Abstract
Despite extensive research into the pathogenesis of acute lung injury and the acute respiratory distress syndrome (ALI/ARDS), mortality remains high at approximately 40%. Current treatment is primarily supportive, with lung-protective ventilation and a fluid conservative strategy. Pharmacologic therapies that reduce the severity of lung injury in experimental studies have not yet been translated into effective clinical treatment options. Therefore, innovative therapies are needed. Recent studies have suggested that bone-marrow-derived multipotent mesenchymal stem cells (MSC) may have therapeutic applications in multiple clinical disorders including myocardial infarction, diabetes, sepsis, hepatic and acute renal failure. Recently, MSC have been studied in several in vivo models of lung disease. This review focuses on first describing the existing experimental literature that has tested the use of MSC in models of ALI/ARDS, and then the potential mechanisms underlying their therapeutic use with an emphasis on secreted paracrine soluble factors. The review concludes with a discussion of future research directions required for potential clinical trials.
Keywords: acute respiratory distress syndrome, keratinocyte growth factor, mesenchymal stem cells, pulmonary edema
1. Introduction
Morbidity and mortality have declined only modestly in patients with clinical acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) in the last decade despite extensive research into its pathophysiology [1-3]. Current treatment remains primarily supportive with lung-protective ventilation and a fluid conservative strategy [4,5]. Pharmacological therapies that reduce the severity of lung injury in vivo and in vitro have not yet been translated to effective clinical treatment options. Innovative therapies are needed. Cell-based therapy with mesenchymal stem cells for the treatment of acute lung injury is very attractive. Mesenchymal stem cells (MSCs), due to their multi-potent nature and their ability to secrete multiple paracrine factors such as growth factors, factors regulating endothelial and epithelial permeability, and anti-inflammatory cytokines can potentially treat the major abnormalities that underlie ALI, including impaired alveolar fluid clearance, altered lung endothelial permeability, and dysregulated inflammation. This review focuses on recent experiments, which support the potential therapeutic use of MSCs in ALI/ARDS.
2. Background
Adult stem cells are tissue-specific cells that have retained the ability to differentiate into a variety of cell lineages thereby making them multipotent. Although adult stem cells do not possess the full range of plasticity of embryonic stem cells (ESCs), they offer practical advantages including ease of isolation and propagation. In addition, they are not associated with the ethical controversy that surrounds ESC research. One class of adult stem cells that has been of particular interest is MSCs. MSCs, also called marrow stromal stem cells, were first discovered in 1968 by Friedenstein and colleagues [6] who found bone marrow stromal cells that were adherent, clonogenic and fibroblastic in appearance. Adult mesenchymal stem cells can be isolated from a variety of human tissues. Bone-marrow-derived MSCs are presumed to reside near the sinusoids and function as support cells for hematopoietic stem cells (HSC). Although MSCs comprise less than 0.1% of all bone marrow cells, they can be isolated from whole bone marrow aspirates by their ability to adhere to plastic and form colonies. Currently, there are no MSC-specific cell surface markers. Consequently, in 2006, the International Society of Cellular Therapy have defined MSCs by three criteria: i) MSCs must be adherent to plastic under standard tissue culture conditions; ii) MSCs must express certain cell surface markers such as CD105, CD90 and CD73, but must not express other markers including CD45, CD34, CD14 or CD11b; and iii) MSCs must have the capacity to differentiate into mesenchymal lineages including osteoblasts, adipocytes and chrondoblasts under in vitro conditions [7]. Use of these cells for therapeutic purposes in a variety of diseases has attracted considerable attention due to their low immunogenicity, their immunomodulatory effects, and their ability to secrete endothelial and epithelial growth factors (Table 1).
Table 1.
Potential criteria for potency assay for MSC based on secretion of paracrine factors alone.
| Soluble factors* | Important in |
|---|---|
| Keratinocyte growth factor | Apoptosis |
| Hepatocyte growth factor | Surfactant synthesis |
| Epidermal growth factor | Alveolar fluid transport Endothelial permeability? |
| Angiopoietin-1 | Epithelial & endothelial permeability? |
| IL-1 receptor antagonist (IL-1RA) | Anti-inflammatory |
| IL-10 | |
| Prostaglandin E2 |
A correlation between the level of cytokines, growth and anti-permeability factors secreted will need to be made with its therapeutic efficacy in animal and human models of ALI/ARDS. For example, will MSC improve alveolar fluid clearance, lung water or lung endothelial permeability to protein or reduce the inflammatory milieu within the injured alveolus.
Secretion of some soluble factors may be dependent on cell-cell contact or the alveolar milieu itself, such as IL-10 or prostaglandin E2.
Allogeneic MSCs are able to evade clearance by the host immune system through a variety of mechanisms including low expression of the MHC I and II proteins and lack of the T cell co-stimulatory molecules, CD80 and CD86 [8]. This property makes MSCs attractive for cell-based therapy because they can be administered to patients without HLA matching. However, recent literature has shown that MSCs can express higher levels of the MHC class proteins than originally thought. Specifically, at low levels of exposure to IFN-γ, MSC upregulates expression of MHC II and do possess some immunostimulatory properties [9-11]. In addition, current studies have demonstrated that infusion of allogeneic MSC can elicit a host response and lead to graft rejection [12]. Therefore, it has become apparent that the original belief that MSC have low immunogenicity is not entirely correct, and that these cells have complex interactions with the innate and adaptive immune systems.
3. Mesenchymal stem cells in animal and human ALI models
Recent studies have suggested that bone-marrow-derived multipotent MSCs may have therapeutic applications in several clinical disorders including myocardial infarction [13-15], diabetes [16], sepsis [17], hepatic failure [18] and acute renal failure [19]. Allogeneic MSC have been investigated in several in vivo models of lung disease [17,20-30]. In bleomycin-induced lung injury and fibrosis, MSC improved survival and lung inflammation when given intravenously. These beneficial effects were not accounted for by lung engraftment rates (< 5%) but rather through a paracrine mechanism [22,25]. In a follow-up study, Ortiz et al. found that a subpopulation of mouse MSC produced IL-1 receptor antagonist (IL-1RA), a paracrine soluble factor that was capable of attenuating the severity of bleomycin-induced lung injury. In the same study, these authors also isolated a subpopulation of human MSC, approximately 5%, that produced high levels of IL-1RA [23]. To determine the effect of MSC on acute lung injury, several groups studied the therapeutic effect of MSC following intra-peritoneal [26] or intra-tracheal Escherichia coli endotoxin [28,30]. Xu et al. found that intravenous administration of MSC following intra-peritoneal LPS prevented endotoxin-induced pulmonary inflammation, injury and edema as well as the influx of neutrophils into the injured alveoli [26]. In addition, Xu et al. and Mei et al. also discovered that transfection of MSC with human angiopoietin-1 (Ang1) further reduced the severity of E. coli endotoxin-induced lung injury [28,30].
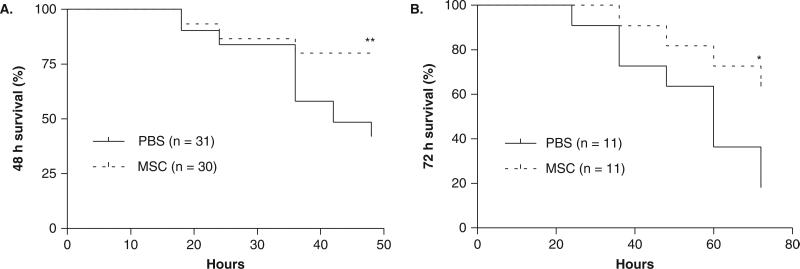
Despite the promising results, little is known regarding the effect of bone-marrow-derived MSC as therapy in experimental models of acute lung injury and pulmonary edema. Most prior studies have not adequately evaluated MSC as a treatment modality; the cells were given concurrently with the injury or before the injury, primarily by the intravenous route of delivery. Recently we reported that intrapulmonary (via the trachea) treatment with MSC 4 h after endotoxin delivery to the lung improved survival and reduced the extent of pulmonary edema formation in E. coli endotoxin-induced acute lung injury in mice (Figure 1) [27]. MSC therapy reduced the plasma and bronchoalveolar lavage levels of pro-inflammatory cytokines and increased the levels of several anti-inflammatory cytokines, including IL-10.
Figure 1. Intratracheal (IT) treatment with MSC improved 48 and 72 h survival in an endotoxin model of ALI in mice.
A. MSC (750,000 cells/30 μl) or PBS (30 μl) was administered IT 4 h after IT instillation of endotoxin (5 mg/kg). 48 h survival was 80% in the MSC group versus 42% in the PBS group (n = 30 for MSC group, n = 31 for PBS group, **p < 0.01 using a log-rank test. B. At 72 h, survival was 64% in the MSC group versus 18% in the PBS group (n = 11 per group, *p < 0.05 using a log-rank test,).
Figure from [27], reprinted with permission, J of Immunology, copyright 2007 The American Association of Immunologists, Inc.
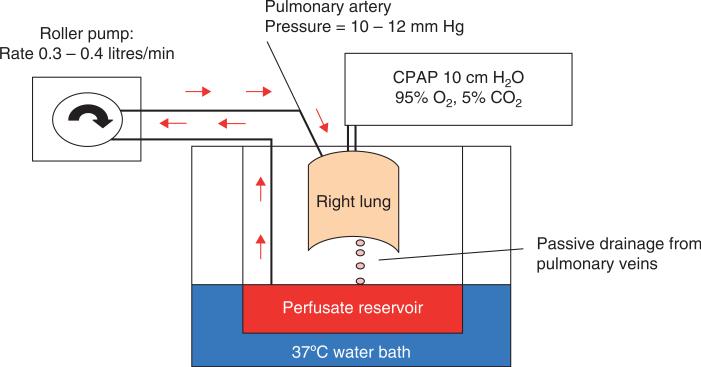
To further define the therapeutic potential of MSC, we have developed two human models of ALI: i) an ex vivo human lung preparation perfused partially with human blood injured by E. coli endotoxin (Figure 2); and ii) primary cultures of human alveolar epithelial type II cells grown in a Transwell plate with an air–liquid interface injured by an inflammatory insult. In the ex vivo perfused human lung, the intra-bronchial instillation of human MSC 1 h following endotoxin-induced lung injury restored alveolar fluid clearance, in part by the secretion of keratinocyte growth factor (KGF) [31]. In primary cultures of human alveolar type II cells, human MSC grown in the bottom chamber of a Transwell plate restored the increase in epithelial permeability to protein caused by exposure to inflammatory cytokines in part by the secretion of angiopoietin-1 [32]. Further studies are in progress to further define the mechanisms of benefit in these models.
Figure 2. Schematic diagram of the ex vivo perfused human lung.
The right or left human lung was selected for perfusion if the total ischemic time was less than 48 h and if the selection criteria were met. The lung was initially rewarmed and perfused with medium (DME H-21) containing 5% albumin over 1 h and inflated and oxygenated with 10 cm H2O continuous positive airway pressure (CPAP) (95% O2). The perfusion rate was set at 0.3 – 0.4 litres/min and the left atrial pressure at 0 mmHg. Following rewarming, alveolar fluid clearance (AFC) was measured in the right or left upper lobe. If AFC > 10%/h, 100 ml fresh human blood was added to the perfusate for a final hematocrit of 4%, and E. coli endotoxin was instilled in the right middle or left lower lobe. Allogeneic human MSCs, MSC-conditioned medium or control lung fibroblasts were added to the endotoxin-injured lung lobe 1 h after the endotoxin instillation (see [31]).
4. Potential mechanism (engraftment)
Much of the initial interest in MSC therapy stemmed from the multipotent properties of the cells. Krause et al. [33] found that a single bone-marrow-derived cell could give rise to cells of multiple different organs including the lung. They reported up to 20% engraftment of bone marrow-derived cells in the lung, including epithelial cells, from a single hematopoietic precursor. Kotton et al. [34] reported that plate-adherent cultured bone marrow cells, when given intravenously in wild-type mice following bleomycin-induced lung injury, engrafted into the recipient lung parenchyma with a morphological and molecular phenotype of alveolar type I pneumocytes. This gave rise to intensive investigation into the possibility that bone-marrow-derived stem cells, MSCs specifically, may be able to regenerate the lung epithelium and/or endothelium [33-37]. However, these results were questioned by multiple groups, who observed only engraftment of leukocyte lineages [38], or low engraftment rates in lung injury models with observed rates of < 1% [22,25,39,40]. Despite initial interest in their multipotent properties, engraftment in the lung now does not appear to play a major beneficial role. The beneficial effect of MSC appears to derive more from their capacity to secrete paracrine soluble factors that modulate immune responses as well as alter the responses of endothelium or epithelium to injury through the release of growth factors [17,18,41-47].
However, the role of stem cell engraftment in repair following lung injury requires further research, as suggested by several recent publications. Sueblinvong et al. [48] found that human umbilical cord MSC when cultured in vitro with specialized growth medium/growth factors expressed Clara cell secretory protein (CCSP), surfactant protein C (SPC) and cystic fibrosis transmembrane conductance regulator (CFTR). More significantly, after systemic administration to immunotolerant, NOD-SCID mice, rare cells were localized in the lung airway epithelium that expressed cytokeratin and human CFTR. Wong et al. [49,50] found a subpopulation of adherent human and murine bone marrow cells that expressed CCSP as well, and when cultured ex vivo with an air-liquid interface, these CCSP+ cells expressed alveolar type I and II markers such as pro-SPC, CFTR and epithelial sodium channel (ENaC). CCSP+ cells preferentially homed to naphthalene-damaged airways when delivered trans-tracheally or intravenously. Interesting, these bone marrow cells expressed CD45 and the mesenchymal stem cell markers CD73, CD90 and CD105 [7].
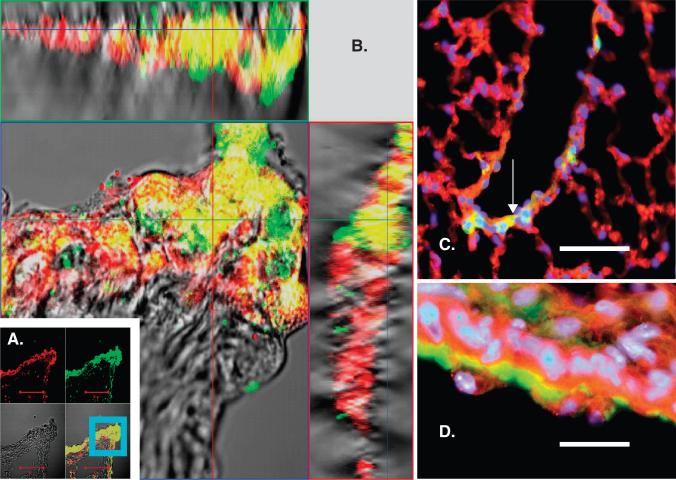
Recently, we obtained and characterized MSC from bone marrow (BM) of GFP transgenic mice and developed an in vitro model to study the endodermal differentiation of MSC using co-cultures of MSC and transformed lung epithelial (A549) cells. MSC in co-culture with A549 were separated by a cell-impermeable membrane to eliminate the possibility of cell fusion. Under these conditions, MSC expressed several lung epithelial markers (cytokeratins 5, 8, 14, 18, 19, pro-surfactant protein C, ZO-1), detected using quantitative reverse transcriptase-PCR and Western blotting. Beta-catenin signaling was activated in MSC. Treatment of MSC with 10 – 20 mM lithium chloride activated the beta-catenin pathway and enhanced expression of epithelial markers, although this activation was transient. We concluded that A549 cells could trigger endodermal epithelial differentiation of MSC by a paracrine mechanism that may include activation of beta-catenin signaling [51]. We further investigated the participation of BM cells in the process of airway epithelial restoration after naphthalene-induced injury. We transplanted sex-mismatched green fluorescent protein (GFP)-tagged MSCs into 5Gy-irradiated C57BL/6 recipients. After 1 month of recovery, experimental animals were subjected to naphthalene IP and killed 2 – 30 days later. By immunofluorescence, immunohistochemistry and in situ hybridization for the Y-chromosome, we observed patches of donor-derived cells in the large and small conducting airways (Figure 3), mostly at 2 – 6 days after injury. GFP+ cells in the epithelium of airways were positive for pancytokeratin and some other epithelial markers. Although rare, GFP+ cells formed clear isolated patches of the bronchial epithelium, consistent with clone formation; as some cells were also positive for proliferating cell nuclear antigen, a marker of proliferating cells [52].
Figure 3. Post-naphthalene injury, rare patches of GFP+ cells in the bronchial epithelium in animals transplanted with GFP+ MSC stained positively for pancytokeratin (PCK).
A, B. Confocal microscopy image of mouse lungs 30 days after naphthalene, paraffin-sectioned and stained for GFP with FITC-labeled antibody (green) and PCK with Alexa Fluor 633-labeled antibody (red). A. Upper left panel – red fluorescence image (Alexa Fluor), upper right panel – green fluorescence image (GFP), lower left – differential interference contrast image of tissue, lower right – combined image. Co-localization of GFP and PCK signal is also clearly seen in combined Z-stack images for the region (B), indicated in panel A by the blue square. Z-stack image B confirms three-dimensional co-localization of GFP and PCK signals (upper panel in B is projections of horizontal slice through the image, and right panel is projection of vertical slice through the image, scale bar is 100 μm). C, D. Fluorescent images of paraffin-sectioned lung 6 days after naphthalene. Immunostaining for GFP (green), PCK (red) and nuclear staining with DAPI (blue). C – merged image for all the stains, the arrow points to a cluster of GFP+ PCK+ nucleated cells in the walls of the small airway. Bar = 50 μm. D - same as C, bar = 10 μm.
Figure from [52], reprinted with permission from Anat Rec (Hoboken), John Wiley & Sons, Inc.
5. Potential mechanism (immunomodulation)
A major characteristic of MSC has been the immunomodulatory properties of the cells. Multiple studies have demonstrated that MSC possess potent immunosuppressive effects by inhibiting the activity of both innate and adaptive immune cells [42,43,53,54]. This immunosuppression has been shown to be mediated by cell-contact-dependent and -independent mechanisms through the release of soluble factors. The list of candidate mediators released or induced by MSC includes TGF-β, PGE2, IDO, IL-10 and IL-1RA among others. In a model of sepsis following cecal ligation and puncture (CLP) in mice, Nemeth et al. [17] found that bone-marrow-derived MSCs, activated by LPS or TNFα, secreted prostaglandin E2, which reprogrammed alveolar macrophages to secrete IL-10. The beneficial effect of MSCs on mortality and improved organ function following sepsis (CLP) was eliminated by macrophage depletion or pretreatment with antibodies to IL-10 or the IL-10 receptor, suggesting an essential role for IL-10. IL-10 is a cytokine secreted predominantly by monocytes that downregulates the expression of TH1 cytokines, MHC class II antigens and costimulatory molecules on macrophages. IL-10 has also been reported to inhibit the rolling, adhesion and transepithelial migration of neutrophils [55]. In co-culture experiments, cell contact between MSC and macrophages was required to stimulate IL-10 production following LPS stimulation; MSC separated by a Transwell plate or MSC-conditioned medium could not induce IL-10 production [17]. In a model of acute lung injury by intratracheal E. coli endotoxin in mice, we [27] found that intrapulmonary MSC improved survival and lung injury in association with a decrease in MIP-2 and TNFα levels in the bronchoalveolar lavage fluid (BAL) and elevated levels of IL-10 in both the plasma and BAL fluids. In bleomycin-induced lung injury and fibrosis in mice, Ortiz et al. [23] found that MSC decreased subsequent lung collagen accumulation, fibrosis and levels of matrix metal-loproteinases in part by IL-1RA secretion; IL-1RA is a cytokine that competitively competes with IL-1β for IL-1 receptor binding. IL-1β is one of the major inflammatory cytokines in pulmonary edema fluid in patients with ALI/ARDS [56]. These results confirmed the anti-inflammatory effect of MSC in multiple lung injury experiments in mice [22,25,26,28,30].
Despite the well documented immunosuppressive effects of MSCs, recent literature described a dual role for MSCs as immunostimulatory cells as well [11]. As explained above, some studies have reported that MSC can upregulate expression of MHC II when exposed to low levels of inflammation and function as antigen-presenting cells stimulating the adaptive immune system [9,10]. Recent evidence has also shown that MSC can secrete IL-6 and induce production of IgG by B lymphocytes in an in vitro setting [57]. In addition, MSCs can prevent neutrophil apoptosis and degranulation in culture without inhibiting their phagocytic or chemotactic capabilities [58]. Thus, recent studies have demonstrated that MSCs have more complex effects on the immune system than their classical role as immune suppressor cells. Understanding the mechanisms responsible for these apparently paradoxical roles that MSCs play in the immune response will be important in developing cell based therapy for clinical use.
A safety concern with MSC-based therapy, particularly in treating ARDS, is their effect on host defense against bacterial infection. Bacterial pneumonia and sepsis from a non-pulmonary cause are two of the most common etiologies of ARDS [2]. Given the preponderance of literature that describes the immunosuppressive effect of MSC, there is concern that this effect may impede the host's ability to clear an infection. However, as mentioned previously, there is new work describing a dual role for MSC in regulating the immune system and their immunostimulatory effects. Furthermore, there has been a recent report demonstrating a protective effect of systemically administered MSC in a mouse model of bacterial sepsis [17] as well as preliminary data from our own group that MSC are associated with a reduction in the number of live bacteria in E. coli pneumonia in mice (unpublished data). Additional work is needed to better define the effects of MSC in the setting of a bacterial infection before MSC based therapy can be used in patients with ALI/ARDS.
6. Potential mechanism (alveolar fluid clearance)
Impaired alveolar fluid clearance (AFC, i.e., the resolution of pulmonary edema) is common in patients with ALI/ARDS. The level of AFC impairment has significant prognostic value in determining morbidity and mortality [59,60]. Several experimental studies have studied the mechanisms that reduce AFC in ALI, and several pathways have been implicated [61,62]. In the alveolar environment, basal AFC is determined predominately by amiloride-sensitive and insensitive sodium channels and the activity of the Na-K ATPase [61,63-66]. Several stimuli can upregulate AFC including beta-adrenergic agonists via cAMP-dependent mechanisms [61,62]. In the mouse and human lung, cAMP dependent alveolar epithelial fluid transport is dependent on CFTR activity, especially in mediating β-adrenergic receptor driven alveolar epithelial fluid transport [67-69].
In ALI, we and other investigators have reported that pulmonary edema fluid contained high levels of several pro-inflammatory cytokines, including IL-1β, IL-8, TNFα and TGFβ1 [70-72]. Several of these pro-inflammatory cytokines have been studied in experimental fluid transport experiments. For example, TNFα decreased the expression of ENaC (α-, β-, γ-subunits) mRNAs and protein levels as well as the amiloride-sensitive current and ouabain-sensitive Rb+ uptake in rat alveolar epithelial cells [73]. Similarly, IL-1β decreased dexamethasone-induced αENaC mRNA and protein levels and the amiloride-sensitive fraction of the transepithelial current and sodium transport across rat type II cell monolayers [74]. More recently, we reported that TGFβ1 decreased the amiloride-sensitive fraction of Na+ uptake and fluid transport across monolayers of rat and human type II cells as well as αENaC mRNA and protein expression [75]. In chronic inflammation associated with nasal polyposis, TGFβ1 downregulated CFTR mRNA and protein expression as well as the cAMP-dependent current in human nasal epithelial cells [76].
Bone marrow derived MSC are known to produce several epithelial specific growth factors, specifically keratinocyte growth factor (KGF), the seventh member of the fibroblast growth factor family. We have been particularly interested in KGF because of work from our group as well as other investigators who have reported that KGF can reduce lung injury in small animal models of pulmonary edema. Recombinant KGF pre-treatment reduced mortality following intra-tracheal instillation of hydrochloric acid [77,78], bleomycin [79,80], hyperoxia [81,82] and Pseudomonas aeruginosa [83]. In rat lung, KGF improved alveolar fluid transport in part by up-regulating αENaC gene expression [84] and Na-K ATPase activity [85].
In the ex vivo perfused human lung, the intra-bronchial instillation of human MSCs 1 h following endotoxin-induced lung injury restored alveolar fluid clearance in part by the secretion of KGF [31]. Several properties of KGF could explain the therapeutic effect of human MSC on restoring AFC, including alveolar epithelial type II cell hyperplasia and differentiation, surfactant production [86], anti-apoptotic effects [87] and increased transcription and/or translation of the major sodium and chloride transport proteins [84,85]. Because the effect of MSC therapy in the E. coli endotoxin-induced lung injury in the ex vivo perfused human lung occurred over a 3 h time period, the therapeutic benefit of KGF in these experiments is less likely to be explained by type II cell hyperplasia or transcriptional effects. Alternatively, an increase in vectorial fluid transport across the alveolar epithelium can be mediated by an increase in trafficking of sodium transport proteins to the cell surface [88,89].
7. Potential mechanism (lung endothelial permeability)
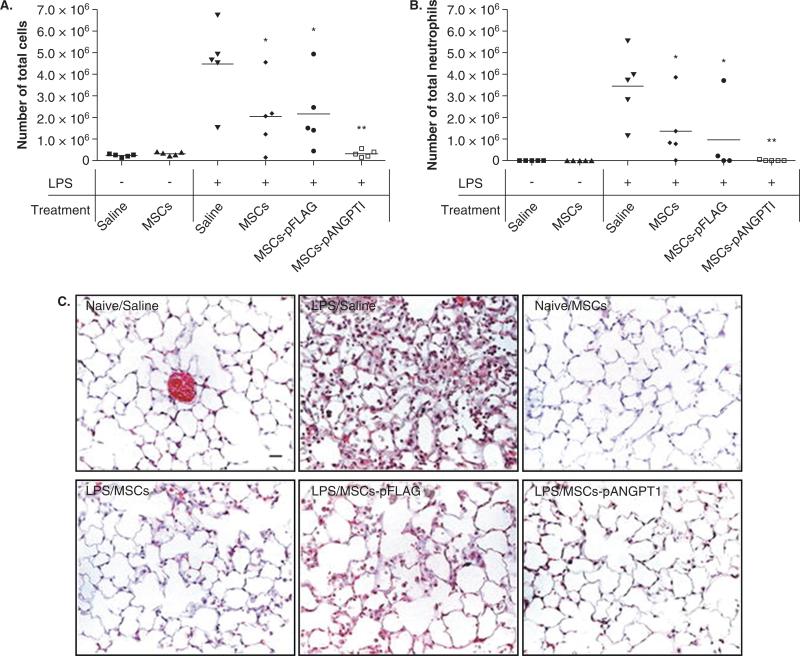
Another possible mechanism through which MSC may be beneficial is through therapeutic effects on the injured lung endothelium. The integrity of the lung microvascular endothelium is essential to prevent the influx of protein-rich fluid from the plasma as well as inflammatory cells which may further aggravate the ability of the lung epithelium to reduce alveolar edema. Several paracrine soluble factors, such as Ang1 and KGF, are potentially important in these effects. Ang1, a ligand for the endothelial Tie2 receptor, is a known endothelial survival [90] and vascular stabilization factor that reduces endothelial permeability and inhibits leukocyte–endothelium interactions by modifying endothelial cell adhesion molecules and cell junctions [91-94]. MSCs or MSCs (used as a vehicle for gene delivery) transfected with the human Ang1 gene reduced both pulmonary vascular endothelial injury and the recruitment of inflammatory cells into the lung in mice injured by LPS-induced lung injury [28,30,95]. In the study by Mei et al. [28], the transfection of Ang1 further reduced lung inflammation and nearly completely reversed the LPS-induced increase in lung permeability (Figure 4). We recently found that allogeneic human MSC secrete a significant amount of Ang1. In addition, using siRNA technology, the secretion of Ang1 produced therapeutic effect on epithelial protein permeability in primary cultures of human alveolar epithelial type II cells injured by an inflammatory insult [32].
Figure 4. Therapeutic potential of MSC combined with angiopoietin 1 (Ang1) in experimental ALI in mice.
MSC administration was associated with a reduction in alveolar inflammation as measured by bronchoalveolar lavage (BAL) total cell count (A) and neutrophil count (B). This effect was augmented in mice given MSC transfected with human Ang1. Histology of the lungs of mice treated with MSC also demonstrated significantly less lung injury and inflammation than control mice (C). Again, this protective effect was even more pronounced in mice receiving MSC transfected with Ang1. Representative images of hematoxylin and eosin stained lung sections from six experimental conditions: (1) Naive/Saline: control mice, (2) LPS/Saline: LPS-injured mice, (3) Naive/MSCs: control MSC mice, (4) LPS/MSCs: LPS-injured mice treated with MSC, (5) LPS/MSCs-pFLAG: LPS-injured mice treated with MSC transfected with a control plasmid, and (6) LPS/MSCs-pANGPT1: LPS-injured mice treated with MSC transfected with a plasmid overexpressing human angiopoietin-1. Lungs were fixed with 4% paraformaldehyde, embedded in paraffin, and then cut into 5 μm thick sections before being stained. Scale bar = 20 μm. Figure from [28].
MSC produce and secrete several epithelial specific growth factors such as KGF. In models of acute permeability edema such as α-naphthylthiourea [96], P. aeruginosa [83] or ventilator-induced lung injury [97], KGF reduced lung edema and bronchoalveolar lavage protein levels. Cultured allogeneic human MSC produced substantial quantities of KGF. The role of KGF is intriguing given the previous studies of ALI in animal models and a recent study by Murakami et al. [98] who reported that fibroblast growth factors (FGF), FGF2, FGF4 and FGF8, which are specific for both FGF receptors 1IIIc and 3IIIc, are responsible for the maintenance of endothelial barrier homeostasis. Another epithelial specific growth factor secreted by MSC is hepatocyte growth factor (HGF). Previously, HGF was found to stabilize the integrity of pulmonary endothelial cells by the inhibition of Rho GTPase and the prevention of actin stress fiber formation and paracellular gaps among pulmonary endothelial cells injured by thrombin [99,100]. In the future, it will be important to understand the contribution of each soluble factor secreted by MSCs to lung endothelial permeability in ALI.
The potential role of other bone-marrow-derived cells such as endothelial progenitor cells (EPC) in ALI as therapy as been studied in animal experiments [101] as well as in some clinical trials [102,103]. In an oleic acid lung injury in rabbits, Lam et al. found that autologous transplantation of EPC preserved not only the pulmonary alveolar-capillary barrier but also pulmonary vascular endothelial-dependent relaxation [101]. In the future, the role of engraftment, trans-differentiation or fusion with both alveolar epithelial or lung endothelial cells by MSC or other progenitor cells will need to be studied further.
8. Conclusions
ALI/ARDS is the most common cause of hypoxemic respiratory failure in critically ill patients. Current treatment for ALI/ARDS is supportive and therefore new treatments are needed. Mesenchymal stem cells (MSC) are adult stem cells most commonly isolated from the bone marrow that possess unique immunomodulatory and paracrine properties which make them attractive for cell based therapy. There has been rapidly emerging literature demonstrating the therapeutic potential of MSC in various organ injury models such as myocardial infarction [13-15], diabetes [16], sepsis [17], hepatic [18] and acute renal failure [19]. Recently, some investigators have also reported that MSC have beneficial effects in experimental models of acute lung injury in both animals [26,28,30] and human tissue [31,32]. Given the promising initial results obtained with the use of MSC in experimental models of ALI/ARDS, there has been enthusiasm to advance cell-based therapy to patients with ALI/ARDS. While clinical trials of MSC based therapy have been initiated in patients with cardiac, renal and auto-immune diseases, there are several questions that need to be addressed before cell-based therapy can be tested in patients with ALI/ARDS. Future research in this field should continue and focus on elucidating the basic mechanisms responsible for the beneficial effects of MSC, as well as determining the practical issues involved in producing a cell-based therapy for patients. In the process, a novel therapy for ALI/ARDS might emerge.
9. Expert opinion
The field of cell-based therapy, involving bone-marrow-derived MSCs, for ALI has stimulated a high level of enthusiasm for its potential therapeutic use clinically in patients with ALI/ARDS based largely on multiple in vivo experiments involving mice [26-28,30]. Recently, in a novel ex vivo perfused human lung preparation, we found that allogeneic human mesenchymal stem cells improved alveolar fluid clearance following endotoxin-induced lung injury, based in part on the secretion of the growth factor, KGF [31]. However, despite these encouraging experimental results, several issues remain which must be addressed prior to any human clinical trials.
First, the isolation and classification of human mesenchymal stem cells must be further defined, particularly concerning the issue of potency. Are all MSCs the same despite different isolation techniques, growth conditions, passage number and use? This is particularly important since MSCs lack a specific cell surface marker that distinguishes them from other cells (based on the classification of the International Society of Cellular Therapy) [7]. A potency assay should be developed to compare and contrast MSCs currently in use in the literature. Some potential parameters that may constitute such a potency assay are included in Table 1.
Second, a more precise understanding of the mechanisms underlying the therapeutic effect of MSCs in models of lung injury is needed. While most investigators have invoked both the immunomodulatory and growth factor production properties of MSCs to explain the protective effects, the exact mechanisms responsible for these effects remain unclear. For example, MSCs secrete or induce production of a variety of soluble factors such as IL-10, PGE2, TGF-β, KGF and others, but it is not known which of these factors is essential to the protection provided by MSCs. In addition, another major question is whether the effect is produced predominantly through cell-contact-dependent or -independent mechanisms or both, and whether or not the functional behavior of the cells changes depending on the alveolar milieu. Answering these questions will determine whether the effect of MSCs can be replicated with a mixture of recombinant soluble factors secreted by MSCs or with MSC-conditioned medium alone. Although MSCs are considered immunoprivileged, one of the primary concerns with administering MSCs to patients is the potential for the MSCs to undergo malignant transformation. While human MSCs have not been shown to cause malignancy, mouse MSCs have been shown to induce malignant tumors in mice [104,105]. Also, there have been reports of MSC enhancing the metastatic potential of solid tumors, such as breast cancer in mouse models [106,107]. Furthermore, there is concern that MSCs may transform after repeated passage in vitro since studies have demonstrated that some of the cells develop abnormal karyotypes, which predispose the cells to malignant transformation [108]. Ironically, because MSCs may have greater immunogenicity this may prove to be beneficial with respect to the concerns of malignancy, since their eventual recognition and clearance by the host immune system would make the development of tumors less likely.
Third, for clinical trials, the optimal dose and route of cell delivery remains to be determined. The experimental literature has utilized doses ranging from 5 × 105 to 106 cells per animal, predominantly in mice. However, dose responses have not been reported in the experimental literature so the optimal dose in mice is unknown as well. In our ex vivo perfused human lung, only 5 × 106 human MSC were required to be instilled into the injured lung lobe following E. coli endotoxin-induced lung injury for efficacy [31]. However, further studies are needed to determine the optimal dose. Prior clinical trials using human MSC may provide a guide for the dose of cells to use, although this is complicated by the fact that these investigators were targeting different organs and diseases as well as different routes of delivery. In addition, the experimental literature has utilized both intravenous and intratracheal routes of delivery with reported beneficial effects [26-28,30]. However, a comparison or even combination of these two routes has not been done. There are potential advantages to each route including relative ease of delivery using the intratracheal route, since this could probably be accomplished with a fibreoptic bronchoscope and could specifically target affected areas of the lung. On the other hand, the intravenous route may offer greater systemic benefit and help alleviate the multiple organ dysfunction that often accompanies ALI/ARDS. Nonetheless, this is an important practical issue that must be resolved before moving forward with clinical trials.
Fourth, the selection of patients for a clinical trial of ALI/ARDS with MSC may be critical for its potential success or failure. Given the uncertainty underlying the mechanism of effect, it may be prudent initially to target patients with a pulmonary source of ALI such as pneumonia or gastric aspiration. In addition, any potential benefit must outweigh the risk to the patient of either intra-bronchial or intravenous instillation of MSCs.
Fifth, although the focus of this review has been predominantly on the potential use of bone marrow derived MSCs, we must always keep in mind that other adult stem cells, such as cells derived from placental or amniotic and endothelial progenitor cells, may have more therapeutic potential. As we began to understand further the mechanisms underlying the therapy efficacy, we will need to expand our experiments to determine the optimal source for the most effective stem cell.
Lastly, despite promising results and public enthusiasm, clinician-scientists involved in the translation of stem cell research into clinical trials must always keep in mind the lessons learned from the field of gene therapy [109]: i) Basic research into the underlying mechanisms of benefit of stem cell must always walk hand in hand with clinical trials; and ii), above all, we must do no harm.
Footnotes
Declaration of interest
The authors declare no conflict of interest and have received no payment for the preparation of this manuscript.
Bibliography
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–27. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 7.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz) 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 9.Chan JL, Tang KC, Patel AP, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–24. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-γ-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–7. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 11.Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69:1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li TS, Hayashi M, Ito H, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-β-preprogrammed bone marrow stem cells. Circulation. 2005;111:2438–45. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 14.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 15.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–06. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekkadan B, van Poll D, Suganuma K, et al. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS ONE. 2007;2:e941. doi: 10.1371/journal.pone.0000941. Published online 26 September 2007, doi:10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 20.Weiss DJ, Kolls JK, Ortiz LA, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–67. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109:3147–51. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–72. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 25.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–52. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Woods CR, Mora AL, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–41. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 28.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. Published online 4 September 2007, doi:10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhen G, Liu H, Gu N, et al. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front Biosci. 2008;13:3415–22. doi: 10.2741/2936. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Qu J, Cao L, et al. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 31.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0907996106. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neyrinck AP, Lee JW, Fang X, et al. Angiopoietin-1 reverses protein permeability in an injured model of cultured human alveolar epithelial type II cells. FASEB J. 2008;22:932.6. [Google Scholar]
- 33.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 34.Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Bunnell BA, Painter RG, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 2005;102:186–91. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spees JL, Pociask DA, Sullivan DE, et al. Engraftment of bone marrow progenitor cells in a rat model of asbestos-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:385–94. doi: 10.1164/rccm.200607-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE. 2007;2:e416. doi: 10.1371/journal.pone.0000416. Published online 2 May 2007, doi:10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 39.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loi R, Beckett T, Goncz KK, et al. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 43.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 44.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 45.Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 46.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. Published online 2 April 2008, doi:10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sueblinvong V, Loi R, Eisenhauer PL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–11. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong AP, Dutly AE, Sacher A, et al. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293:L740–52. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- 50.Wong AP, Keating A, Lu WY, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–48. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popov BV, Serikov VB, Petrov NS, et al. Lung epithelial cells induce endodermal differentiation in mouse mesenchymal bone marrow stem cells by paracrine mechanism. Tissue Eng. 2007;13:2441–50. doi: 10.1089/ten.2007.0001. [DOI] [PubMed] [Google Scholar]
- 52.Serikov VB, Popov B, Mikhailov VM, et al. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–45. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- 53.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 54.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 55.Ajuebor MN, Das AM, Virag L, et al. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–91. [PubMed] [Google Scholar]
- 56.Geiser T, Atabai K, Jarreau PH, et al. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1β-dependent mechanism. Am J Respir Crit Care Med. 2001;163:1384–8. doi: 10.1164/ajrccm.163.6.2006131. [DOI] [PubMed] [Google Scholar]
- 57.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–43. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 58.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–62. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 59.Matthay MA, Wiener-Kronish JP. Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis. 1990;142:1250–7. doi: 10.1164/ajrccm/142.6_Pt_1.1250. [DOI] [PubMed] [Google Scholar]
- 60.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–83. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 61.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 62.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol. 2006;35:10–9. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol. 1999;61:627–61. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 64.Eaton DC, Chen J, Ramosevac S, et al. Regulation of Na+ channels in lung alveolar type II epithelial cells. Proc Am Thorac Soc. 2004;1:10–6. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- 65.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685–95. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 66.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol. 2002;93:1852–9. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 67.Fang X, Song Y, Hirsch J, et al. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L242–9. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 68.Brochiero E, Dagenais A, Prive A, et al. Evidence of a functional CFTR Cl- channel in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L382–92. doi: 10.1152/ajplung.00320.2002. [DOI] [PubMed] [Google Scholar]
- 69.Mutlu GM, Adir Y, Jameel M, et al. Interdependency of β-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res. 2005;96:999–1005. doi: 10.1161/01.RES.0000164554.21993.AC. [DOI] [PubMed] [Google Scholar]
- 70.Pugin J, Verghese G, Widmer MC, Matthay MA. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–12. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 71.Olman MA, White KE, Ware LB, et al. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1β-induced IL-6 expression. J Immunol. 2004;172:2668–77. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- 72.Lee JW, Fang X, Dolganov G, et al. Acute lung injury edema fluid decreases net fluid transport across human alveolar epithelial type II cells. J Biol Chem. 2007;282:24109–19. doi: 10.1074/jbc.M700821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dagenais A, Frechette R, Yamagata Y, et al. Downregulation of ENaC activity and expression by TNF-α in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L301–11. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- 74.Roux J, Kawakatsu H, Gartland B, et al. Interleukin-1β decreases expression of the epithelial sodium channel α-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–89. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- 75.Frank J, Roux J, Kawakatsu H, et al. Transforming growth factor-β1 decreases expression of the epithelial sodium channel αENaC and alveolar epithelial vectorial sodium and fluid transport via an ERK1/2-dependent mechanism. J Biol Chem. 2003;278:43939–50. doi: 10.1074/jbc.M304882200. [DOI] [PubMed] [Google Scholar]
- 76.Pruliere-Escabasse V, Fanen P, Dazy AC, et al. TGF-β1 downregulates CFTR expression and function in nasal polyps of non-CF patients. Am J Physiol Lung Cell Mol Physiol. 2005;288:L77–83. doi: 10.1152/ajplung.00048.2004. [DOI] [PubMed] [Google Scholar]
- 77.Nemzek JA, Ebong SJ, Kim J, et al. Keratinocyte growth factor pretreatment is associated with decreased macrophage inflammatory protein-2α concentrations and reduced neutrophil recruitment in acid aspiration lung injury. Shock. 2002;18:501–6. doi: 10.1097/00024382-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Yano T, Deterding RR, Simonet WS, et al. Keratinocyte growth factor reduces lung damage due to acid instillation in rats. Am J Respir Cell Mol Biol. 1996;15:433–42. doi: 10.1165/ajrcmb.15.4.8879176. [DOI] [PubMed] [Google Scholar]
- 79.Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol. 1998;186:90–8. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 80.Yi ES, Salgado M, Williams S, et al. Keratinocyte growth factor decreases pulmonary edema, transforming growth factor-beta and platelet-derived growth factor-BB expression, and alveolar type II cell loss in bleomycin-induced lung injury. Inflammation. 1998;22:315–25. doi: 10.1023/a:1022304317111. [DOI] [PubMed] [Google Scholar]
- 81.Barazzone C, Donati YR, Rochat AF, et al. Keratinocyte growth factor protects alveolar epithelium and endothelium from oxygen-induced injury in mice. Am J Pathol. 1999;154:1479–87. doi: 10.1016/S0002-9440(10)65402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panos RJ, Bak PM, Simonet WS, et al. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest. 1995;96:2026–33. doi: 10.1172/JCI118250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viget NB, Guery BP, Ader F, et al. Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1199–209. doi: 10.1152/ajplung.2000.279.6.L1199. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Folkesson HG, Jayr C, et al. Alveolar epithelial fluid transport can be simultaneously upregulated by both KGF and β-agonist therapy. J Appl Physiol. 1999;87:1852–60. doi: 10.1152/jappl.1999.87.5.1852. [DOI] [PubMed] [Google Scholar]
- 85.Guery BP, Mason CM, Dobard EP, et al. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997;155:1777–84. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 86.Yano T, Mason RJ, Pan T, et al. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1146–58. doi: 10.1152/ajplung.2000.279.6.L1146. [DOI] [PubMed] [Google Scholar]
- 87.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–40. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 88.Dada LA, Sznajder JI. Mechanisms of pulmonary edema clearance during acute hypoxemic respiratory failure: role of the Na,K-ATPase. Crit Care Med. 2003;31:S248–52. doi: 10.1097/01.CCM.0000057895.22008.EC. [DOI] [PubMed] [Google Scholar]
- 89.Planes C, Blot-Chabaud M, Matthay MA, et al. Hypoxia and β2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem. 2002;277:47318–24. doi: 10.1074/jbc.M209158200. [DOI] [PubMed] [Google Scholar]
- 90.Kwak HJ, So JN, Lee SJ, et al. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–53. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 91.Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 92.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 93.Kim I, Moon SO, Park SK, et al. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–9. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 94.Pizurki L, Zhou Z, Glynos K, et al. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–36. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCarter SD, Mei SH, Lai PF, et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014–26. doi: 10.1164/rccm.200609-1370OC. [DOI] [PubMed] [Google Scholar]
- 96.Mason CM, Guery BP, Summer WR, Nelson S. Keratinocyte growth factor attenuates lung leak induced by alpha-naphthylthiourea in rats. Crit Care Med. 1996;24:925–31. doi: 10.1097/00003246-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 97.Welsh DA, Summer WR, Dobard EP, et al. Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med. 2000;162:1081–6. doi: 10.1164/ajrccm.162.3.9908099. [DOI] [PubMed] [Google Scholar]
- 98.Murakami M, Nguyen LT, Zhang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–66. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates throm bin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J. 2007;21:2776–86. doi: 10.1096/fj.06-7660com. [DOI] [PubMed] [Google Scholar]
- 100.Singleton PA, Salgia R, Moreno-Vinasco L, et al. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem. 2007;282:30643–57. doi: 10.1074/jbc.M702573200. [DOI] [PubMed] [Google Scholar]
- 101.Lam CF, Liu YC, Hsu JK, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology. 2008;108:392–401. doi: 10.1097/ALN.0b013e318164ca64. [DOI] [PubMed] [Google Scholar]
- 102.Burnham EL, Taylor WR, Quyyumi AA, et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–60. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 103.Yamada M, Kubo H, Ishizawa K, et al. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60:410–3. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aguilar S, Nye E, Chan J, et al. Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung. Stem Cells. 2007;25:1586–94. doi: 10.1634/stemcells.2006-0762. [DOI] [PubMed] [Google Scholar]
- 105.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 106.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 107.Corcoran KE, Trzaska KA, Fernandes H, et al. Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS ONE. 2008;3:e2563. doi: 10.1371/journal.pone.0002563. Published online 25 June 2008, doi:10.1371/journal.pone.0002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Izadpanah R, Kaushal D, Kriedt C, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–38. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson JM. A history lesson for stem cells. Science. 2009;324:727–8. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]