Abstract
In this study the sensitivity of the S2-SSFP (steady-state free precession) signal for functional MRI (fMRI) at 7 Tesla was investigated. In order to achieve the necessary temporal resolution, a 3D acquisition scheme with acceleration along two spatial axes was employed. Activation maps based on S2-SSFP data showed similar spatial localisation of activation and sensitivity as spin-echo echo-planar imaging (SE-EPI) but data can be acquired with substantially lower power deposition. The functional sensitivity estimated by the average z-values was not significantly different for SE-EPI compared to the S2-signal, however slightly lower for the S2-signal (6.74 ± 0.32 for the TR = 15 ms protocol, and 7.51 ± 0.78 for the TR = 27 ms protocol) compared to SE-EPI (7.49 ± 1.44 and 8.05 ± 1.67) using the same activated voxels, respectively. The relative signal changes in these voxels upon activation were slightly lower for SE-EPI (2.37%± 0.18%) compared to the TR = 15 ms S2-SSFP protocol (2.75%± 0.53%) and significantly lower than the TR = 27 ms protocol (5.38% ± 1.28%) in line with simulations results. The large relative signal change for the long TR SSFP protocol can be explained by contributions from multiple coherence pathways, and the low intrinsic intensity of the S2 signal. In conclusion, whole-brain T2-weighted fMRI with negligible image distortion at 7 Tesla is feasible using the S2-SSFP sequence and partially parallel imaging.
Keywords: BOLD, 7 Tesla, brain, neuroimaging, steady-state free procession
Introduction
T2-weighted BOLD fMRI is attractive for functional imaging at very high static magnetic field strengths such as 7 T. This is because the sensitivity of T2-weighted blood oxygenation level dependent (BOLD) fMRI increases rapidly with increasing main magnetic field strength and indeed at 7 T some articles show a superior spatial specificity for T2-weighted BOLD as compared to a T2*-weighting (1–3). Unfortunately at 7 T the application of multi-slice spin-echo EPI for fMRI with whole-brain coverage can be limited by power deposition (4). A known alternative method for obtaining T2-weighted images is the use of the S2-signal component of a non-balanced steady-state free precession (SSFP) sequence (5). This sequence is attractive because of its low RF power deposition and lack of image distortion.
The use of other types of coherent steady state sequences, i.e. balanced SSFP, has already been explored both in terms of off-resonance sensitivity to deoxyhaemoglobin-induced frequency shifts (transition-band SSFP) (6–8) and with regard to BOLD and diffusion induced signal changes in passband SSFP (8–10). Non-balanced SSFP sequences that acquire S1- and/or S2-signals (either separately (5) or in common (11)) have hardly been used for fMRI, one reason being that sequence parameters for optimum contrast lead to rather long volume acquisition times. To our knowledge only one study has tried to use the S2-signal for fMRI (12). The main advantage of this family of sequences compared to balanced SSFP is that no banding artefacts occur. Also other short TR, low flip angle sequences are of relevance in a number of situations: the FLASH sequence is still occasionally used to obtain (high spatial resolution) undistorted fMRI images (13,14); the echo-shifted PRESTO technique (15) combines elements of both FLASH and EPI and makes it possible to acquire T2*-weighted images with TE > TR.
Although the S2-signal of non-balanced SSFP sequence has a T2-like contrast this method is known to suffer from low SNR, and the magnitude of the BOLD signal change has not been fully explored. The practicability of this method for fMRI depends on achieving both sufficient speed and sensitivity. This approach should thus provide:
sufficient temporal resolution: this requires that the BOLD response be sampled once every2–3 s (16), a requirement that is incompatible with the classic version of this sequence if whole-brain coverage is required. The development of multi-channel receiver coils and partially parallel imaging (17,18) with excellent stability in the temporal domain makes it possible to acquire 3D whole brain data in less than 3 s using high acceleration factors (AF);
sufficient functional contrast per unit time: by examining the differential contrast of the SSFP signal with respect to changes in T2 it is possible in theory to obtain optimum values for the flip angles and repetition times for signal changes in grey matter. In practice the complex nature of the BOLD contrast mechanisms and the lack of knowledge concerning the impact of physiological noise on this sequence led us to examine two plausible protocols for fMRI.
The purpose of this article is to explore whether highly accelerated 3D S2-SSFP represents a viable technique to acquire T2-weighted BOLD fMRI at 7 T. If a comparable sensitivity to SE-EPI can be achieved then this approach will offer the benefits of whole-brain acquisition, low power deposition, and negligible image distortion within an acceptable TR.
Materials and Methods
Simulations
In order to be able to compare the performance of S2-SSFP and SE-EPI we calculated the sequence parameters that give maximum BOLD contrast for both SE-EPI and S2-SSFP. This is straightforward and well-known for the spin-echo signal which is given by:
| [1] |
The maximum contrast can be calculated by optimising the differential contrast between activated and resting state. The differential contrast is obtained by differentiating with respect to R2 and the maximum contrast occurs when the differential with respect to TE is equal to zero.
| [2] |
This means that spin-echo contrast has a maximum at TE ≈ T2. Using T2 of 60 ms for grey matter at 7 T (19) results in a maximum BOLD contrast at a TE of 60 ms.
For the SSFP sequence, the calculations are not that straightforward. Following established theory (20,21) the S2-signal intensity is given by:
| [3] |
with
Equation 3 was differentiated with respect to T2 using Mathematica (Wolfram research, Champaign, IL, USA) to obtain the differential BOLD contrast in the same way as for SE (eq. 2). The contrast values are normalised to unit magnetisation and thus the unit is [s]. The optimum values of TR and flip angle which maximise this contrast were then obtained numerically. The considerable algebraic complexity of the expressions made it impossible for Mathematica to solve this problem analytically. For the contrast calculation grey matter relaxation times of 60 ms (T2, (19)) and 2000 ms (T1, (22)) at 7 T were used. The results of these simulations show that maximum contrast would be obtained using TR~14 ms and a flip angle α of 25 degrees. This approach makes the assumption that BOLD signal changes in grey matter can be approximated by a simple change in T2 that contains contributions from all coherence pathways. However, if the higher coherence pathways would be disturbed by physiological fluctuations, mainly the first coherence pathway, i.e. the primary S2-echo arising from the previous two RF pulses would contribute to the functional signal change. To assess this possibility we also included an experiment that resembles the SE experiment in that the primary S2-echo would be close to the original spin echo, resulting in TRSSFP ~ TESE/2. If physiological fluctuations and dynamic averaging effects were to be considered correctly then it would be necessary to model these for all coherence pathways contributing to the SSFP signal. This would represent a considerable undertaking, far beyond the scope of the present article.
fMRI measurements
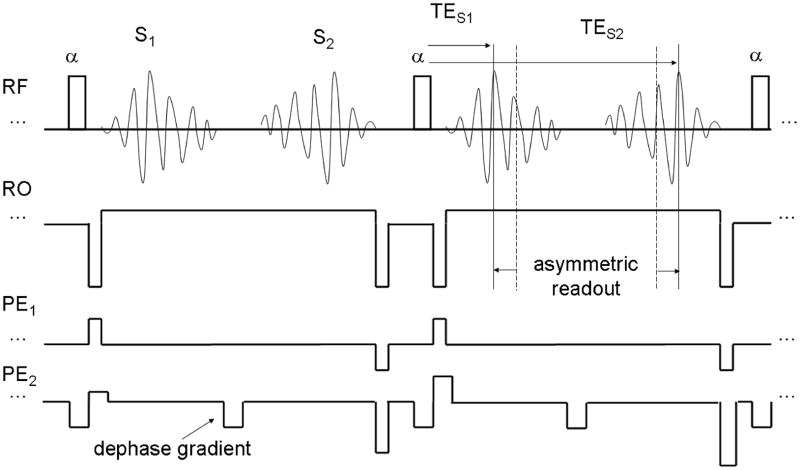
We performed two different S2-SSFP fMRI protocols: For one we used the sequence parameters that gave maximum contrast per unit T2 change as assessed in the simulations (TR = 15 ms, alpha = 25 degrees) and for the second set of S2-SSFP fMRI we used those that should give maximum contrast under the assumption that only the primary S2-echo contributes to the contrast (TR = 27 ms). The S2-signals were acquired using the second contrast of a 3D, fast acquired double echo in the steady state (FADESS) sequence (Fig. 1). We chose to use an implementation that also acquires the S1-signal as the readout bandwidth becomes unreasonably low (< 50 Hz/pixel for the TR27 protocol) when using the maximum possible readout time which leads to severe image artefacts. The functional contrast is not purely T2-weighted so we have not included it in our comparison with SE-EPI, but it could be used to increase sensitivity and SNR.
Fig. 1.
Schematic pulse sequence diagram of the proposed FADESS sequence in steady state. TES1 and TES2 denote the echo times of the S1 and S2 signals, respectively. The displacement from the previous (for S1) and next RF pulse (for S2) determines the respective T2* weighting which can be reduced by using an asymmetric readout.
The 3D acquisition scheme made it possible to use parallel acceleration in both phase encoding directions. The reference lines for parallel reconstruction were acquired once at the start of the functional run. The readout bandwidth (BW) was chosen as low as possible considering the TR used. In order to reduce T2* weighting, i.e. to keep the echo as close as possible to the RF-pulse without reducing the readout window an asymmetric readout was used (see Fig. 1).
In total, data of 12 healthy volunteers were acquired (2 female, 10 male) using a whole body MR scanner (Magnetom 7 T, Siemens). Written informed consent was obtained from the subjects according to the local guidelines.
S2-SSFP parameters (TR15 protocol): 32-channel phased array receive coil that uses a birdcage transmit coil (23), TR = 15 ms, TE(S2) = 12 ms, α = 25° (slab selective binomial pulse), matrix size (MA) = 64 × 48 × 24, FOV = 224 mm × 168 mm, oversampling (OS) in slice direction = 25%, slice thickness (TH) = 5 mm, acceleration factor (AF) = 3 × 3, asymmetric readout = 35% (i.e. the echo occurs at 35% of the readout window, is thus shifted 15% closer to the respective RF-pulse, see also Fig. 1), BW = 150 Hz/pixel, acquisition time/volume (TA) = 2.5 s. The specific absorption rate (SAR) level was 23% of the allowed maximum. Slices were positioned in a transverse orientation and slightly tilted to cover the whole brain. The reconstruction of the partially parallel acquired data sets was performed based on the GRAPPA implementation of the manufacturer (software version syngo MR B15) with a calibration data matrix of 24 × 21 and a reconstruction kernel size of 3 × 2. This protocol was performed in eight subjects.
S2-SSFP parameters (TR27 protocol): the same parameters were used as in the TR15 protocol but were adapted to the longer TR of 27 ms, i.e. TE(S2) = 22 ms, no OS in the slice direction, PF = 6/8, BW = 75 Hz/pixel, TA = 2.7 s, SAR level of 13%. This protocol was also performed in eight subjects.
For comparison of functional sensitivitya functional SE-EPI run on the same subjects was performed using the same hardware setup as well as matched geometrical parameters, a TR of 2.7 s matched to the TR27 protocol, and an acceleration factor of three in the PE direction (BW = 1594 Hz/pixel, echo spacing = 0.71 ms). The SAR level was 68%. A TE of 60 ms was used for optimal contrast. This SE protocol was used for all subjects for a direct comparison with the S2 protocols. In three subjects no acceleration was used resulting in a slightly longer TR (3 s). One of the SE-EPI data sets at 7 T was acquired with the birdcage coil surrounding the 32 channel receive array due to technical difficulties. This subject was not included in the analysis.
The stimulation paradigm consisted of blocks of rest and blocks of an 8 Hz flickering checkerboard (20 s per block) which were repeated for five minutes. The functional data were analysed using a GLM as implemented in FEAT v5.98 (FSL 4.1 (24), FMRIB, Oxford, UK) using McFlirt motion correction, 5 mm spatial smoothing, a low pass filter (2.8 s HWHM Gaussian kernel) and a high pass filter (Gaussian-weighted least-squares straight line fitting, with a sigma of 50.0 s). To obtain clusters of significantly activated voxels a z-threshold of 5.3 was used and at cluster level each cluster’s significance level as estimated from Gaussian random field theory was compared with the cluster probability threshold of p < 0.00005. One subject showed excessive motion in all protocols and was therefore not included in the further analysis. In cases where more than one cluster was found, the one located within the occipital cortex was used in the further analysis. For the analysis of activated pixels common to both SE and S2-SSFP a linear co-registration algorithm as implemented in FEAT was used to co-register the SE-EPI data to the S2-SSFP. A paired t-test was performed to assess significant differences between SE-EPI and S2-SSFP.
Results
Simulations
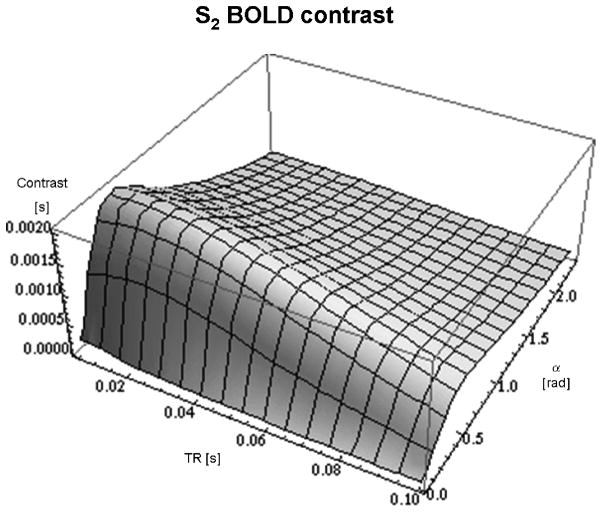
Figure 2 shows the results of the simulations, depicting the differential T2-contrast of the S2-signal of both α and TR generated for tissue with relaxation times similar to those of grey matter 7 T (T1 = 2000 ms; T2 = 60 ms). The simulation results show that the S2-contrast does not show a very strong dependency on TR (the contrast changes only by a few percent up to a TR of 50 ms), however the dependency on α is more pronounced. The maximum contrast for S2 occurs at a TR that is about one quarter of the T2 value. The contrast values are normalised to unit magnetisation and thus the unit is [s].
Fig. 2.
shows the simulation results for the S2 contrast as a function of TR and flip angle α at 7 Tesla. The z-axis shows the BOLD contrast per unit magnetisation [s]. Maximum contrast is achieved for TR = 14 ms and α = 25°.
fMRI Measurements
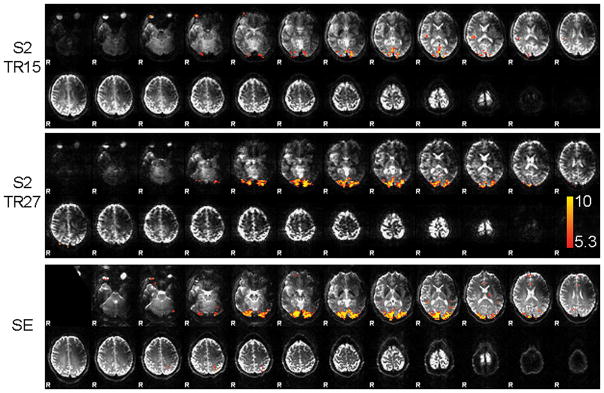
The activation maps of a single subject are shown in Fig. 3 for both S2 protocols and the SE-EPI run. The image contrast of the S2 protocols is very similar to the SE-EPI image contrast as both are heavily T2-weighted. Clear activation is found for SE-EPI and S2-SSFP (TR27) in the visual cortex, but only four out of seven subjects show activation for the TR15 protocol. Table 1 lists the number of activated voxels for the separate analyses, the number of commonly activated voxels between S2 and SE-EPI, the average z-value, and the average relative signal change in these voxels. The number of activated voxels and average z-values for SE-EPI are not significantly different from the S2-SSFP results. The functional sensitivity of the S2-signal as estimated by the average z-values in the activated area is slightly lower compared to SE-EPI (6.74 ± 0.32 versus 7.49 ± 1.44 for TR15 (N=4); 7.51 ± 0.78 versus 8.05 ± 1.67 for TR27). The relative S2-signal change of 2.75% ± 0.53% (TR15) is similar to that of SE-EPI (2.37% ± 0.18%), whereas the relative S2-signal change of 5.38% ± 1.28% (TR27) is significantly larger than for SE-EPI (2.53% ± 0.75%).
Fig. 3.
Activation maps of a single subject of the S2 signal with TR = 15 ms (top), TR = 27 ms (middle), and SE-EPI (bottom) overlaid on the respective mean image. The same colorscale for the z-values ranging from 5.3 to 10 was used for all functional overlays.
Table 1.
fMRI activation results at 7 Tesla for the two S2 protocols compared to SE-EPI.
| number of voxels | number of common voxels | average z-value (common voxels) | relative signal change (common voxels) | |
|---|---|---|---|---|
| S2 (TR15)1 | 333 ± 265 | 156 ± 114 | 6.74 ± 0.32 | 2.75% ± 0.53% |
| SE | 470 ± 235 | 7.49 ± 1.44 | 2.37% ± 0.18% | |
| S2 (TR27) | 278 ± 196 | 151 ± 133 | 7.51 ± 0.78 | 5.38% ± 1.28% * |
| SE | 470 ± 235 | 8.05 ± 1.67 | 2.53% ± 0.75% * | |
Using the data of 4 subjects as in 3 subjects no activation was found in the TR15 protocol
Significantly different (p < 0.05)
Discussion
Our results show that S2-SSFP is a viable technique for T2-weighted whole brain fMRI at 7 T. The functional sensitivity of the S2-signal at 7 T – as estimated by the average z-values in the activated area – is not significantly different from SE-EPI. The fact that no activation was found in three subjects for the TR15 protocol and that the average z-value was lower indicates a lower sensitivity of the TR15 protocol compared to the TR27 protocol. As the simulated contrast for the TR27 protocol is only reduced by 3% compared to the TR15 protocol, this might be due to higher sensitivity to motion in case of the short TR protocol as the measured signal is a composition of more components from past excitations (25).
Due to the fact that this sequence is – in essence – spin-echo like and refocuses static magnetic field inhomogeneities, the small residual signal dropout is due to the residual T2*-weighting caused by the necessary separation of the S2-echo from the following RF pulse. In our study this was reduced as much as possible without reducing the readout window by using an asymmetric readout. The remaining T2*-weighting in the 7 T experiment was thus reduced to that corresponding to a TE of about 3 to 5 ms for the different TRs used, which should be minimal assuming a T2* of about 33 ms of grey matter at 7 T (26). A further reduction of T2*- weighting and signal dropout could be achieved by using a higher readout bandwidth at the cost of increased thermal noise. Also, in the case of SE-EPI some T2*-weighting is present due to the EPI readout window.
A major advantage of T2-contrast images at high field strengths obtained by S2-SSFP is the reduced power deposition due to the low flip angles required. At 7 T, the SE-EPI protocol used had an about 3 times higher SAR value than the TR15 protocol and an about 5 times higher one than the TR27 protocol, which would lead to less volume coverage or longer measurement times in cases where less SAR efficient coils or pulses are used.
Another advantage is the reduced distortion compared to EPI methods when using no acceleration for EPI. When using an AF of 3 it becomes comparable to the distortion of S2-SSFP which occurs in the readout direction with the relatively low readout BW of 75 Hz/px of the TR27 protocol. A higher readout bandwidth (150 Hz/px) as used in the TR15 protocol due to the short TR further reduced geometric distortions.
While the use of a non-EPI readout has several above mentioned advantages it leads to long measurement times. The use of a 3D acquisition scheme enabled us to use 2D parallel acceleration and the combination of coil arrays with many elements and a high field resulted in a good image quality with a nine-fold acceleration. The advantage of parallel imaging at high fields has already been shown (27,28). However, going to higher resolution is a limitation of the SSFP implementation in its current form as a higher resolution leads to a longer measurement time which is less of a problem for SE-EPI.
When performing the simulations using Eqs. 1 and 3 for a grey matter T2 of 60 ms (7 T) assuming a T2 change of 1.5 ms between rest and activation (estimated from the SE-EPI experiment at 7 T in (2)), we obtain signal changes of 2.47% (SE), and 2.37% (S2, TR15) and 3.05% (S2, TR27) for our experimental parameters. This is in good agreement with the corresponding experimental results of 2.37% and 2.53% (SE), and 2.75% (S2, TR15), respectively, however less so for the S2-signal changes of the TR27 protocol (5.38%). As these calculations are based on a simple T2 change in grey matter this discrepancy at the longer TR protocol might be explained by partial volume effects of other tissues. The reason that the relative signal changes for S2 are larger than for SE, can be explained by the fact that the S2-signal is intrinsically smaller and contains contributions from multiple coherence pathways where also higher order pathways contribute. These higher order contributions can be viewed as spin echoes with longer echo times which then have higher functional signal changes but a lower intensity which results in higher relative signal changes.
We conclude that whole brain fMRI using S2-SSFP is feasible at 7 T. Due to low power deposition and decreased susceptibility induced image artifacts, it may constitute an alternative to SE-EPI.
Acknowledgments
Two authors (J.R.P. and L.L.W.) acknowledge support from National Institutes of Health grants: NCRR P41RR14075, NIBIB R01EB006847 and research support from Siemens Medical Solutions. One of the authors (LLW) has obtained consulting income from Siemens Healthcare.
References
- 1.Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med. 2003;49(6):1019–1027. doi: 10.1002/mrm.10472. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub E, Duong TQ, Van De Moortele PF, Lindquist M, Adriany G, Kim SG, Ugurbil K, Hu X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003;49(4):655–664. doi: 10.1002/mrm.10433. [DOI] [PubMed] [Google Scholar]
- 3.Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37(4):1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yacoub E, Van De Moortele PF, Shmuel A, Ugurbil K. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24(3):738–750. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Gyngell ML. The application of steady-state free precession in rapid 2DFT NMR imaging: FAST and CE-FAST sequences. Magn Reson Imaging. 1988;6(4):415–419. doi: 10.1016/0730-725x(88)90478-x. [DOI] [PubMed] [Google Scholar]
- 6.Miller KL, Hargreaves BA, Lee J, Ress D, deCharms RC, Pauly JM. Functional brain imaging using a blood oxygenation sensitive steady state. Magn Reson Med. 2003;50(4):675–683. doi: 10.1002/mrm.10602. [DOI] [PubMed] [Google Scholar]
- 7.Scheffler K, Seifritz E, Bilecen D, Venkatesan R, Hennig J, Deimling M, Haacke EM. Detection of BOLD changes by means of a frequency-sensitive trueFISP technique: preliminary results. NMR Biomed. 2001;14(7–8):490–496. doi: 10.1002/nbm.726. [DOI] [PubMed] [Google Scholar]
- 8.Miller KL, Smith SM, Jezzard P, Wiggins GC, Wiggins CJ. Signal and noise characteristics of SSFP FMRI: a comparison with GRE at multiple field strengths. Neuroimage. 2007;37(4):1227–1236. doi: 10.1016/j.neuroimage.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Bowen C, Mason J, Menon R, Gati J. High field balanced-SSFP FMRI: Examining a diffusion contrast mechanism using varied flip angles. ISMRM. 2006:665. [Google Scholar]
- 10.Zhong K, Leupold J, Hennig J, Speck O. Systematic investigation of balanced steady-state free precession for functional MRI in the human visual cortex at 3 Tesla. Magn Reson Med. 2007;57(1):67–73. doi: 10.1002/mrm.21103. [DOI] [PubMed] [Google Scholar]
- 11.Redpath TW, Jones RA. FADE--a new fast imaging sequence. Magn Reson Med. 1988;6(2):224–234. doi: 10.1002/mrm.1910060211. [DOI] [PubMed] [Google Scholar]
- 12.Auerbach EJ, Heberlein K, Hu X. High-Resolution T2 fMRI at High Magnetic Fields using PSIF. Proceedings of the 10th Annual Meeting of ISMRM; 2002. p. 2345. [Google Scholar]
- 13.Barth M, Norris DG. Very high-resolution three-dimensional functional MRI of the human visual cortex with elimination of large venous vessels. NMR Biomed. 2007;20(5):477–484. doi: 10.1002/nbm.1158. [DOI] [PubMed] [Google Scholar]
- 14.Voit D, Frahm J. Echo train shifted multi-echo FLASH for functional MRI of the human brain at ultra-high spatial resolution. NMR Biomed. 2005;18(8):481–488. doi: 10.1002/nbm.998. [DOI] [PubMed] [Google Scholar]
- 15.Duyn JH, Mattay VS, Sexton RH, Sobering GS, Barrios FA, Liu G, Frank JA, Weinberger DR, Moonen CT. 3-dimensional functional imaging of human brain using echo-shifted FLASH MRI. Magn Reson Med. 1994;32(1):150–155. doi: 10.1002/mrm.1910320123. [DOI] [PubMed] [Google Scholar]
- 16.Norris DG. Principles of magnetic resonance assessment of brain function. J Magn Reson Imaging. 2006;23(6):794–807. doi: 10.1002/jmri.20587. [DOI] [PubMed] [Google Scholar]
- 17.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 18.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 19.Michaeli S, Garwood M, Zhu XH, DelaBarre L, Andersen P, Adriany G, Merkle H, Ugurbil K, Chen W. Proton T2 relaxation study of water, N-acetylaspartate, and creatine in human brain using Hahn and Carr-Purcell spin echoes at 4T and 7T. Magn Reson Med. 2002;47(4):629–633. doi: 10.1002/mrm.10135. [DOI] [PubMed] [Google Scholar]
- 20.Zur Y, Wood ML, Neuringer LJ. Motion-insensitive, steady-state free precession imaging. Magn Reson Med. 1990;16(3):444–459. doi: 10.1002/mrm.1910160311. [DOI] [PubMed] [Google Scholar]
- 21.Buxton RB. Signal Intensity in Fast NMR Imaging with Short Repetition Times. J Magn Reson. 1989;83:576–585. [Google Scholar]
- 22.Wright PJ, Mougin OE, Totman JJ, Peters AM, Brookes MJ, Coxon R, Morris PE, Clemence M, Francis ST, Bowtell RW, Gowland PA. Water proton T1 measurements in brain tissue at 7, 3, and 1.5 T using IR-EPI, IR-TSE, and MPRAGE: results and optimization. Magma. 2008;21(1–2):121–130. doi: 10.1007/s10334-008-0104-8. [DOI] [PubMed] [Google Scholar]
- 23.Wiggins GC, Wiggins CJ, Potthast A, Alagappan V, Kraff O, Reykowski A, Wald LL. A 32 Channel Receive-only Head Coil And Detunable Transmit Birdcage Coil For 7 Tesla Brain Imaging. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, Washington, USA. 2006. p. 415. [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 25.Denolin V, Metens T. Three-dimensional BOLD fMRI with spin-echo characteristics using T2 magnetization preparation and echo-planar readouts. Magn Reson Med. 2003;50(1):132–144. doi: 10.1002/mrm.10516. [DOI] [PubMed] [Google Scholar]
- 26.Koopmans PJ, Manniesing R, Niessen WJ, Viergever MA, Barth M. MR venography of the human brain using susceptibility weighted imaging at very high field strength. Magma. 2008;21(1–2):149–158. doi: 10.1007/s10334-007-0101-3. [DOI] [PubMed] [Google Scholar]
- 27.Ohliger MA, Grant AK, Sodickson DK. Ultimate intrinsic signal-to-noise ratio for parallel MRI: electromagnetic field considerations. Magn Reson Med. 2003;50(5):1018–1030. doi: 10.1002/mrm.10597. [DOI] [PubMed] [Google Scholar]
- 28.Wiesinger F, Van de Moortele PF, Adriany G, De Zanche N, Ugurbil K, Pruessmann KP. Potential and feasibility of parallel MRI at high field. NMR Biomed. 2006;19(3):368–378. doi: 10.1002/nbm.1050. [DOI] [PubMed] [Google Scholar]