Abstract
BACKGROUND/OBJECTIVE:
Methicillin-resistant Staphylococcus aureus (MRSA) colonization is associated with a significant risk of subsequent MRSA infection in the hospital setting. The use of decolonization as an infection control strategy remains highly controversial despite publications evaluating more than 40 different decolonization regimens over the past 60 years. The present study describes the benefits and potential drawbacks of such an approach in the patient population.
METHODS:
A retrospective cohort study was performed to assess the efficacy and subsequent outcome for patients with newly identified MRSA colonization at the Horizon Health Network in Moncton, New Brunswick.
RESULTS:
A total of 241 patients with MRSA colonization or infection during the study period (2000 to 2005 inclusive) were identified. Eighty-nine MRSA-positive patients were decolonized according to a standardized regimen (hospital protocol group), and 98 received an alternative decolonization regimen (other treatment group). No attempt at decolonization was made for 54 patients (no treatment group). The hospital protocol group demonstrated superior overall successful decolonization compared with the other treatment group (67 of 84 [80%] versus 48 of 89 [54%]; OR 3.3; 95% CI 1.6 to 7.1; P=0.0004) and the no treatment group (four of 43 [9%]; OR 36.9; 95% CI 11.2 to 161.7; P<0.000001). The mean observed duration of culture negativity for the subgroup who remained MRSA culture negative over the long term was 419±398 days (range one to 1817 days). Successful decolonization occurred in 115 patients and permitted subsequent release from contact isolation for 4530 patient-days. The rate of clinical infection with MRSA was significantly lower in the hospital protocol group versus the other treatment group (16 of 89 [18%] versus 37 of 98 [38%]; OR 0.38; 95% CI 0.18 to 0.78; P=0.003).
CONCLUSION:
The present study supports recent reports indicating that MRSA decolonization can be successful using a multifactorial approach (chlorhexidine soap, enhanced hygiene/housekeeping and combination oral/topical antimicrobial therapy) in hospitalized patients, both over the short and long term. Unlike previous studies, decolonization appeared to be effective in a relatively unselected population, including patients with lines and catheters. Inability to decolonize was most closely associated with failure to use a standardized decolonization protocol.
Keywords: Decolonization, Efficacy, Long term, MRSA
Abstract
HISTORIQUE ET OBJECTIF :
La colonisation du Staphylococcus aureus résistant à la méthicilline (SARM) s’associe à un risque important d’infection subséquente par le SARM en milieu hospitalier. Le recours à la décolonisation comme stratégie de contrôle de l’infection demeure hautement controversé malgré des publications évaluant plus de 40 schémas de décolonisation depuis 60 ans. La présente étude visait à décrire les bienfaits et les inconvénients potentiels d’une telle démarche auprès de la population de patients.
MÉTHODOLOGIE :
Les auteurs ont effectué une étude de cohorte rétrospective pour évaluer l’efficacité et l’issue subséquente pour les patients présentant une colonisation par le SARM nouvellement décelée au Réseau de santé Horizon de Moncton, au Nouveau-Brunswick.
RÉSULTATS :
Au total, les auteurs ont repéré 241 patients présentant une colonisation ou une infection par le SARM pendant la période de l’étude (de 2000 à 2005, inclusivement). Quatre-vingt-neuf patients positifs au SARM ont été décolonisés selon un schéma normalisé (groupe de protocole hospitalier) et 98 ont reçu un autre schéma de décolonisation (autre groupe de traitement). Enfin, 54 patients n’ont subi aucune tentative de décolonisation (groupe non traité). Le groupe de protocole hospitalier a démontré une décolonisation globale plus réussie que l’autre groupe de traitement (67 sur 84 [80 %] par rapport à 48 sur 89 [54 %]; RR 3,3; 95 % IC 1,6 à 7,1; P=0,0004) et que le groupe non traité (quatre sur 43 [9 %]; RR 36,9; 95 % IC 11,2 à 161,7; P<0,000001). La durée moyenne observée de négativité des cultures dans le sous-groupe qui demeurait négatif à la culture du SRAM à long terme correspondait à 419±398 jours (plage de un à 1 817 jours). Cent quinze patients ont profité d’une décolonisation réussie, ce qui a permis de mettre un terme à l’isolation des contacts pour 4 530 jours-patient. Le taux d’infection clinique par le SRAM était considérablement plus faible dans le groupe de protocole hospitalier que dans l’autre groupe de traitement (16 sur 89 [18 %] par rapport à 37 sur 98 [38 %]; RR 0,38; 95 % IC 0,18 à 0,78; P=0,003).
CONCLUSION :
La présente étude étaye les rapports récents indiquant que la décolonisation par le SRAM peut réussir au moyen d’une démarche multifocale (savon de chlorhexidine, meilleure hygiène et meilleur entretien ménager et association d’antimicrobiens oraux et topiques) chez les patients hospitalisés, tant à court terme qu’à long terme. Contrairement aux études précédentes, la décolonisation semble efficace dans une population relativement non sélectionnée, y compris les patients munis de sondes et de cathéters. L’incapacité de décoloniser s’associait plus étroitement au défaut d’utiliser un protocole de décolonisation normalisé.
Staphylococcus aureus is one of the most common causes of nosocomial infection worldwide. Over the past 30 years, there has been a pandemic spread of methicillin-resistant S aureus (MRSA), occurring primarily in the hospital setting. MRSA now accounts for more than 50% of all bloodstream S aureus isolates in the United States (1). Instead of replacing endemic methicillin-sensitive S aureus, these MRSA clones have added to the baseline endemic prevalence of methicillin-sensitive S aureus (2). This has increased the overall burden of S aureus in the hospital setting (3,4). This phenomenon may be due to MRSA virulence factors that enhance bacterial adhesion (5). This may also account for the prolonged duration of colonization noted in these patients, which has an estimated half-life of 40 months (6).
MRSA colonization is associated with a significant risk of subsequent MRSA infection in the hospital setting. Recent acquisition of MRSA colonization is associated with a 30% risk of subsequent infection (7). This enhanced risk appears to persist, with 23% of long-term carriers (carriage for one year or longer) developing late-onset MRSA infection (8).
The principal infection control measures for limiting the spread of nosocomial MRSA infection involve performing admission screening cultures for MRSA, isolating colonized/infected patients, using barrier precautions and optimizing hand hygiene among health care workers (9). While these measures are very effective when stringently applied, nosocomial outbreaks of MRSA continue to occur, mostly due to breakdown in adherence to these measures. These measures are also taxing on patients, their families and health care institutions. MRSA decolonization has been proposed as a potential infection control strategy to further reduce nosocomial spread of MRSA colonization and the subsequent risk of MRSA infection (10,11). Decolonization could also enhance patient well-being for individuals in whom successful decolonization permits the discontinuation of oppressive isolation and barrier precautions.
The use of decolonization as an infection control strategy remains highly controversial despite publications evaluating more than 40 different decolonization regimens over the past 60 years (12). A recent Cochrane collaboration review (13) of six randomized controlled trials studying MRSA decolonization concluded that there was insufficient evidence to support the use of decolonization. Despite these data, decolonization in selected patients is frequently performed in many hospitals (14).
There is now a significant body of evidence (15–17) suggesting that decolonization using topical agents alone is often inadequate for current circulating nosocomial MRSA clones. However, a recent randomized controlled trial (18) using combined topical and systemic decolonization therapy has demonstrated 74% successful decolonization at three months in the active treatment group.
A similar protocol has been used selectively for some patients with MRSA colonization at The Moncton Hospital (Moncton, New Brunswick), and the present cohort study was performed to describe the benefits and potential drawbacks of such an approach in the patient population. The primary outcome measure was successful MRSA decolonization both over the short and long term. Secondary outcome measures included the identification of potential factors associated with successful decolonization, and the incidence of subsequent MRSA infection and mortality.
METHODS
A retrospective cohort study was conducted to assess the efficacy and subsequent outcome for patients with newly identified MRSA colonization at The Moncton Hospital. The Moncton Hospital, a teaching hospital affiliated with the Faculty of Medicine at Dalhousie University (Halifax, Nova Scotia) and the Horizon Health Network, has a catchment area of 180,000 people and an average of 16,000 admissions per year. The research protocol was approved by the South-East Regional Health Authority Research Ethics Board.
Since December 1997, screening cultures for MRSA have been obtained on admission to the hospital from all at-risk patients (ie, patients hospitalized in the previous nine months or those who live in a long-term care facility). Patients colonized or infected with MRSA were also identified through cultures obtained as part of routine care during their hospital stay (ie, nonscreening cultures). When a patient with a positive culture was identified, the hospital infection control department and the attending physician were notified. Patients were placed under contact precautions, and patient data were entered into an infection control MRSA patient database. The hospital infection control service provided a decolonization protocol (hospital protocol) on the chart of every colonized patient to guide physicians who believed that decolonization could be beneficial to their patient. The attending physician could then make a clinical decision on whether to attempt to decolonize the patient using the hospital protocol, an alternative protocol of their choice or to provide no decolonization. The hospital protocol was not a standing order, and provided no guidance regarding who could benefit from a decolonization trial. All patients who were successfully decolonized (defined as a minimum of two consecutive weekly sets of MRSA screening cultures) were removed from contact isolation.
The study population consisted of all MRSA-positive patients between 2000 and 2005 (inclusive) found in the hospital microbiology laboratory database and the infection control MRSA patient database. Patient demographic data, comorbid conditions (including Charlson age-adjusted comorbidity index), a description of the specific MRSA decolonization regimen received (if any) and outcomes, were obtained from the MRSA database and the hospital chart.
Three patient groups were defined based on the decolonization regimen received – identified as the hospital protocol, other treatment and no treatment groups. The hospital protocol consisted of 2% mupirocin cream to both nares and all open or colonized wounds three times a day, trimethoprim/sulfamethoxazole (160 mg trimethoprim/800 mg sulfamethoxazole) one tablet twice daily, and oral rifampin 300 mg twice daily, all for seven days. The other treatment group included all patients prescribed any alternative decolonization regimen. Examples of alternative decolonization regimens were oral cotrimoxazole plus topical mupirocin, or topical mupirocin alone. Patients in whom no decolonization regimen was prescribed were classified as the no treatment group. All three patient groups were prescribed chlorhexidine soap for bathing at the time MRSA was first identified. Patients presenting with de novo MRSA infection were not considered for MRSA decolonization until their infection had been successfully treated.
Baseline and follow-up cultures for MRSA were obtained from both anterior nares, perianal region, vascular catheter exit sites, tracheostomy sites, wounds/incisions and urine (if a Foley catheter was present). These cultures were repeated a minimum of three days after decolonization therapy was completed, and were repeated once weekly or at least twice if the patient remained hospitalized. Screening cultures were then repeated during any subsequent hospitalizations.
Laboratory methods
Clinical specimens were plated onto mannitol salt agar (PML Microbiologicals, USA) with oxacillin (6 μg/mL) and incubated at 35°C for 72 h. Methicillin resistance was confirmed using oxacillin agar screen plates as per the Clinical Laboratory Standards Institute (USA) guidelines, with confirmation using a latex agglutination assay for detection of penicillin-binding protein 2a (Denka Seiken Ltd, Japan). Susceptibility testing of MRSA isolates for other antimicrobial agents was performed using the MicroScan panel system (Pos Combo 21, Dade Behring, USA); mupirocin susceptibility testing was not performed. Susceptibility testing for other antimicrobials was always performed on initial isolates and would only be repeated at the request of a physician or if an isolate was obtained in the context of MRSA infection.
Outcome measures
Short-term decolonization success was defined as two successive negative cultures during a one-month period following treatment. Long-term decolonization success was defined as successive negative cultures obtained at least three months after successful short-term decolonization. Secondary outcome measures included the identification of potential factors associated with successful decolonization and the incidence of subsequent MRSA infection. The diagnosis of MRSA infection and determination of the primary infection site was assigned on the basis of the National Nosocomial Infections Surveillance criteria. Thirty-day mortality was measured from the time of the first positive MRSA culture. Cause of death was determined by chart review. The potential impact of MRSA infection on patient mortality was categorized as unrelated, unlikely, possible, probable and definitely by a reviewer blinded to the treatment received. MRSA infection was classified as having a definite impact on mortality if patients were bacteremic or had active MRSA infection at the time of death.
Statistical analysis
Data were analyzed for each group, and results were expressed as proportions or means, according to the type of variable. The hospital protocol group was compared separately to the other treatment and to the no treatment group, excluding missing values. For dichotomous variables, comparisons between groups were summarized by the OR with its two-sided 95% CI. Statistical significance was tested using the two-tailed Fisher’s exact test for 2×2 tables. For nondichotomous categorical variables, statistical significance was tested using the equivalent to the Fisher’s exact test for larger tables if low cell frequencies were present and the χ2 test otherwise. For continuous data, the two-sided Kruskal-Wallis nonparametric test was used. Empirical significance levels (P values) were reported to allow the reader to apply any desired significance threshold. Statistical diagnostic methods were applied to verify the suitability of the methods; the potential impact of missing values on the conclusions was explored, but detailed results of these due diligence measures are not reported. The database was created in Excel 2003 (Microsoft, USA), and computations were performed in Matlab 2006b (The MathWorks Inc, USA), SYSTAT 11 (SYSTAT Software Inc, USA) and R 2.9.0 (R Foundation for Statistical Computing, Austria).
RESULTS
A total of 241 patients with MRSA colonization or infection during the study period (2000 to 2005 inclusive) were identified. Patient characteristics are summarized in Table 1. Eighty-nine MRSA-positive patients were treated according to the hospital protocol and 98 received an alternative decolonization regimen (other treatment). No attempt at decolonization was made for 54 patients (no treatment). There was no difference in sex between the three groups of patients. The hospital protocol group was slightly younger by approximately five years. The comorbidity burden was similar among groups, except for a relative excess of cardiac conditions in the no treatment group. The majority of patients had at least one or more indwelling lines or tubes; the no treatment group were least likely to have indwelling lines or tubes. The hospital protocol group were more likely to have MRSA colonization at multiple sites.
TABLE 1.
Baseline characteristics of patients colonized with methicillin-resistant Staphylococcus aureus (MRSA)
| Characteristic | Hospital protocol, n (%) | Other treatment, n (%) | No treatment, n (%) | Hospital protocol vs other treatment, P | Hospital protocol vs no treatment, P |
|---|---|---|---|---|---|
| Number of patients (n=241) | 89 (37) | 98 (41) | 54 (22) | – | – |
| Female sex | 36 (40) | 48 (49) | 24 (44) | 0.527* | 0.158* |
| Age, years (mean ± SD) | 68.6±16.8 | 73.2±14.3 | 73.8±19.2 | 0.048* | 0.008* |
| Comorbidity (% of group)† | |||||
| Cardiac | 30 (34) | 28 (29) | 25 (46) | 0.527* | 0.034* |
| Diabetic | 16 (18) | 17 (17) | 7 (13) | 0.643* | 1.000* |
| Hepatic | 6 (7) | 3 (3) | 4 (7) | 0.246* | 0.313* |
| Oncological | 19 (21) | 21 (21) | 11 (20) | 1.000* | 1.000* |
| Renal | 12 (13) | 14 (14) | 6 (11) | 0.627* | 1.000* |
| Respiratory | 20 (22) | 24 (24) | 13 (24) | 1.000* | 0.863* |
| Vascular | 25 (28) | 21 (21) | 14 (26) | 0.551* | 0.312* |
| Neurological | 7 (8) | 6 (6) | 7 (13) | 0.224* | 0.776* |
| Charlson age-adjusted comorbidity index (mean ± SD) | 3.9±2.5 | 4.3±2.0 | 4.9±2.9 | 0.119‡ | 0.014‡ |
| Number of lines/tubes | |||||
| None | 10 (11) | 6 (6) | 18 (33) | – | – |
| 1 | 31 (35) | 42 (43) | 25 (47) | 0.319* | 0.0005* |
| 2 or more | 48 (54) | 50 (51) | 11 (20) | – | – |
| MRSA source† | |||||
| Nares | 54 (61) | 60 (61) | 33 (61) | 0.655* | 0.709* |
| Perirectal/rectal | 46 (52) | 52 (53) | 25 (46) | 0.883* | 0.858* |
| Wound | 27 (30) | 38 (39) | 11 (20) | 0.442* | 0.322* |
| Urine | 34 (38) | 11 (11) | 5 (9) | 0.007* | 0.027* |
| Devices | 17 (19) | 15 (15) | 3 (6) | 0.438* | 0.043* |
| Respiratory | 9 (10) | 6 (6) | 0 (0) | 0.290* | 0.026* |
| Nares only | 10 (11) | 18 (18) | 15 (28) | 0.305* | 0.010* |
| MRSA colonization at 2 or more sites | 63 (71) | 59 (60) | 20 (37) | 0.034* | <0.0001* |
Two-sided Fisher’s exact test, its generalization or χ2 test;
Total greater than 100% due to multiple classification;
Two-sided Kruskal-Wallis test. vs Versus
Details of patient decolonization rates are described in Table 2. The overall rate of successful decolonization for the hospital protocol was 80%. The hospital protocol group had a superior short-term decolonization success rate compared with the other treatment group (63 of 82 [77%] versus 46 of 78 [59%]; OR 2.3; 95% CI 1.1 to 4.9; P=0.018) and the no treatment group (three of 38 [8%]; OR 37.2; 95% CI 10.1 to 209.6; P<0.000001) (Table 2). Considering patients with unknown outcomes, even assuming the extreme scenario that decolonization for all unknowns failed in the hospital protocol group but succeeded in the other two groups, the hospital protocol group was still significantly more successful than either the other treatment group (P=0.01) or no treatment group (P<0.0000001). The hospital protocol group also demonstrated superior long-term decolonization success compared with the other treatment group (33 of 62 [53%] versus (18 of 55 [33%]; OR 2.4; 95% CI 1.0 to 5.3; P=0.039) and the no treatment group (two of 32 [6%]; OR 16.2; 95% CI 3.7 to 155.5; P=0.000003). The mean ± SD observed duration of culture negativity for the subgroup who remained MRSA culture-negative over the long term (measured as time between first and last negative culture) was 419±398 days (range one to 1817 days). The actual duration of culture negativity may be much longer because repeat MRSA cultures would only be obtained if patients were rehospitalized.
TABLE 2.
Methicillin-resistant Staphylococcus aureus (MRSA) decolonization rates and patient outcomes
| Outcome | Hospital protocol, n=89 | Other treatment, n=98 | No treatment, n=54 | Hospital protocol vs other treatment OR (95% CI); P | Hospital protocol vs no treatment OR (95% CI); P |
|---|---|---|---|---|---|
| Overall decolonization, success/known (%) | 67/84 (80) | 48/89 (54) | 4/43 (9) | 3.3 (1.6–7.1); P=0.0004* | 36.9 (11.2–161.7); P<0.000001* |
| Short-term decolonization, success/known (%) | 63/82 (77) | 46/78 (59) | 3/38 (8) | 2.3 (1.1–4.9); P=0.018* | 37.2 (10.1–209.6); P<0.000001* |
| Long-term decolonization, success/known (%) | 33/62 (53) | 18/55 (33) | 2/32 (6) | 2.4 (1.0–5.3); P=0.039* | 16.2 (3.7–155.5); P=0.000003* |
| MRSA infection rate, present/known (%) | 16/89 (18) | 37/98 (38) | 5/42 (12) | 0.38 (0.18–0.78); P=0.003* | 1.66 (0.53–6.24); P=0.452* |
| Length of stay (days) | |||||
| Mean ± SD | 80±108 | 61±110 | 30±81 | P=0.001† | P<0.0000001† |
| First quartile | 22 | 8.5 | 3 | – | – |
| Median | 55 | 27 | 8 | – | – |
| Third quartile | 110 | 70 | 17.5 | – | – |
| Disposition | P=0.023* | P=0.097* | |||
| Expired | 11 (12) | 19 (19) | 9 (17) | – | – |
| Extramural (home hospital service) | 9 (10) | 11 (11) | 3 (5) | – | – |
| Home | 31 (35) | 44 (45) | 29 (54) | – | – |
| Nursing home | 14 (16) | 7 (7) | 8 (15) | – | – |
| Other hospitals | 11 (12) | 5 (5) | 2 (4) | – | – |
| Special care home | 0 (0) | 5 (5) | 2 (4) | – | – |
| Unknown | 13 (15) | 7 (7) | 1 (2) | – | – |
| 30-day mortality (from first-positive MRSA culture and sensitivity) | 3/89 (3) | 9/98 (9) | 7/54 (13) | 0.35 (0.06–1.45); P=0.139* | 0.24 (0.04–1.10); P=0.042* |
| MRSA-associated mortality, probable/definite (%) | 0/89 (0) | 4/98 (4) | 1/54 (2) | 0 (0.00–1.65); P=0.123* | 0 (0.00–23.66); P=0.378* |
Two-sided Fisher’s exact test, its generalization or χ2 test;
Two-sided Kruskal-Wallis test. vs Versus
Potential factors associated with successful decolonization were analyzed using baseline patient characteristics. Overall, use of the hospital protocol was the only significant positive predictor of successful decolonization (P<0.001) based on univariate analysis. Under the hospital protocol, there was a significant association between the primary MRSA colonization site and decolonization success (P=0.022), driven by a lower success rate when the primary colonization site was a wound. Under the hospital protocol, the only other factor that approached significance as a predictor of inability to decolonize was cancer comorbidity (P=0.082).
Decolonization was successful for 115 patients, permitting release from contact isolation for 4530 patient-days, which represented 45% of the total hospital stay.
Twenty-four per cent (58 of 241) of all culture-positive patients developed an MRSA infection during their initial or subsequent hospitalizations (Table 2). The rate of clinical infection with MRSA was significantly lower in the hospital protocol group versus the other treatment group (16 of 89 [18%] versus 37 of 98 [38%]; OR 0.38; 95% CI 0.18 to 0.78; P=0.003). Table 3 details the various types of MRSA infections for these two groups. The lowest rate of infection was noted in the no treatment group (five of 42 [12%]).
TABLE 3.
Types of methicillin-resistant Staphylococcus aureus infections
| Hospital protocol, n (%) | Other treatment, n (%) | |
|---|---|---|
| Skin and soft tissue | 9 (56.3) | 23 (62.2) |
| Pneumonia | 4 (25.0) | 5 (13.5) |
| Surgical site infection | 2 (12.5) | 4 (10.8) |
| Urinary tract infection | 1 (6.2) | 3 (8.1) |
| Bloodstream | 0 (0.0) | 2 (5.4) |
| Total | 16 (100.0) | 37 (100.0) |
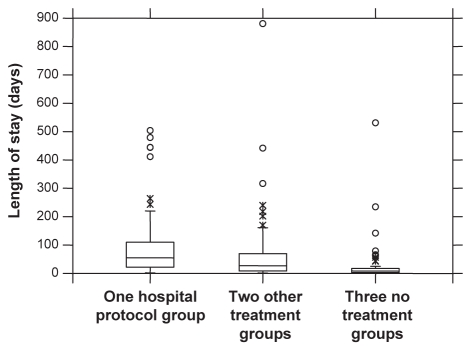
The patients treated by hospital protocol had significantly longer lengths of stay than either the other treatment group (average 80 versus 61 days; P<0.001; median 55 versus 27 days) or the no treatment group (average 80 versus 30 days; P<0.0000001; median 55 versus eight days) (Table 2, Figure 1).
Figure 1).
Box and whiskers plot of the length of stay according to treatment. In all three groups, the distribution has a long right tail (ie, short stays dominate, while some very long stays push up the average). The hospital protocol group showed longer stays followed by the other treatment group and then the no treatment group
Overall mortality was similar among the three groups (Table 2). There were 11 deaths in the hospital protocol group (10 unrelated to MRSA infection, one unlikely related), 19 deaths in the other treatment group (15 unrelated to MRSA infection, three probable and one definite) and nine in the no treatment group (seven unrelated to MRSA infection, one unlikely related, one definite). For all groups combined, the mortality rate was lower among patients successfully decolonized (12 of 105 [11.4%] versus 20 of 91 [22.0%]; OR 0.46; 95% CI 0.19 to 1.06; P=0.054). Patients in the hospital protocol group were more likely to be discharged to another hospital or long-term care facility (Table 2). Clostridium difficile infection developed in one patient in both treatment groups within four weeks of antibiotic therapy. No case of secondary antibiotic resistance was documented postdecolonization in the subgroup with persistent or recurrent MRSA colonization.
During the study, the rate of nosocomial MRSA infection at the hospital fell from one case per 1000 admissions in 2003 to 0.6 cases per 1000 admissions in 2005, and fell further to 0.3 cases per 1000 admissions in 2007.
DISCUSSION
The current retrospective study describing the experience with MRSA decolonization in hospitalized patients again supports the observation that a strategy using combined topical and oral antimicrobial therapy is superior to alternative regimens, many of which either used topical mupirocin alone or topical mupirocin in combination with only one other oral antimicrobial.
The failure of topical therapy alone is not surprising considering that the majority of patients in our study, as in other series, were colonized at multiple sites, with gastrointestinal colonization documented in more than 50% of patients. The robust efficacy of the regimen used in the present study was reflected by the high rate of short- and long-term decolonization success despite the fact that most patients had multiple risk factors for decolonization failure, including the presence of chronic wounds, multiple comorbidities and multiple indwelling devices (eg, vascular catheters, Foley catheters, tracheostomy tubes and gastrostomy tubes). Topical therapy remains an important component of a decolonization regimen because mupirocin resistance is a significant risk factor for regimen failure (18,19). We did not measure mupirocin resistance in our study population.
Many previous studies (13–18,20,21) investigating MRSA decolonization strategies have been compromised by small sample size, tendency to exclude patients with multiple indwelling catheters and limited follow-up. The present study highlights the fact that even in a relatively unselected cohort, the mean duration of successful decolonization exceeded one year. The adjunctive use of rigorous housekeeping measures at The Moncton Hospital likely contributed to decolonization success, because S aureus localized to fomites can maintain viability in excess of 38 weeks (22). One previous study (18) demonstrated that 18% of decolonization failures are the result of recolonization with new strains.
The decision to decolonize in the present study was made by the attending physician and was influenced by multiple factors. Patients with longer lengths of stay were more likely to be decolonized for several reasons. These patients were believed to be a significant persistent nidus for nosocomial MRSA transmission; their quality of care and emotional health were believed to be compromised by long-term contact isolation and, in many circumstances, nursing homes would not accept these patients unless they had been successfully decolonized. This contrasts with the group who received no decolonization treatment, which was largely comprised of patients receiving palliative care, or the relatively healthy with short-term lengths of stay – both situations in which the benefits of decolonization would be less tangible. This epidemiology is reflected by shorter lengths of stay, reduced vascular catheter use and higher mortality in this group, in which no attempt was made to decolonize.
The presence of a colonized wound was the only factor identified in the present study that was associated with failure to decolonize, a finding observed by previous investigators (12). This may reflect occult MRSA wound infection, which would be unlikely to be eradicated by a one-week course of oral therapy. Poor antibiotic penetration into wound granulation tissue and biofilm has also been demonstrated (23).
The risk of MRSA colonization must be weighed against the risk of decolonization therapy. The total cost of medications associated with our treatment protocol was estimated to be $32.00 per treatment course. This is similar to other estimates of cost across Canada and the United States (24,25). Canadian data have estimated a cost of $14,360 per patient when MRSA colonization progresses to infection (25). This total has been derived from increased hospital stay, isolation costs and management. With an estimated MRSA infection rate of 20% to 60% reported in the literature, there is a significant cost savings for decolonization therapy versus treatment of infection.
Adverse effects associated with antibiotic therapy include common side effects, allergic drug reactions, antibiotic resistance and superinfection. One of the most significant adverse events associated with MRSA decolonization is C difficile colitis. One patient in our treatment group developed C difficile infection. The average cost for one C difficile infection has been estimated at $8,000, the majority of which would be for the cost of a longer hospital stay (26). With one case of colitis in the present study, we had an absolute risk increase of 0.01 and with a number needed to harm of 1000 patients. We believe that the benefits afforded by decolonization treatment, which include discontinuation of contact isolation and reduced MRSA infection, outweigh the aforementioned drawbacks.
There are several methodological limitations in our study. Because the present study was a retrospective cohort analysis, selection bias clearly influenced the decision to decolonize, because patients who received no decolonization treatment had shorter lengths of stay and higher rates of nares colonization alone. Similar selection bias may have influenced the subsequent difference in infection rates seen between patients in the hospital protocol and those in the other treatment group. Selection bias may not fully explain the large difference in MRSA rates between the two groups. Both groups were similar in age and comorbidity burden, and after excluding all patients who were successfully decolonized, the MRSA infection rate was identical between the two groups.
Differences in the MRSA infection rates could have been due to observation bias because a significant proportion of patients were lost to follow-up. On the one hand, we may have missed infections managed at other institutions. However, we expect the numbers to be small because infected patients were likely to be readmitted to The Moncton Hospital because it is the tertiary care centre for the region. Missed infections, if any, would be more likely in the other treatment or no treatment groups because these patients tended to have shorter lengths of stay. The dependence on hospital admission to measure outcomes would also have diminished our ability to measure total duration of successful decolonization. Therefore, our study likely underestimated both the rate and duration of successful decolonization, because this could only be measured in patients with prolonged lengths of stay or frequent readmission to our institution – reflecting poorer health status.
CONCLUSION
The present study supports recent reports indicating that MRSA decolonization can be successful using a multifactorial approach (chlorhexidine soap, enhanced hygiene/housekeeping and combination oral/topical antimicrobial therapy) in hospitalized patients, both in the short and long term. Unlike previous studies, decolonization appeared to be effective in a relatively unselected population, including patients with lines and catheters. Inability to decolonize was most closely associated with failure to use a standardized decolonization protocol.
Acknowledgments
The authors thank Candace MacLoughlin, clinical research nurse for data collection; Lynn Dow; Kim Larracy; Rebecca Close and Dr Magda Kuhn for laboratory information and technical assistance.
Footnotes
FINANCIAL SUPPORT/CONFLICTS OF INTEREST: No financial support was received, and no conflicts of interest were declared.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial blood stream infections in US hospitals: Analysis of 24,179 cases from a prospective national surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Herwaldt LA. Control of methicillin-resistant Staphylococcus aureus in the hospital setting. Am J Med. 1999;106:115–85. doi: 10.1016/s0002-9343(98)00350-7. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous Report: Deaths involving MRSA: England and Wales, 2000–2004. Health Stat Q. 2006;29:63–8. [PubMed] [Google Scholar]
- 4.Simor AE, Ofner-Agostini M, Gravel D, et al. Surveillance for methicillin-resistant Staphylococcus aureus in Canadian hospitals in a report update from the Canadian Nosocomial Infection Surveillance Program. Can Commun Dis Rep. 2005;31:33–40. [PubMed] [Google Scholar]
- 5.Bisognano C, Vaudaux PE, Rohner P, Lew DP, Hooper DC. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1428–37. doi: 10.1128/aac.44.6.1428-1437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 1994;19:1123–8. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 7.Huang S, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–5. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 8.Datta R, Huang S. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis. 2008;47:176–81. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbarth S. Control of endemic methicillin-resistant Staphylococcus aureus – recent advances and future challenges. Clin Microbiol Infect. 2006;12:1154–62. doi: 10.1111/j.1469-0691.2006.01572.x. [DOI] [PubMed] [Google Scholar]
- 10.Boyce JM, Jackson MM, Pugliese G, et al. Methicillin-resistant Staphylococcus aureus (MRSA): A briefing for acute care hospitals and nursing facilities. Infect Control Hosp Epidemiol. 1994;15:105–15. doi: 10.1086/646870. [DOI] [PubMed] [Google Scholar]
- 11.Vriens M, Blok H, Gigengack-Baars A, Mascini E, van der Werken C, Verhoef J, Troelstra A. Methicillin-resistant Staphylococcus aureus carriage among patients after hospital discharge. Infect Control Hosp Epidemiol. 2005;26:629–33. doi: 10.1086/502592. [DOI] [PubMed] [Google Scholar]
- 12.Boyce JM. MRSA patients: Proven methods to treat colonization and infection. J Hosp Infect. 2001;48(Suppl A):S9–14. doi: 10.1016/s0195-6701(01)90005-2. [DOI] [PubMed] [Google Scholar]
- 13.Loeb M, Main C, Walker-Dilks C, Early A. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340. doi: 10.1002/14651858.CD003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung S, Louie M, Simor A. Combined topical and oral antimicrobial therapy for the eradication of methicillin-resistant Staphylococcus aureus (MRSA) colonization in hospitalized patients. Can J Infect Dis. 2002;13:287–92. doi: 10.1155/2002/567090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallen AJ, Wilson CT, Larson RJ. Perioperative intranasal mupirocin for the prevention of surgical site infections: Systematic review of the literature and meta-analysis. Infect Control Hosp Epidemiol. 2005;26:916–22. doi: 10.1086/505453. [DOI] [PubMed] [Google Scholar]
- 16.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM, et al. Surgical site infections in orthopedic surgery: The effect of mupirocin nasal ointment in a double-blind randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353–8. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 17.Wertheim HF, Vos MC, Ott A, et al. Mupirocin prophylaxis against nosocomial Staphylococcus aureus infections in nonsurgical patients. A randomized study. Ann Intern Med. 2004;140:419–25. doi: 10.7326/0003-4819-140-6-200403160-00007. [DOI] [PubMed] [Google Scholar]
- 18.Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178–85. doi: 10.1086/510392. [DOI] [PubMed] [Google Scholar]
- 19.Cookson BD. The emergence of mupirocin resistance: A challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11–8. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 20.Tomic V, Svetina Sorli P, Trinkaus D, Sorli J, Widmer AF, Trampuz A. Comprehensive strategy to prevent nosocomial spread of methicillin-resistant Staphylococcus aureus in a highly endemic setting. Arch Intern Med. 2004;164:2038–43. doi: 10.1001/archinte.164.18.2038. [DOI] [PubMed] [Google Scholar]
- 21.Darouiche R, Wright C, Hamill R, Koza M, Lewis D, Markowski J. Eradication of colonization by methicillin-resistant Staphylococcus aureus by using oral minocycline-rifampin and topical mupirocin. Antimicrob Agents Chemother. 1991;35:1612–5. doi: 10.1128/aac.35.8.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietze B, Rath A, Wendt C, Martiny H. Survival of MRSA on sterile goods packaging. J Hosp Infect. 2001;49:255–61. doi: 10.1053/jhin.2001.1094. [DOI] [PubMed] [Google Scholar]
- 23.Ryan DM. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J Antimicrob Chemother. 1993;31:1–16. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- 24.Shorr A, Tabak Y, Gupta V, Johannes R, Liu L, Kollef M. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit Care. 2006;10:97. doi: 10.1186/cc4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim T, Oh PI, Simor AE. The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals. Infect Control Hosp Epidemiol. 2000;22:99–104. doi: 10.1086/501871. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox MH, Cunniffe JG, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect. 1996;34:23–30. doi: 10.1016/s0195-6701(96)90122-x. [DOI] [PubMed] [Google Scholar]