Abstract
Objectives
Studies in rats showed that the pharmacokinetics of the tricarbonyl core radiopharmaceutical 99mTc(CO)3-nitrilotriacetic acid, 99mTc(CO)3(NTA), were essentially identical to those of 131I ortho-iodohippuran (131I-OIH), the clinical gold standard for the measurement of effective renal plasma flow. Our objective was to compare the pharmacokinetics of these two tracers in healthy volunteers.
Methods
99mTc(CO)3(NTA) was prepared with commercially available NTA and a commercially available IsoLink kit (Covidien) and isolated by reversed-phased high-performance liquid chromatography. Approximately 74 MBq (2.0 mCi) of 99mTc(CO)3(NTA) were coinjected with 9.25 MBq (250 μCi) of 131I-OIH in nine volunteers, and simultaneous imaging of each tracer was performed for 24 min. Plasma clearances were determined from 8 blood samples obtained 3–90 min after injection using the single-injection, two-compartment model. Plasma protein binding, red cell uptake, and percentage injected dose in the urine at 30 and 180 minutes were determined.
Results
There was no difference in the plasma clearances of 99mTc(CO)3(NTA) and 131I-OIH, 475 ± 105 mL/min versus 472 ± 108 mL/min, respectively. The plasma protein binding and red cell uptake of 99mTc(CO)3(NTA) were 43 ± 5% and 9 ± 6%, respectively; both values were significantly lower (P < 0.001) than the plasma protein binding (75 ± 3%) and red cell uptake (17 ± 5%) of 131I-OIH. There was no significant difference in the percent injected dose recovered in the urine at 30 min and at 3 h; for comparison, the percent dose in the urine at 3 h was 91 ± 4% for 99mTc(CO)3(NTA) and 91 ± 6% for 131I-OIH (P = 0.96). Image quality with 99mTc(CO)3(NTA) was excellent, and the renogram parameters were similar to those of 131I-OIH.
Conclusions
Preliminary results in healthy volunteers suggest that the pharmacokinetic behavior of 99mTc(CO)3(NTA) is comparable to that of 131I-OIH.
Keywords: 99mTc-tricarbonyl, renal radiopharmaceuticals, 99mTc(CO)3(NTA), 131I-ortho-iodohippurate (131I-OIH), 99mTc-mercaptoacetyltriglycine (99mTc-MAG3)
INTRODUCTION
Although 131I-orthoiodohippuran (131I-OIH) has excellent pharmacokinetic properties as a renal tracer, its use has been compromised because of the suboptimal imaging characteristics of the 364-keV photon of 131I and the delivery of relatively high radiation doses to kidney and thyroid in patients with impaired renal function (1). The limitations of 131I-OIH led to the introduction of 99mTc-mercaptoacetyltriglycine (99mTc-MAG3) as a 99mTc replacement for 131I-OIH in 1986 (2,3). The image quality of the 99mTc-MAG3 images was superior to that of 131I-OIH (4); moreover, the 40%-60% extraction fraction of 99mTc-MAG3 is substantially higher than the 20% extraction fraction of 99mTc-diethyltriaminepentaacetic acid (99mTc-DTPA) (5,6). The higher extraction of 99mTc-MAG3 led to its superior performance compared to 99mTc-DTPA in adult and pediatric patients with suspected obstruction (7,8). Currently an estimated 70% of all the renal scans in the United States are performed with 99mTc-MAG3 and 131I-OIH has been withdrawn from the market even though it had a higher extraction fraction than 99mTc-MAG3 (5,9).
Nevertheless, despite of improved image quality and diagnostic superiority to 99mTc-DTPA, 99mTc-MAG3 still has limitations. A small percentage of 99mTc-MAG3 is eliminated via the hepatobiliary pathway, and this percentage increases in patients with reduced renal function; the resulting activity in the gallbladder has been mistaken for activity in the kidney (10,11). A larger issue is the fact that the clearance of 99mTc-MAG3 is only 50%-60% of the clearance of 131I-OIH (3,4,12); the fact that 99mTc-MAG3 does not provide a direct measurement of effective renal plasma flow (ERPF) led Jafri et al (12) to conclude that 99mTc-MAG3 is not suitable as a replacement for 131I-OIH for the measurement of ERPF. Another reported problem is the reproducibility of the 99mTc-MAG3 clearance based on plasma sample measurements. Kotzerke et al. evaluated the reproducibility of the plasma sample 99mTc-MAG3 clearance and concluded that it was not precise enough to evaluate a change in kidney function (i.e. after surgery or chemotherapy) (13). Piepsz et al. also observed marked differences in repeat 99mTc-MAG3 plasma clearance measurements and warned that changes in the 99mTc-MAG3 plasma clearance should be interpreted with caution in general clinical practice (14).
These limitations stimulated continuing efforts to develop a 99mTc renal radiopharmaceutical with both reduced hepatobiliary excretion and superior pharmacokinetic properties that would allow a more accurate and precise measurement of ERPF. For almost 20 years, these synthetic efforts exclusively used the {TcO}3+ core with technetium in its +5 oxidation state (2,15–19); the most successful of these agents were the 99mTc-LL- and DD- ethylenedicysteine (99mTc-EC) isomers. These agents have clearances comparable to that of 99mTc-MAG3 but less than that of 131I-OIH (18). The limited success using the {TcO}3+ core coupled with the numerous synthetic advantages of the 99mTc water-stable organometallic tricarbonyl precursor, [99mTc(CO)3(H2O)3]+, led us to shift our focus to 99mTc renal radiopharmaceuticals based on a fac-{99mTc(CO)3}+ core with 99mTc in its +1 oxidation state (20–23).
The first class of renal radiopharmaceuticals with a tricarbonyl core tested in humans were the 99mTc(CO)3-lanthionine complexes (21). Although their clearances and rates of renal excretion were still less than those of 131I-OIH, they proved to be excellent renal imaging agents in healthy volunteers and demonstrated the potential of renal radiopharmaceutical development based on this core. We subsequently chose 99mTc-tricarbonyl-nitrilotriacetic acid, 99mTc(CO)3(NTA), for further investigation because it would be formed as a single species, would be dianionic at physiological pH, and, similarly to 99mTc-MAG3, would have a dangling carboxylate group favoring tubular transport (23). Initial studies of 99mTc(CO)3(NTA) showed that it was a stable complex with pharmacokinetic properties in Sprague-Dawley rats equivalent or superior to those of 131I-OIH (23). This report compares the pharmacokinetic properties of 99mTc(CO)3(NTA) and 131I-OIH in healthy volunteers.
MATERIALS AND METHODS
General
Nitrilotriacetic acid (NTA) was purchased from Aldrich. 99mTc-pertechnetate (99mTcO4−) was eluted from a 99Mo/99mTc generator (Amersham Health) with 0.9% saline. IsoLink vials were obtained as a gift from Covidien. [99mTc(CO)3(H2O)3]+ was prepared according to the manufacturer’s insert by adding 99mTcO4− generator eluent [1 mL, 1.11–3.7 GBq (30–100 mCi)] to the IsoLink vial, heating for 40 min at 100 °C and adding 1 N HCl (120 μL) to neutralize the solution. The radiolabeled compound was analyzed on a high-performance liquid chromatography (HPLC) instrument (System Gold Nouveau, Beckman Coulter) equipped with a model 170 radiometric detector and a model 166 ultraviolet light-visible light detector, 32 Karat chromatography software (Beckman Coulter), and an octyldecyl silane column (C18 RP Ultrasphere; 5-μm, 4.6 × 250 mm; Beckman Coulter). The solvent system was 0.05 M TEAP buffer pH 2.5 (solvent A) and ethanol (solvent B), and the flow rate was 1 mL/min. The gradient method was the same as reported previously (22). Urine, plasma and red blood cell radioactivity was measured with a gamma counter (Packard Cobra II Gamma Counter; Perkin Elmer) with correction for 131I scatter into the 99mTc window.
Radiosynthesis of 99mTc(CO)3(NTA) and 131I-OIH
The NTA ligand was labeled as previously described (23,24). Briefly, 0.5 mL of a freshly prepared solution of the [99mTc(CO)3(H2O)3]+ precursor (pH ~ 7–8) was added to a vial containing ~1.0 mg of the NTA ligand in 0.2 mL of water. The pH of the solution was adjusted to about 7 with 1 M NaOH, heated at 70 °C for 15 min, and cooled to room temperature, yielding the dianion, fac-[99mTc(CO)3(NTA)]2−, which we describe as 99mTc(CO)3(NTA). 99mTc(CO)3(NTA) was separated from unlabeled ligand by HPLC; the radiochemical purity was more than 99%. Ethanol was partially removed by N2 gas, and the collected solution of 99mTc(CO)3(NTA) was buffered in a physiological phosphate buffer (PBS) at pH 7.4. The HPLC-purified complex in PBS (pH 7.4) was passed through a Sep-Pak Plus C18 cartridge (Waters Co.) (primed with 4 mL of ethanol) and sterile Millex-GS 22 μm filter (Millipore Co.) (primed with 4 mL of saline) into a sterile, pyrogen-free empty vial. The final concentration was 37 MBq/mL (1 mCi/mL) and the final pH was 7.4. Test samples were sent for analysis and determined to be sterile and pyrogen free.
131I-OIH was prepared by the isotope exchange reaction between non-radioactive hippuran (OIH) and radioactive sodium iodide (Na131I) according to the method reported by Anghileri (25) and modified as previously described (23). 131I-OIH was obtained with a 98–99% labeling yield.
Healthy Volunteer Studies
All studies were performed with the approval of the Radioactive Drug Research Committee and the Emory University Institutional Review Board; signed consent was obtained from each volunteer. Nine healthy volunteers (3 man, 6 women; mean age ± SD, 33.8 ± 12.4 y; range 20–55 y) participated in this study. Inclusion criteria required the absence of any history of kidney or bladder diseases and a normal finding on a review of systems. Pregnancy was excluded by means of a urine pregnancy test. Measurements of blood pressure, heart rate, and temperature were obtained before and after injection for each volunteer; in addition a complete blood cell count, standard chemistry panel and urinalysis were obtained before and 24 h after injection.
Approximately 74 MBq (2 mCi) of 99mTc(CO)3(NTA) was coinjected with 9.25 MBq (250μCi) of 131I-OIH, and imaging was performed using for 24 minusing a Infinia (GE Healthcare) camera with a 9.5 mm (3/8-in) crystal fitted with a high-energy collimator; a 20% window was centered over the 365-KeV photopeak of 131I, and a second 20% window was centered over the 140-keV photopeak of 99mTc. Data were acquired in a 128 × 128 matrix using a 3-phase dynamic acquisition and processed on a Xeleris computer (GE Healthcare) using an in-house upgrade of the QuantEM 2.0 renal software; QuantEM is licensed to GE Healthcare by Emory University and provides a validated camera-based 99mTc-MAG3 clearance based on the integral of the injected dose from 1.0 to 2.5 min after injection (26). Renogram curves were generated using cortical (parenchymal) and whole kidney regions of interest, and the time to peak height of the renogram curve (TTP) and 20 min-to-maximum count ratios (20min/max) were calculated for both sets of renogram curves. Blood samples were obtained at 3, 5, 10, 20, 30, 45, 60, and 90 min after injection, and plasma clearances for 131I-OIH and 99mTc(CO)3(NTA) were determined using the single-injection, two-compartment model of Sapirstein et al. (27). The volunteers voided at 30, 90 and 180 min after injection to determine the percentage dose in the urine. Plasma protein binding was determined by ultracentrifugation (Centrifree® micropartition system; Amicon Inc.) of 1 mL of plasma: PPB = (1.0 − [ultrafiltrate concentration/plasma concentration]) × 100. The percentage uptake in the erythrocytes was calculated from the whole blood (counts/g) and packed cells (counts/g). Percent erythrocyte uptake = [(counts/g in erythrocytes × hematocrit)/counts/g in whole blood]. No correction was made for plasma trapped in the red blood cells sample. Plasma protein binding and erythrocyte uptake were calculated using duplicate samples, and the mean values were reported.
To determine whether the complex was metabolized or was excreted unchanged in the urine, a 1-mL urine sample from the 30-min urine collection from one volunteer was analyzed by reversed phase HPLC and the tracing compared to reversed-phase HPLC analysis of the purified complex.
Statistical Analysis
All results are expressed as the mean ± SD. To determine the statistical significance of differences between the 2 groups, comparisons were made with the 2-tailed Student t test for paired data; P < 0.05 was considered to be statistically significant.
RESULTS
Normal Volunteer Studies
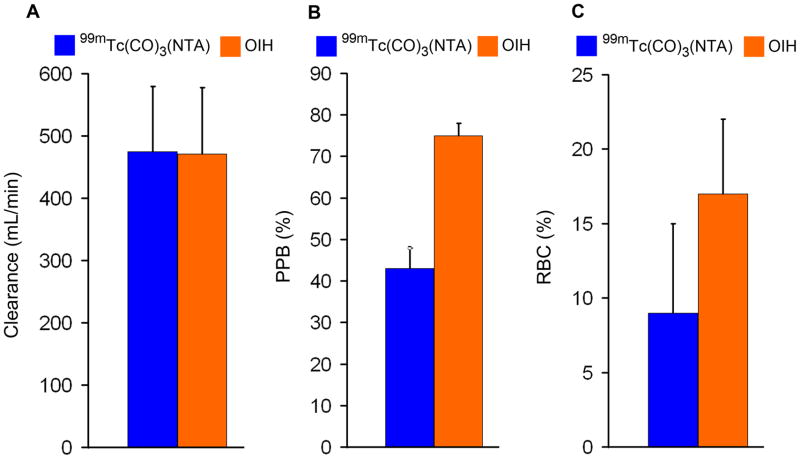
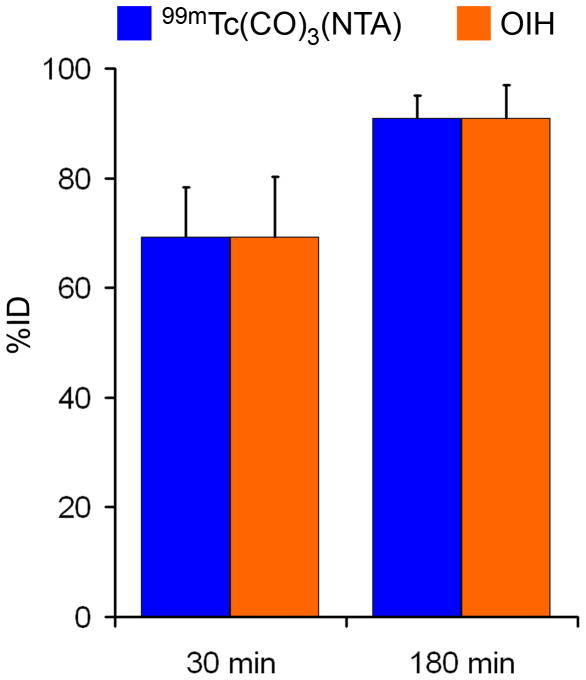
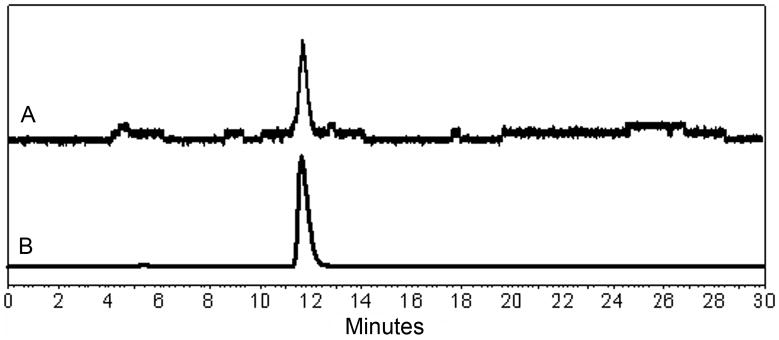
There was no evidence of any toxicity based on measurements of blood pressure, heart rate, temperature, complete blood cell count, standard chemistry panel, or urine analysis for any of the volunteers. The clearance of 99mTc(CO)3(NTA) averaged 475 ± 105 (SD) mL/min, compared to 472 ± 108 mL/min for 131I-OIH (Fig. 1). The plasma protein binding of 99mTc(CO)3(NTA), 43 ± 5%, was significantly less than that of 131I-OIH, 75 ± 3% (P < 0.001); similarly, red cell uptake for 99mTc(CO)3(NTA), 9 ± 6%, was also significantly less than that of 131I-OIH, 17 ± 5% (P < 0.001) (Fig. 1). Both tracers were rapidly excreted in the urine, with 69 ± 9% and 69 ± 11% in the urine at 30 min for 99mTc(CO)3(NTA) and 131I-OIH, respectively (P = 0.95), and 91 ± 4% and 91 ± 6% at 180 min (P = 0.96), respectively (Fig. 2). Reversed-phase HPLC analysis of an aliquot from the 30-min urine sample of one of the volunteers showed a single peak (Fig. 3A) with the same retention time as the purified complex (Fig. 3B) indicating that the tracer is excreted unchanged in the urine (Fig. 3).
Figure 1.
Bar graphs comparing the clearance (A), plasma protein binding (PPB) (B) and red cell uptake (RBC) (C) of 99mTc(CO)3(NTA) and 131I-OIH in healthy volunteers.
Figure 2.
Bar graphs comparing the urine excretion of 99mTc(CO)3(NTA) and 131I-OIH at 30 and 180 min in healthy volunteers.
Figure 3.
A urine sample from healthy volunteer injected with 99mTc(CO)3(NTA) was subjected to reversed-phase HPLC analysis (A) and compared to a reference HPLC 99mTc(CO)3(NTA) tracing (B). Both tracings show single peak with same retention times indicating that 99mTc(CO)3(NTA) is excreted unchanged in urine.
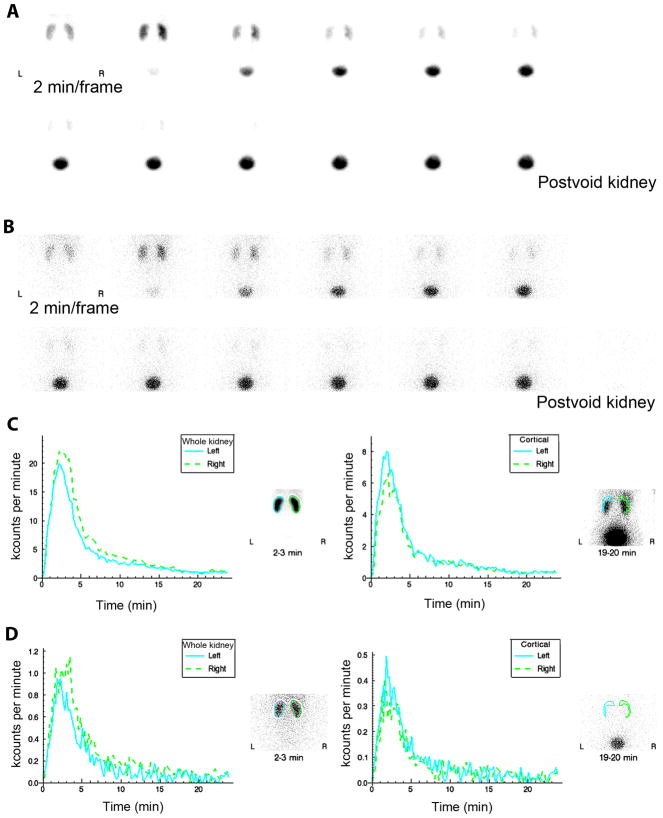
99mTc(CO)3(NTA) image quality was excellent, compared with that of 131I-OIH, and the renogram curves were almost identical (Fig. 4). There was no significant difference in relative uptake in the left kidney (49.8 ± 3.4% for 99mTc(CO)3(NTA), compared to 49.0 ± 4.1% for 131I-OIH (P = 0.17; Table 1)). The TTP and 20 min/max of 99mTc(CO)3(NTA) and 131I-OIH for whole kidney and for cortical (parenchymal) regions of interest are shown Table 1. For the cortical regions of interet, TTP for the left kidney was 2.52 ± 0.51 min for 99mTc(CO)3(NTA), compared with 2.46 ± 0.73 for 131I-OIH (P = 0.71, not statistically significant); for the right kidney, the cortical TTP was 2.60 ± 0.60 for 99mTc(CO)3(NTA) and was greater than that of 131I-OIH, 1.98 ± 0.60 (P = 0.02). The cortical 20 min/max ratio was slightly greater for 99mTc(CO)3(NTA) than for 131I-OIH for both the left and right kidneys (0.13 ± 03 vs. 0.10 ± 0.04 for the left kidney [P = 0.03] and 0.13 ± 0.02 vs. 0.10 ± 0.02 for the right kidney [P = 0.046]) (Table 1). The camera-based clearance of 99mTc(CO)3(NTA) was 456 ± 130 mL/min/1.73 m2 (Fig. 5).
Figure 4.
Two-minute kidney images after simultaneous injection of 79.2 MBq (2.14 mCi) of 99mTc(CO)3(NTA) (A) and 8.9 MBq (240 uCi) of 131I-OIH (B) in a healthy 22-y-old female volunteer. Whole-kidney and cortical (parenchymal) renogram curves are displayed in C for 99mTc(CO)3(NTA)andin D for 131I-OIH. Renogram curves for 131I-OIH are quite noisy because of relatively low count rate resulting from a lower administered dose and the poor capture of the 364-keV photon of I-131 by 9.5 mm (3/8 in) crystal.
TABLE 1.
Renogram Parameters for Whole-Kidney and Cortical Regions of Interest of 99mTc(CO)3(NTA) Compared with 131I-OIH in Humans (n = 9).
| Left kidney |
Right kidney |
Left cortical |
Right cortical |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Agent | Uptake % | TTP (min) | 20 min/max | Uptake % | TTP (min) | 20 min/max | TTP (min) | 20 min/max | TTP (min) | 20 min/max |
|
99mTc(CO)3(NTA) (SD) |
49.8 (3.4) |
3.3 (1.6) |
0.15 (0.04) |
50.2 (3.4) |
3.4 (1.9) |
0.16 (0.04) |
2.52 (0.51) |
0.13 (0.03) |
2.6 (0.6) |
0.13 (0.02) |
|
131I-OIH (SD) |
49.0 (4.1) |
2.6 (0.6) |
0.11 (0.03) |
51.0 (4.1) |
2.8 (1.6) |
0.11 (0.19) |
2.46 (0.73) |
0.10 (0.04) |
1.98 (0.6) |
0.10 (0.02) |
| P | 0.17 | 0.13 | 0.01 | 0.17 | 0.02 | 0.004 | 0.71 | 0.03 | 0.02 | 0.046 |
Data are mean ± SD.
Figure 5.
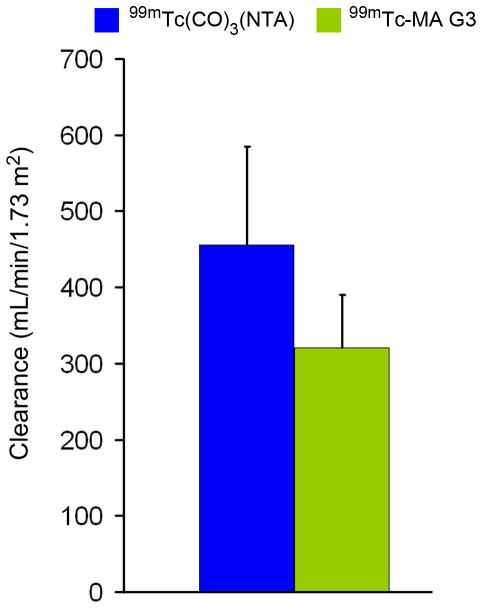
Camera-based clearance (mL/min/1.73 m2) for 99mTc(CO)3(NTA) and 99mTc-MAG3.
DISCUSSION
In our study, the 99mTc(CO)3(NTA) plasma clearance was 100% of the 131I-OIH clearance; in contrast, multiple studies have shown that the 99mTc-MAG3 plasma clearance is only about 50–60% that of 131I-OIH (3,4, 12,15). Although our study did not directly compare 99mTc-MAG3 and 99mTc(CO)3(NTA), the camera-based clearance measurement developed for 99mTc-MAG3 provides an indirect comparison (26); using this equation, the camera-based clearance of 99mTc(CO)3(NTA) in our volunteers was 456 ± 130 mL/min/1.73 m2 compared to a camera-based 99mTc-MAG3 clearance of 321 ± 69 mL/min/1.73 m2 obtained in a prior study of 106 subjects referred for potential kidney donation (Fig. 5) (28). These results indicate that 99mTc(CO)3(NTA) is accumulated more rapidly by the kidney than 99mTc-MAG3, implying that 99mTc(CO)3(NTA) has a higher clearance than 99mTc-MAG3.
The mean clearance of 131I-OIH in our healthy volunteers (472 mL/min) was slightly lower than the mean 131I-OIH clearance we obtained in an earlier study of healthy volunteers (530 mL/min) (3). The possibility of clearance variations points out the need for an internal standard such as 131I-OIH in evaluations of the clearance of new 99mTc renal tracers. Overall, the differences between 131I-OIH and 99mTc(CO)3(NTA) were minimal. 99mTc(CO)3(NTA) has the advantages of a slightly lower protein binding and red cell binding than does 131I-OIH. A lower protein binding may increase the extraction fraction since unbound tracer can be filtered by the glomerulus as well as extracted by the tubules. The reduction in red cell binding is advantageous in obtaining an accurate measure of extraction fraction; the extraction fraction is based on the difference in the plasma concentration of the tracer in arterial and renal venous blood. The tracer bound to red cells is in equilibrium with the plasma; when a renal vein blood sample is obtained, tracer associated with the red cells reequilibrates with the plasma before the plasma can be separated from the red cells and results in an overestimation of the plasma tracer concentration.
131I-OIH has slightly lower time to peak and 20 min/max than 99mTc(CO)3(NTA); however, these differences are small (0.13 for 99mTc(CO)3(NTA) vs. 0.10 for 131I-OIH), and the accuracy of the 20 min/max for 131I-OIH may have been reduced because of the low 131I counting rate at 20 min. For comparison, the cortical 20 min/max for 99mTc(CO)3(NTA) was 0.13 and for 99mTc-MAG3 was 0.19 in an earlier study of 106 subjects referred for potential kidney donation (28).
In summary, 99mTc(CO)3(NTA) is a very promising renal tracer. It is a stable complex (23) with pharmacokinetic properties that are almost identical to those of 131I-OIH in rats and healthy human. The kinetics and metabolism of the NTA ligand itself have already been investigated in several species, including humans (29–31). The oral median lethal dose of Na3NTA·H2O in rodents is about 2000 mg/kg of body weight (32). No adverse effects were noted following ingestion of a single dose of 10 mg of NTA by eight human volunteers (29), and Health Canada has determined the acceptable daily intake of NTA in drinking water to be 10 μg/kg of body weight per day (30). In our studies, no free NTA ligand was injected because the ligand was separated from the complex by HPLC before injection, and the administrated dose of the 99mTc(CO)3(NTA) complex was extremely small (less than 0.2 μg/kg of body weight). In a typical kit formulation, free NTA ligand would undoubtedly be injected, but the injected dose of NTA would likely still be below the daily acceptable limit established by Health Canada.
Our study did not evaluate hepatobiliary excretion. Hepatobiliary excretion tends to be more pronounced when renal function is compromised. One of the isomers of an early 99mTc renal tracer introduced by Fritzberg et al., 99mTc-N,N′-bis-(mercaptoacetyl)-2,3-diaminopropionate (99mTc-carboxyDADS), performed well in rodents and healthy subjects but had a greater diminution in clearance relative that of 131I-OIH in patients with reduced renal function (33,34). Future studies will need to evaluate 99mTc(CO)3(NTA) in a patient population with impaired renal function.
CONCLUSION
Initial results in normal volunteers showed that 99mTc(CO)3(NTA) is cleared from the blood and excreted in the urine as rapidly as 131I-OIH. Previous studies have shown that 99mTc(CO)3(NTA) is stable, forms as a single species and is amenable to kit formulation. Moreover, 99mTc(CO)3(NTA) has less protein binding and less activity associated with red cells than does 131I-OIH. These results strongly suggest that 99mTc(CO)3(NTA) will prove to be a superior 99mTc renal tubular imaging agent and a superior 99mTc tracer for the measurement of effective renal plasma flow.
Acknowledgments
This research was supported by a grant from National Institute of Health (NIH/NIDDK R37 DK38842). We thank Dr. Patricia A. Marzilli for her valuable comments during the preparation of the article and Eugene Malveaux for his technical assistance. Covidien is gratefully acknowledged for providing the IsoLink kits.
References
- 1.Marcus CS, Kuperus JH. Pediatric renal iodine-123 orthoiodohippurate dosimetry. J Nucl Med. 1985;26:211–214. [PubMed] [Google Scholar]
- 2.Fritzberg AR, Kasina S, Eshima D, Johnson DL. Synthesis and bilogical evaluation of technetium-99m MAG3 as a hipurran replacement. J Nucl Med. 1986;27:111–116. [PubMed] [Google Scholar]
- 3.Taylor A, Eshima D, Fritzberg AR, Christian PE, Kasina S. Comparison of iodine-131 OIH and technetium-99m MAG3 renal imaging in volunteers. J Nucl Med. 1986;27:795–803. [PubMed] [Google Scholar]
- 4.Russell CD, Thorstad B, Yester MV, Stutzman M, Baker T, Dubovsky EV. Comparison of technetium-99m MAG3 with iodine-131 hippuran by a simultaneous dual channel technique. J Nucl Med. 1988;29:1189–1193. [PubMed] [Google Scholar]
- 5.Schaap GH, Alferink THR, de Jong RBJ, et al. 99mTc-MAG3: Dynamic studies in patients with renal disease. Eur J Nucl Med. 1988;14:28–31. doi: 10.1007/BF00252614. [DOI] [PubMed] [Google Scholar]
- 6.Volkmann R, Jakobsson L, Jonsson B-A, Friberg P, Jensen G, Moonen M. Single-Kidney 99mTc-mercatoacetyltriglycine extraction and clearance as c ompared with para-aminohippurate. In: O’Reilly PH, Taylor A, Nally JV, editors. Radionuclides in Nephrourology. Blue Bell, Pennsylvania, USA: Field & Wood, Medical Periodicals, Inc; 1994. pp. 21–26. [Google Scholar]
- 7.O’Reilly P, Aurell M, Britton K, et al. Consensus on diuresis renography for investigating the dilated upper urinary tract. J Nucl Med. 1996;37:1872–1876. [PubMed] [Google Scholar]
- 8.Gordon I, Colarinha P, Fettich J, et al. Guidelines for standard and diuretic renogram in children. Eur J Nucl Med. 2001;28:BP21–30. [PubMed] [Google Scholar]
- 9.Bubeck B, Brandau W, Weber E, et al. Pharmacokinetics of technetium-99m-MAG3 in humans. J Nucl Med. 1990;31:1285–1293. [PubMed] [Google Scholar]
- 10.Sanchez J, Friedman S, Kempf J, Abdel-Dayem H. Gallbladder activity appearing 6 minutes after the intravenous injection of Tc99m MAG3 simulating a picture of obstructive uropathy of the right kidney. Clin Nucl Med. 1993;18:30–34. doi: 10.1097/00003072-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Rosen JM. Gallbladder uptake simulating hydronephrosis on Tc-99m MAG3 scintigraphy. Clin Nucl Med. 1993;18:713–714. doi: 10.1097/00003072-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Jafri RA, Britton KE, Nimmon CC, et al. Technetium-99m MAG3. A comparison with iodine-123 and iodine-131 orthoiodohippurate in patients with renal disorders. J Nucl Med. 1988;29:147–158. [PubMed] [Google Scholar]
- 13.Kotzerke J, Glatz S, Grillenberger Reproducibility of a single-sample method for 99mTc-MAG3 clearance under clinical conditions. Nucl Med Commun. 1997;18:352–357. doi: 10.1097/00006231-199704000-00175. [DOI] [PubMed] [Google Scholar]
- 14.Piepsz A, Tondeur M, Kinthaert J, et al. Reproducibility of technetium-99m mercaptoacetylglycine clearance. Eur J Nucl Med. 1996;23:195–198. doi: 10.1007/BF01731844. [DOI] [PubMed] [Google Scholar]
- 15.Eshima D, Fritzberg AR, Taylor A. Tc-99m renal tubular function agents: current status. Semin Nucl Med. 1990;20:28–40. doi: 10.1016/s0001-2998(05)80174-6. [DOI] [PubMed] [Google Scholar]
- 16.Van Nerom CG, Bormans GM, De Roo MJ, Verbruggen AM. First experience in healthy volunteers with technetium-99m L,L-ethylenedicysteine, a new renal imaging agent. Eur J Nucl Med. 1993;20:738–746. doi: 10.1007/BF00180902. [DOI] [PubMed] [Google Scholar]
- 17.Padhy AK, Bormanji J, Nimmon CC, Solanki KK, Chiwatanratan T, Britton KE. Tc-99m diaminocyclohexane (DACH), a cationic renal tubular agent: first results in man. In: O’Reilly PH, Taylor A, Nally JV, editors. Radionuclides in Nephrology. Blue Bell, Pennsylvania, USA: Field & Wood Medical Periodicals, Inc; 1994. pp. 1–3. [Google Scholar]
- 18.Taylor A, Hansen L, Eshima D, et al. Comparison of technetium-99m-LL-EC isomers in rats and humans. J Nucl Med. 1997;38:821–826. [PubMed] [Google Scholar]
- 19.Taylor AT, Lipowska M, Hansen L, Malveaux E, Marzilli LG. 99mTc-MAEC complexes: new renal radiopharmaceuticals combining characteristics of 99mTc-MAG3 and 99mTc-EC. J Nucl Med. 2004;45:885–891. [PubMed] [Google Scholar]
- 20.Lipowska M, Cini R, Tamasi G, Xu X, Taylor AT, Marzilli LG. Complexes having the fac-{M(CO)3}+ core (M = Tc, Re) useful in radiopharmaceuticals: X-ray and NMR structural characterization and density functional calculations of species containing two sp3 N donors and one sp3 O donor. Inorg Chem. 2004;43:7774–7783. doi: 10.1021/ic049544i. [DOI] [PubMed] [Google Scholar]
- 21.Lipowska M, He H, Xu X, Marzilli LG, Taylor A. First evaluation of a 99mTc-tricarbonyl complex, 99mTc(CO)3(LAN), as a new renal radiopharmaceutical in humans. J Nucl Med. 2006;47:1032–1040. [PMC free article] [PubMed] [Google Scholar]
- 22.He H, Lipowska M, Christoforou AM, Marzilli LG, Taylor AT. Initial evaluation of new 99mTc(CO)3 renal imaging agents having carboxyl-rich thioether ligands and chemical characterization of Re(CO)3 analogues. Nucl Med Biol. 2007;34:709–716. doi: 10.1016/j.nucmedbio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipowska M, Marzilli LG, Taylor AT. 99mTc(CO)3-nitrilotriacetic acid: a new renal radiopharmaceutical showing pharmacokinetic properties in rats comparable to those of 131I-OIH. J Nucl Med. 2009;50:454–460. doi: 10.2967/jnumed.108.058768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rattat D, Eraets K, Cleynhens B, Knight H, Fonge H, Verbruggen A. Comparison of tridentate ligands in competition experiments for their ability to form a [99mTc(CO)3] complex. Tetrahedron Lett. 2004;45:2531–2534. [Google Scholar]
- 25.Anghileri LJ. A simplified method for preparing high specific activity 131I-labeled hippuran. Int J Appl Radiat Isotopes. 1964;15:95. [Google Scholar]
- 26.Taylor A, Manatunga A, Morton K, et al. Multicenter trial validation of a camera based method to measure Tc-99m mercaptoacetyltriglycine, or Tc-99m MAG3, clearance. Radiology. 1997;204:47–54. doi: 10.1148/radiology.204.1.9205222. [DOI] [PubMed] [Google Scholar]
- 27.Sapirstein L, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearance of intravenously injected creatinine in the dog. Am J Physiol. 1955;181:330–336. doi: 10.1152/ajplegacy.1955.181.2.330. [DOI] [PubMed] [Google Scholar]
- 28.Esteves FP, Taylor A, Manatunga A, et al. Normal values for camera-based 99mTc-MAG3 clearance, MAG3 curve parameters, excretory parameters and residual urine volume. Am J Roentgenol. 2006;187:W610–W617. doi: 10.2214/AJR.05.1550. [DOI] [PubMed] [Google Scholar]
- 29.Budny JA, Arnold JD. Nitrilotriacetate (NTA): human metabolism and its importance in the total safety evaluation program. Toxicol Appl Pharmacol. 1973;25:48–53. doi: 10.1016/0041-008x(73)90161-0. [DOI] [PubMed] [Google Scholar]
- 30.Health Canada, Environmental and Workplace Health. [Accessed September 9, 2009];Nitrilotriacetic acid (NTA) 1990 Available at: http://www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/nitrilotriacetic_acid/index-eng.php.
- 31.World Health Organization. Health criteria and other supporting information. 2. Vol. 2. Geneva, Switzerland: World Health Organization; 1996. Nitrilotriacetic acid in drinking-water. Guidelines for drinking-water quality. [Google Scholar]
- 32.Anderson RL, Bishop WE, Campbell RL. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. Crit Rev Toxicol. 1985;15:1–102. doi: 10.3109/10408448509023766. [DOI] [PubMed] [Google Scholar]
- 33.Fritzberg AR, Kuni CC, Klingensmith WC, III, Stevens J, Whitney WP. Synthesis and biological evaluation of Tc-99m N,N′-bis-(mercaptoacetyl)-2,3-diaminopropionate: a potential replacement of I-131 hippuran. J Nucl Med. 1982;23:592–598. [PubMed] [Google Scholar]
- 34.Klingensmith WC, III, Fritzberg AR, Spitzer VM, Johnson DL, Kuni CC, Williamson MR, Washer G, Weil R., III Clinical evaluation of T c-99m N,N′-bis(mercaptoacetyl)-2,3-diaminopropanoate as a replacement for I-131 hippurate: Concise Communication. J Nucl Med. 1984;25:42–48. [PubMed] [Google Scholar]