippocampus and cerebral cortex are well documented to play an important role in learning, memory, and attentional processes (98,99), and there is strong evidence that impairments in basal forebrain cholinergic function contribute to age-related cognitive decline and to the behavioral and psychological symptoms of dementia (100,101,102). Initially, Drachman and co-workers (103,104,105) demonstrated that systemic administration of the cholinergic blocker scopolamine (a muscarinic receptor antagonist) produced deficits in humans, consistent with the suspected role of central cholinergic projections in learning and memory. Subsequent studies on nonhuman primates demonstrated similar delay-dependent memory impairments after systemic administration of either scopolamine or atropine (a muscarinic receptor blocker), comparable to memory deficits seen in normal aged monkeys (106,107,108,109,110). Notably, deficits were not produced by methylscopolamine (109), a muscarinic antagonist that does not cross the blood-brain barrier, suggesting that the impairments observed were due specifically to the disruption of central cholinergic processes.
A. Alzheimer’s disease
Evidence for the specific involvement of basal forebrain cholinergic projections in the etiology of memory dysfunction grew significantly after the discovery that Alzheimer’s disease (AD) is associated with a significant loss of cholinergic neurons in the MS and NBM (111,112,113,114). AD is an age-dependent neurodegenerative disease characterized by a progressive neuropathology with a corresponding loss of learning and memory and other cognitive processes. One of the most consistent neurotransmitter abnormalities associated with the disease is the degeneration of cholinergic neurons in the nucleus basalis of Meynert and the loss of cholinergic inputs to the neocortex and hippocampus (115). Numerous studies have shown decreases in ChAT, acetylcholine (ACh) release, acetylcholinesterase, as well as nicotinic and muscarinic receptors in the cerebral cortex and hippocampus of postmortem AD brains (116,117,118,119). These cholinergic deficits have been shown to correlate positively with the cognitive impairments observed in AD (reviewed in Ref. 115). Many of these markers appear to be preserved, however, during early stages of AD (120,121,122). This does not mean that the cholinergic projections are functioning normally. Indeed, disturbance of axonal transport in cholinergic neurons has been identified as one of the earliest signs of disease in humans and in transgenic mice (123). Geula (124) reported a loss of calbindin (a calcium binding protein) in basal forebrain cholinergic neurons that corresponds with the appearance of neurofibrillary tangles before manifestation of dementia. There is also evidence for early impairment of presynaptic cholinergic receptors and cholinesterase activity in the cerebral cortex of patients with mild dementia (125). Collectively, these studies suggest that a progressive decline in basal forebrain cholinergic function, culminating in the loss of cholinergic neurons, contributes significantly to cognitive impairment associated with advanced age and with AD-related dementia.
Animal studies are consistent with these findings. Specific mouse models of AD have been shown to develop deficits in basal forebrain cholinergic function (particularly muscarinic receptor signaling), in addition to deficits in hippocampal function and cognitive performance (126,127,128). These effects may be due in part to effects of β-amyloid on the JAK2/STAT3 signaling pathway (129).
B. Cholinergic lesion studies
Studies show that cholinergic lesions in the MS and NBM as well as selective muscarinic antagonists impair performance on a variety of cognitive tasks. Early studies focused on working memory deficits produced by scopolamine or by neurochemical lesions in the MS and NBM as assessed using Morris water maze, radial arm maze, and simple T-maze alternation tasks (see Ref. 99 for review). Many of the neurochemical lesions were not entirely selective, however, and more recent studies have shown that cholinergic deficits cannot account for all of the cognitive deficits observed. Using much more selective lesioning methods, several studies have shown relatively little effect of cholinergic lesions on working memory performance (130,131,132,133); however, significant and reproducible deficits in visual attention have been observed (134,135,136). It is now well accepted that cholinergic projections from the NBM to the frontal cortex play an important role in attentional processes (137,138). One recent study also showed a substantial increase in perseverative behavior after basal forebrain cholinergic lesions (139). At present, the full range of cognitive deficits associated with cholinergic impairment is not altogether clear.
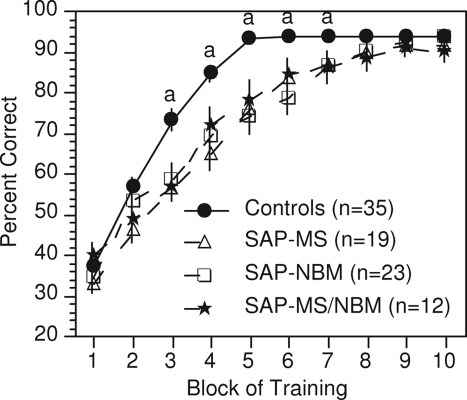
Because of our interest in the effects of estradiol on DMP acquisition, we recently evaluated the effects of cholinergic lesions in the MS and NBM on acquisition of the DMP task (140,141). Rats received selective cholinergic lesions in the MS, the NBM, or both the MS and NBM. Results showed that cholinergic lesions in either brain region (resulting in cholinergic denervation of either the hippocampus or the frontal cortex) increased significantly the number of trials required for rats to learn the task (Fig. 3). Once animals had learned the task, increasing the intertrial delay did not differentially affect task performance, suggesting that the learning deficit was not due to an effect on short-term spatial memory. Nor did the individual lesions appear to affect ultimate strategy selection. What the lesions did affect, however, was the predisposition to adopt a persistent turn, defined as selecting the same arm of the maze in 15 of 16 trials over a 2-d period. This could be interpreted as an increase in perseveration. The duration of this behavior also was affected, suggesting difficulty in switching from this behavioral pattern to a more effective strategy. The net result is that rats required more time to reach criterion.
Figure 3.
Effects of cholinergic lesions on acquisition of the DMP task. Ovariectomized rats received injections of 192IgG-saporin (SAP) into the MS, the NBM, or both to produce selective cholinergic lesions in each brain region. Values indicate mean percentage correct ± sem. Each block represents three consecutive days of training. a, Controls differ significantly from all other groups; P < 0.05. [Adapted from R. B. Gibbs and D. A. Johnson: Neurobiol Learn Mem 88:19–32, 2007 (140) © Elsevier].
Rats can use a variety of strategies to solve spatial navigation tasks (142). Place learning refers to acquisition of a navigational task by means of an allocentric strategy in which extramaze visual cues are used to identify which direction is correct. Response learning refers to acquisition of a similar navigational task by means of an egocentric strategy in which rats use internal kinetic cues to identify which direction is correct. Using the DMP task, Fitz et al. (143) showed that when rats begin to perseverate (e.g., adopt a persistent turn), they use a response-based strategy; however, they ultimately abandon this strategy and adopt a place-based strategy to reach criterion. This results in a significant increase in the number of trials required to learn the task. Hence, deficits in DMP acquisition produced by cholinergic lesions appear to be due to an increase in perseveration as well as a decrease in cognitive flexibility (i.e., the ability to shift from a response strategy to a more effective place strategy). Notably, this effect could be completely abolished by providing a mild stress shortly before training each day (144), which suggests that the development of a persistent turn is related to arousal or attention.
C. Effects on visuospatial attention and stimulus processing
The idea that cholinergic lesions impair DMP acquisition by affecting visuospatial attention, and thereby the predisposition to use a place vs. a response learning strategy, makes sense when one considers the effects of muscarinic antagonists and cholinergic lesions on visuospatial attention and stimulus processing. For example, performance of monkeys on a visuospatial cuing task is sensitive to cholinergic manipulation, and studies have shown that both scopolamine administration and cholinergic lesions disrupt performance on this task (145,146,147). Similarly, cholinergic lesions of the NBM disrupt sustained visual attention in rats (134). More recently, cholinergic projections to the neocortex have been shown to enhance signal-to-noise ratios for sensory processing (148,149,150,151). In addition, evidence suggests that cholinergic inputs enhance the maintenance of selective attention and modulate the effects of sensory interference in a manner that interacts with stimulus salience (152,153). According to Furey et al. (153), “the manipulation of cholinergic activity modulates the relative salience of competing stimuli during selective attention to result in specific and selective effects on performance.” For example, when performing a selective visual attention task, scopolamine increased reaction time to relevant stimuli, and physostigmine (a cholinesterase inhibitor) decreased reaction time to relevant stimuli, but only when the stimulus was the less salient of two competing stimuli. This suggests that ACh release increases the relative salience of sensory cues in a manner related to the behavioral relevance of the cues. Viewed in this context, it follows that cholinergic lesions of the MS and NBM should result in a decrease in the salience of relevant sensory and temporal/spatial cues. In the case of a navigational task such as the DMP task, use of a place strategy relies on the ability to identify and incorporate extramaze visual and spatial cues into an effective learning strategy. Any decrease in the saliency of relevant visuospatial cues would likely result in a greater reliance on egocentric learning strategies (i.e., strategies that do not rely on extramaze visual cues). In the case of the DMP task, this results in an increase in perseveration and in the adoption of a response-based turning strategy, thereby delaying acquisition. This interpretation is consistent with the idea that cholinergic impairment reduces the ability to utilize allocentric visual cues, thereby increasing the reliance on egocentric learning strategies.
This hypothesis, which has not yet been tested, provides some provocative clues as to how estrogen therapy may affect performance on certain tasks, particularly navigational tasks that rely on specific learning strategies. For example, assuming that estradiol enhances cholinergic function and that enhancing cholinergic function favors the use of a place-based learning strategy, then one would predict that estradiol treatment would enhance performance on tasks that benefit from such a strategy. Two studies have, in fact, demonstrated that ovariectomy impairs and estradiol restores performance on a place learning task (154,155). Conversely, one would predict that estradiol would have little effect on a spatial task that does not rely on extramaze visual cues. Wang et al. (61) recently showed that estradiol impairs rather than enhances performance on an operant test of delayed spatial alternation. Likewise, the configural association task (described in Section III.A), which is not affected by ovariectomy and estrogen treatment, also does not rely on extramaze visual cues. This is in contrast to the positive effects detected on navigational and visual recognition tasks. We hypothesize that these differences relate directly to estradiol effects on basal forebrain cholinergic function.
V. Effects of Estrogen Therapy on Basal Forebrain Cholinergic Function
Many studies have shown that ovariectomy and estrogen therapy significantly affect basal forebrain cholinergic function. Luine (91) reported that 10 d of continuous estradiol treatment produced a significant increase in ChAT activity in the medial aspect of the horizontal limb of the DBB, the frontal cortex, and CA1 of the dorsal hippocampus. O'Malley et al. (156) subsequently reported that 3 wk after ovariectomy, there was a significant decrease in high-affinity choline uptake (HACU) in the rat frontal cortex relative to gonadally intact controls, and that this effect was reversed by estradiol. HACU reflects activity of the high-affinity choline transporter located on the presynaptic membrane of cholinergic terminals. The activity of this transporter regulates choline uptake at the synapse, which in turn regulates the levels of presynaptic choline (157,158). In addition, the activity of this transporter varies in association with ACh release (159). When cholinergic neurons are active, the availability of choline becomes a rate-liming factor in the production of ACh (158). Therefore, increases in HACU indirectly reflect an increase in both ACh production and release.
Additional studies have since confirmed that estradiol increases HACU in both hippocampus and frontal cortex (160) and also increases levels of ChAT mRNA and protein (160,161,162,163,164,165) and trkA mRNA within cholinergic neurons (166,167), and the density of cholinergic fibers in specific regions of prefrontal cortex (168,169). Increases in ChAT mRNA typically range between 20 and 30%, whereas increases in HACU have been reported to range from 46% in the hippocampus to 82% in the frontal cortex (164). Levels of ChAT mRNA in the MS and NBM also fluctuate over the course of the estrous cycle (170), suggesting that changes in circulating gonadal hormones contribute to normal physiological fluctuations in basal forebrain cholinergic function. In response to an acute injection of estradiol, peak levels of ChAT mRNA were detected after 24 h in the MS and after 72 h in the NBM (170). Progesterone enhanced the effects of estradiol by decreasing the time to achieve peak levels of ChAT mRNA in the NBM and by prolonging the effects of estradiol on ChAT mRNA in the MS. These findings suggest that both estradiol and progesterone play a role in the normal physiological regulation of basal forebrain cholinergic neurons.
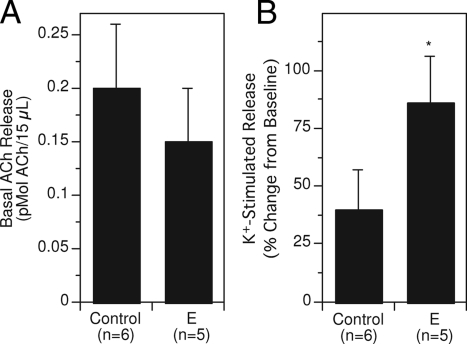
Consistent with the effects on ChAT and HACU, more recent studies have documented the ability of estradiol to increase potassium-stimulated ACh release in the hippocampus of young ovariectomized rats (47,171,172). This is one of the more robust effects of estradiol on cholinergic function. Estradiol produces a 2-fold increase in potassium-stimulated ACh release that persists with long-term treatment (Fig. 4). Estradiol does not appear to affect basal ACh release.
Figure 4.
Effects of estradiol treatment on basal (A) and potassium-stimulated (B) ACh release. Ovariectomized rats received estradiol (E; SILASTIC capsule containing 17ß-estradiol and implanted sc) or a control treatment (empty SILASTIC capsule implanted sc). After several weeks, in vivo microdialysis was used to assess basal and potassium-stimulated ACh release. Note that E-treatment had no significant effect on basal release but increased potassium-stimulated release approximately 2-fold. Panels A and B represent data from the same sets of rats. *, P < 0.05 relative to controls. [Adapted from R. Gabor et al.: Brain Res 962:244–247, 2003 (171) © Elsevier].
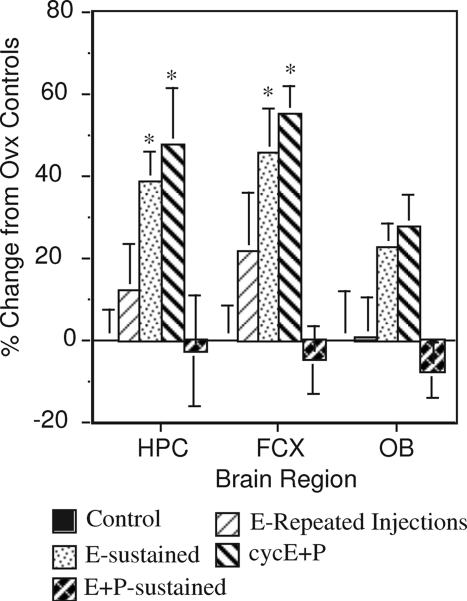
Some of the effects on cholinergic measures also are dependent on the dose and duration of estrogen treatment. For example, increased numbers of ChAT-like immunoreactive cells were detected in the MS and NBM after short-term treatment with low levels of estradiol, but not after longer-term treatment or treatment with higher doses (161,162). Effects also can vary based on the regimen of treatment. For example, one study (160) showed that the effects of 2 wk of estradiol treatment on HACU in the hippocampus and frontal cortex were greatest in rats receiving continuous estradiol, or repeated (cyclical) injections of estradiol + progesterone, and were less in rats receiving repeated injections of estradiol alone (Fig. 5). In contrast, continuous simultaneous administration of estradiol + progesterone produced no increase in HACU. Likewise, we have shown that daily oral administration of CEE + MPA produced no significant increase in ChAT in the hippocampus or frontal cortex of cynomologus monkeys (173). Note that this regimen is most comparable to the combination regimen used in the Women’s Health Initiative trial. Two other studies found that sustained estradiol treatment was more effective than intermittent treatment at enhancing performance on a radial arm maze task by young adult rats (174) and a water-based radial arm maze task by aged mice (175), whereas Bimonte-Nelson et al. (176) reported beneficial effects of both low-dose tonic and intermittent treatment on a Morris water maze task. This is an understudied area, and more information about the importance of dose and regimen of treatment to effects on performance is needed.
Figure 5.
Effects of different hormone treatment regimens on HACU in the hippocampus, frontal cortex, and olfactory bulbs. Ovariectomized rats were treated for 2 wk with either repeated injections of estradiol (one sc injection of 10 μg 17ß-estradiol bezoate in sesame oil every 3 d), sustained estradiol (3 mm SILASTIC capsule containing 17ß-estradiol crystals implanted sc), cyclical administration of estradiol + progesterone (repeated administration of 17ß-estradiol benzoate followed 2 d later with a single administration of 500 μg progesterone on a repeating 3-d cycle), or sustained simultaneous administration of estradiol + progesterone (one 3 mm SILASTIC capsule containing 17ß-estradiol and two 3 mm SILASTIC capsules containing progesterone implanted sc). Controls received implants of empty capsules. All rats that received SILASTIC capsules also received repeated injections of sesame oil on the same 3-d schedule as the other groups. n = 5–6 rats/group. Note that continuous estradiol and cyclical treatment with estradiol + progesterone had the greatest positive effects, whereas simultaneous continuous administration of estradiol and progesterone produced negative effects, albeit not significant. *, P < 0.05 relative to controls. [Adapted from R. B. Gibbs: Neuroscience 101:931–938, 2000 (160) © Elsevier].
VI. Evidence That Effects on Basal Forebrain Cholinergic Projections Are Relevant to Estrogen Effects on Cognitive Performance
Converging evidence suggests that the effects of estradiol on basal forebrain cholinergic function play an important role in mediating effects on cognitive performance. In addition to the effects on place and response learning mentioned in Section IV.B, studies show that decrements in T-maze and radial arm maze performance induced by either systemic or intrahippocampal administration of scopolamine can be reduced or prevented by estradiol (41,42,49). Dumas et al. (177) recently reported similar results in humans, such that estradiol reduced cognitive impairments produced by scopolamine and mecamylamine (a nicotinic receptor antagonist) in postmenopausal women. Further study confirmed that estradiol reduced the effects of anticholinergic drugs on a test of episodic memory, but only in younger postmenopausal women (age 50–62 yr) (178). Packard (179) showed that memory-enhancing effects produced by injecting estradiol directly into the hippocampus of rats can be blocked by systemic administration of scopolamine, thereby demonstrating cholinergic modulation of estrogen effects in the hippocampus. Marriott and Korol (180) showed that effects of estradiol on ACh release in the hippocampus correlated with effects on place learning. These studies demonstrate that many effects of estradiol on cognitive performance involve an important interaction with basal forebrain cholinergic projections.
Voytko (63) showed that effects of scopolamine on a visuospatial cuing task were greater in young adult ovariectomized monkeys treated with estradiol than in monkeys treated with placebo. In this case, scopolamine affected the disengaging component of the visuospatial cuing task, which relies on the integrity of the posterior parietal cortex (181,182). In contrast, the tasks that have been evaluated in rats and mice rely heavily on either hippocampal or prefrontal circuits. This suggests that the interaction between estradiol and cholinergic afferents with respect to cognitive performance also is task specific and may vary according to the specific brain regions involved.
Some have argued that effects of estrogens on cognitive performance are more directly related to effects on hippocampal connectivity and function. It is true that estradiol has many well-documented effects on hippocampal structure and function. Some of these effects include increasing the number of dendritic spines and new contacts on the apical dendrites of CA1 pyramidal cells, increasing N-methyl-d-aspartate (NMDA) receptor expression and responses in CA1 neurons, increasing long-term potentiation and decreasing long-term depression at CA1 synapses, increasing presynaptic proteins synaptophysin and syntaxin and postsynaptic proteins spinophilin and PSD-95 in the CA1 region, and activating microtubule-associated kinases and cAMP response element binding protein (CREB) (reviewed in Refs. 2 and 183). There is evidence that the number of dendritic spines on CA1 dendrites is affected via modulation of γ-aminobutyric acid (GABA)-mediated inhibitory input, resulting in the disinhibition of CA1 pyramidal cells (184). Notably, this effect is reduced significantly by cholinergic denervation (185). Studies also suggest that the effects of estradiol on dendritic spines and NMDA receptors in CA1 are mediated via cholinergic inputs. For example, Lâm and Leranth et al. (186) showed that cholinergic lesions in the MS prevent the estradiol-mediated increase in spine density on CA1 dendrites, and Daniel and Dohanich (187) showed that treating rats with an M2 muscarinic receptor antagonist blocks the ability of estradiol to increase NMDA receptor binding in CA1. This was associated with a loss of estradiol’s ability to enhance working memory performance on a radial arm maze. Conversely, treating with a cholinesterase inhibitor increased NMDA receptor binding in CA1 independent of estradiol. Daniel et al. (188) subsequently showed that direct administration of an M2 muscarinic receptor antagonist into the hippocampus of ovariectomized rats significantly reduced the ability of estradiol to enhance working memory performance on a Morris water maze task. These data suggest that specific effects of estradiol on hippocampal structure and function are mediated, at least in part, by cholinergic inputs, and that ACh acting at M2 muscarinic receptors located in the hippocampus plays an important role in mediating the positive effects of estradiol on working memory. Significant effects of estradiol on dendritic spine density in the prefrontal cortex of both rats and monkeys also have been described (189,190,191). Estradiol increases the density of cholinergic and monoaminergic fibers in prefrontal cortex (168,169); however, whether the effects on spine density in the prefrontal cortex rely upon specific cholinergic or monoaminergic inputs has not yet been investigated.
Recently, we tested whether cholinergic projections from the MS are necessary for hormone-mediated enhancement of DMP acquisition (48,192). Our data show that cholinergic lesions block the ability of estradiol alone, as well as estradiol + progesterone, to enhance DMP acquisition in rats. Based on our analysis of this and other cholinergic lesion studies and on the importance of cholinergic inputs to visuospatial processing, we hypothesize that estradiol enhances DMP acquisition by enhancing the facility to incorporate extramaze visuospatial information into an effective learning strategy, which in turn increases cognitive flexibility (e.g., the ability to switch strategies) and decreases perseveration. On navigational tasks, this is reflected by an increase in the predisposition and ability to use place vs. response learning strategies (154,155,193).
Evidence for effects of estrogen therapy on cognitive flexibility and perseveration has also recently been described in nonhuman primates. Voytko et al. (194) evaluated the effects of estradiol and estradiol + progesterone on visuospatial attention and the ability to perform a cognitive set switching task in middle-aged ovariectomized rhesus monkeys. These were the same monkeys in which both hormone therapies were shown to enhance performance on a delayed matching to sample task, but not on a delayed response task (64). Set shifting was evaluated using a modified Wisconsin card sort task, and visuospatial attention was evaluated using a visual cued reaction time task. Ovariectomy produced significant deficits on both tasks, consistent with the idea that loss of ovarian function disrupts processes involved with visuospatial attention. The set-shifting impairment was associated with a significant increase in perseverative errors (similar to effects of cholinergic lesions on DMP acquisition) resulting in increased time to acquire a new set. The authors comment that this may be due either to an inability to release attention from a relevant perceptual dimension (resulting in perseveration) or to an inability to refocus attention to a previously irrelevant perceptual dimension (cognitive flexibility). Notably, treatment with either estradiol or estradiol + progesterone significantly reduced the impairment in set-shifting ability. This is consistent with the role of corticopetal cholinergic projections in modulating attention to biologically relevant visuospatial stimuli and with the idea that estrogens can affect components of executive function by interacting with these cholinergic projections. Note that postmenopausal women receiving hormone therapy likewise demonstrate better performance on the Wisconsin card sorting task than women not receiving therapy (9,195,196), although the specific ability to shift visuospatial attention has not been tested. One study showed that hormone therapy for up to 10 yr was associated with fewer perseverative errors on the task as well as preservation of gray matter in prefrontal cortex, both of which were dependent on the duration of treatment and the degree of aerobic fitness (9). Another study showed that estrogen therapy reduced perseveration during verbal recall in recently postmenopausal women (197).
Collectively, these studies provide strong evidence that effects of estradiol on cognitive performance involve important interactions with basal forebrain cholinergic projections and that the behavioral effects relate in part to effects on visuospatial attention and the predisposition to use specific learning strategies.
A. Relevance to navigational performance in humans
Corresponding effects of gonadal hormones in humans may account for some of the differences in navigational performance that have been observed in women vs. men. For example, Astur et al. (198,199) reported substantial sex differences in performance of a virtual water maze task, which reflect strong male-female differences in navigational strategy. Newhouse et al. (200) showed that these differences can be detected even in childhood before puberty, suggesting that the differences may be due to an organizational effect of gonadal hormones on brain development. Sandstrom et al. (201) showed that when navigating a virtual maze, females rely predominantly on landmark information, whereas males rely on both landmark and geometric information. Saucier et al. (202) tested the abilities of men and women to follow either landmark- or Euclidean-based instructions during a navigation task. Men performed best when using Euclidean information, whereas women performed best when using landmark information, suggesting a sex difference in the ability to use these two types of spatial information. Grön et al. (203) reported that during visuospatial navigation, males showed greater activation of the left parahippocampal gyrus, whereas females showed greater activation of the right parietal and prefrontal cortex (Broadman’s area 9/46). Grön hypothesized that activation of the prefrontal cortex in females reflects the working memory demand to hold landmark cues “on-line,” whereas activation of the left parahippocampal gyrus in males reflects their reliance on geometric information. This is consistent with the idea that sex differences in performance reflect differences in predisposition toward certain learning strategies, based on the selective activation of hippocampal and cortical circuits. We hypothesize that estradiol biases these predispositions by affecting the cholinergic modulation of the corresponding circuits.
Recent studies suggest that loss of cholinergic inputs abolishes the ability of estradiol to produce these effects. Hence, as cholinergic function declines (e.g., with advanced age or AD), one would predict that beneficial effects of estradiol on cognitive performance also decline. As discussed in Section VII, this has important implications for the potential use of estrogen therapies in postmenopausal women to prevent or reduce cognitive decline associated with aging and AD.
VII. Estrogen Therapy for the Prevention and Treatment of Cognitive Decline Associated with Aging and AD
Clearly, loss of ovarian function and hormone replacement can significantly affect cognitive performance in both animals and humans. An important question is whether hormone therapies also can prevent or reduce the development of cognitive impairment associated with aging and neurodegenerative disease. Animal studies show that treatment with estradiol, or in some cases with a cyclical regimen of estradiol + progesterone, can prevent or reduce age-related cognitive decline on specific tasks (46,60,67,204,205,206,207,208,209) (also reviewed in Ref. 4). Human observational studies also suggest that postmenopausal hormone therapy can significantly reduce the risk of AD-related dementia in women, although prospective randomized clinical trials, most notably the WHIMS trial, have been less promising (reviewed in Ref. 210). The ability of hormone therapy to improve cognitive measures in women diagnosed with AD likewise has been less promising, with three studies reporting no lasting effects of estrogen therapy on cognitive performance (211,212,213). In each of these studies, estrogen therapy consisted of daily oral CEE, and the cognitive assessments, although appropriate for assessing global dementia, were not ideal for assessing effects in discrete cognitive domains. Failure to achieve adequate levels of estradiol may have been particularly critical (21,22,214). Even with a high dose of CEE (1.25 mg/d), circulating levels of estradiol can approach a mean of only approximately 40 pg/ml in some women, which is far less than the levels attained over the course of a normal menstrual cycle (mean levels, ∼70–100 pg/ml; peak levels, ∼300 pg/ml) (22). Wolf et al. (214) evaluated the effects of short-term (2 wk) transdermal estradiol on cognitive performance in a small group of elderly healthy women. No overall effects of treatment were observed; however, within the estradiol-treated group, those subjects who reached higher estradiol levels (>29 pg/ml; mean, 51.9 pg/ml) had significantly better verbal memory scores than subjects with lower levels. In this case, women were postmenopausal for an average of 17 yr before entering the study, which may have limited the effectiveness of the estrogen treatment. A separate small, placebo-controlled, double-blind study showed that transdermal estradiol (0.05 mg/d) administered to women with AD significantly improved performance on both attention and verbal memory tasks and that these effects correlated with plasma concentrations of estradiol (215). A follow-up study using a higher dose of estradiol (0.1 mg/d) reported similar effects on measures of verbal memory, semantic memory, and visual memory (216). These findings suggest that beneficial effects of estrogen therapy on specific cognitive measures can be achieved in women with early stages of AD when an appropriate dose and regimen of estradiol is used. More recently, two observational studies reported that women with a genetic risk factor for AD (APOEε4 positive genotype) who received long-term, low-dose estradiol + progesterone showed preservation of hippocampal volume and hippocampal metabolism relative to subjects that did not receive hormone therapy (10,217). This is consistent with the animal literature showing that estrogen deficiency exacerbates and estradiol treatment reduces β-amyloid deposition and neuropathology in mouse models of AD (218,219,220). These findings are the latest to offer a direct link between menopausal hormone therapy and protection from early brain changes associated with AD. Another factor that may play a critical role in affecting the ability of hormone therapy to confer protection from aging- and AD-related cognitive decline is the timing of therapy relative to age and the onset of menopause (210).
A. The importance of timing
Evidence has accumulated that the ability of estradiol to enhance cognitive performance declines as a function of age and time after the loss of ovarian function. Our own studies were the first to show that when rats were ovariectomized at middle-age, hormone therapy initiated either immediately or within 3 months prevented a significant decline in DMP acquisition when tested at advanced age, whereas hormone therapy initiated 10 months after ovariectomy was significantly less effective (204). Similarly, Markowska and Savonenko (205) showed that estradiol treatment initiated in middle-aged and aged rats within 6 months after ovariectomy significantly enhanced performance on a delayed alternation task, whereas treatment initiated 9 months after ovariectomy did not. The importance of timing was shown more definitively by Daniel et al. (46) who reported that estradiol treatment initiated immediately after ovariectomy in rats at either 12 or 17 months of age significantly improved acquisition of an eight-arm radial maze task, whereas treatment initiated at 17 months of age, 5 months after ovariectomy, was not effective. More recently, Talboom et al. (208) reported that the effects of ovariectomy and estradiol treatment on a reference memory version of a Morris water maze task decline with age, and we recently confirmed that the ability of estradiol to enhance acquisition of the DMP task is lost in advanced age when rats are ovariectomized as young adults (50). These findings confirm that the effects of estradiol on cognitive performance decline with age and time after ovariectomy.
Human clinical trials also support the idea that early, but not late, hormone therapy can protect against AD-related dementia in women. Before WHIMS, a 3-yr prospective observational study was conducted in Cache County, Utah (221). A total of 1866 women with a mean age of approximately 74.4 yr were included in the analysis. Eight hundred of these women had never used estrogen therapy. Of those that had used estrogen therapy, most (72%) used an unopposed oral CEE. Analyses showed that past estrogen use, but not current use, was associated with a significant reduction in the risk of developing AD [odds ratio, 0.33; 95% confidence interval (CI), 0.15–0.65]. In addition, the reduction in risk among past users increased with increasing duration of treatment, such that the apparent likelihood of developing AD was reduced 42, 68, and 83% among women who had used estrogen therapy for less than 3 yr, 3–10 yr, and more than 10 yr, respectively. These findings suggest that women who initiated estrogen therapy at an earlier age (i.e., closer to the onset of menopause) and who remained on therapy for 10 yr were far less likely to develop AD than women who never used estrogen therapy or who initiated therapy much later in life.
A more recent study examined cognitive function in elderly women who had participated 5–10 yr earlier in a randomized clinical trial of estrogen therapy for osteoporosis (222). Subjects received estrogen therapy or placebo for a period of 2–3 yr, beginning shortly after menopause, and were evaluated for cognitive performance 5–15 yr later. Results showed that among women who received 2–3 yr of therapy around the time of menopause, the risk of cognitive impairment was decreased by 64% compared with women who received placebo. These results suggest that estrogen therapy administered around the time of menopause can provide long-term protection against cognitive impairment.
Further support for the importance of timing comes from the recent report of the Multi-Institutional Research in Alzheimer’s Genetic Epidemiology (MIRAGE) study (223). This report examined the relation between estrogen therapy used for more than 6 months and AD risk in 971 postmenopausal women. Notably, a significant reduction (65%) in the risk of developing AD was detected, but only in the youngest age tertile (50–63 yr), again suggesting that estrogen therapy initiated during the early postmenopause, but not later in life, may reduce the risk of AD.
Most recently, a reanalysis of the WHIMS data showed that the effects of estrogen therapy on dementia were vastly different for women who reported prior use of estrogen therapy vs. those who did not (224). For example, the likelihood of developing all-cause dementia in prior users was approximately half of that in women who had never used estrogen therapy (pooled adjusted hazard ratio, 0.54; 95% CI, 0.32–0.91), and the risk of developing AD in prior users was less than half of that in never-users (hazard ratio, 0.36; 95% CI, 0.16–0.85).
Collectively, both the animal and human studies suggest that the ability of estrogen therapy to confer protection from cognitive decline decreases with age and time after menopause. In other words, something happens over the course of aging and long-term hormone deprivation that reduces responsiveness to estrogen therapy. This is referred to as the Critical Period Hypothesis and is currently the focus of much study and debate (225,226). We hypothesize that an underlying cause of the critical period is a substantial decrease in basal forebrain cholinergic function.
VIII. The Cholinergic Basis of the Critical Period Hypothesis
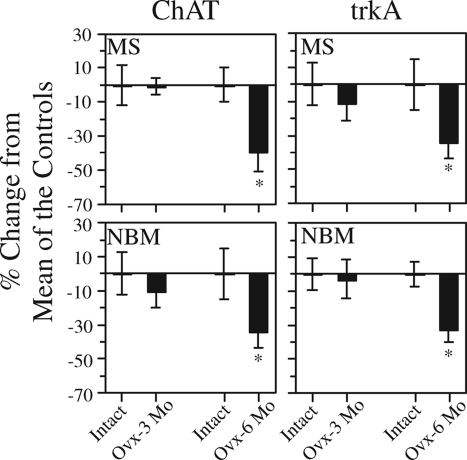
As discussed above, specific effects of estrogen therapy on the hippocampus, as well as beneficial effects on specific cognitive tasks, are lost in response to selective cholinergic lesions or treatment with specific cholinergic antagonists. Many studies have demonstrated significant age-related decreases in cholinergic parameters in the brains of both rodents and nonhuman primates, which correlate with age-related reductions in cognitive performance. Included are decreases in the number and size of cholinergic neurons in the MS, DBB, and NBM (227,228,229,230,231), decreases in HACU in the hippocampus and frontal cortex (232,233), as well as decreases in ACh synthesis (234,235,236), ACh release (237,238,239,240), and cholinergic synaptic transmission (241). In addition, studies show that long-term loss of ovarian function has negative effects on basal forebrain cholinergic neurons beyond the effects of normal aging. In one study, we compared levels of ChAT and trkA mRNA in the MS and NBM of rats killed 3 or 6 months after ovariectomy with levels in age-matched gonadally intact controls (242). TrkA is a nerve growth factor receptor responsible for mediating the trophic effects of nerve growth factor on basal forebrain cholinergic neurons (243). Significant reductions in both ChAT (34- 39%) and trkA (32–34%) mRNA in the MS and NBM of animals killed 6 months (at 19 months of age), but not 3 months (at 16 months of age), after ovariectomy were observed relative to age-matched gonadally intact controls (Fig. 6). A second study showed that in animals killed 16–17 months after ovariectomy (at 29–30 months of age), the levels of trkA mRNA were decreased by 50–60%, and levels of ChAT mRNA were decreased by 29–49%, in the MS and NBM relative to much younger (6-month-old) ovariectomized controls (244). This is consistent with the reductions in ACh release that have been reported in aged rats (240). Note that Chu et al. (245) reported a significant correlation between reductions in trkA mRNA within cholinergic neurons in the NBM and the development of mild cognitive impairment in women. Hence, a contributing cause of mild cognitive impairment could be a reduction in basal forebrain cholinergic function mediated by a loss of neurotrophic support. Our studies suggest that loss of ovarian function exacerbates this effect, leading to even greater loss of cholinergic function with time after menopause.
Figure 6.
Graphs showing that ovariectomy has negative effects on the levels of ChAT and trkA mRNA in the MS and NBM beyond the effects of normal aging. Rats received ovariectomy or sham surgery at 13 months of age and were euthanized 3 or 6 months later. Levels of ChAT and trkA mRNA within cholinergic neurons in the MS and NBM were analyzed by quantitative in situ hybridization histochemistry. Bars represent percentage change ± sem from the mean of the corresponding age-matched gonadally intact controls. Note the decrease in ChAT and trkA mRNA detected at 6 months, but not at 3 months, after ovariectomy. n = 4 and n = 7 gonadally intact rats euthanized at 16 and 19 months of age. n = 7 and n = 5 ovariectomized rats euthanized at 16 and 19 months of age, 3 and 6 months following ovariectomy. *, P < 0.05 relative to age-matched gonadally intact controls. [Adapted from R. B. Gibbs: Exp Neurol 151:289–302, 1998 (242) © Academic Press].
The ability of estradiol to affect basal forebrain cholinergic function also appears to decrease with age and time after loss of ovarian function. For example, the ability of estradiol to increase ChAT and trkA mRNA in young rats (166,167) is significantly reduced in aged ovariectomized rats (244). Effects of estradiol on ChAT protein levels in the hippocampus and frontal cortex of middle-aged rats also change with time after ovariectomy (165). Specifically, treatment with estradiol increased levels of ChAT in the hippocampus, but not the frontal cortex, of middle-aged rats when administered immediately after ovariectomy, whereas the reverse was seen after a 5-month delay (165). These data parallel the effects of ovariectomy and immediate vs. delayed estradiol treatment on ERα protein expression in the hippocampus (246). Our own studies show that estradiol increases ChAT activity in the hippocampus of middle-aged rats that were ovariectomized as young adults but that this effect is lost in aged rats in conjunction with a loss of estradiol effect on DMP acquisition (50).
Hence, animal studies suggest that ovariectomy has negative effects on basal forebrain cholinergic neurons beyond the effects of normal aging and that this exacerbates the effects of age on cholinergic function. In addition, studies suggest that the ability of estradiol to enhance cholinergic function decreases with age and time after menopause. Because the ability of estradiol to enhance performance on certain cognitive tasks relies, at least in part, on these cholinergic projections, it makes sense that the effects on cognitive performance decline with age and time after menopause. We refer to this as the cholinergic basis of the critical period hypothesis—i.e., the critical period is defined in large part by the significant decline in basal forebrain cholinergic function associated with age and with loss of ovarian hormones.
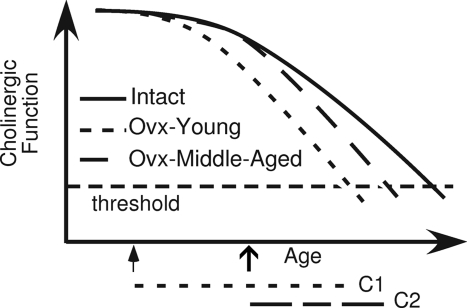
One possible model is illustrated in Fig. 7. This model assumes that cholinergic function is relatively stable in young adulthood but declines steadily after middle-age, and that the cholinergic neurons are further impaired by the loss of ovarian function, both of which are supported by experimental evidence. In addition, the model assumes that loss of estrogen effect occurs when basal forebrain cholinergic function decreases below a critical threshold. In this case, the model predicts that ovariectomy earlier in life would be associated with a longer critical period. This is because the time it takes for cholinergic function to reach threshold will be greater after ovariectomy at a young age than after ovariectomy at middle-age (Fig. 7). In addition, the model predicts that ovariectomy earlier in life would be associated with a greater longitudinal risk for cognitive decline and dementia. This makes sense if one considers that ovariectomy at an earlier age would increase the likelihood that a woman’s basal forebrain cholinergic function will have declined below threshold by a given time in advanced age. This would result in an overall increase in risk for this population. Consistent with this model, McLay et al. (247) reported that early menopause in women, whether due to surgery or to natural menopause, is associated with greater declines in cognitive function later in life. In addition, Rocca et al. (248) reported that ovariectomy before menopause is associated with increased risk for cognitive decline and dementia and that the risk increases as the age at ovariectomy decreases. These findings are consistent with our model and with the idea that the length of the critical period is determined both by age and time after loss of ovarian function.
Figure 7.
Proposed model in which the critical period for attaining beneficial effects of estrogen therapy on cognitive performance is determined by a decline in basal forebrain cholinergic function. The model assumes that cholinergic function declines with age and that the rate of decline is accelerated by loss of ovarian function. Threshold refers to the minimum level of cholinergic function necessary to confer beneficial effects of estrogen therapy on a cognitive task. The critical period is defined as the time between the loss of ovarian function and the decline in cholinergic function to threshold. Based on this model, loss of ovarian function at middle-age (open arrow) is associated with a shorter critical period (C2) than is loss of ovarian function at a younger age (filled arrow; C1). [Adapted from R. B. Gibbs et al.: Horm Behav 56:73–83, 2009 (50) © Elsevier].
Note that whereas data suggest ovariectomy has a significant negative impact on basal forebrain cholinergic function, we did not in fact detect a significant effect of ovariectomy on the total number of cholinergic neurons detected in the MS and NBM of aged rats (244). This suggests that loss of ovarian function does not by itself lead to a loss of cholinergic neurons, but merely a reduction in cholinergic function. If correct, then it is possible that enhancing cholinergic function could restore beneficial effects of estrogen therapy on cognitive performance.
IX. Testing the Cholinergic Hypothesis
Two mechanisms could explain why loss of basal forebrain cholinergic function leads to a loss of estradiol effect on cognitive performance. One possibility is that estradiol enhances performance by increasing cholinergic activity in the hippocampus and cerebral cortex. This is consistent with the many studies showing that ovariectomy impairs and estradiol enhances cholinergic function. Another possibility is that cholinergic activity modulates the effects of estradiol on hippocampal and cortical neurons, enabling estradiol to produce lasting changes in cortical connectivity and function. This is consistent with the ability of estradiol to increase the number of dendritic spines located on the apical dendrites of CA1 pyramidal cells, provided that cholinergic inputs are intact. If the second possibility is correct, then enhancing cholinergic function could restore estrogen effects by enabling estrogens to exert specific effects on hippocampal and cortical circuits.
We have begun to test this by testing whether donepezil, a cholinesterase inhibitor used in the treatment of AD, could restore effects of estradiol on DMP acquisition in aged ovariectomized rats. Rats were ovariectomized at 3 months of age. At middle-age (12–17 months) and advanced age (22–27 months), we then tested the ability of estradiol to enhance DMP acquisition and, most importantly, the ability to restore estradiol effects by administering daily injections of donepezil. Estradiol alone significantly enhanced DMP acquisition in middle-aged rats, but not in aged rats, demonstrating the loss of estradiol effect with advanced age (50). Donepezil alone had no effect on DMP acquisition in either age group; however, donepezil treatment restored the ability of estradiol to enhance DMP acquisition in aged rats (Fig. 8). Whether donepezil restores the ability of estradiol to enhance cholinergic activity is currently being tested. Further analysis showed that aging was associated with a significant increase in the number of rats that adopted a persistent turn during DMP acquisition (50). This is consistent with the effects of cholinergic lesions (discussed in Section IV.B), and is further evidence that a decline in cholinergic function contributes to an age-related deficit on this task. Furthermore, our analysis suggests that the significant effect of donepezil + estradiol on DMP acquisition in aged rats was due entirely to a substantial reduction in perseveration, i.e., in the predisposition to adopt a persistent turn (33.3% of aged rats that received both donepezil + estradiol adopted a persistent turn vs. 92.3% of aged controls). These findings demonstrate the feasibility of using cholinesterase inhibitor treatment to reinstate beneficial effects of estrogen therapy on cognitive performance, even when therapy is initiated many months after loss of ovarian function. Note that this result is consistent with a report by Schneider et al. (249), which showed that postmenopausal women with AD and who also received estrogen therapy responded better to tacrine (a cholinesterase inhibitor formerly used to treat AD) than women not on estrogen therapy. Much more study is needed; however, these findings raise the possibility that cholinergic therapies could reinstate beneficial effects of estrogen therapy on cognitive performance in postmenopausal women, even in older women who have not used estrogen therapy for many years.
Figure 8.
Plots summarizing the effects of donepezil (Don) (3 mg/kg/d administered ip) and estradiol (E) (SILASTIC capsule containing 17ß-estradiol and implanted sc) on the number of days required to reach criterion (DTC) on the DMP task for middle-aged (A) and aged (B) rats. Rats were ovariectomized at 2 months of age, and treatments were initiated at either 12–25 or 22–27 months of age. Bars represent the mean ± sem. Numbers in parentheses indicate group sizes. Note that estradiol reduced DTC in middle-aged rats, but not in aged rats. Donepezil alone had no effect at either age; however, donepezil restored the effect of estradiol on DTC in aged rats. a, Significant main effect of E treatment (P < 0.05). *, P < 0.05 relative to controls and to Don-treated rats. [Adapted from R. B. Gibbs et al.: Horm Behav 56:73–83, 2009 (50) © Elsevier].
X. Cellular Mechanisms Underlying Estrogen Effects on Cholinergic Function and Cognitive Performance
A. Hippocampus
There is still much work to be done toward identifying the cellular mechanisms responsible for the effects of estrogen therapy on cholinergic function and cognitive performance. As mentioned in Section VI, many studies have documented acute effects of estradiol on hippocampal neurons (see Refs. 2 and 183 for reviews), although the extent to which these effects are responsible for the effects of sustained estrogen therapy on the performance of specific tasks is far from clear. Because this review focuses on the cholinergic hypothesis, readers are referred to these other reviews for an extensive discussion of effects on hippocampal function. Two issues, however, are discussed here: 1) the relationship between effects on dendritic spine density in CA1 and spatial memory; and 2) mechanisms underlying effects of estradiol on object recognition memory.
1. Relationship between effects on dendritic spine density in CA1 and spatial memory
Woolley (250) showed that 2 d of estradiol treatment (10 μg/d) in young adult ovariectomized rats produces a significant increase (∼30%) in the number of dendritic spines on apical dendrites of CA1 pyramidal cells, which peaks at approximately 3 d and then decays slowly to baseline over the next 10–14 d. This effect has been replicated in other laboratories and is associated with an increase in excitatory synaptic contacts with these cells, and specifically with an increase in new contacts as opposed to a strengthening of existing contacts (251). Progesterone, administered 24 h after the last injection of estradiol, enhances the effect of estradiol on spine density within 2 h but causes a rapid return to baseline within 24 h. Similar effects of estradiol also have been observed in monkeys (252). As discussed above, evidence suggests that, in rats, these estrogen-induced changes in spine density rely upon forebrain cholinergic inputs.
There is evidence to suggest that effects on spine density underlie effects of hormone therapy on cognitive performance; however, not all studies agree, and the relationship appears complex. Sandstrom and Williams (253) showed that treating rats with estradiol and progesterone produced effects on short-term spatial memory that paralleled the changes in dendritic spine density reported by Woolley. Performance increased in response to estradiol, was greatest 2–3 d after administration, and then decreased gradually toward baseline over the next 5 d. When progesterone was administered 24 h after 2 consecutive days of estradiol, performance again increased in response to estradiol, was greatest in animals tested several hours after progesterone administration when CA1 spine density is at its peak, and returned to baseline 24 h later when spine density is reduced. The time-course of these events suggests that increases in dendritic spine density in CA1 may underlie effects of estrogen therapy on short-term spatial memory.
McLaughlin et al. (254) likewise showed that estradiol, administered as two 10 μg pulses 72 and 48 h before testing, increased spatial memory on a placement recognition task and increased spine density on the apical, but not the basal, dendrites of CA1 pyramidal cells. This same regimen, however, did not improve spatial memory on a Morris water maze task. A lower dose of estradiol (two 5 μg pulses administered 72 and 48 h before testing) improved performance on the water maze task; however, this regimen did not increase spine density on the apical dendrites of CA1 pyramidal cells. In fact, this regimen decreased spine density on the basal dendrites of these cells. Neither regimen affected performance on a Y-maze task.
Frick et al. (255) also evaluated the effects of estradiol on spatial memory and on spine density in CA1. Contrary to McLaughlin et al. (254), they reported that 10 μg estradiol administered 72 and 48 h before behavioral testing impaired spatial memory during acquisition of a Morris water maze task, and did not increase spine density on the apical dendrites of CA1 pyramidal cells. Further study showed that estradiol did increase spine density in behaviorally naive rats, but not in rats trained on the water maze, suggesting that training mitigated the effect of estradiol on dendritic spines.
Collectively, these data demonstrate that the relationship between effects on spine density and cognitive performance is not straightforward and can be very complex. We conclude from this that a better mechanistic understanding of the relationship between spine density in CA1 and performance on specific tasks is needed, and that one cannot assume that effects on spines will translate to a positive or negative effect on a specific cognitive task.
2. Mechanisms underlying effects of estradiol on object recognition memory
Several laboratories have shown that estradiol, as well as specific regimens of estradiol + progesterone, can enhance memory consolidation using a novel object recognition task in mice (56,256,257). This task has been very useful because it clearly involves memory consolidation and requires only one trial for learning. Recent studies by Frick and co-workers (55) have shown that effects of estradiol and progesterone on object recognition memory are mediated in the dorsal hippocampus and involve rapid activation of the p42 isoform of ERK. In addition, the effect was produced by intrahippocampal injection of estradiol bound to BSA. Estradiol-BSA does not cross the cell membrane, suggesting the involvement of membrane-associated ERs. Effects on NMDA receptors and the activation of protein kinase A also have been implicated (258). These data suggest that estradiol acting at the cell membrane of hippocampal cells enhances memory consolidation for a novel object in mice via a mechanism dependent on the activation of MAPK signaling, protein kinase A signaling, and NMDA receptor function. Interestingly, treatment with estradiol + progesterone, which also enhanced memory consolidation for novel objects, was not associated with increased phosphorylated ERK in the dorsal hippocampus (57), suggesting that adding progesterone either alters the time-course of ERK activation or invokes a different mechanism for enhancing memory consolidation on this task. Whether cholinergic inputs play a role in any of these responses is unknown.
B. The role of specific estrogen receptors
Effects of estradiol are mediated via binding to specific receptors that, when activated, either activate specific signaling pathways or affect the expression of specific genes. This discussion would be incomplete without some mention of the receptors that are responsible for mediating these effects.
1. ERα and ERß
Two nuclear ERs have been identified, ERα and ERß (259,260). These receptors are part of a large superfamily of nuclear receptors that regulate gene transcription, but which also can associate with specific membrane compartments and activate specific second messenger signaling pathways such as MAPK, CamKII, and CREB (261,262). Both receptors are expressed in the brain in a variety of isoforms, studied mainly at the mRNA level (263,264,265). In the adult brain, ERα is highly expressed in areas of the hypothalamus that are responsible for reproduction, as well as in areas involved in learning, memory, emotionality, and attention such as the hippocampus and amygdala. ERß is more widely distributed throughout the brain and, in addition to the hypothalamus, hippocampus, and amygdala, is expressed in many regions of the neocortex (266,267,268). Understanding which receptors are involved in mediating the effects of estradiol on cognitive function is important because it will lead to the development of highly selective ER modulators that can provide beneficial effects on brain in the absence of adverse side effects on other tissues such as heart, breast, and endometrium.
A small number of studies have attempted to identify which of the ERs is involved in mediating effects of estradiol on specific cognitive tasks. Results are somewhat conflicting. For example, Walf et al. (269) reported that diarylpropiolnitrile (DPN; an ERß-selective agonist) enhances object recognition memory in wild-type mice, but not in ERß knockout mice, demonstrating a selective role for ERß on this task. In contrast, Frye et al. (54) reported that propylpyrazole triol (PPT; an ERα-selective agonist), but not DPN, enhances performance on a placement recognition task (a spatial task) in rats. This suggests differential involvement of ERß and ERα in circuits supporting memory for objects vs. those supporting memory for spatial locations. Rhodes and Frye (270), however, reported that DPN was effective at enhancing memory for the location of a hidden platform in a water maze, whereas PPT was not. Several studies have shown that activity at ERß can reduce measures of anxiety and depression (271,272,273,274,275), and some of the cognitive tasks that have been used (e.g., inhibitory avoidance, contextual fear conditioning, water maze tasks) include a significant anxiety or stress-related component. Recently, we showed that both PPT and DPN enhance acquisition of the DMP task in rats, similar to effects of estradiol (276). Whether any of these effects are related specifically to effects on basal forebrain cholinergic function still needs to be determined.
With respect to mechanisms by which estradiol affects the cholinergic neurons, studies show that approximately 30% of cholinergic neurons in the MS express ERα, but not ERß (277,278). Using cultures of primary embryonic basal forebrain, Pongrac et al. (279) showed that estradiol increases HACU in these cultures via activation of MAPK (279). In addition, this effect was blocked by ICI182,780, which inhibits estradiol binding to both ERα and ERß. More recently, Szego et al. (280) showed that estradiol stimulates CREB activation in basal forebrain cholinergic neurons of adult mice. This effect was replicated using an acute brain slice preparation and was extended by showing that effects were blocked by inhibition of MEK1/2, and by treating with ICI182,780. In addition, effects were observed in ERß knockout mice, but not in ERα knockout mice. This suggests that estradiol, acting at ERα, activates MAPK signaling leading to CREB phosphorylation in at least a subpopulation of the cholinergic neurons.
2. GPR30
Evidence is accumulating rapidly for a novel membrane-bound G protein-coupled ER (GPR30), which is unrelated to ERα or ERß (see Refs. 281 and 282 for reviews). This receptor also may play an important role in mediating effects of estrogen therapy on cholinergic function and cognitive performance. GPR30 is a seven-transmembrane-spanning receptor that is localized to both intracellular membranes and plasma membrane (283), and has been shown to promote rapid estrogen signaling in a variety of cell types including breast cancer cells, uterine epithelial cells, keratinocytes, thymus, and selected cell lines (284,285,286,287). GPR30 is one member of a large superfamily of seven-transmembrane-spanning receptors and is structurally unrelated to the classical ERα and ERß receptors. Standard radioreceptor assays have shown that GPR30 is associated with estrogen binding activity characteristic of steroid hormone receptors. In SKBR3 cells, GPR30 confers high affinity (Kd, 2.7 nm), saturable, low capacity (Bmax, 8 pm) estrogen binding (284). In Cos-7 cells, transfection with GPR30 confers estradiol binding with a Ki of 6.6 nm and a Kd similar to that calculated for 17ß-estradiol binding in SKBR3 cells (288). Heterologous expression studies and RNA interference or antisense knockdown experiments confirm that binding is associated specifically with GPR30 and does not require classical ER. Competitive binding assays show that steroid binding to GPR30 is specific for estradiol and for certain phytoestrogens, whereas other steroid hormones (e.g., 17α-estradiol, progesterone, cortisol, and testosterone) show little binding at concentrations up to 10 μm. In contrast, the antiestrogens ICI182,780 and tamoxifen compete effectively, with relative binding affinities approximately 10% that of estradiol (284).
Functional studies using breast cancer cells lacking classical ERs show that GPR30 mediates up-regulation of c-fos protooncogene by estradiol, as well as by other estrogen-like compounds such as the phytoestrogens genistein and quercetin (289). Using Ishikawa cells (which express ERα) and human endometrial cancer cells (HEC1A cells; which do not express functional ERα), Vivacqua et al. (286) demonstrated that 17ß-estradiol and the major antiestrogenic metabolite of tamoxifen (4-hydroxytamoxifen) up-regulate c-fos expression specifically through interaction with GPR30. In MCF7 cells, which express both ERα and GPR30, induction of c-fos can be mediated by both receptor systems.
Characteristics that distinguish GPR30-mediated effects from those produced by ERα or ERß are: 1) a classical estrogen response element in the target gene is not required for GPR30-mediated effects; 2) effects of estradiol mediated by ERα are blocked by hydroxytamoxifen, whereas effects of estradiol mediated by GPR30 are mimicked by hydroxytamoxifen; 3) effects of estradiol mediated by GPR30 are blocked by the epidermal growth factor receptor kinase inhibitor tyrphostin AG 1478 and by the G protein inhibitor pertusis toxin, whereas effects of estradiol mediated by ERα and ERß are not; and 4) effects mediated by GPR30 are blocked by antisense knockdown of GPR30 expression and are conferred by transfection with a GPR30 expression vector. These studies provide strong evidence that GPR30 is a novel membrane ER distinct from classical nuclear ERs and capable of conferring both estradiol binding and estradiol effects.
Both Northern blot and Western blot studies have confirmed that GPR30 is expressed in many tissues, including brain. It is well known that estradiol, as well as specific phytoestrogens and even tamoxifen, can produce rapid effects in specific neurons, although the mechanism that underlies these effects is still unclear. It is likely that some of these effects are mediated by GPR30. GPR30 was cloned from hippocampus more than 10 yr ago, and recent immunohistochemical staining has demonstrated GPR30 expression not only in hippocampus, but also in many other regions of the brain including the striatum, specific regions of the hypothalamus, and the midbrain (290). GPR30 also is expressed in spinal cord and in dorsal root ganglion neurons, and G-1 (a selective GPR30 agonist) has been shown to depolarize spinal neurons in culture (291) and to stimulate protein kinase Cξ-dependent hyperalgesia in rats (292).
Recently, we evaluated GPR30 expression in the rat forebrain and have detected intense GPR30 immunostaining in the vast majority of cholinergic neurons in the MS, DBB, and NBM, as well as in noncholinergic neurons in deep layers of the frontal cortex, the hippocampus, hypothalamus, and other forebrain structures. In contrast, most parvalbumin (GABAergic) neurons in the MS are GPR30 negative. In addition, we recently showed that the specific GPR30 agonist G-1 enhanced DMP acquisition in ovariectomized rats as effectively as estradiol (276). Whether the effects of G-1 on performance are due specifically to effects on basal forebrain cholinergic neurons needs to be tested; however, these data suggest that GPR30 could play a role in mediating direct effects of estradiol on cholinergic neurons in the basal forebrain, with corresponding effects on cognitive performance. The recent development of a selective GPR30 antagonist (293) will greatly facilitate progress in evaluating the role of GPR30 in mediating these effects.
3. Estrogen receptor summary
Studies show that all three receptors, ERα, ERß and GPR30, can play a significant role in mediating effects of estradiol on cognitive performance. We predict that the contribution of any one of these receptors will depend on the cognitive components being tested and the degree to which specific neural circuits are involved. For example, tasks that involve stress or negative reinforcement may be particularly sensitive to activation of ERß. Tasks that are sensitive to cholinergic modulation or that involve rapid activation of cell signaling in hippocampal neurons may be particularly sensitive to activation of ERα or GPR30. Further studies are needed to determine how activation of receptors within specific regions of the brain contribute to effects on specific tasks. The fact that GPR30 is expressed by the majority of cholinergic neurons projecting to the hippocampus and neocortex and that a GPR30 agonist enhances acquisition of the DMP task highlights the need for further studies on the role of GPR30 in modulating basal forebrain cholinergic function.
C. Mood/affect and effects on monoamines
This review has focused on the cholinergic hypothesis and on tasks pertaining to learning, memory, and executive function. One topic that has not been discussed is the effect of gonadal hormones on affect and mood. Gonadal hormones can have significant and reproducible effects on affect and mood (294,295,296,297,298), which in turn can influence performance on cognitive tasks, particularly tasks involving working memory, decision making, and attention. These effects are mediated in part by monoaminergic projections originating in the brainstem and projecting throughout the prefrontal and parietal cortices, and by local GABAergic inhibitory circuits. Particularly relevant are the effects of estradiol on serotonergic projections (299), which play a role in clinical depression. Equally important are effects on noradrenergic and dopaminergic projections to the prefrontal and parietal cortices (168,300,301,302), which have a role in working memory, executive function, and cortical arousal. These effects are discussed in other recent reviews (6,303,304,305). They are mentioned here to acknowledge that, in addition to the effects on cholinergic projections, estrogens affect other neurotransmitter systems that have important roles in modulating cognitive performance. Readers are referred to these other reviews for a detailed discussion.
XI. Concluding Remarks
It is well established that basal forebrain cholinergic neurons play an important role in learning, memory, and attentional processes and contribute to cognitive decline associated with aging and AD. Evidence presented in this review demonstrates that estrogen therapy has important interactions with basal forebrain cholinergic neurons that contribute significantly to estrogenic effects on cognitive performance. Two types of interactions have been described: 1) the ability of estradiol to increase basal forebrain cholinergic function; and 2) the importance of functional cholinergic projections for enabling estrogens to affect hippocampal plasticity and performance on specific tasks. Evidence for direct effects of estradiol on cholinergic neurons in the MS and NBM and on cholinergic function in the hippocampus and cortex have been described. Current evidence suggests that these effects are mediated via ERα and possibly GPR30, and involve activation of MAPK and CREB. The effects on cholinergic function may enhance performance in part by increasing visuospatial attention, cognitive flexibility, and the ability to incorporate visual cues into an effective learning strategy. In addition, data from several laboratories demonstrate that cholinergic inputs to the hippocampus are necessary for enabling estradiol to produce effects on hippocampal structure and function, as well as the ability to enhance performance on specific tasks. The significance of this is that deficits in basal forebrain cholinergic function (such as occur with aging and AD) are predicted to result in a significant decrease in the ability of estrogen therapy to enhance cognitive performance. This, I believe, explains at least in part why the effects of estradiol on cognitive performance diminish with advanced age and time after ovariectomy and provides a molecular basis for the critical period hypothesis. A model is proposed in which loss of estrogen effect occurs when basal forebrain cholinergic function decreases below a critical threshold. Recent evidence suggests that drugs that enhance cholinergic function could provide an effective therapeutic strategy for reinstating beneficial effects of estrogen therapy on cognitive performance. This could be of tremendous benefit to older postmenopausal women, particularly women who have not used hormone therapy for many years and are beginning to show signs of mild cognitive impairment. These ideas are currently being tested. Future progress must also focus on identifying the specific neural circuitries and molecular events that underlie the effects of chronic estrogen therapy on performance within specific cognitive domains. This will result in the development of more effective and targeted therapies that benefit the aging brain and prevent or reduce cognitive impairment associated with aging and AD.
Acknowledgments
I acknowledge the excellent technical assistance of Douglas Nelson and Rhiannon Mauk, who contributed significantly to recent publications from the Gibbs laboratory; long-time collaborator David Johnson at Duquesne University; and graduate students both at the University of Pittsburgh and at Duquesne University, who have contributed to this research. I also thank Dr. Tony Plant at the University of Pittsburgh for providing expert feedback on the manuscript, and the National Institutes of Health and the National Science Foundation, which have funded much of the work described in this review.
Footnotes
Disclosure Summary: R.B.G. served on a scientific advisory panel for Wyeth Pharmaceuticals, received funding from Organon, the Netherlands, and received product for preclinical research from Lilly Pharmaceuticals and from AstraZeneca. R.B.G. currently receives funding from the National Institutes of Health.
First Published Online December 17, 2009
Abbreviations: ACh, Acetylcholine; AD, Alzheimer’s disease; CEE, conjugated equine estrogen; ChAT, choline acetyltransferase; CI, confidence interval; CREB, cAMP-response element binding protein; DBB, diagonal band of Broca; DMP, delayed matching-to-position; DPN, diarylpropiolnitrile; ER, estrogen receptor; GABA, γ-aminobutyric acid; GPR30, G protein-coupled ER; HACU, high-affinity choline uptake; MPA, medroxyprogesterone acetate; MS, medial septal nucleus; NBM, nucleus basalis magnocellularis; NMDA, N-methyl-d-aspartate; PPT, propylpyrazole triol; SI, substantia innominata.
References
- Suzuki S, Brown CM, Wise PM 2006 Mechanisms of neuroprotection by estrogen. Endocrine 29:209–215 [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS 2008 Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol 29:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM 2006 Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol 18:787–795 [DOI] [PubMed] [Google Scholar]
- Frick KM 2009 Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav 55:2–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R 2006 Preclinical data relating to estrogen’s effects on cognitive performance. In: Rasgon NL, ed. The effects of estrogen on brain function. Baltimore, MD: The Johns Hopkins University Press; 9–45 [Google Scholar]
- Sherwin BB, Henry JF 2008 Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29:88–113 [DOI] [PubMed] [Google Scholar]
- Greene RA 2000 Estrogen and cerebral blood flow: a mechanism to explain the impact of estrogen on the incidence and treatment of Alzheimer’s disease. Int J Fertil Womens Med 45:253–257 [PubMed] [Google Scholar]
- Resnick SM, Maki PM 2001 Effects of hormone replacement therapy on cognitive and brain aging. Ann NY Acad Sci 949:203–214 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Elavsky S, McAuley E, Korol DL, Scalf PE, Kramer AF 2007 Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging 28:179–185 [DOI] [PubMed] [Google Scholar]
- Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS 2006 Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J 21:214–218 [PubMed] [Google Scholar]
- Henderson VW 2006 Estrogen-containing hormone therapy and Alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience 138:1031–1039 [DOI] [PubMed] [Google Scholar]
- Berrodin TJ, Chang KC, Komm BS, Freedman LP, Nagpal S 2009 Differential biochemical and cellular actions of Premarin estrogens: distinct pharmacology of bazedoxifene-conjugated estrogens combination. Mol Endocrinol 23:74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Chen FP 2009 Postmenopausal hormone therapy and risk of breast cancer. Chang Gung Med J 32:140–147 [PubMed] [Google Scholar]
- Wren BG 2009 The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med J Aust 190:321–325 [DOI] [PubMed] [Google Scholar]
- Lacey Jr JV, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, Schatzkin A, Schairer C 2002 Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288:334–341 [DOI] [PubMed] [Google Scholar]
- Miller J, Chan BK, Nelson HD 2002 Postmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 136:680–690 [DOI] [PubMed] [Google Scholar]
- L'hermite M, Simoncini T, Fuller S, Genazzani AR 2008 Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas 60:185–201 [DOI] [PubMed] [Google Scholar]
- Wharton W, Asthana S, Gleason CE 2009 The use of transdermal 17β-estradiol in the treatment of Alzheimer’s disease. In: Hogervorst E, Henderson V, Gibbs RB, Brinton R, eds. Hormones, cognition and dementia: state of the art and emergent therapeutic strategies. Cambridge, UK: Cambridge University Press; 307–332 [Google Scholar]
- Gleason CE, Carlsson CM, Johnson S, Atwood C, Asthana S 2005 Clinical pharmacology and differential cognitive efficacy of estrogen preparations. Ann NY Acad Sci 1052:93–115 [DOI] [PubMed] [Google Scholar]
- Squire LR 2004 Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem 82:171–177 [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE 2004 The medial temporal lobe. Annu Rev Neurosci 27:279–306 [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER 1998 Cognitive neuroscience and the study of memory. Neuron 20:445–468 [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR 1996 A neostriatal habit learning system in humans. Science 273:1399–1402 [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ 2002 Learning and memory functions of the basal ganglia. Annu Rev Neurosci 25:563–593 [DOI] [PubMed] [Google Scholar]
- Thompson RF 2005 In search of memory traces. Annu Rev Psychol 56:1–23 [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE 1997 Temporal dynamics of brain activation during a working memory task. Nature 386:604–608 [DOI] [PubMed] [Google Scholar]
- Miller EK 2000 The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65 [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM 2000 Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483 [DOI] [PubMed] [Google Scholar]
- Koechlin E, Summerfield C 2007 An information theoretical approach to prefrontal executive function. Trends Cogn Sci 11:229–235 [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA 1998 Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA 95:876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC 1993 Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA 90:878–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Masdeu JC, Sastre-Garriga J, Goñi J, Vélez-de-Mendizábal N, Duque B, Pastor MA, Bejarano B, Villoslada P 2008 Mapping the brain pathways of declarative verbal memory: evidence from white matter lesions in the living human brain. Neuroimage 42:1237–1243 [DOI] [PubMed] [Google Scholar]
- McGaugh JL 2004 The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28 [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Roozendaal B 2009 Drug enhancement of memory consolidation: historical perspective and neurobiological implications. Psychopharmacology (Berl) 202:3–14 [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L 2009 Sex influences on the neurobiology of learning and memory. Learn Mem 16:248–266 [DOI] [PubMed] [Google Scholar]
- Dohanich GP 2002 Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, eds. Hormones, brain and behavior. San Diego: Academic Press; 265–327 [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP 1997 Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32:217–225 [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP 1998 Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem 69:225–240 [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP 1999 Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav 62:711–717 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 1999 Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24:161–173 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC 2003 Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging 24:37–48 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM 2007 Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav 52:237–243 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL 2006 Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology 147:607–614 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA 2004 Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology 29:741–748 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2002 Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav 42:245–257 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1999 Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav 36:222–233 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA 2009 Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav 56:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA 2008 Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague-Dawley rats. Endocrinology 149:3176–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ 2003 Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144:2836–2844 [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS 2004 Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA 101:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA 2007 Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem 88:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM 2008 Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci 28:8660–8667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM 2006 Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 84:112–119 [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM 2009 Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience 160:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW 2006 Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 49:197–205 [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM 2000 Acute administration of estrogen and progesterone impairs the acquisition of the spatial Morris water maze in ovariectomized rats. Horm Behav 38:234–242 [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Frick KM 2004 Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci 118:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL 2008 Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci 122:794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R 2003 Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643 [DOI] [PubMed] [Google Scholar]
- Voytko ML 2002 Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis). Behav Neurosci 116:187–197 [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R 2008 Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience 154:1205–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG 2002 Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging 23:589–600 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chhabra RK, Hall MJ, Herndon JG 2004 Executive function is less sensitive to estradiol than spatial memory: performance on an analog of the card sorting test in ovariectomized aged rhesus monkeys. Behav Processes 67:313–319 [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA 2003 Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neuroscience 23:5708–5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Yaffe K, Richards M, Huppert F 2002 Hormone replacement therapy for cognitive function in postmenopausal women (Cochrane Review). Cochrane Database Syst Rev 3 [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB 1992 Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 17:485–495 [DOI] [PubMed] [Google Scholar]
- Halpern D 1992 Sex differences in cognitive abilities. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- Krug R, Born J, Rasch B 2006 A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology 31:965–975 [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T 1996 “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab 81:2545–2549 [DOI] [PubMed] [Google Scholar]
- Hackman BW, Galbraith D 1976 Replacement therapy with piperazine oestrone sulphate (‘Harmogen’) and its effect on memory. Curr Med Res Opin 4:303–306 [DOI] [PubMed] [Google Scholar]
- Krug R, Mölle M, Dodt C, Fehm HL, Born J 2003 Acute influences of estrogen and testosterone on divergent and convergent thinking in postmenopausal women. Neuropsychopharmacology 28:1538–1545 [DOI] [PubMed] [Google Scholar]
- Linzmayer L, Semlitsch HV, Saletu B, Böck G, Saletu-Zyhlarz G, Zoghlami A, Gruber D, Metka M, Huber J, Oettel M, Gräser T, Grünberger J 2001 Double-blind, placebo-controlled psychometric studies on the effects of a combined estrogen-progestin regimen versus estrogen alone on performance, mood and personality of menopausal syndrome patients. Arzneimittelforschung 51:238–245 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Naftolin F, Zelterman D, Marchione KE, Holahan JM, Palter SF, Shaywitz BA 2003 Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause 10:420–426 [DOI] [PubMed] [Google Scholar]
- Sherwin BB 1988 Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357 [DOI] [PubMed] [Google Scholar]
- Ditkoff EC, Crary WG, Cristo M, Lobo RA 1991 Estrogen improves psychological function in asymptomatic postmenopausal women. Obstet Gynecol 78:991–995 [PubMed] [Google Scholar]
- LeBlanc ES, Neiss MB, Carello PE, Samuels MH, Janowsky JS 2007 Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause 14:191–202 [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E 2000 Sex steroids modify working memory. J Cogn Neurosci 12:407–414 [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Birge SJ, Williams DB, Kohrt WM 2001 Effects of hormone replacement therapy on cognitive performance in elderly women. Maturitas 38:137–146 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D 1993 Estrogen replacement therapy and cognitive function in older women. JAMA 269:2637–2641 [PubMed] [Google Scholar]
- Kang JH, Weuve J, Grodstein F 2004 Postmenopausal hormone therapy and risk of cognitive decline in community-dwelling aging women. Neurology 63:101–107 [DOI] [PubMed] [Google Scholar]
- Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E 2002 Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med 113:543–548 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J 2000 The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas 34:S35–S52 [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu HP 2000 The Women’s Health Initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol Aging 21:475–496 [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD 2006 Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci 7:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2008 Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport 19:789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA 2009 Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav 55:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason CE, Schmitz TW, Hess T, Koscik RL, Trivedi MA, Ries ML, Carlsson CM, Sager MA, Asthana S, Johnson SC 2006 Hormone effects on fMRI and cognitive measures of encoding: importance of hormone preparation. Neurology 67:2039–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN 1985 Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol 89:484–490 [DOI] [PubMed] [Google Scholar]
- Luine VN, McEwen BS 1983 Sex differences in cholinergic enzymes of diagonal band nuclei in the rat preoptic area. Neuroendocrinology 36:475–482 [DOI] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS 1975 Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res 86:293–306 [DOI] [PubMed] [Google Scholar]
- Mesulam MM 1996 The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer’s disease. Prog Brain Res 109:285–297 [DOI] [PubMed] [Google Scholar]
- Woolf NJ 1991 Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37:475–524 [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE 1997 GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol 383:163–177 [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH 1983 Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA 1999 Cognitive functions of the basal forebrain. Curr Opin Neurobiol 9:178–183 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW 1997 Central cholinergic systems and cognition. Annu Rev Psychol 48:649–684 [DOI] [PubMed] [Google Scholar]
- von Linstow Roloff E, Platt B 1999 Biochemical dysfunction and memory loss: the case of Alzheimer’s dementia. Cell Mol Life Sci 55:601–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH 1999 Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Natl Acad Sci USA 96:10893–10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L 2006 Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev 127:158–165 [DOI] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J 1974 Human memory and the cholinergic system: a relationship to aging? Arch Neurol 30:113–121 [DOI] [PubMed] [Google Scholar]
- Drachman DA 1977 Memory and cognitive function in man: does the cholinergic system have a specific role? Neurol 27:783–790 [DOI] [PubMed] [Google Scholar]
- Drachman DA, Noffsinger D, Sahakian BJ, Kurdziel S, Fleming P 1980 Aging, memory and the cholinergic system: a study of dichotic listening. Neurobiol Aging 1:39–43 [DOI] [PubMed] [Google Scholar]
- Aigner TG, Mishkin M 1986 The effects of physostigmine and scopolamine on recognition memory in monkeys. Behav Neural Biol 45:81–87 [DOI] [PubMed] [Google Scholar]
- Aigner TG, Mitchell SJ, Aggleton JP, DeLong MR, Struble RG, Price DL, Wenk GL, Pettigrew KD, Mishkin M 1991 Transient impairment of recognition memory following ibotenic-acid lesions of the basal forebrain in macaques. Exp Brain Res 86:18–26 [DOI] [PubMed] [Google Scholar]
- Bartus RT 1978 Short-term memory in the rhesus monkey: effects of dopamine blockade vs acute haloperidol administration. Pharmacol Biochem Behav 9:353–357 [DOI] [PubMed] [Google Scholar]
- Bartus RT, Johnson HR 1976 Short-term memory in the rhesus monkey: disruption from the anti-cholinergic scopolamine. Pharmacol Biochem Behav 5:39–46 [DOI] [PubMed] [Google Scholar]
- Penetar DM, McDonough Jr JH 1983 Effects of cholinergic drugs on delayed matching-to-sample performance of rhesus monkeys. Pharmacol Biochem Behav 19:963–967 [DOI] [PubMed] [Google Scholar]
- Bartus RT 2000 On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 163:495–529 [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ 1976 Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2:1403 [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Blessed G, Tomlinson BE 1977 Necropsy evidence of central cholinergic deficits in senile dementia. Lancet 1:189 [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR 1982 Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239 [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T 2006 The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113:1625–1644 [DOI] [PubMed] [Google Scholar]
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R 2002 Alzheimer’s disease and the basal forebrain cholinergic system: relations to β-amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68:209–245 [DOI] [PubMed] [Google Scholar]
- Giacobini E 2003 Cholinergic function and Alzheimer’s disease. Int J Geriatr Psychiatry 18:S1–S5 [DOI] [PubMed] [Google Scholar]
- Koch HJ, Haas S, Jürgens T 2005 On the physiological relevance of muscarinic acetylcholine receptors in Alzheimer’s disease. Curr Med Chem 12:2915–2921 [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Gould TJ 2002 Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer’s disease and schizophrenia. Behav Cogn Neurosci Rev 1:5–20 [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V 1999 Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 281:1401–1406 [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI 1999 Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol 411:693–704 [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ 2002 Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 51:145–155 [DOI] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS 2005 Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307:1282–1288 [DOI] [PubMed] [Google Scholar]
- Geula C 1998 Abnormalities of neural circuitry in Alzheimer’s disease: hippocampus and cortical cholinergic innervation. Neurology 51(Suppl 1):S18–29; discussion S65–S67 [DOI] [PubMed] [Google Scholar]
- Herholz K, Weisenbach S, Kalbe E 2008 Deficits of the cholinergic system in early AD. Neuropsychologia 46: 1642–1647 [DOI] [PubMed] [Google Scholar]
- Goto Y, Niidome T, Hongo H, Akaike A, Kihara T, Sugimoto H 2008 Impaired muscarinic regulation of excitatory synaptic transmission in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Eur J Pharmacol 583:84–91 [DOI] [PubMed] [Google Scholar]
- Wang Y, Greig NH, Yu QS, Mattson MP 2009 Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging 30:1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machová E, Jakubík J, Michal P, Oksman M, Iivonen H, Tanila H, Dolezal V 2008 Impairment of muscarinic transmission in transgenic APPswe/PS1dE9 mice. Neurobiol Aging 29:368–378 [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S 2009 Amyloid-β causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol Psychiatry 14:206–222 [DOI] [PubMed] [Google Scholar]
- McMahan RW, Sobel TJ, Baxter MG 1997 Selective immunolesions of hippocampal cholinergic input fail to impair spatial working memory. Hippocampus 7:130–136 [DOI] [PubMed] [Google Scholar]
- Chappell J, McMahan R, Chiba A, Gallagher M 1998 A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacol 37:481–487 [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M 1995 Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci 109:714–722 [DOI] [PubMed] [Google Scholar]
- Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL 1996 Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behav Brain Res 82:93–101 [DOI] [PubMed] [Google Scholar]
- Robbins TW 2002 The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Bouger P, Chudasama Y, Cardinal RN, Robbins TW 2004 Cortical cholinergic function and deficits in visual attentional performance in rats following 192 IgG-saporin-induced lesions of the medial prefrontal cortex. Cereb Cortex 14:922–932 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Dalley JW, Nathwani F, Bouger P, Robbins TW, Nathwani F 2004 Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn Mem 11:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B 2003 Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem 80:245–256 [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B 2005 Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev 48:98–111 [DOI] [PubMed] [Google Scholar]
- Cutuli D, Foti F, Mandolesi L, De Bartolo P, Gelfo F, Federico F, Petrosini L 2009 Cognitive performances of cholinergically depleted rats following chronic donepezil administration. J Alzheimers Dis 17:161–176 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA 2007 Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem 88:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DA, Zambon NJ, Gibbs RB 2002 Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res 943:132–141 [DOI] [PubMed] [Google Scholar]
- Dudchenko PA 2001 How do animals actually solve the T maze? Behav Neurosci 115:850–860 [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA 2008 Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull 77:356–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA 2006 Aversive stimulus attenuates impairment of acquisition in a delayed match to position T-maze task caused by a selective lesion of septo-hippocampal cholinergic projections. Brain Res Bull 69:660–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Cutrell EB, Marrocco RT 1999 Scopolamine slows the orienting of attention in primates to cued visual targets. Psychopharmacology (Berl) 142:1–8 [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, Marrocco RT 1997 Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology (Berl) 132:324–334 [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL 1994 Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci 14:167–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM 1991 Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience 40:13–20 [DOI] [PubMed] [Google Scholar]
- Sato H, Hata Y, Hagihara K, Tsumoto T 1987 Effects of cholinergic depletion on neuron activities in the cat visual cortex. J Neurophysiol 58:781–794 [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA 1983 Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res 289:143–155 [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV 2000 Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290:2315–2319 [DOI] [PubMed] [Google Scholar]
- Alenda A, Nuñez A 2007 Cholinergic modulation of sensory interference in rat primary somatosensory cortical neurons. Brain Res 1133:158–167 [DOI] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC 2008 Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology 33:913–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ 2005 Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem 84:132–137 [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL 2002 Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116:411–420 [DOI] [PubMed] [Google Scholar]
- O'Malley CA, Hautamaki RD, Kelley M, Meyer EM 1987 Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res 403:389–392 [DOI] [PubMed] [Google Scholar]
- Jope RS 1979 High affinity choline transport and acetylCoA production in brain and their roles in the regulation of acetylcholine synthesis. Brain Res 180:313–344 [DOI] [PubMed] [Google Scholar]
- Trommer BA, Schmidt DE, Wecker L 1982 Exogenous choline enhances the synthesis of acetylcholine only under conditions of increased cholinergic neuronal activity. J Neurochem 39:1704–1709 [DOI] [PubMed] [Google Scholar]
- Vaca K, Pilar G 1979 Mechanisms controlling choline transport and acetylcholine synthesis in motor nerve terminals during electrical stimulation. J Gen Physiol 73:605–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB 2000 Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience 101:931–938 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW 1992 Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol 116:23–39 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1997 Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res 757:10–16 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW 1994 Effects of estrogen replacement on relative levels of ChAT, TrkA and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol 129:70–80 [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW 1994 Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res 644:305–312 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM 2008 Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol 20:1023–1027 [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM 1996 The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of adult female Sprague-Dawley rat. J Neurosci 16:1860–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB 1998 Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement. Brain Res 787:259–268 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG 1999 Ovarian hormones differentially influence immunoreactivity for dopamine β-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 409:438–451 [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML 2004 Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol 469:507–521 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1996 Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci 16:1049–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB 2003 Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res 962:244–247 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA 1997 Effects of estrogen on potassium-evoked acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res 749:143–146 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB 2002 Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologous monkeys. Neuroscience 113:907–914 [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puoliväli J, Helisalmi S, Hiltunen M, Soininen H, Tanila H 2006 Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen α and β mRNA levels in ovariectomized female mice. Neuroscience 137:1143–1152 [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM 2006 Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res 1115:135–147 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC 2006 Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci 24:229–242 [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P 2006 Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology 31:2065–2078 [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P 2008 Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav 53:159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG 1998 Posttraining estrogen and memory modulation. Horm Behav 34:126–139 [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL 2003 Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem 80:315–322 [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC 1998 Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18:7426–7435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE 1993 A PET study of visuospatial attention. J Neurosci 13: 1202–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS 2007 Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol 47:657–680 [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS 2001 Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci 21:6532–6543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS 2003 A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci 23:4479–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lâm TT, Leranth C 2003 Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci 17:1997–2005 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP 2001 Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci 21:6949–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD 2005 Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience 132:57–64 [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH 2004 Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223 [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH 2007 Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA 104:11465–11470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH 2006 Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci 26:2571–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB 2007 Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav 52:352–359 [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J 2004 Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav 45:330–338 [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ 2009 Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci 29:10362–10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R, Boccardi M, Benussi L, Testa C, Villa A, Pievani M, Gigola L, Sabattoli F, Barbiero L, Frisoni GB, Binetti G 2006 Effects of estrogens on cognition and brain morphology: involvement of the cerebellum. Maturitas 54:222–228 [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Stern Y 2007 Effects of hormone replacement therapy and aging on cognition: evidence for executive dysfunction. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14:301–328 [DOI] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA 2006 Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 13:411–422 [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ 1998 A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res 93:185–190 [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ 2004 Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res 151:103–115 [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS 2007 Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res 183:1–7 [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA 1998 Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res 6:351–360 [DOI] [PubMed] [Google Scholar]
- Saucier DM, Green SM, Leason J, MacFadden A, Bell S, Elias LJ 2002 Are sex differences in navigation caused by sexually dimorphic strategies or by differences in the ability to use the strategies? Behav Neurosci 116:403–410 [DOI] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW 2000 Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci 3:404–408 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2000 Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21:107–116 [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV 2002 Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci 22:10985–10995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM 2002 Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav 42:284–293 [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KB 2002 Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging 23:87–95 [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA 2008 Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem 90:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Dudek B 2005 Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res 1036:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW 2009 The critical window hypothesis: hormone exposures and cognitive outcomes after menopause. In: Hogervorst E, Henderson V, Gibbs RB, Brinton R, eds. Hormones, cognition and dementia: state of the art and emergent therapeutic strategies. Cambridge, UK: Cambridge University Press; 131–177 [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ 2000 Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease. JAMA 283:1007–1015 [DOI] [PubMed] [Google Scholar]
- Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC 2000 Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 54:2061–2066 [DOI] [PubMed] [Google Scholar]
- Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR 2000 Estrogen for Alzheimer’s disease in women. Randomized, double-blind, placebo-controlled trial. Neurology 54:295–301 [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Törber S, McEwen BS, Kirschbaum C 1999 Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology 24:727–741 [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR 1999 Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo- controlled, double-blind, pilot study. Psychoneuroendocrinology 24:657–677 [DOI] [PubMed] [Google Scholar]
- Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR 2001 High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 57:605–612 [DOI] [PubMed] [Google Scholar]
- Yue Y, Hu L, Tian QJ, Jiang JM, Dong YL, Jin ZY, Cheng YH, Hong X, Ge QS, Zuo PP 2007 Effects of long-term, low-dose sex hormone replacement therapy on hippocampus and cognition of postmenopausal women of different apoE genotypes. Acta Pharmacol Sin 28:1129–1135 [DOI] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R 2005 Brain estrogen deficiency accelerates A β plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci USA 102:19198–19203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ 2007 Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Pike CJ 2008 Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology 149:2607–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC 2002 Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County study. JAMA 288:2123–2129 [DOI] [PubMed] [Google Scholar]
- Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C 2005 Early postmenopausal hormone therapy may prevent cognitive impairment later in life. Menopause 12:12–17 [DOI] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA 2005 Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry 76:103–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Espeland MA, Hogan PE, Rapp SR, Stefanick ML, Wactawski-Wende J, Johnson KC, Wassertheil-Smoller S, Freeman R, Curb D, Prior use of hormone therapy and incident Alzheimer’s disease in the Women’s Health Initiative Memory Study. Proc 59th Annual Meeting of the American Academy of Neurology, Boston, MA, 2007 [Google Scholar]
- Sherwin BB 2007 The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol 19:77–81 [DOI] [PubMed] [Google Scholar]
- Maki PM 2006 Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience 138:1027–1030 [DOI] [PubMed] [Google Scholar]
- Altavista MC, Rossi P, Bentivoglio AR, Crociani P, Albanese A 1990 Aging is associated with a diffuse impairment of forebrain cholinergic neurons. Brain Res 508:51–59 [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Björklund A 1992 Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging 13:9–23 [DOI] [PubMed] [Google Scholar]
- Fischer W, Gage FH, Björklund A 1989 Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur J Neurosci 1:34–45 [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Rogers J 1987 Age-related shrinkage of cortically projecting cholinergic neurons: a selective effect. Ann Neurol 22:31–36 [DOI] [PubMed] [Google Scholar]
- Stroessner-Johnson HM, Rapp PR, Amaral DG 1992 Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged rhesus monkey. J Neurosci 12:1936–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristofiková Z, Klaschka J, Tejkalová H, Benesová O 1992 High-affinity choline uptake and muscarinic receptors in rat brain during aging. Arch Gerontol Geriatr 15:87–97 [DOI] [PubMed] [Google Scholar]
- Sherman KA, Friedman E 1990 Pre- and post-synaptic cholinergic dysfunction in aged rodent brain regions: new findings and an interpretative review. Int J Dev Neurosci 8:689–708 [DOI] [PubMed] [Google Scholar]
- Gibson GE, Peterson C, Jenden DJ 1981 Brain acetylcholine declines with senescence. Science 213:674–676 [DOI] [PubMed] [Google Scholar]
- Sherman KA, Kuster JE, Dean RL, Bartus RT, Friedman E 1981 Presynaptic cholinergic mechanisms in brain of aged rats with memory impairments. Neurobiol Aging 2:99–104 [DOI] [PubMed] [Google Scholar]
- Sims NR, Bowen DM, Allen SJ, Smith CC, Neary D, Thomas DJ, Davison AN 1983 Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem 40:503–509 [DOI] [PubMed] [Google Scholar]
- Takei N, Nihonmatsu I, Kawamura H 1989 Age-related decline of acetylcholinesterase release evoked by depolarizing stimulation. Neurosci Lett 101:182–186 [DOI] [PubMed] [Google Scholar]
- Wu CF, Bertorelli R, Sacconi M, Pepeu G, Consolo S 1988 Decrease of brain acetylcholine release in aging freely-moving rats detected by microdialysis. Neurobiol Aging 9:357–361 [DOI] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R 1990 Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. J Neurosci 10:3069–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Stuckman S, Sarter M, Bruno JP 1996 Potassium, but not atropine-stimulated cortical acetylcholine efflux, is reduced in aged rats. Neurobiol Aging 17:565–571 [DOI] [PubMed] [Google Scholar]
- Taylor L, Griffith WH 1993 Age-related decline in cholinergic synaptic transmission in hippocampus. Neurobiol Aging 14:509–515 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1998 Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp Neurol 151:289–302 [DOI] [PubMed] [Google Scholar]
- Barbacid M 1993 Nerve growth factor: a tale of two receptors. Oncogene 8:2033–2042 [PubMed] [Google Scholar]
- Gibbs RB 2003 Effects of ageing and long-term hormone replacement on cholinergic neurones in the medial septum and nucleus basalis magnocellularis of ovariectomized rats. J Neuroendocrinol 15:477–485 [DOI] [PubMed] [Google Scholar]
- Chu Y, Cochran EJ, Bennett DA, Mufson EJ, Kordower JH 2001 Down-regulation of trkA mRNA within nucleus basalis neurons in individuals with mild cognitive impairment and Alzheimer’s disease. J Comp Neurol 437:296–307 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM 2009 The ability of oestradiol administration to regulate protein levels of oestrogen receptor α in the hippocampus and prefrontal cortex of middle-aged rats is altered following long-term ovarian hormone deprivation. J Neuroendocrinol 21:640–647 [DOI] [PubMed] [Google Scholar]
- McLay RN, Maki PM, Lyketsos CG 2003 Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci 15:161–167 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton 3rd LJ 2007 Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 69:1074–1083 [DOI] [PubMed] [Google Scholar]
- Schneider LS, Farlow MR, Henderson VW, Pogoda JM 1996 Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology 46:1580–1584 [DOI] [PubMed] [Google Scholar]
- Woolley CS 2000 Effects of oestradiol on hippocampal circuitry. Novartis Found Symp 230:173–180; discussion 181–177 [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS 2001 Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA 98:3525–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH 2003 Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol 465:540–550 [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL 2001 Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 115:384–393 [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD 2008 Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav 54:386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C 2004 Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci 19:3026–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM 2007 Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging 28:602–610 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA 2006 Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM 2008 Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci 122:716–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD 2004 Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology 145:1069–1074 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Nat Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavathi B, Kumar R 2006 Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol 207:594–604 [DOI] [PubMed] [Google Scholar]
- McEwen B 2002 Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384 [DOI] [PubMed] [Google Scholar]
- Lewandowski S, Kalita K, Kaczmarek L 2002 Estrogen receptor β. Potential functional significance of a variety of mRNA isoforms. FEBS Lett 524:1–5 [DOI] [PubMed] [Google Scholar]
- Pfeffer U 1996 Estrogen receptor mRNA variants. Do they have a physiological role? Ann NY Acad Sci 784:304–313 [DOI] [PubMed] [Google Scholar]
- Pfeffer U, Fecarotta E, Arena G, Forlani A, Vidali G 1996 Alternative splicing of the estrogen receptor primary transcript normally occurs in estrogen receptor positive tissues and cell lines. J Steroid Biochem Mol Biol 56:99–105 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA, Hurd YL 1998 Differential distribution and regulation of estrogen receptor-α and -β mRNA within the female rat brain. Brain Res Mol Brain Res 54:175–180 [DOI] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF 1997 Distribution of estrogen receptor-β-like immunoreactivity in rat forebrain. Neuroendocrinology 66:63–67 [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA 2008 Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor β knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem 89:513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA 2006 ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem 85:183–191 [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF 2006 New roles for estrogen receptor β in behavior and neuroendocrinology. Front Neuroendocrinol 27:217–232 [DOI] [PubMed] [Google Scholar]
- Day M, Sung A, Logue S, Bowlby M, Arias R 2005 β-Estrogen receptor knockout (BERKO) mice present attenuated hippocampal CA1 long-term potentiation and related memory deficits in contextual fear conditioning. Behav Brain Res 164:128–131 [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2006 A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 31:1097–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA 2007 Administration of estrogen receptor β-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav 86:407–414 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA 2004 Antidepressant effects of ERβ-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav 78:523–529 [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB 2009 Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav 56:309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I 2000 Estrogen binding and estrogen receptor characterization (ERα and ERß) in the cholinergic neurons of the rat basal forebrain. Neuroscience 96:41–49 [DOI] [PubMed] [Google Scholar]
- Miettinen RA, Kalesnykas G, Koivisto EH 2002 Estimation of the total number of cholinergic neurons containing estrogen receptor-α in the rat basal forebrain. J Histochem Cytochem 50:891–902 [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Gibbs RB, Defranco DB 2004 Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience 124:809–816 [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabás K, Balog J, Szilágyi N, Korach KS, Juhász G, Abrahám IM 2006 Estrogen induces estrogen receptor α-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci 26:4104–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty K, Kim KH, Bender JR 2006 Minireview: estrogen receptor-mediated rapid signaling. Endocrinology 147:5557–5563 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ 2008 Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y 2006 G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P 2005 GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–367 [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M 2007 G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ 2007 Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol 193:311–321 [DOI] [PubMed] [Google Scholar]
- Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, Oprea TI, Dun NJ 2009 Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res 87:1610–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T 2008 GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci 27:1700–1709 [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER 2009 In vivo effects of a GPR30 antagonist. Nat Chem Biol 5:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL 2009 Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol 52:516–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CN, Poitras JR, Prouty J 2003 Effect of reproductive hormones and selective estrogen receptor modulators on mood during menopause. Drugs Aging 20:85–100 [DOI] [PubMed] [Google Scholar]
- Graziottin A, Serafini A 2009 Depression and the menopause: why antidepressants are not enough? Menopause Int 15:76–81 [DOI] [PubMed] [Google Scholar]
- Harsh V, Meltzer-Brody S, Rubinow DR, Schmidt PJ 2009 Reproductive aging, sex steroids, and mood disorders. Harv Rev Psychiatry 17:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Zappert LN, Williams KE 2006 Clinical data on estrogen’s effects on mood. In: Rasgon NL, ed. The effects of estrogen on brain function. Baltimore, MD: The Johns Hopkins University Press; 95–115 [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM 2002 Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 23:41–100 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Bethea CL 2003 Ovarian hormone influences on the density of immunoreactivity for tyrosine hydroxylase and serotonin in the primate corpus striatum. Neuroscience 122:757–772 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM 2008 Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci 28:9525–9535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG 1998 Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 395:1–17 [PubMed] [Google Scholar]
- Koenig AM, Thase ME 2009 First-line pharmacotherapies for depression—what is the best choice? Pol Arch Med Wewn 119:478–486 [PubMed] [Google Scholar]
- Osterlund MK 2009 Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta doi: 10.1016/j.bbagen.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Maki PM, Dumas J 2009 Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med 27:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]